Abstract
Purpose
We asked if retinal image quality is maximum during accommodation, or sub-optimal due to accommodative error, when subjects perform an acuity task.
Methods
Subjects viewed a monochromatic (552nm), high-contrast letter target placed at various viewing distances. Wavefront aberrations of the accommodating eye were measured near the endpoint of an acuity staircase paradigm. Refractive state, defined as the optimum target vergence for maximising retinal image quality, was computed by through-focus wavefront analysis to find the power of the virtual correcting lens that maximizes visual Strehl ratio.
Results
Despite changes in ocular aberrations and pupil size during binocular viewing, retinal image quality and visual acuity typically remain high for all target vergences. When accommodative errors lead to sub-optimal retinal image quality, acuity and measured image quality both decline. However, the effect of accommodation errors of on visual acuity are mitigated by pupillary constriction associated with accommodation and binocular convergence and also to binocular summation of dissimilar retinal image blur. Under monocular viewing conditions some subjects displayed significant accommodative lag that reduced visual performance, an effect that was exacerbated by pharmacological dilation of the pupil.
Conclusions
Spurious measurement of accommodative error can be avoided when the image quality metric used to determine refractive state is compatible with the focusing criteria used by the visual system to control accommodation. Real focusing errors of the accommodating eye do not necessarily produce a reliably measurable loss of image quality or clinically significant loss of visual performance, probably because of increased depth-of-focus due to pupil constriction. When retinal image quality is close to maximum achievable (given the eye’s higher-order aberrations), acuity is also near maximum. A combination of accommodative lag, reduced image quality, and reduced visual function may be a useful sign for diagnosing functionally-significant accommodative errors indicating the need for therapeutic intervention.
Keywords: accommodation, image quality, aberrations, refractive state
Introduction
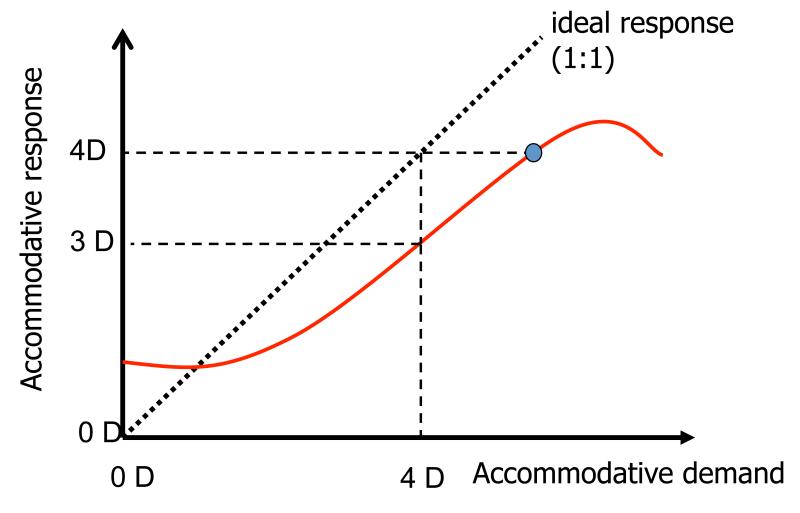
According to classical models of accommodation1, 2, the human eye typically accommodates too much for distant targets (“accommodative lead”) and too little for near targets (“accommodative lag”). Between these two extremes, accommodative response is proportional to stimulus vergence but the constant of proportionality is generally less than one (Fig. 1). This classical model implies that accommodative errors are the rule rather than the exception. Experimental evidence of reduced visual acuity at near and distance that is correlated with accommodative lag and lead3, but eliminated when the accommodative lags are optically corrected4, clearly support the classic model of accommodation. However, the story seems more complicated because other studies report examples of little or no decline in visual acuity with reduced viewing distance5 in subjects with significant accommodative lags6.
Figure 1.
Classic accommodative response curve in which accommodative response (refractive state of relaxed eye – refractive state of accommodated eye) in dioptres is plotted as a function of accommodative demand (refractive state of relaxed eye – target vergence) in dioptres. For this example an accommodative demand of 4D elicits an accommodative response of 3D, indicating a lag (demand-response) of 1 D. Although this eye has the ability to accommodate 4D (small circle), lag persists even though improved focusing would improve retinal image quality by reducing defocus blur.
Several contemporary studies have challenged the classical model of static accommodation by presenting evidence that a stimulus-response slope other than unity does not necessarily imply a focusing error that degrades image quality7-9. Instead, these studies suggest that part of the measured leads and lags are spurious results produced when the method used to determine the eye’s refractive state is different from the method used by the visual system to control accommodation10, 11. For example, in the experiment of Buehren & Collins7, most of the observed errors of accommodation could be explained by assuming subjects accurately focused their eyes using paraxial rays, whereas the minimum RMS refraction method focuses a more marginal location in the pupil. Distinguishing between real and false accommodative errors is further complicated by the fact that, in aberrated eyes, accommodative demand varies with the visual stimulus. For example, optimal focus for high spatial frequencies is achieved by focusing a near-paraxial region of the pupil11-14, whereas optical focus for a low spatial frequency targets or high contrast point of light requires focus of the more marginal optics10, 11, 15.
The literature contains clear evidence that some individuals fail to focus the retinal image optimally even when the eye is capable of accommodating sufficiently8,9,8, 9. Although these data support the idea that retinal image quality can be sub-optimal in the accommodating eye, several aspects of these data indicate that subjects may be employing the eye’s depth of focus (DoF) to achieve adequate image quality with minimum accommodative effort. Thus the increase in accommodative lag associated with increased accommodation (Fig. 1) may reflect the increased DoF associated with accommodative pupil miosis1. Also, accommodation leads and lags can increase dramatically for stimuli with large DoF (e.g. low spatial frequencies, blurred targets16-18. Thus it is possible that some part of the accommodative lag is real, but of no consequence for performing a visual task.
The goal of our study was to develop experimental methods that could reveal functionally significant losses of retinal image quality due to real accommodative error. Most of our subjects demonstrated normal acuity (logMAR ≤ 0.0, Snellen better than 6/6 or 20/20) over a range of target vergences, which indicates relatively high levels of image quality when performing an acuity task. However, some subjects demonstrated reduced acuity and sub-optimal image quality associated with functionally significant levels of accommodative error. These effects of accommodation error were partially mitigated by pupillary constriction associated with accommodation and binocular convergence.
Methods
Apparatus and procedure
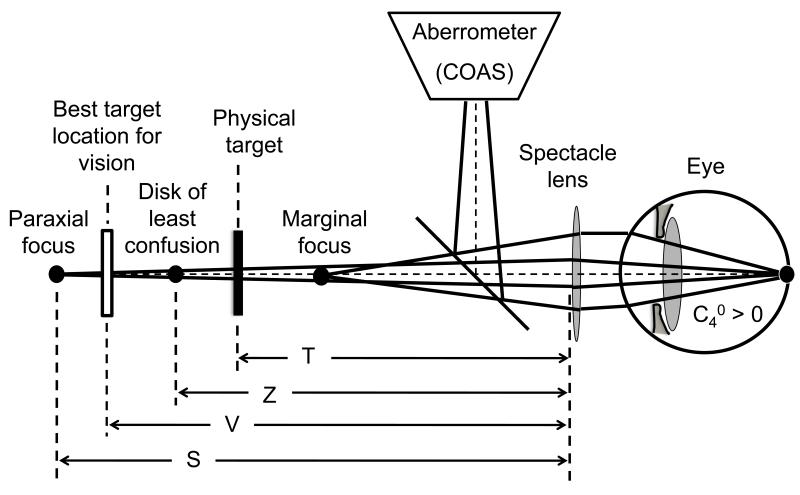
Wavefront aberrations were measured for the right eye of 10 young adults (ages 29.6±7.7 years) while the subject viewed letters displayed on a micro computer display [LiteEye by eMagin, www.emagin.com] with 852×600 monochrome square pixels (15 micron pixel width). The display was mounted on an optical rail that allowed positioning of the stimulus at selected viewing distances ranging from 2m to 22cm from spectacle lens to target (Fig.2). Target vergence (TV) is the negative inverse of this viewing distance. Individual pixels in the microdisplay subtended 0.068 milliradian = 0.23 arcmin when viewed at 22.2 cm. Thus the 1 arcmin gap in a 0.0 logMAR (Snellen 6/6 or 20/20) tumbling-C letter was represented by at least 4 pixels at all viewing distances. A narrow-band interference filter (552nm centre wavelength) placed immediately in front of the display made the visual stimulus appear as black letters on a green background of luminance 35.5 cd/m2. The bandwidth of the interference filter (typically less than 30nm) was not a critical parameter since visual acuity is known to be little affected by ocular chromatic aberration19. Our main reason for selecting 552nm was to maximize target luminance when viewed through the narrow-band interference filter. Although source luminance is independent of viewing distance for an extended diffuse reflector or emitter, retinal illuminance will vary as pupil diameter changes in response to changes in viewing distance. Thus, retinal illuminance was not constant in our experiment, but it varied in the same naturalistic way as would be expected when viewing the same object in daily life.
Figure 2.
Schematic of the experimental apparatus and the location of several reference planes, all of which are conjugate to the retina according to some metric of image quality.
An initial clinical refraction was used to prescribe spectacle lenses to correct the relaxed eye’s sphero-cylindrical refractive error. An additional +1 D was added to the prescription so that the corrected eye had a nominal far point 1m from the spectacle lens. This arrangement allowed placement of an optically-distant visual-target at 2m, which is 0.5D beyond the nominal far point. Viewing distances closer than the near point were not tested. For each target vergence, visual acuity was measured three times in a randomised sequence. Acuity was measured with the Freiburg forced choice staircase procedure for a Landolt-C target with 8 possible orientations20. Aberrometry measurements of the corrected eye were obtained near the end of the staircase when stimulus letters close to the subject’s acuity limit were being identified correctly by the subject.
A wavefront aberrometer (COAS, www.wavefrontsciences.com) measured the aberrations of the corrected eye for 840 nm radiation reflected into the eye by a hot-mirror serving as a beam splitter. The wavefront sensor in the aberrometer was made optically conjugate to the spectacle plane by an internal telescopic relay that was included in the instrument’s calibration. Wavefront measurements were adjusted to 552nm using a model of ocular chromatic aberration that included second-order and higher-order chromatic aberrations21, 22. Thus all measurements of wavefront error refer to the eye+spectacle system for the wavelength of light used to measure visual performance. No correction was made for small myopic shifts (0.25 D on average) that can occur if infrared radiation penetrates deeper into the fundus compared to visible light23. As illustrated in Fig. 2, for an eye with positive spherical aberration, the retinal conjugate plane for paraxial rays is distal to the conjugate plane for marginal rays and the conjugate plane for the disk of least confusion lies between these limits. Accommodative errors are indicated when the optimum target location for maximising visual performance and image quality does not coincide with the physical target.
Letter acuity and static aberrometry were measured for stimulus vergences ranging from +0.5 D to −4.5 D in steps of 0.5 D, each presented in a random sequence. The optical bench was positioned such that targets moved along the right eye’s primary line of sight for all distances, so no rotation of the right eye was required as the object’s viewing distance changed and thus no repositioning of the aberrometer was required. To avoid interference by the aberrometer’s probe beam on acuity measurements, the aberrometer measurement axis was rotated approximately 1 degree downward from the line-of-sight. For monocular viewing experiments the left eye was occluded, but for binocular viewing experiments the left eye viewed the stimulus through correcting lenses prescribed from the initial clinical exam. A control experiment designed to reduce the effect of pupillary constriction on acuity measurements was performed by instilling one or two drops of 1% phenylephrine in the measured eye.
Data Analysis
A schematic drawing of the experimental apparatus and the location of several reference planes is shown in Fig. 2. .The stimulus to accommodation provided by a visual target located at viewing distance T causes the eye to refocus, thereby changing its refractive state. We measure refractive state by the vergence of by adopting an appropriate refractive state. Conceptually, the retinal conjugate point in object space (i.e. the image of the entrance apertures of cone photoreceptors). An implicit assumption of wavefront aberrometry is that measurement light from the probe beam is reflected from a plane very close to the cone apertures. Under this assumption, the retinal conjugate point may be envisioned as the point in object space where the image of the retinal beacon produced by light reflected from the fundus is optimally focused. In a system free of higher-order aberrations, this focus point is unambiguously specified as the axial location where the image has maximum quality. However, ocular aberrations render the exact location of the focus point ambiguous because image quality is ambiguous. Aberrations cause different metrics of image quality to be maximised at different axial locations so the retinal conjugate is not uniquely located. Choosing an appropriate metric of image quality for locating the retinal conjugate is therefore a critical step in our experimental design. We selected the metric called visual Strehl ratio because it is known to make unbiased predictions of optimum focusing of letters near the acuity limit24. This choice of metric is further supported by evidence that visual Strehl ratio is monotonically related to visual acuity over a large range of aberration magnitude in normal25, 26 and abnormal eyes27, 28. According to these studies, a 0.22 change in log visual Strehl ratio corresponds on average to a clinically significant change (0.1 logMAR = 1 line on a letter chart) in visual acuity. Visual Strehl ratio can be computed several ways in the spatial and frequency domains29. In this study we used the metric VSMTF (visual Strehl ratio computed in the spatial frequency domain) because it provided smoother through-focus curves for which a global maximum was more evident.
Measurement of refractive state based on retinal image quality is contrasted with three conventional measures of refractive state based on geometrical optics (Fig. 2). Paraxial rays focus at distance S, marginal rays focus at distance M, and a disk of least confusion associated with minimum RMS wavefront error is focused at distance Z. In this report, we refer to the vergence 1/S for paraxial rays as the Seidel refractive state, 1/M as the marginal refractive state and 1/Z as the Zernike refractive state. By standard sign conventions, all three of these vergences are negative for real far points and, moreover, they may be interpreted as the power of an additional correcting lens needed to make the system emmetropic according to each respective focusing method. These measures of refractive state may be calculated directly from Zernike coefficients Cnm for a pupil of radius R using the following equations30:
| [1] |
| [2] |
| [3] |
A fourth measure of refractive state illustrated in Fig. 2 is 1/V, where V is the viewing distance that maximizes the image quality metric VSMTF. We anticipate that the actual target distance T might be different from all four of the focal distances illustrated. However, we argue that when visual acuity is maximum, retinal image quality for the visual target must also be maximum because maximum acuity cannot be achieved with sub-optimal image quality31. Therefore we also anticipate that when visual acuity is maximised, VSMTF (and similarly unbiased metrics) is maximised by stimuli in the target plane, and therefore V and T are equal.
To determine absolute refractive state using the VSMTF metric from measured Zernike coefficients for a given state of accommodation, computer software optimised the defocus coefficient C20 needed to maximize VSMTF for an image screen at infinity. This optimization process used standard Fourier optics29 to compute the point spread function (PSF) and optical transfer function (OTF) at infinity, from which VSMTF was computed by weighting with published neural contrast sensitivity function32 and integrating the result over spatial frequency29. Target vergence in dioptres computed from the value of C20 that optimised VSMTF was our measure of absolute refractive state of the eye+spectacle for pupil radius R.
VSMTF is a normalised measure of image quality defined as the volume under the visually-weighted modulation transfer function (MTF) for an aberrated eye divided by the corresponding volume for an optically perfect eye. When pupil diameter varies during an experiment, VSMTF becomes difficult to interpret because the normalization factor varies with pupil diameter. To make VSMTF a useful measure for comparing retinal image quality even when pupil size varies, we modified the definition by normalising by the diffraction-limited volume for a fixed pupil size of 3mm, which is essentially the same as for all larger pupils. This re-normalization (designated VSMTF*) has no impact on the measurement of refractive state yet retains the advantage of a unitless measure that varies between 0 and 1 for assessing image quality in the accommodating eye.
Results
The goal of our study was to test two competing models of static accommodation that make opposite predictions about retinal image quality in the accommodating eye. To determine if image quality is sub-optimal, we conducted two experimental tests. First, we measured retinal image quality at each state of accommodation and used post-hoc optical analysis to determine if image quality could have been improved by refocusing the target’s retinal image. If refocusing doesn’t help, then we will conclude accommodation is accurate and image quality is optimum for that target vergence. Second, we measured visual acuity simultaneously with aberrometry to determine if acuity suffers during accommodation. If accommodative lag increases significantly as targets come closer, as predicted by the classical model, then a loss of visual acuity should presumably be evident.
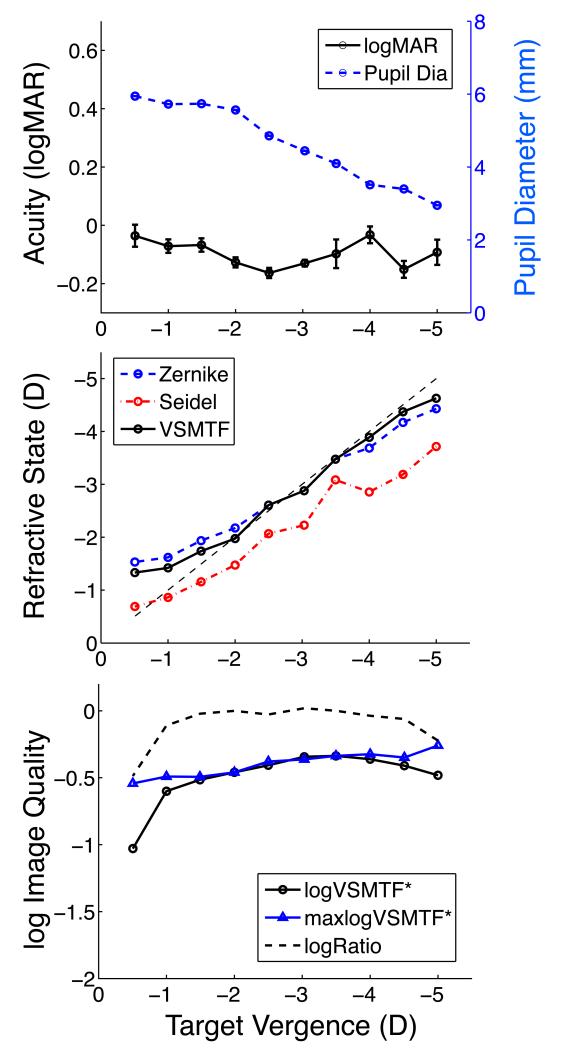
Experimental measurements obtained from observer JM who maintained accurate accommodation over a large range of target vergences under binocular viewing conditions are shown in Fig. 3. Visual acuity was normal (logMAR < 0.0, Snellen better than 6/6 or 20/20) for all target vergences tested while pupil diameter decreased twofold as a result of accommodation and convergence due to binocular fixation (top panel). Refractive state that maximizes VSMTF for each target vergence was nearly optimal over most of the target range, becoming slightly overpowered (lead) for the most distant targets and slightly underpowered (lag) for the closest targets (middle panel). Larger accommodative leads and lags were reported by the minRMS (Zernike) and paraxial (Seidel) measures of refractive state, but we judged these accommodative errors to be spurious when acuity was optimum. VSMTF was stable over most of the vergence range (bottom panel) and computational refocusing only improved VSMTF significantly at the ends of this range. The VSMTF ratio (actual / computationally refocused) was approximately 1 for all states of accommodation, which implies accommodative errors were negligible. These results show that neither retinal image quality nor visual performance suffered as a result of accommodation over a 3.5 D range of target vergences when this subject viewed the target binocularly.
Figure 3.
Example of accurate accommodation and normal acuity for observer JM. Upper panel shows variation of binocular visual acuity (black line, left ordinate, symbol = mean, error bar = +/− 1 standard error of the mean) and pupil diameter (blue line, right ordinate) with target vergence. Large values of negative target vergence correspond to a near target. Middle panel shows absolute refractive state of the accommodating eye + spectacle system computed three ways as a function of target vergence. Dashed line indicates ideal focusing (optimum target vergence matches the physical target vergence). Lower panel shows absolute image quality (VSMTF*) for the measured wavefront (circles) relative to the maximum possible value (triangles) achieved by optimum focusing of the wavefront. Dashed curve is the ratio of VSMTF* to maximum VSMTF*. Ordinate for the bottom panel is logarithmic.
A counter example of accommodative performance that resembles the classical model of increasing lag errors as the object approaches is shown in Fig. 4. Interestingly, these results were obtained from the same subject as in Fig. 3 but under different viewing conditions. In this experiment, viewing was monocular and pupillary constriction was retarded by the application of phenylephrine. The effect of these changes in test conditions was to reduce accommodative accuracy and concomitantly reduce visual acuity. Refractive state was optimal only for targets from 2/3 to 1 m, and the eye became increasingly underpowered (lag) and image quality declined for closer targets. Accommodative errors for the VSMTF refractions were substantial (>1D) for near targets (TV ≤ −3.5D). The VSMTF ratio was approximately 1 for more distant targets (−1, −1.5 D) but declined by up to 2 orders of magnitude for near targets, which confirms the visual effect of substantial accommodative errors. A comparison of these results with those in Fig. 3 suggests that accommodation of the measured eye under binocular viewing conditions was more accurate than under monocular conditions for this subject. Moreover, with increased pupil miosis due to convergence, the small accommodative errors for binocular viewing failed to significantly degrade image quality or binocular visual acuity. This mitigating effect of pupil constriction on image quality and acuity for near targets is absent in the control experiment employing pharmacological dilation (Fig. 3) and to a lesser extent without dilation in some subjects.
Figure 4.
Example of inaccurate accommodation (lag) and reduced acuity as the visual target approaches the eye. Graphical format and conventions are the same as in Fig. 3. Monocular viewing with phenylephrine by same subject (JM) as in Fig. 3.
Accommodative behaviour for other subjects in the test population under our three experimental protocols (binocular, monocular, monocular with pupil dilation) ranged between the two previous examples of accurate accommodation (Fig. 3) and increasing accommodative error (Fig. 4). In summarising the major trends in the results our aim was to reveal the causal train of events from (1) changes in target vergence to (2) accommodative errors to (3) degradation of retinal image quality to (4) loss of visual acuity. Step #1 in this train can be observed in graphs relating target vergence and refractive state obtained by metric VSMTF, like those in the middle panel of Figs 3 and 4 showing the amount and sign of accommodative error for each target vergence. To reveal the remaining steps we constructed a 4-quadrant plot of the kind shown in Fig. 5. Each subject contributed 10 points (one for each target vergence) to each quadrant graph. Quadrant Q4 shows how accommodative errors affect retinal image quality as specified by metric VSMTF*. Quadrant Q1 shows how retinal image quality affects visual acuity. Combining Q1 and Q4 graphically eliminates the intermediate step of retinal image quality to reveal the functional relationship between accommodative error and visual acuity in quadrant Q2. Quadrant Q3 serves to link the common axis shared by Q2 and Q4. Thus, each data point in Q4 has a corresponding point in Q1 and Q2 as illustrated by two examples (the red and blue boxes) showing how a given level of accommodative error for one subject and one viewing distance reduces image quality and compromises visual acuity.
Figure 5.
Summary of the effect of accommodative error on retinal image quality and visual acuity during binocular viewing by all subjects. Each symbol in each quadrant represents the results for one viewing distance by one subject. Blue symbols indicated accommodative lead (error > 0) and red symbols indicate accommodative lag (error < 0). The blue box connects corresponding points in all 4 quadrants for which accommodative lead was greatest and the red box connects corresponding points when accommodative lag was greatest.
The effect of accommodative error on retinal image quality and visual acuity when subjects viewed the target binocularly is summarised in Fig. 5. Accommodative leads (blue symbols) of up to +1 D occurred, indicating the target was located beyond the eye’s far-point. Accommodative lags (red symbols) of up to −1.5 D occurred, mainly for near targets (but not closer than the eye’s near-point). Positive and negative accommodative errors reduced retinal image quality (Q4) associated with losses of visual acuity (Q1). As expected, the general trend was for reduced image quality to produce increased logMAR (Q2) but the large amount of scatter in the data suggests the presence of other factors affecting acuity besides the magnitude of focusing error. One such factor is the sign of accommodative error, which (according to Q2) produced a greater loss of acuity for accommodative lead than for lag. Investigation of two possible explanations for this striking asymmetry in the visual effects of accommodative leads and lags are described below.
Although large amounts of accommodative lag in the measured eye led to correspondingly large losses of image quality (Fig 5, Q4), it is possible that refractive state was slightly different in the fellow eye33. Therefore, the fellow eye (or binocular summation of both eyes) may have contributed to measured acuity34 enabling maintenance of good performance (Q2) in spite of accommodative error in the measured eye. If this explanation is correct then repeating the experiment under monocular viewing conditions should eliminate the potentially confounding effects of binocular summation. As predicted, Fig. 6 shows that monocular viewing yielded fewer examples of large accommodative lag associated with low logMAR values. In this experiment the association between image quality and monocular visual acuity (Q1) is clearer with less scatter in the data, which again suggests that binocular summation may have allowed subjects to achieve good acuity during binocular viewing even when the measured eye suffered from large amounts of accommodative lag (Fig 5). Nevertheless, asymmetry in Q2 persisted, which suggests other factors remain unaccounted.
Figure 6.
Summary of the effect of accommodative error on retinal image quality and visual acuity during monocular viewing by all subjects. Graphical conventions are the same as in Fig. 5.
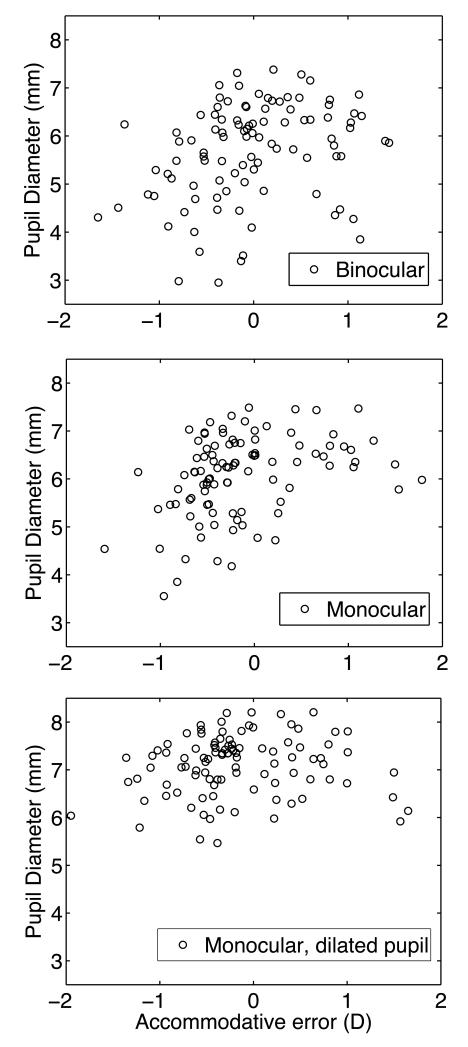
Another possible explanation for the asymmetric effect of the sign of accommodative error on acuity is that lag typically occurred for near targets, for which the eye’s pupil is relatively small as shown for a typical subject in Fig. 3. Thus pupil tended to be smaller for lags (negative accommodation errors) as shown for the population of subjects in Fig. 7. Greater pupil constriction would mitigate optical blur caused by accommodative error and other optical aberrations35, 36 thereby allowing the subject to achieve better acuity. This explanation is consistent with the observation that good acuity (logMAR < 0.1, Snellen better than 6/7.5 or 20/25) was frequently measured during accommodative lag (when pupils are smaller) but on only one occasion during accommodative lead (Fig. 6, Q2). If pupil constriction is an important factor mitigating the effects of accommodative lag, then pharmacological dilation of the pupil should reduce or eliminate this asymmetric effect of positive and negative accommodative error on acuity. Inspection of Fig. 8 confirms these predictions. Image quality changed by about the same amount for equal amounts of accommodative lead and lag (Q4), which produced about the same increase in logMAR (Q1). The overall result was less asymmetric in the effect of accommodative error on monocular visual acuity (Q2), but we should not expect total symmetry since pupil dilation with phenylephrine was incomplete (Fig. 7).
Figure 7.
Association between accommodative error and pupil diameter. Upper panel is for binocular viewing with normal pupil constriction. Middle panel is for monocular viewing with normal pupil constriction. Lower panel is for monocular viewing with pharmacologically dilated pupil. Each symbol represents the mean pupil diameter for 3 repetitions of one viewing distance by one subject.
Figure 8.
Summary of the effect of accommodative error on retinal image quality and visual acuity during monocular viewing with pupils dilated pharmacologically. Graphical conventions are the same as in Fig. 5.
Discussion
The goal of our study was to develop experimental methods that could distinguish between real and false errors of accommodation, and to distinguish accommodative errors that degrade visual acuity from those that are small enough to lie within the eye’s DoF. The classical model of accommodation predicts sub-optimal optical quality and sub-optimal vision at most target distances due to real accommodative leads and lags. An alternative model predicts that some apparent accommodative errors are spurious, and that optimal or near optimal (within a DoF) image quality and vision is maintained at all viewing distances between the far point and near point7, 37. We hypothesised that if measured accommodative errors are visually significant, then retinal image quality and visual acuity should both suffer. Our results confirm that when accommodation is insufficient to optimize retinal image quality, visual acuity declines. This decline in image quality and the associated loss of visual acuity are more evident for accommodative lead than for lag probably because of the mitigating effects of pupillary constriction associated with increased accommodation and binocular convergence35, 36. It is also possible that interocular differences in optical aberrations (including defocus) reduced the effects of accommodative errors on binocular acuity, which is a similar phenomenon to that observed with monovision corrections34. In support of this hypothesis, we found that monocular accommodation was slightly more accurate than binocular accommodation for our study population, with fewer examples of large values of accommodative lag (Fig. 6). However, for some subjects (e.g. Fig. 3, 4) accommodation was much more accurate binocularly, presumably due to supplemental accommodative drive due to ocular convergence.
About 60% of our visual acuity measurements achieved normal levels (logMAR of less than or close to 0.0, Snellen better than or close to 6/6 or 20/20) for most target vergences, monocularly and binocularly, which indicates relatively high levels of image quality when performing an acuity task at near. This is consistent with a model of accurate accommodation that produces sufficiently high levels of retinal image quality to support normal visual acuity throughout the normal range of accommodation. This model allows for increased tolerance to accommodative errors for near targets because of pupillary constriction and a concomitant increase in DoF38. A minority of measurements (<10%) demonstrated significant errors of accommodation (>1D) under monocular viewing conditions that were consistent with the classical model of increasingly large lags of accommodation to approaching targets.
We chose to measure visual acuity with nearly monochromatic light in order to avoid the complicating effects of chromatic aberration on the optical analysis. The disadvantage imposed by this choice is that natural light sources are typically polychromatic so our results must be extrapolated to be relevant to daily life. This extrapolation is justified by prior work showing visual acuity to be little affected by ocular chromatic aberration19.”
No attempt was made to correct for possible bias in the wavefront measurements that will occur if the fundus layer that reflects infrared light from the aberrometer’s probe beam lies posterior to the cone entrance apertures. Such bias would make the eye appear longer and therefore relatively myopic to the aberrometer. If that happened in our subject population, then it would have produced an apparent accommodative lead (i.e. an over-powered eye) when, in fact, the eye was optimally focused for the visual target and visual acuity was optimum. Our results were opposite to this prediction: the general trend was for accommodative lag even when acuity was maximum (Figs. 5,6,8).
To correct for longitudinal chromatic aberration between the aberrometry wavelength (840nm) and the vision testing wavelength (552nm) we used a theoretical model based on population means. Since individual variation of ocular chromatic aberration is reportedly small39, we do not expect a large error when using the model to correct refractive state measurements for individual eyes. Moreover, since there is no reason to expect systematic error in applying this model to the current population, the effect of individual variation in chromatic aberration should be confined to increased variance, which might explain some of the variance evident in the reported data.
Recent reports describing the effect of spherical aberration on accommodation errors have suggested a need to abandon the notion that the 1:1 accommodation curve is ideal. However, when false accommodation errors are avoided by using an appropriate measure of accommodation response, our approach shows this “gold standard” reference line need not be abandoned, provided refractive state is defined as the optimum target vergence for maximising retinal image quality10, 11, 40. Clear examples of this can be seen in the modelling results of Thibos et al 2013, and the experimental data of Collins 2001 and Beuren 20067, 37, 41. However, because DoF increases when the pupil constricts42, 43, slopes <1 may reflect less-than-optimal yet adequate image quality1. The impact of DOF on gain of accommodation is easily seen in studies where DOF is increased by either decreasing the spatial bandwidth of the stimulus16-18, 44, making the stimulus larger45, decreasing stimulus luminance4, or reducing pupil size46. The increased DOF associated with larger stimuli45 may be the explanation for overestimates of accommodative amplitude obtained with the push-up test47 because as the target is brought closer its size increases and thus its bandwidth is reduced.
We chose to represent accommodative behaviour by the change in absolute refractive state as a function of target vergence (e.g. Figs 3, 4). It is more common in the literature for both of these variables to be referenced to the far point, in which case the independent variable is called “accommodative demand” and the dependent variable is “accommodative response”. That traditional convention presupposes knowledge of the far-point, which is not always obvious or uniquely determined in an eye with higher order aberrations11, 12, 44, 48. The refractive state data of Fig. 4, for example, suggests the far-point vergence could be anywhere between −1 and −2 D depending on the metric used for measuring refractive state. The acuity data for that subject may suggest −1.5 D, but the loss of acuity for target vergence −1 D was evidently not caused by defocus blur since accommodation error was negligible. Instead the loss of acuity was probably due to increased aberrations as indicated by the lower overall image quality. The target vergence that minimizes acuity might be considered the far point, but it too is problematic because the complex change of aberrations and pupil size that occur during accommodation can lead to subtle changes in image quality with concomitant effects on visual acuity49.
Several studies have shown that maximum acuity occurs when the eye is slightly (1-3 D) accommodated4, 50-52. This phenomenon is evident in Figs.3, 4 as a slight minimum in logMAR in the central part of the target vergence range and a corresponding slight maximum in VSMTF*. This result probably reflects a combination of reduced accommodative errors, accommodative pupil miosis and a decrease in spherical aberration, which is usually minimum between 1.5 and 3 D of accommodation7, 8, 53.
The literature suggests that binocular summation will have relative little effect on acuity54 provided anisometropia is carefully eliminated for each state of accommodation. Our experimental design did not include a provision for eliminating anisometropia beyond that measured in a standard clinical exam with accommodation relaxed. Thus we felt the onus was upon us to conduct a control experiment comparing results under monocular and binocular conditions. Although these acuity differences were not large, less scatter was present in the monocular measurements (Fig. 6) compared to binocular measurements (Fig. 5), which is consistent with binocular summation improving acuity slightly for differentially blurred retinal images in the two eyes.
In conclusion, we find that classical models of increasing lag with increasing target vergence do not necessarily imply significant loss of image quality or loss of visual performance. Mitigating factors such as pupil miosis and binocular summation can reduce the impact of accommodative errors to achieve sufficient retinal image quality for observers to obtain normal levels of acuity (logMAR ≤ 0, Snellen better than 6/6 or 20/20) while accommodating. Nevertheless, despite these mitigating factors, some observers demonstrate abnormally low acuity during accommodation caused by reduced image quality produced by accommodation errors of focus and their interaction with higher order aberrations. We suggest that a combination of accommodative lag, reduced image quality, and reduced visual function may be a useful sign for diagnosing functionally-significant accommodative-insufficiency indicating the need for therapeutic intervention.
Acknowledgements
This study was partially supported by the Fundación Séneca (Region de Murcia), Spain, Grant: 15312/PI/10 and by grant R01-EY05109 from the National Eye Institute of the US National Institutes of Health. Technical support was provided by NEI Core Grant P30EY019008.
References
- 1.Charman WN. The eye in focus: accommodation and presbyopia. Clinical & experimental optometry: journal of the Australian Optometrical Association. 2008;91(3):207–25. doi: 10.1111/j.1444-0938.2008.00256.x. Epub 2008/03/14. [DOI] [PubMed] [Google Scholar]
- 2.Ciuffreda KJ. Accommodation and its anomalies. In: Cronly-Dillon J, editor. Vision and Visual Dysfunction. CRC press; London: 1991. pp. 231–79. [Google Scholar]
- 3.Subbaram MV, Bullimore MA. Visual acuity and the accuracy of the accommodative response. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians. 2002;22(4):312–8. doi: 10.1046/j.1475-1313.2002.00037.x. Epub 2002/08/07. [DOI] [PubMed] [Google Scholar]
- 4.Johnson CA. Effects of luminance and stimulus distance on accommodation and visual resolution. J Opt Soc Am. 1976;66(2):138–42. doi: 10.1364/josa.66.000138. Epub 1976/02/01. [DOI] [PubMed] [Google Scholar]
- 5.Luckiesh M, Moss F. The Variation in Visual Acuity with Fixation-Distance. J Opt Soc Am. 1941;31:594–5. [Google Scholar]
- 6.Heron G, Furby HP, Walker RJ, Lane CS, Judge OJ. Relationship between visual acuity and observation distance. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians. 1995;15(1):23–30. Epub 1995/01/01. [PubMed] [Google Scholar]
- 7.Buehren T, Collins MJ. Accommodation stimulus-response function and retinal image quality. Vision research. 2006;46(10):1633–45. doi: 10.1016/j.visres.2005.06.009. Epub 2005/07/26. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Gil N, Fernandez-Sanchez V. The change of spherical aberration during accommodation and its effect on the accommodation response. Journal of vision. 2010;10(13):12. doi: 10.1167/10.13.12. Epub 2010/11/16. [DOI] [PubMed] [Google Scholar]
- 9.Tarrant J, Roorda A, Wildsoet CF. Determining the accommodative response from wavefront aberrations. Journal of vision. 2010;10(5):4. doi: 10.1167/10.5.4. Epub 2010/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Gil N, Peixoto-de-Matos SC, Thibos LN, Gonzalez-Meijome JM. Shedding light on night myopia. Journal of vision. 2012;12(5):4. doi: 10.1167/12.5.4. Epub 2012/05/18. [DOI] [PubMed] [Google Scholar]
- 11.Xu R, Bradley A, Thibos L. Impact of Primary Spherical Aberration, Spatial Frequency and Stiles Crawford Apodization on Wavefront determined Refractive Error: Computational Study. Ophthalmic and Physiological Optics. 2012 doi: 10.1111/opo.12072. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black G, Linfoot EH. Spherical Aberration and the Information Content of Optical Images. Mathematical,Physical&Engineering Sciences. 1957;239:522–40. [Google Scholar]
- 13.Charman WN, Jennings JA, Whitefoot H. The refraction of the eye in the relation to spherical aberration and pupil size. Br J Physiol Opt. 1978;32:78–93. Epub 1978/01/01. [PubMed] [Google Scholar]
- 14.Cheng X, Bradley A, Ravikumar S, Thibos LN. Visual impact of Zernike and Seidel forms of monochromatic aberrations. Optom Vis Sci. 2010;87(5):300–12. doi: 10.1097/OPX.0b013e3181d95217. Epub 2010/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouroulis P. Aberration and image quality representation for visual optical systems. In: Mouroulis P, editor. Visual Instrumentation Optical Design and Engineering Principles. McGraw Hill; New York: 1999. [Google Scholar]
- 16.Heath GG. The influence of visual acuity on accommodative responses of the eye. American journal of optometry and archives of American Academy of Optometry. 1956;33(10):513–24. doi: 10.1097/00006324-195610000-00001. Epub 1956/10/01. [DOI] [PubMed] [Google Scholar]
- 17.Kotulak JC, Schor CM. The effects of optical vergence, contrast, and luminance on the accommodative response to spatially bandpass filtered targets. Vision research. 1987;27(10):1797–806. doi: 10.1016/0042-6989(87)90108-8. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 18.Owens DA. A comparison of accommodative responsiveness and contrast sensitivity for sinusoidal gratings. Vision research. 1980;20(2):159–67. doi: 10.1016/0042-6989(80)90158-3. Epub 1980/01/01. [DOI] [PubMed] [Google Scholar]
- 19.Bradley A. Perceptual manifestations of imperfect optics in the human eye: attempts to correct for ocular chromatic aberration. Optometry and Vision Science. 1992;69(7):515–21. doi: 10.1097/00006324-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Bach M. The Freiburg Visual Acuity test--automatic measurement of visual acuity. Optom Vis Sci. 1996;73(1):49–53. doi: 10.1097/00006324-199601000-00008. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 21.Nam J, Rubinstein J, Thibos L. Wavelength adjustment using an eye model from aberrometry data. Journal of the Optical Society of America A, Optics, image science, and vision. 2010;27(7):1561–74. doi: 10.1364/JOSAA.27.001561. Epub 2010/07/03. [DOI] [PubMed] [Google Scholar]
- 22.Thibos LN, Ye M, Zhang X, Bradley A. The chromatic eye: a new reduced-eye model of ocular chromatic aberration in humans. Applied optics. 1992;31(19):3594–600. doi: 10.1364/AO.31.003594. Epub 1992/07/01. [DOI] [PubMed] [Google Scholar]
- 23.Warren DF, Jacobs RJ, Thibos LN. Investigating the disparity between objective and subjective measures of spherical refractive error made with infrared light; Annual meeting of American Academy of Optometry; 2006. [Google Scholar]
- 24.Martin J, Vasudevan B, Himebaugh N, Bradley A, Thibos L. Unbiased estimation of refractive state of aberrated eyes. Vision research. 2011;51(17):1932–40. doi: 10.1016/j.visres.2011.07.006. Epub 2011/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng X, Bradley A, Thibos LN. Predicting subjective judgment of best focus with objective image quality metrics. J Vis. 2004;4(4):310–21. doi: 10.1167/4.4.7. Epub 2004/05/12. [DOI] [PubMed] [Google Scholar]
- 26.Ravikumar A, Sarver EJ, Applegate RA. Change in visual acuity is highly correlated with change in six image quality metrics independent of wavefront error and/or pupil diameter. Journal of vision. 2012;12(10):11. doi: 10.1167/12.10.11. Epub 2012/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Sanchez V, Ponce ME, Lara F, Montes-Mico R, Castejon-Mochon JF, Lopez-Gil N. Effect of 3rd-order aberrations on human vision. Journal of cataract and refractive surgery. 2008;34(8):1339–44. doi: 10.1016/j.jcrs.2008.04.017. Epub 2008/07/29. [DOI] [PubMed] [Google Scholar]
- 28.Pesudovs K, Coster DJ. Penetrating keratoplasty for keratoconus: the nexus between corneal wavefront aberrations and visual performance. Journal of refractive surgery. 2006;22(9):926–31. doi: 10.3928/1081-597X-20061101-18. Epub 2006/11/28. [DOI] [PubMed] [Google Scholar]
- 29.Thibos LN, Hong X, Bradley A, Applegate RA. Accuracy and precision of objective refraction from wavefront aberrations. J Vis. 2004;4(4):329–51. doi: 10.1167/4.4.9. Epub 2004/05/12. [DOI] [PubMed] [Google Scholar]
- 30.Thibos LN, Bradley A, Lopez-Gil N. Modeling the impact of spherical aberration on accommodation. Ophthalic and Physiological Optics. 2013 doi: 10.1111/opo.12047. submitted. [DOI] [PubMed] [Google Scholar]
- 31.Ravikumar S, Thibos LN, Bradley A. Calculation of retinal image quality for polychromatic light. J Opt Soc Am A Opt Image Sci Vis. 2008;25(10):2395–407. doi: 10.1364/josaa.25.002395. Epub 2008/10/03. [DOI] [PubMed] [Google Scholar]
- 32.Campbell FW, Green DG. Optical and retinal factors affecting visual resolution. J Physiol. 1965;181:576–93. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullimore MA, Jacobs RJ. Subjective and objective assessment of soft bifocal contact lens performance. Optom Vis Sci. 1993;70(6):469–75. doi: 10.1097/00006324-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Legras R, Hornain V, Monot A, Chateau N. Effect of induced anisometropia on binocular through-focus contrast sensitivity. Optom Vis Sci. 2001;78(7):503–9. doi: 10.1097/00006324-200107000-00013. Epub 2001/08/16. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Gil N, Iglesias I, Artal P. Retinal image quality in the human eye as a function of the accommodation. Vision research. 1998;38(19):2897–907. doi: 10.1016/s0042-6989(98)00086-8. Epub 1998/11/03. [DOI] [PubMed] [Google Scholar]
- 36.Radhakrishnan H, Charman WN. Age-related changes in ocular aberrations with accommodation. Journal of vision. 2007;7(7):11, 1–21. doi: 10.1167/7.7.11. Epub 2007/08/10. [DOI] [PubMed] [Google Scholar]
- 37.Thibos L, Bradley A, Lopez-GIl N. Modeling the impact of spherical aberration on accommodation. Ophthalmic and Physiological Optics. 2013 doi: 10.1111/opo.12047. submitted. [DOI] [PubMed] [Google Scholar]
- 38.Chateau N, De Brabander J, Bouchard F, Molenaar H. Infrared pupillometry in presbyopes fitted with soft contact lenses. Optom Vis Sci. 1996;73(12):733–41. doi: 10.1097/00006324-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Thibos LN, Ye M, Zhang X, Bradley A. The chromatic eye: a new reduced-eye model of ocular chromatic aberration in humans. Applied Optics. 1992;31:3594–600. doi: 10.1364/AO.31.003594. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Gil N, Fernadez-Sanchez V, Thibos L, Montes-Mico R. Objective Amplitude of Accommodation Computed from Optical Quality Metrics Applied to Wavefront Outcomes. J Optometry. 2009;2:223–34. [Google Scholar]
- 41.Collins M. The effect of monochromatic aberrations on Autoref R-1 readings. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians. 2001;21(3):217–27. doi: 10.1046/j.1475-1313.2001.00568.x. Epub 2001/06/09. [DOI] [PubMed] [Google Scholar]
- 42.Tucker J, Charman WN. The depth-of-focus of the human eye for Snellen letters. Am J Optom Physiol Opt. 1975;52:3–21. doi: 10.1097/00006324-197501000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Legge GE, Mullen KT, Woo GC, Campbell FW. Tolerance to visual defocus. J Opt Soc Am A. 1987;4(5):851–63. doi: 10.1364/josaa.4.000851. [DOI] [PubMed] [Google Scholar]
- 44.Charman WN, Tucker J. Dependence of accommodation response on the spatial frequency spectrum of the observed object. Vision research. 1977;17(1):129–39. doi: 10.1016/0042-6989(77)90211-5. Epub 1977/01/01. [DOI] [PubMed] [Google Scholar]
- 45.Atchison DA, Charman WN, Woods RL. Subjective depth-of-focus of the eye. Optom Vis Sci. 1997;74(7):511–20. doi: 10.1097/00006324-199707000-00019. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 46.Ward PA, Charman WN. Effect of pupil size on steady state accommodation. Vision research. 1985;25(9):1317–26. doi: 10.1016/0042-6989(85)90047-1. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 47.Win-Hall DM, Ostrin LA, Kasthurirangan S, Glasser A. Objective accommodation measurement with the Grand Seiko and Hartinger coincidence refractometer. Optom Vis Sci. 2007;84(9):879–87. doi: 10.1097/OPX.0b013e3181559ace. Epub 2007/09/18. [DOI] [PubMed] [Google Scholar]
- 48.Green DG, Campbell FW. Effect of focus on the visual response to a sinusoidally modulated spatial stimulus. J Opt Soc Am. 1965;55(9):1154–7. [Google Scholar]
- 49.van den Brink G. Measurements of the geometrical aberrations of the eye. Vision Research. 1962;2:233–44. [Google Scholar]
- 50.Geddes M, McLean J, McMonnies C, Woodward P. The variation of visual acuity with observation distance. Australian Journal of Optometry. 1966;49:164–9. [Google Scholar]
- 51.Otero JM, Plaza L, Rios M. Influencia del la aberracion monocromatica de apertura en la miopia nocturna. Anales de Fisica y Quimica. 1948:293–304. [Google Scholar]
- 52.Otero JM, Aguilar M. La agudeza visual mínima de los valores umbrales. Anales de Fisica y Quimica. 1950;46(A):197–202. [Google Scholar]
- 53.Plainis S, Ginis HS, Pallikaris A. The effect of ocular aberrations on steady-state errors of accommodative response. Journal of vision. 2005;5(5):466–77. doi: 10.1167/5.5.7. Epub 2005/08/16. [DOI] [PubMed] [Google Scholar]
- 54.Cagenello R, Arditi A, Halpern DL. Binocular enhancement of visual acuity. J Opt Soc Am A Opt Image Sci Vis. 1993;10(8):1841–8. doi: 10.1364/josaa.10.001841. Epub 1993/08/01. [DOI] [PubMed] [Google Scholar]