Abstract
Rationale
Acute systemic administration of salvinorin A, a naturally occurring κ-opioid receptor (KOPr) agonist, decreases locomotion and striatal dopamine (DA) overflow.
Objectives
Conventional and quantitative microdialysis techniques were used to determine whether salvinorin A infusion into the dorsal striatum (DSTR) decreases DA overflow by altering DA uptake or release. The influence of repeated salvinorin A administration on basal DA dynamics and cocaine-evoked alterations in DA overflow and locomotion was also assessed.
Materials and methods
Salvinorin A was administered via the dialysis probe (0; 20–200 nM) or via intraperitoneal (i.p.) injection (1.0 or 3.2 mg/kg per day×5 days). The effects of a challenge dose of cocaine were examined 48 h after repeated salvinorin treatment.
Results
Retrodialysis of salvinorin A produced a dose-related, KOPr antagonist reversible, decrease in DA levels. Extracellular DA levels were decreased whereas DA extraction fraction, which provides an estimate of DA uptake, was unaltered. In contrast to its acute administration, repeated salvinorin A administration did not modify dialysate DA levels. Similarly, neither basal extracellular DA levels nor DA uptake was altered. Unlike synthetic KOPr agonists, prior repeated administration of salvinorin A did not attenuate the locomotor activating effects of an acute cocaine (20 mg/kg, i.p.) challenge. However, cocaine-evoked DA overflow was enhanced.
Conclusions
These data demonstrate that acute, but not repeated, salvinorin A administration decreases mesostriatal neurotransmission and that activation of DSTR KOPr is sufficient for this effect. Differences in the interaction of salvinorin and synthetic KOPr agonists with cocaine suggest that the pharmacology of these agents may differ.
Keywords: Salvinorin A, Dopamine, κ-opioid receptor, Dorsal striatum, No net flux microdialysis, Rat
Introduction
Salvia divinorum is a member of the mint family that possesses psychoactive and hallucinogenic effects. Recent studies examining the binding and function of salvinorin A, the psychoactive component of S. divinorum, have demonstrated that this compound is a potent and selective κ-opioid receptor (KOPr) agonist (Roth et al. 2002; Sheffler and Roth 2003; Chavkin et al. 2004). Salvinorin A is the first naturally occurring KOPr agonist to be identified; however, its structure differs from that of synthetic KOPr agonists.
Recreational use of salvinorin A has increased recently as products containing salvinorin A have become widely available through a variety of sources including the internet (Hazelden 2004; Babu et al. 2005; Pavarin 2006). The increased availability and marketing of salvinorin A as a “legal” hallucinogen has led to concern about its abuse potential (Babu et al. 2005).
Microdialysis studies have shown that acute systemic administration of synthetic KOPr agonists such as U69593 and U50488H decreases dopamine overflow in the dorsal striatum (DSTR; Di Chiara and Imperato 1988; Donzanti et al. 1992; Spanagel et al. 1992). This effect has been attributed to a decrease in dopamine (DA) release and an increase in transporter-mediated DA uptake (Thompson et al. 2000). Repeated agonist administration produced no effect on basal extracellular DA concentrations or DA uptake in the dorsal striatum (Acri et al. 2001). Alterations in the behavioral and DA response to a subsequent challenge injection of cocaine are also seen.
The locomotor stimulant effects of an acute cocaine challenge are attenuated in animals with a prior history of repeated KOPr agonist administration. The cocaine-induced increase in DA levels in the DSTR is augmented (Heidbreder et al. 1998). These effects have been attributed to KOPr-mediated decreases in postsynaptic D2 receptor number and downregulation of presynaptic D2 receptors that regulate DA uptake and release (Acri et al. 2001; Izenwasser et al. 1998). Cocaine-antagonist-like effects of synthetic KOPr agonists are also observed following their acute administration. KOPr agonist pretreatment attenuates cocaine-evoked locomotor activation and the conditioning of cocaine reward (Crawford et al. 1995). Other studies have shown that acute KOPr agonist administration attenuates intravenous cocaine self-administration in rats (Glick et al. 1995; Schenk et al. 1999) and the reinstatement of cocaine seeking produced by experimenter-administered cocaine (Schenk et al. 1999, 2000). However, studies examining the interaction of salvinorin A with cocaine are lacking.
Behavioral effects of salvinorin A in humans include hallucinations and out-of-body experiences (Siebert 1994; Valdes III 1994; Hazelden 2004). Doses of 200–500 µg produce visions that last from 30 min to 1 or 2 h, while doses over 1 mg have longer-lasting effects (Siebert 1994; Valdes III 1994). Salvinorin A, like synthetic KOPr agonists, produced psychotomimesis in humans and aversive effects in experimental animals (Pfeiffer et al. 1986; Shippenberg and Herz 1987; Zhang et al. 2005). There is evidence that the aversive effects of synthetic KOPr agonists result from a decrease in DA neurotransmission in brain regions subserving incentive motivation (Shippenberg et al. 1993). Microdialysis studies have shown that acute systemic administration of salvinorin A decreases dialysate DA levels in the mouse DSTR (Zhang et al. 2005) and rat nucleus accumbens (NAC; Carlezon et al. 2006). Whether these effects reflect alterations in release and/or uptake is unknown. Such information is critical to understanding the mechanisms by which KOPr agonists modulate presynaptic DA neurotransmission and exert control over behavior.
Accordingly, the present studies examined: (1) the influence of systemic and intrastriatal salvinorin A administration on DA levels in the DSTR and (2) the influence of repeated administration of salvinorin A on basal DA dynamics and cocaine-evoked alteration in DA overflow and locomotor activity. Conventional microdialysis techniques were used to determine if perfusion of salvinorin A into the DSTR decreases DA levels in this region via a KOPr-dependent mechanism and to evaluate the effect of repeated administration of salvinorin A on cocaine-evoked DA overflow in the DSTR. Quantitative microdialysis under transient conditions (Olson and Justice 1993; Chefer et al. 2003) was conducted to monitor alterations in DA uptake and extracellular DA levels as a function of time after acute salvinorin A administration.
Materials and methods
Animals
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA, USA; weighing approximately 400 g) were housed two to three per cage for at least 1 week before use in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. They were maintained in a temperature- and humidity-controlled environment under a 12-h light–dark cycle with food and water available ad libitum. Experiments were conducted during the light cycle. All experiments were conducted in accordance with the guidelines of the National Institutes of Health–National Institute on Drug Abuse Institutional Care and Use Committee (Rockville, MD, USA).
Surgical procedures
Rats were anesthetized with Equithesin (sodium pentobarbital, chloral hydrate, and magnesium sulfate; 9.72 mg/ml; 3 ml/kg) and implanted unilaterally with a guide cannula (CMA/11; CMA/Microdialysis, Acton, MA, USA) aimed at the DSTR using standard stereotaxic techniques. Each rat was mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) with the upper incisor bar set at 3.5 mm below the interaural line. The coordinates (anterior +1.0 relative to bregma; lateral, −2.5; ventral, −3.5 from the dura surface) were based on the atlas of Paxinos and Watson (1998).
Drug treatments
For microdialysis studies assessing the effects of acute systemic administration of salvinorin A on DA overflow, rats were injected with vehicle (75% dimethyl sulfoxide (DMSO), 25% H20, 1.0 ml/kg intraperitoneal (i.p.)) and either 1.0 or 3.2 mg/kg salvinorin A (i.p.). For studies assessing the effects of DSTR salvinorin A perfusion, rats received vehicle or salvinorin A (20, 60, or 200 nM) in artificial cerebrospinal fluid (aCSF) through the probe. These concentrations were chosen based on binding data and studies examining the effects of synthetic KOPr agonists following their intracranial infusion (Spanagel et al. 1992; Roth et al. 2002; Chefer et al. 2005; Ford et al. 2006). Additional animals received the selective KOPr antagonist, nor-binaltorphimine (nor-BNI; 10 mg/kg, subcutaneous (s.c.)), or vehicle 24 h prior to salvinorin A perfusion (200 nM). The 24-h pretreatment regimen was used based on findings that nor-BNI antagonizes the µ opioid receptor in the first few hours after pretreatment (Endoh et al. 1992).
For studies assessing the effects of repeated salvinorin A administration, rats were allowed 5–7 days of recovery from surgery. They then received daily injections of salvinorin A (1.0 or 3.2 mg/kg, i.p.) or vehicle (75% DMSO, 25% H20, 1.0 ml/kg i.p.) for 5 days. The 1.0 and 3.2-mg/kg doses of salvinorin A were administered in volumes of 1.0 and 2.0 ml/kg, respectively. Microdialysis was conducted 48 h after the last injection. Salvinorin A was kindly supplied by Thomas Prisinzano (University of Iowa). Cocaine hydrochloride was supplied by the Research Technology Branch of the National Institute on Drug Abuse and prepared in sterile saline.
Microdialysis procedures
Microdialysis was conducted as described previously (Chefer and Shippenberg 2006). A CMA/11 microdialysis probe (outer membrane dimension 0.24×2-mm length) was manually inserted into the microdialysis cannula ~12 h before experiments and the rat was placed into a Plexiglas test chamber (26×26×33 cm). The microdialysis probe was flushed overnight at a flow rate of 0.6 µl/min with aCSF, containing (in millimolar): 145 NaCl, 2.8 KCl, 1.2 MgCl2, 1.2 CaCl2, 0.25 ascorbic acid (to minimize oxidation of DA), and 5.4 D-glucose, adjusted to pH 7.0 using NaOH (high-performance liquid chromatography (HPLC) grade) and filtered through a 0.22-µm filter (Bioanalytical Systems). On the day of the experiment, perfusate was replaced with fresh aCSF and samples were collected in microcentrifuge tubes every 10 min at a flow rate of 0.6 µl/min after a 60-min equilibration period.
Conventional microdialysis
After collection of six basal samples, rats were injected i.p. with vehicle. Thirty minutes later, six consecutive 10-min samples were collected. The 30-min time lag was used in this and other experiments to account for the dead volume of the dialysate tubing. Rats then received 1.0 or 3.2-mg/kg salvinorin A (i.p.), and another six samples were collected. For retrodialysis studies, four basal samples were initially collected. The aCSF was then replaced with that containing vehicle or salvinorin A (20, 60, or 200 nM). Four consecutive samples were collected. The solutions were then replaced with aCSF, and another eight samples were collected.
In studies assessing the effects of repeated salvinorin A administration (1.0 or 3.2 mg/kg, i.p.×5 days) on basal and cocaine-evoked DA dialysate levels, ten consecutive basal samples were collected. Rats were then injected with either saline or cocaine (20 mg/kg, i.p.), and another ten samples were collected.
Quantitative microdialysis
Quantitative (no net flux) microdialysis under transient conditions was used to determine the effects of intra-DSTR perfusion of salvinorin A (200 nM) on extracellular DA levels (DAext), and the DA extraction fraction (Ed) as a function of time after acute drug administration. Previous studies have shown that changes in Ed provide an estimate of changes in transporter-mediated DA uptake (Olson and Justice 1993). The use of this technique enables quantification of drug-induced changes in extracellular DA and transporter-mediated uptake. It also provides information as to drug-induced alterations in DA release. Separate groups of rats were perfused with 20- or 40-nM DA in aCSF for the entire experiment. Following collection of four consecutive dialysate samples, the aCSF-containing DA was replaced with that containing the same concentration of DA with salvinorin A. Thirty minutes later, four consecutive samples were obtained.
Quantitative (no net flux) microdialysis experiments were conducted 48 h after repeated administration of vehicle or salvinorin A (1.0 mg/kg, i.p.×5 days) to determine changes in basal DAext and Ed. The 48-h time point was chosen based on previous research investigating the effects of repeated administration of U-69593 (Heidbreder et al. 1998; Acri et al. 2001). For these experiments, the normal aCSF was replaced with one of five concentrations of DA prepared in aCSF (DA in 0, 2, 5, 10, 20 nM). Three 10-min samples were collected. The aCSF was then replaced with that containing a different DA concentration and three 10-min samples were collected. This procedure was repeated three times with three samples collected for each of the five concentrations. All samples and DA standards were frozen on dry ice and placed at −80°C until analyzed.
Assessment of locomotor activity
Locomotor activity produced by an acute challenge dose of saline or cocaine was evaluated in separate animals 48 h following the repeated administration of vehicle or salvinorin A. Experiments were conducted in the same chambers that were used for microdialysis. Locomotor activity was measured using the Tru Scan Photobeam Linc system (Coulbourn). Following a 3-h habituation period, baseline locomotor activity was measured at 10-min intervals for 100 min. Rats were then injected with saline or cocaine (20 mg/kg, i.p.) and activity was measured for another 100 min.
Chromatographic analysis of brain microdialysates
DA was determined by HPLC coupled to electrochemical detection as described previously (Chefer et al. 2003; Chefer et al. 2005). The retention time for DA was 2.8–3.3 min, and the limit of detection was <0.5 nM.
Histology
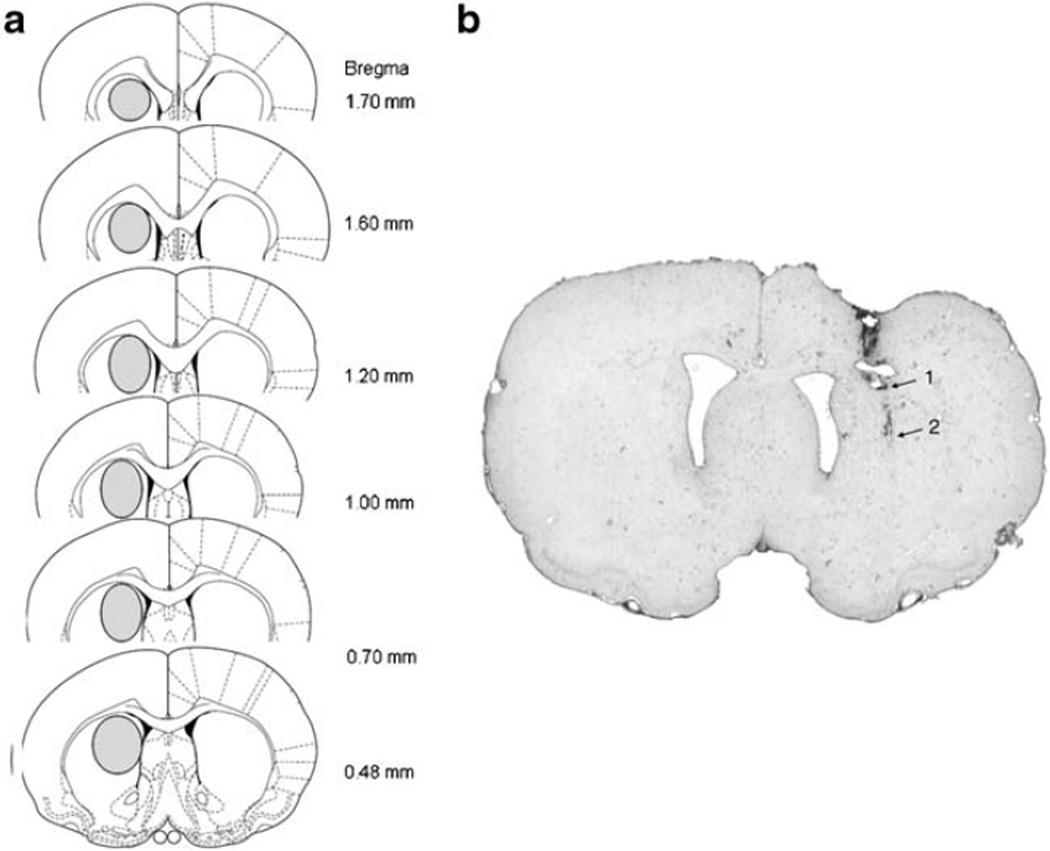
After the completion of microdialysis experiments, all animals were deeply anesthetized with Equithesin (3 ml/kg) and killed by decapitation. The brains were removed and frozen for histological analysis. Only data from animals with correct placements were used for subsequent analysis. The location of the dialysis probe in the DSTR is illustrated in Fig. 1.
Fig. 1.
Location of microdialysis probes in the DSTR. a The areas of the DSTR where the probes were located are shown with an oval in each brain section. b Photo of representative probe placement in the DSTR. Arrow 1 indicates the end of the probe shaft and arrow 2 indicates the location of the tip of the probe membrane
Data analysis
Data from conventional microdialysis experiments are expressed as a percent of baseline. The mean±the standard error of the mean (SEM) were used for analyses and are depicted in graphs. Analyses of variance (ANOVA) were conducted for conventional microdialysis and behavioral data. Subsequent F tests were conducted to compare data between groups. The accepted value of significance was p<0.05.
For quantitative microdialysis experiments under transient conditions, DAext and Ed were assessed from the linear regression equation as described previously (Olson and Justice 1993; Chefer et al. 2003). To compare the Ed and DAext for perfusion of salvinorin A to baseline samples, the mean and SEM of the four baseline samples was compared separately to the mean and SEM of each of the four salvinorin samples using unpaired t-tests. For quantitative microdialysis following repeated administration of salvinorin A, DAext and Ed were calculated as described previously (Parsons et al. 1991).
Results
Effects of acute systemic administration of salvinorin A on DA levels in the DSTR
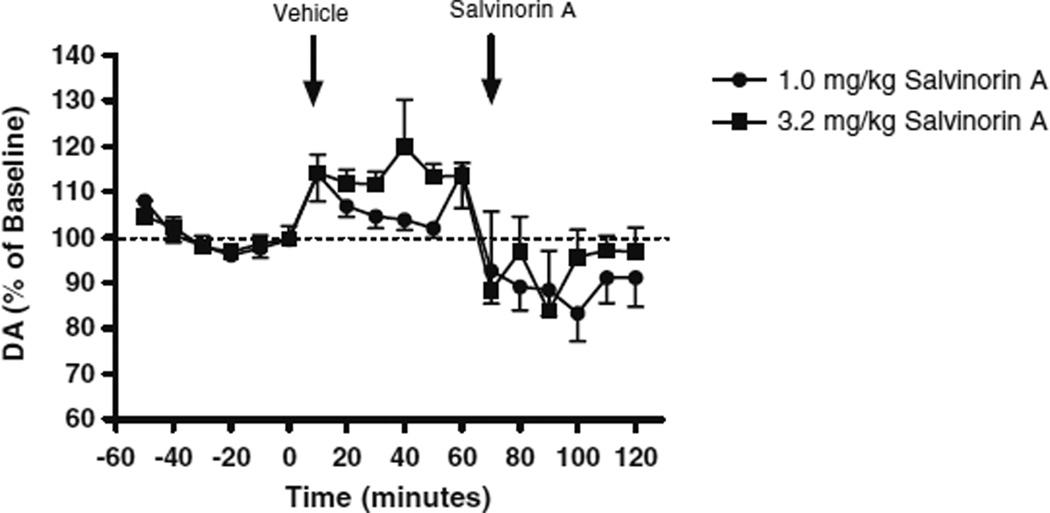
Figure 2 shows the influence of systemic administration of vehicle or salvinorin A (1.0 or 3.2 mg/kg, i.p.) on DA overflow in the DSTR (Fig. 2). A three-way repeated-measures ANOVA comparing basal and vehicle-evoked DA overflow indicated a significant increase in DA levels following vehicle administration (F(1, 18)=16.70, p=0.001). In confirmation of a previous study (Zhang et al. 2005), administration of salvinorin A (1.0 or 3.2 mg/kg) significantly decreased DA levels relative to that produced by vehicle injection (F(1, 18)=32.69, p=0.001). There was no significant difference between salvinorin A doses in the magnitude of these effects.
Fig. 2.
Effects of acute systemic administration of salvinorin A (1.0 or 3.2 mg/kg, i.p.) on DA levels in the DSTR
Effects of local perfusion of salvinorin A on DA levels in the DSTR
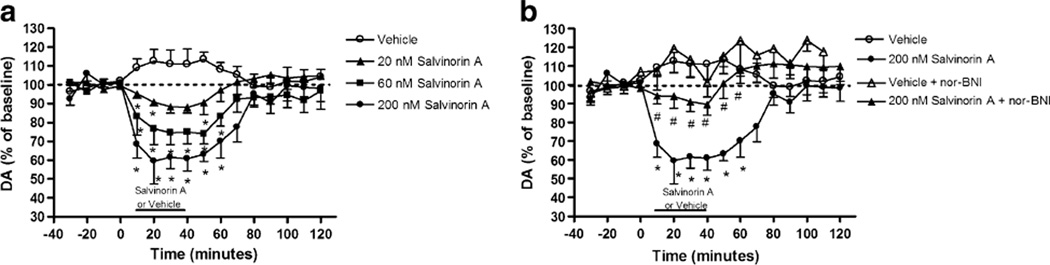
Figure 3a shows the effects of DSTR perfusion of vehicle or salvinorin A (20, 60, or 200 nM) on DA overflow as revealed by conventional microdialysis techniques. Perfusion of salvinorin A produced a concentration related decrease in DA levels as demonstrated by a significant main effect of concentration (F(3, 11)=10.11, p=0.002) and a time × concentration interaction (F(45, 165)=2.05, p=0.001). Subsequent F tests revealed that concentrations of ≥20 nM significantly decreased DA overflow. To determine whether this decrease is KOPr mediated, nor-BNI (10 mg/kg, s.c.) was administered 24 h prior to perfusion of 200-nM salvinorin A (Fig. 3b). A three-way repeated-measures ANOVA revealed a significant main effect of pretreatment (F(1, 11)=7.63, p=0.019) and significant interactions for time × pretreatment (F(15, 165)= 2.41, p=0.004) and time × pretreatment × concentration (vehicle or 200 nM; F(15, 165)=2.10, p=0.012), indicating that nor-BNI attenuated the salvinorin-A-evoked decrease in DA. Subsequent F tests revealed significant differences between nor-BNI- and salvinorin-A-treated rats relative to those that received vehicle prior to salvinorin A.
Fig. 3.
Effects of acute local perfusion of salvinorin A on DSTR DA levels. a Concentration curve of intra-DSTR salvinorin A; asterisk indicates significant difference from the vehicle group. b Effect of systemic administration of nor-BNI 24-hrs prior to perfusion of 200-nM salvinorin A number sign indicates significant difference from the 200-nM salvinorin A group
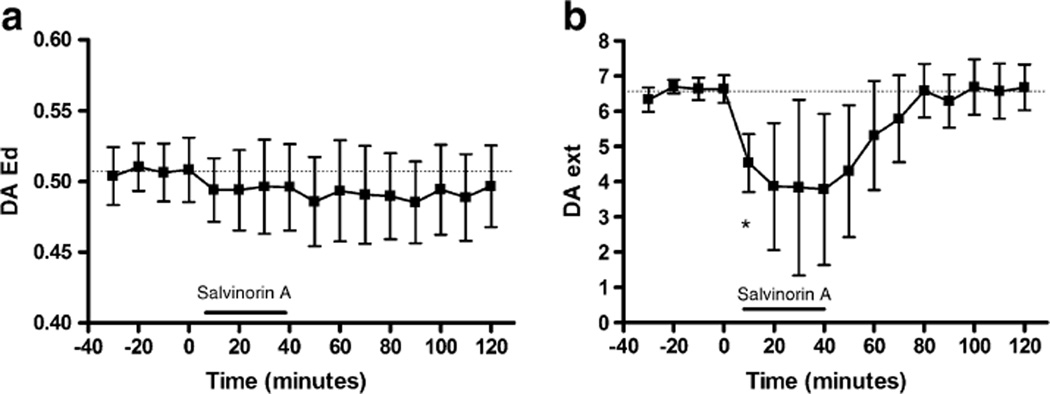
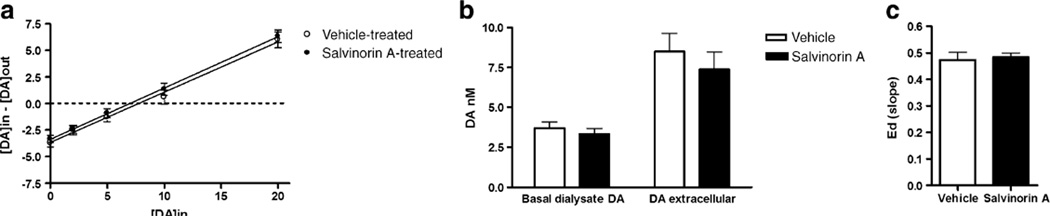
Quantitative (no net flux) microdialysis under transient conditions was conducted to determine whether the decrease in DA overflow produced by DSTR perfusion of salvinorin A was a result of changes in DA release, DA uptake, or both (Fig. 4). Salvinorin A perfusion did not modify the Ed for DA (Fig. 4a). However, a significant decrease in DAext was seen (t(22)=2.47, p=0.022; Fig. 4b). The decrease in extracellular DA levels in the absence of changes in Ed indicates that DA release is decreased in response to intra-DSTR perfusion of salvinorin A.
Fig. 4.
Influence of acute DSTR perfusion of salvinorin (200 nM) on basal DA dynamics as assessed using quantitative microdialysis under transient conditions. a Ed (slope), b DAext asterisk indicates significant difference from the mean of the baseline samples. The dotted line represents the mean of the baseline samples
Effects of repeated systemic administration of salvinorin A on DA dynamics
To determine whether repeated systemic administration of salvinorin A is associated with prolonged changes in basal DA dynamics, quantitative microdialysis was conducted 48 h after the cessation of a 5-day salvinorin A (0; 1.0 mg/kg) treatment regimen (Fig. 5). The results of the linear regression analysis are shown in Fig. 5a. Basal DA overflow was unaltered in response to repeated salvinorin A administration (Fig. 5b). Determination of DA levels during aCSF perfusion revealed no difference between vehicle (3.7±0.4 nM) and salvinorin (3.3±0.3) pretreatment. Similarly, analysis of the y-intercept of the linear regression plot (Fig. 5a), which corresponds to dialysate levels obtained in a conventional microdialysis experiment, revealed no difference between treatment groups. The lack of a difference between treatment groups indicates that DA overflow is unaltered. No difference between treatment groups in DAext (DAin–DAout=0) was seen (Fig. 5b). Similarly, there was no difference between groups in the slope of the regression line which represents the Ed (Fig. 5a). Thus, DA uptake was unaltered (Fig. 5c).
Fig. 5.
Effects of repeated systemic administration of salvinorin A (1.0 mg/kg) on basal DAext and Ed in DSTR as determined by quantitative microdialysis. Linear regression plot (a), basal dialysate and extracellular concentrations of DA (b), and Ed (slope) of linear regression (c)
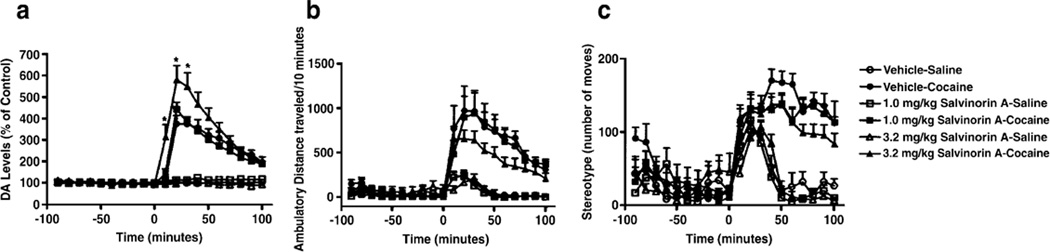
Effects of repeated systemic administration of salvinorin A on cocaine-evoked DA levels in the DSTR and locomotor activity
DA overflow in response to an acute cocaine (20 mg/kg) challenge was quantified 48 h after the cessation of repeated vehicle or salvinorin A (1.0 or 3.2 mg/kg) administration (Fig. 6a). A four-way ANOVA (pretreatment × drug (saline vs. cocaine) × drug challenge (pre vs. post cocaine) × time] revealed a significant main effect for cocaine (F(1, 34)=183.32, p=0.001), indicating that administration of cocaine significantly increased DA levels. While there was no significant main effect for pretreatment, there were multiple interactions: pretreatment × drug (F(2, 34)=5.13, p=0.011); pretreatment × drug × cocaine challenge × time (F(18, 306)=4.093, p=0.001). Subsequent F tests conducted at each time point revealed that repeated administration of 3.2 mg/kg salvinorin A significantly increased cocaine-evoked DA levels.
Fig. 6.
Effects of repeated systemic salvinorin A administration on cocaine-evoked DA levels in the DSTR (a), locomotor activity (b), stereotypy (c). Asterisk indicates significant difference from the vehicle–cocaine group
Cocaine-evoked locomotor activity was measured in parallel groups of animals (Fig. 6b). Administration of cocaine significantly increased activity as revealed by a main effect of drug (F(1, 31)=52.55, p=0.001). However, the magnitude of this effect did not differ between control animals and those with a prior history of repeated salvinorin A administration. Cocaine significantly increased stereotypy (F(1, 31)=155.88, p=0.001; Fig. 6c). There was no difference between pretreatment groups in this effect.
Discussion
The current study demonstrates that both acute systemic administration and acute intra-DSTR perfusion of salvinorin A decrease DA overflow in rat DSTR. Quantitative microdialysis revealed that intra-DSTR perfusion of salvinorin A decreased DAext levels but did not affect the Ed; an indirect measure of DA uptake. Following repeated administration of salvinorin A, DA overflow and basal DA dynamics were unaltered. The ability of cocaine to increase DA overflow in the DSTR was enhanced whereas the locomotor activating effects of cocaine were unchanged.
Consistent with a recent study in the mouse (Zhang et al. 2005), the current results demonstrate that systemic salvinorin A administration decreases dialysate DSTR DA levels in the rat. Perfusion of salvinorin A into the DSTR produced a concentration-dependent decrease in dialysate DSTR DA levels indicating that the actions of salvinorin A in this region are sufficient to modulate striatal DA overflow. Previous studies demonstrated a decrease in DA levels produced by local perfusion of synthetic KOPr agonists into either the DSTR or NAC (Donzanti et al. 1992; Spanagel et al. 1992; You et al. 1999). The decrease of DA in the DSTR produced by intrastriatal salvinorin A is most likely due to KOPr activation because pretreatment with the selective KOPr antagonist nor-BNI attenuated the salvinorin-A-evoked decrease in DA overflow. Our findings, thus, indicate that the activation of DSTR KOPr is sufficient for the inhibitory effects of salvinorin A on mesostriatal DA transmission.
Quantitative microdialysis under transient conditions was conducted to determine whether the decrease in DA overflow associated with DSTR perfusion of salvinorin reflects alterations in DA reuptake or release. Acute administration of salvinorin A decreased DAext but did not in modify Ed, indicating that salvinorin A decreases DA release but does not alter DA uptake in the DSTR. Analogous findings have been obtained with a synthetic KOPr agonist. U-69593 produced a concentration-dependent, nor-BNI-reversible, inhibition of K+-evoked [3H] DA release from rat striatal synaptosomes (Ronken et al. 1993). Decreased DA overflow has been observed in response to intra-NAC perfusion of U-69593 in mice (Chefer et al. 2005). However, acute U-69593 administration also increased DA uptake in this region (Thompson et al. 2000). Systemic administration of salvinorin A decreases dialysate DA levels of DA in the NAC (Carlezon et al. 2006). Therefore, it is possible that although salvinorin A does not alter DA uptake in the DSTR, its acute administration may affect DA uptake in the NAC.
Quantitative microdialysis revealed no alteration in DAext or Ed following repeated salvinorin A administration. These results are consistent with a previous study showing that repeated administration of U-69593 does not affect basal DAext or DA uptake in the DSTR (Acri et al. 2001). However, the findings in the DSTR differ from those showing that repeated administration of U-69593 decreases DA uptake but does not affect extracellular DA levels in the NAC (Thompson et al. 2000). Therefore, both acute and repeated administration of KOPr agonists appears to alter DA uptake in the NAC but not the DSTR. Future studies are needed to determine whether acute or repeated administration of salvinorin A would change DA uptake in the NAC.
Repeated administration of salvinorin A (3.2 mg/kg) significantly increased cocaine-evoked DA levels in the DSTR. Increases in cocaine-evoked DA levels have been reported in the NAC and DSTR following repeated U-69593 administration (Heidbreder et al. 1998). Furthermore, repeated U-69593 administration significantly increased K+-evoked DA levels in the NAC (Fuentealba et al. 2006), and chronic treatment of cultured mesencephalic DA neurons with U-69593 increased K+-evoked [3H] DA release (Ronken et al. 1994). Taken together, the present findings and those reported previously with U-69593 suggest that prolonged or repeated KOPr activation in the striatum or NAC sensitizes DA neurons to evoked release.
A possible mechanism for the enhancement of cocaine-and K+-evoked DA release by KOPr agonists could be a loss of D2 autoreceptors that regulate DA release. Repeated U-69593 pretreatment attenuated the decrease in striatal DA dialysate levels produced by acute administration of the D2 receptor agonist quinpirole and reduced the locomotor stimulant effects of quinpirole (Acri et al. 2001). Additionally, in the study where repeated administration of U-69593 significantly increased K+-evoked DA levels, quinpirole perfusion decreased basal and K+-evoked DA levels in control rats but not in rats pretreated with U-69593 (Fuentealba et al. 2006). The impairment of D2-mediated inhibition of DA release may result from a downregulation of DA D2 autoreceptors. Repeated administration of U-69593 decreased the number of DA D2 receptors in the caudate putamen by 40% but did not affect binding affinity (Izenwasser et al. 1998).
The current results indicate that repeated administration of salvinorin A increases cocaine-evoked DA release without significantly attenuating cocaine-evoked locomotor activity. These results contrast with previous research showing that repeated administration of U-69593 attenuates cocaine-evoked locomotor activity (Heidbreder et al. 1998; Collins et al. 2001). It is possible that testing of a different dose of cocaine or time point would have yielded different findings. However, the present findings suggest that, while salvinorin A produces many of the same effects as synthetic KOPr agonists, the pharmacology of salvinorin A may differ from that of synthetic KOPr agonists. Interestingly, Chavkin et al. (2004) found that salvinorin A was more potent and efficacious than either U-69593 or U-50488 in human embryonic kidney-293 cells expressing the human KOPr. Furthermore, although the potency of salvinorin A in stimulating [35S] guanosine gamma thio-phosphate (GTPγS) binding in Chinese hamster ovary cells expressing the cloned human KOPr is similar to that of U-50488, it is approximately 40-fold less potent than U-50488 in promoting KOPr internalization (Wang et al. 2005). Another study demonstrated that salvinorin A (10–50 µM) partially inhibited µ receptor binding, affected the Kd and Bmax of the µ receptor, and acted as a noncompetitive inhibitor of DAMGO-stimulated [35S]GTPγS binding. These findings indicate that salvinorin A allosterically modulates the µ-opioid receptor (Rothman et al. 2007). In contrast to the partial µ receptor inhibition by salvinorin A, U-50488 completely inhibited µ receptor binding, producing inhibition curves consistent with competitive inhibition (Rothman et al. 2007). Thus, salvinorin A may interact differently with the µ receptor than other KOPr agonists.
In summary, the present findings show that salvinorin A, like other KOPr agonists, decreases mesostriatal neurotransmission by affecting DA release but not DA uptake. Additionally, this study is the first to assess the effects of repeated salvinorin A administration on cocaine-evoked DA release and locomotor activity. The increase in cocaine-evoked DA release in the DSTR produced by salvinorin A is consistent with previous research examining the effects of U-69593 (Heidbreder et al. 1998). However, a difference in the interaction of salvinorin A and synthetic KOPr agonists with the behavioral response to cocaine was seen, suggesting that the pharmacology of these agents may not be identical.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse. We thank Thomas Prisinzano, Ph.D. for his discussions and support of these studies. We also thank Eric Oh for his technical assistance and Jennifer Davis for her help with editing.
Footnotes
The experiments comply with the current laws of the US, and the authors have no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- Acri JB, Thompson AC, Shippenberg T. Modulation of pre-and postsynaptic dopamine D2 receptor function by the selective kappa-opioid receptor agonist U69593. Synapse. 2001;39:343–350. doi: 10.1002/1098-2396(20010315)39:4<343::AID-SYN1018>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Babu K, Boyer EW, Hernor C, Brush DE. Emerging drugs of abuse. Clin Ped Emerg Med. 2005;6:81–84. [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious {kappa}-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Paradoxical effects of prodynorphin gene deletion on basal and cocaine-evoked dopaminergic neurotransmission in the nucleus accumbens. Eur J Neurosci. 2006;23:229–238. doi: 10.1111/j.1460-9568.2005.04525.x. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci. 2003;23:3076. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Gerdes RM, D’addario C, Izenwasser S. Kappa opioid agonists alter dopamine markers and cocaine-stimulated locomotor activity. Behav Pharmacol. 2001;12:237–245. doi: 10.1097/00008877-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF. Kappa agonist-induced reduction in dopamine release: site of action and tolerance. Res Commun Chem Pathol Pharmacol. 1992;78:193–210. [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba JA, Gysling K, Magendzo K, Andres ME. Repeated administration of the selective kappa-opioid receptor agonist U-69593 increases stimulated dopamine extracellular levels in the rat nucleus accumbens. J Neurosci Res. 2006;84:450–459. doi: 10.1002/jnr.20890. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Hazelden Foundation. Drug abuse trends. 2004 Jun; http://www.research.hazelden.org.
- Heidbreder CA, Schenk S, Partridge B, Shippenberg TS. Increased responsiveness of mesolimbic and mesostriatal dopamine neurons to cocaine following repeated administration of a selective kappa-opioid receptor agonist. Synapse. 1998;30:255–262. doi: 10.1002/(SICI)1098-2396(199811)30:3<255::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Acri JB, Kunko PM, Shippenberg T. Repeated treatment with the selective kappa opioid agonist U-69593 produces a marked depletion of dopamine D2 receptors. Synapse. 1998;30:275–283. doi: 10.1002/(SICI)1098-2396(199811)30:3<275::AID-SYN5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Olson RJ, Justice JB., Jr Quantitative microdialysis under transient conditions. Anal Chem. 1993;65:1017–1022. doi: 10.1021/ac00056a012. [DOI] [PubMed] [Google Scholar]
- Pavarin RM. Substance use and related problems: a study on the abuse of recreational and not recreational drugs in Northern Italy. Ann Ist Super Sanità. 2006;42:477–484. [PubMed] [Google Scholar]
- Parsons L, Smith A, Justice J. Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Ronken E, Mulder AH, Schoffelmeer AN. Interacting pre-synaptic kappa-opioid and GABAA receptors modulate dopamine release from rat striatal synaptosomes. J Neurochem. 1993;61:1634–1639. doi: 10.1111/j.1471-4159.1993.tb09797.x. [DOI] [PubMed] [Google Scholar]
- Ronken E, Mulder AH, Schoffelmeer AN. Chronic activation of mu-and kappa-opioid receptors in cultured catecholaminergic neurons from rat brain causes neuronal supersensitivity without receptor desensitization. J Pharmacol Exp Ther. 1994;268:595–599. [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Murphy DL, Xu H, Godin JA, Dersch CM, Partilla JS, Tidgewell K, Schmidt M, Prisinzano TE. Salvinorin A: allosteric interactions at the mu-opioid receptor. J Pharmacol Exp Ther. 2007;320:801–810. doi: 10.1124/jpet.106.113167. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. Reinstatement of extinguished drug-taking behavior in rats: effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology (Berl) 2000;151:85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Roth BL. Salvinorin A: the ‘magic mint’ hallucinogen finds a molecular target in the kappa opioid receptor. Trends Pharmacol Sci. 2003;24:107–109. doi: 10.1016/S0165-6147(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu-and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg T. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. {kappa}-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes LJ., III Salvia divinorum and the unique diterpene hallucinogen, salvinorin (divinorin) A. J Psychoactive Drugs. 1994;26:277–283. doi: 10.1080/02791072.1994.10472441. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct kappa ligands (salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Terenius L. Modulation of neurotransmitter release in the basal ganglia of the rat brain by dynorphin peptides. J Pharmacol Exp Ther. 1999;290:1307–1315. [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]