Summary
Bacillus thuringiensis ssp. israelensis (Bti) has been used worldwide for the control of dipteran insect pests. This bacterium produces several Cry and Cyt toxins that individually show activity against mosquitoes but together show synergistic effect. Previous work demonstrated that Cyt1Aa synergizes the toxic activity of Cry11Aa by functioning as a membrane-bound receptor. In the case of Cry toxins active against lepidopteran insects, receptor interaction triggers the formation of a pre-pore oligomer that is responsible for pore formation and toxicity. In this work we report that binding of Cry11Aa to Cyt1Aa facilitates the formation of a Cry11Aa pre-pore oligomeric structure that is capable of forming pores in membrane vesicles. Cry11Aa and Cyt1A point mutants affected in binding and in synergism had a correlative effect on the formation of Cry11Aa pre-pore oligomer and on pore-formation activity of Cry11Aa. These data further support that Cyt1Aa interacts with Cry11Aa and demonstrate the molecular mechanism by which Cyt1Aa synergizes or suppresses resistance to Cry11Aa, by providing a binding site for Cry11Aa that will result in an efficient formation of Cry11Aa pre-pore that inserts into membranes and forms ionic pores.
Introduction
Bacillus thuringiensis is a Gram-positive bacterium that produces crystal inclusions during the sporulation phase that are toxic to different insect species and to other non-vertebrate animals as nematodes (Schnepf et al., 1998). These crystal inclusions are generally composed of different δ-endotoxins known as Cry or Cyt proteins (de Maagd et al., 2003). The Cry and Cyt are considered as pore-forming toxins that target midgut epithelium cells on susceptible insects (Schnepf et al., 1998; de Maagd et al., 2003). The Cry and Cyt proteins are used worldwide for the control of different insect species especially crop pests and mosquitoes that are vectors of human diseases.
The mode of action of Cry proteins has been principally studied in Cry1A toxins that are selectively active against lepidopteran larvae. The Cry toxins ingested by susceptible larvae dissolve in the alkaline environment of the gut, thereby releasing soluble protoxins. The inactive Cry protoxins are then cleaved at specific sites by midgut proteases yielding active protease-resistant fragments of about 60 kDa (Schnepf et al., 1998; Bravo et al., 2007). In the case of Cry1A toxins a sequential interaction with two different receptor molecules has been proposed (Bravo et al., 2004). Receptors for Cry1A are located on the apical microvilli membrane of the midgut epithelium columnar cells (Bravo et al., 1992). The interaction of Cry1Ab toxin with its primary receptor, a 210 kDa membrane-bound cadherin protein, facilitates further proteolytic cleavage of the N-terminal, removing helix α-1; this cleavage induces the formation of an oligomeric pre-pore that is membrane insertion competent (Gómez et al., 2002). The pre-pore oligomer then binds to a second GPI-anchored receptor, either aminopeptidase-N (APN) or alkaline phosphatase (ALP) depending on the lepidopteran insect species (Knight et al., 1994; Jurat-Fuentes and Adang, 2006), leading finally to insertion of the toxin into membrane lipid rafts (Zhuang et al., 2002; Bravo et al., 2004). Recently it was shown that the interaction of Cry1Ac pre-pore with the GPI-anchored receptor facilitates membrane insertion (Pardo-López et al., 2006).
Bacillus thuringiensis ssp. israelensis (Bti) has been used worldwide for the control of different mosquito species (Aedes, Culex and Anopheles) and black fly that are vectors of many human diseases (Schnepf et al., 1998). This bacterium produces at least six toxins that individually show activity against mosquitoes: Cry4Aa, Cry4Ba, Cry10Aa, Cry11Aa, Cyt1Aa and Cyt2Ba (Crickmore et al., 1995). Ligand blot experiments revealed that in Aedes aegypti, Cry11Aa binds to at least three midgut brush border membrane proteins of 200, 100 and 65 kDa (Fernandez et al., 2006). In particular the 100 and the 65 kDa are GPI-anchored, and the 65 kDa was identified as an ALP molecule involved in the toxicity of Cry11Aa (Fernandez et al., 2006). Cyt proteins, in contrast to Cry toxins, do not interact with protein receptors in the microvilli of midgut cells epithelium, binding directly to membrane lipids, resulting in membrane insertion and pore formation (Knowles et al., 1989).
One of the most interesting features of Bti is that Cyt1Aa toxin synergizes activity of Cry toxins and suppresses resistance in mosquitoes to Cry toxins (Chang et al., 1993; Wu et al., 1994; Wirth et al., 1997). Cry11Aa is toxic to A. aegypti, but its activity is greatly increased in the presence of sublethal levels of Cyt1Aa (Wirth et al., 1997). Also, Cyt1Aa overcomes the resistance of Culex quinquefasciatus population resistant to Cry11Aa (Wirth et al., 2000). Recently, we demonstrated that Cyt1Aa synergizes the toxic activity of Cry11Aa by functioning as a membrane-bound receptor of Cry11Aa (Pérez et al., 2005). The proposed mechanism is that Cyt1Aa inserts into midgut epithelium membranes and exposes protein regions that recognize Cry11Aa thereby facilitating the insertion of Cry11Aa into membranes and pore formation (Pérez et al., 2005). Cry11Aa binds Cyt1Aa using the loop α-8 that is also involved in Cry11Aa–ALP receptor interaction, and some mutations in this region affected the specific binding of Cry11Aa to Cyt1Aa and reduced synergism between these proteins (Pérez et al., 2005). In Cry11Aa there are other regions of domain II involved in the interaction of this toxin with ALP and with other proteins present in the mosquito brush border membrane vesicles (BBMV) (Fernandez et al., 2006). In this report we analysed the effect of the interaction of Cry11Aa with Cyt1Aa on the formation of a pre-pore structure. Our data show that binding of Cry11Aa to Cyt1Aa facilitates the formation of a pre-pore oligomeric structure that is capable of forming pores in synthetic lipid membrane vesicles, explaining the mechanism of synergism of Cyt1Aa on Cry11Aa toxicity.
Results
Cyt1Aa enhances Cry11Aa oligomer formation in brush border membranes from A. aegypti midgut cells
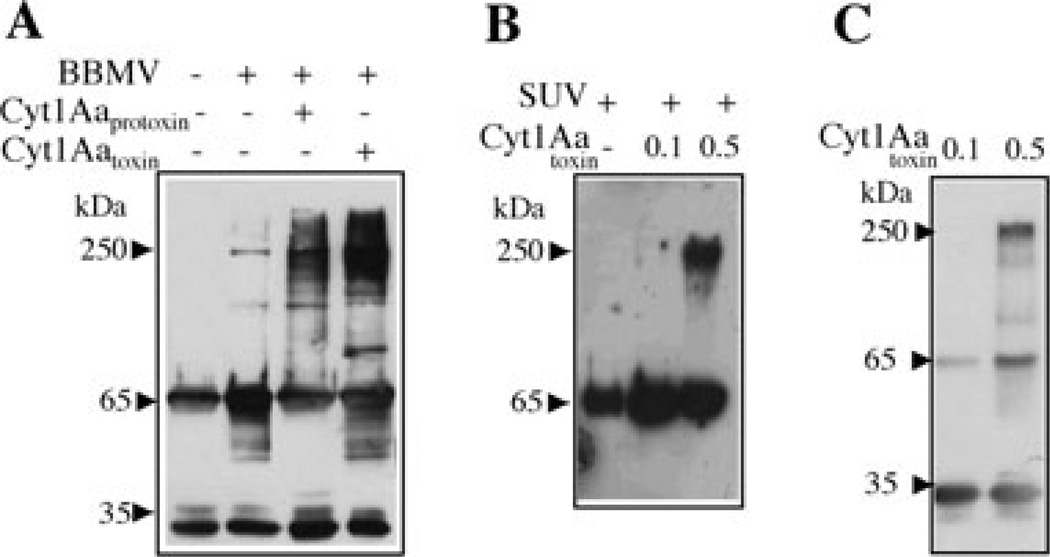
Cry1A and Cry3A toxins form oligomeric structures when they are activated in the presence of their receptors located in the midgut microvilli of target midgut cells (Gómez et al., 2002; Rausell et al., 2004a; Muñoz-Garay et al., 2006). In order to determine if Cry11Aa is also able to form oligomeric structures when it is activated in the presence of its native receptors, we purified BBMV from A. aegypti midgut cells that contain membrane receptors for Cry11Aa (Fernandez et al., 2006). The soluble Cry11Aa protoxin was then activated with trypsin in the presence of BBMV. Figure 1A shows that oligomeric structures of high molecular size, around 250 kDa, were formed when the Cry11Aa protoxin was activated in the presence of its receptors located in A. aegypti BBMV.
Fig. 1.
Analysis of Cry11Aa oligomer formation by Western blot using polyclonal anti-Cry11A antibody.
A. Activation of soluble Cry11Aa protoxin with trypsin in BBMV isolated from A. aegypti. Incubation was performed for 1 h at 37°C in absence or in the presence of Cyt1Aa-solubilized protoxin or Cyt1Aa-activated toxin. The membrane fraction was separated by centrifugation, suspended in 20 µl of 10 mM CHES, 150 mM KCl, pH 9, loaded in SDS-PAGE and visualized by Western blot. Control without BBMV was also included; this sample was not centrifuged.
B. Activation of soluble Cry11Aa protoxin with trypsin for 1 h at 37°C in SUV (PC : Ch : S mixture) in the presence or absence of different concentrations of Cyt1Aa activated toxin. The membrane fraction was separated by centrifugation as above and Cry11Aa protein was visualized by Western blot.
C. Activation of soluble Cry11Aa protoxin with trypsin in solution for 1 h at 37°C in the presence of different concentrations of Cyt1Aa-activated toxin. Oligomerization of Cry11A was analysed by Western blot. Size of proteins was estimated from molecular pre-stained plus standard, all blue (Bio-Rad).
We previously showed that the Cyt1Aa toxin interacts with Cry11Aa toxin (Pérez et al., 2005). To analyse if the Cyt1A can also facilitate formation of the Cry11A oligomer, the soluble protoxin of Cry11Aa was activated with A. aegypti BBMV in the presence of Cyt1Aa protein, either as protoxin or as activated toxin. Figure 1A shows that both forms of Cyt1Aa increased substantially the amount of Cry11Aa oligomer formation.
Cyt1Aa induces oligomerization of Cry11A in liposomes composed of synthetic lipids
We analysed if Cyt1Aa was able to induce the formation of Cry11Aa oligomers in the absence of BBMV receptors. The soluble Cry11Aa protoxin was incubated with trypsin and small unilamellar vesicles (SUV), composed of a 10:3:1 mixture of phosphatidyl choline : cholesterol : sterylamine, respectively, in the absence or presence of different concentrations of activated Cyt1Aa toxin. Figure 1B shows that Cyt1Aa was able to induce oligomerization of Cry11Aa when used at a 1:5 ratio (Cyt1Aa : Cry11Aa w/w). Finally, we analysed if Cyt1Aa was able to induce oligomerization of Cry11Aa in the absence of synthetic liposomes. Figure 1C shows that activated Cyt1Aa induces oligomerization of Cry11Aa in solution when the mixture is treated with trypsin.
To determine if Cyt1Aa was part of the 250 kDa oligomer observed with anti-Cry11Aa antibody in Fig. 1B and C, we analyse these activated mixtures by Western blot using a specific anti-Cyt1Aa antibody. The immunodetection of Cyt1Aa did not reveal the 250 kDa band, indicating that 250 kDa oligomer composes only of Cry11Aa (data not shown).
Pore-formation activity of the oligomeric Cry11Aa structure
To determine if the Cry11Aa 250 kDa oligomer has pore-formation activity, soluble Cry11Aa protoxin was activated with trypsin in SUV in the presence of Cyt1Aa-activated toxin as described above for Fig. 1B. As controls we performed similar activation treatment but using only Cry11Aa protoxin in the absence of Cyt1Aa toxin or using Cyt1Aa in the absence of Cry11Aa protoxin.
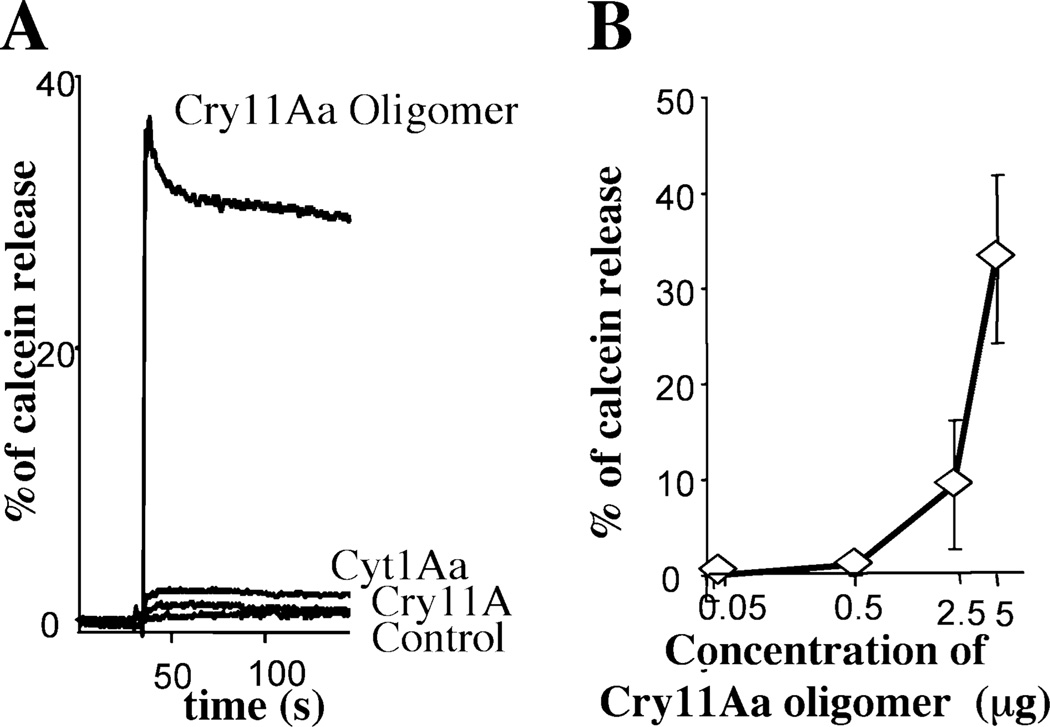
Figure 2A shows the positive and negative controls of the K+ permeability assay performed with a fluorescent dye sensitive to changes in membrane potential. The positive control is the K+-specific ionophore valinomycin and the negative control is the same buffer used to suspend the activated toxin samples. The fluorescence responses to changes in the K+-diffusion potential by varying external K+ concentration are presented (Fig. 2A). Control traces corresponding to KCl permeability of SUV showed no permeability in the absence of valinomycin. Figure 2B shows that the Cry11Aa oligomer had high pore-forming activity. In contrast with the protein samples containing either Cry11Aa alone activated with trypsin or only activated Cyt1Aa that showed marginal pore-formation activity.
Fig. 2.
Analysis of pore-formation activity of Cry11Aa oligomeric structure using the membrane potential assay. The Cry11Aa oligomeric structure was obtained as described in Fig. 1B by activation of Cry11Aa protoxin with trypsin in the presence of SUV and Cyt1Aa toxin in 1:5 ratio (Cyt1Aa : Cry11Aa). After incubation, SUV were centrifuged and the pellet was suspended in 20 µl of 150 mM MeGluCl, 2 mM CaCl2, 10 mM Hepes pH 7 buffer, boiled for 4 min and used in the pore-forming assay. Controls of Cry11Aa protoxin or Cyt1Aa activated in identical conditions were also assayed. The KCl additions (labelled as 1–6) were 1, 2.5, 6, 12.5, 26, 52 mM final concentration respectively.
A. Traces of fluorescence response induced by positive control, valinomycin or negative control, buffer used to suspend the toxin samples.
B. Traces of fluorescence response induced by protein samples containing oligomeric Cry11Aa, monomeric Cry11Aa and monomeric Cyt1Aa are shown. m, slope of percentage of changes in AFU versus external K+ concentration. AFU, arbitrary fluorescence units.
To further demonstrate that a pore-formation activity was associated with the oligomeric structure of Cry11Aa, we analysed the effect of Cry11Aa oligomers on the integrity of lipid membrane vesicles using calcein-release experiments. The release of entrapped calcein from SUV was measured as dequenching of the calcein fluorescence, and was thereby monitored continuously as an increase in the fluorescence intensity. Data are expressed as percentage of the maximal fluorescence release, obtained with the positive control Triton X-100. Figure 3A shows that the Cry11Aa oligomeric structure induces a fast release of encapsulated calcein. A similar fast release of calcein was induced with soluble oligomeric Cry11Aa structure obtained in the absence of SUV (data not shown). In contrast, samples of the Cry11Aa monomeric structure or Cyt1Aa toxin had minimal effect on the calcein-loaded SUV. Finally, Fig. 3B shows the dose–response curve of Cry11Aa oligomer in the calcein-release experiments, showing a direct correlation of calcein released from the SUV with Cry11Aa oligomer concentration.
Fig. 3.
Analysis of pore-formation activity of Cry11Aa oligomeric structure using calcein leakage assay. The Cry11Aa oligomeric structure was obtained as described in Fig. 2, but SUV containing oligomeric Cry11Aa were suspended in 150 mM KCl, 10 mM CHES, pH 9. Controls of Cry11Aa protoxin or Cyt1Aa activated in identical conditions were also assayed. Maximal leakage at the end of each experiment was assessed with 0.1% Triton X-100.
A. The effect of oligomeric Cry11Aa or monomeric samples of Cry11Aa and Cyt1Aa on the integrity of lipid membrane vesicles. Control is the same buffer used to suspend the toxin samples.
B. Dose–response curve of Cry11Aa oligomer using the calcein-release assay.
Mutations that affect toxin synergism have a correlative effect on Cry11Aa oligomer formation
To determine if oligomer formation of Cry11Aa correlates with the binding interaction between Cyt1Aa and Cry11Aa, we analysed the olimerization of Cry11Aa using single-point mutants of these proteins that are affected in their interaction and correlated with their synergistic activity against A. aegypti insect larvae (Pérez et al., 2005). Previously, we reported that mutants Cry11Aa-S259A and Cyt1Aa-K225A showed reduced binding with their corresponding Cyt1Aa or Cry11Aa wild-type toxin that correlated with lower synergism. In contrast Cyt1Aa-K198A mutant showed increased binding to Cry11Aa and higher synergism (Pérez et al., 2005). Figure 4A shows that activation of the mutant Cry11Aa-S259A with trypsin in PC : Ch : S SUV in the presence of wild-type Cyt1Aa resulted in a severe reduction of Cry11Aa oligomer formation. Similarly, the activation of Cyt1Aa-K225A mutant with wild-type Cry11Aa resulted in reduction of oligomer formation. The mixture of both mutant toxins, Cry11Aa-S259A with Cyt1Aa-K225A, resulted in complete lost of Cry11Aa oligomer formation. In contrast, the activation of Cry11Aa protoxin performed in the presence of Cyt1Aa-K198A mutant was very effective to produce the Cry11Aa oligomer, and similar to that observed with Cyt1Aa. Finally, the pore-formation activity of these protein samples was analysed using the calcein-release assay as described above. Figure 4B shows that protein mixtures affected in oligomer formation were also severely affected in pore-formation activity showing a low response in calcein release from the SUV. In contrast, the activation of Cry11Aa performed in the presence of Cyt1Aa-K198A mutant that had efficient oligomer formation showed high pore formation, similar to the pore-formation activity obtained with wild-type toxins.
Fig. 4.
Analysis of oligomer-formation and pore-formation activity, induced by mutant proteins affected in Cry11Aa–Cyt1Aa binding interaction.
A. Analysis of oligomer formation after trypsin activation of different protein mixtures performed in SUV as described in Fig. 1B.
B. Analysis of pore-formation activity by calcein-release assay of same protein samples presented in (A). Controls including individual toxins are shown including SUV in the absence of protein sample.
Discussion
Bacteria rely on various strategies for pathogenesis, and many vertebrate and invertebrate pathogens use pore-forming toxins to exert their effects on their host cellular targets, for example, in the case of enterohaemorrhagic Escherichia coli, which inject a TIR receptor into host cells that then aids in attachment of the bacteria to the injected host cells (Kenny et al., 1997). A similar strategy is used by the bacterial pathogen of mosquitoes, Bti, which produces multiple crystal proteins, one of which can then serve as a midgut receptor for the other toxins (Pérez et al., 2005). In this work we demonstrate that the Bti Cyt1Aa enhances Cry11Aa toxicity by facilitating the formation of a pre-pore oligomeric structure that is efficient in forming pores.
Cry toxins are pore-forming toxins that are secreted as water-soluble proteins and undergo conformational changes in order to insert into the membrane of their target insect cells. In other bacterial pore-forming toxins, the membrane channel state was obtained after formation of an oligomeric pre-pore structure that is membrane insertion competent. This oligomeric structure is produced after receptor binding (Parker and Feil, 2005). Different lepidopteran-, coleopteran- and dipteran-specific Cry toxins form oligomeric pre-pore structures after interaction with their receptor located in insect midgut cells: Cry1Ab (Tigue et al., 2001; Gómez et al., 2002), Cry1Ac (Pardo-López et al., 2006), Cry1Ca (Herrero et al., 2004; Muñoz-Garay et al., 2006), Cry1Da, Cry1Ea, Cry1Fa (Muñoz-Garay et al., 2006), Cry3Aa, Cry3Ba, Cry3Ca (Rausell et al., 2004a) and Cry4Ba (Likitvivatanavong et al., 2006).
In this report we analysed the oligomerization of Cry11Aa induced by its native receptors located in BBMV of A. aegypti or induced by Cyt1Aa. We show that Cyt1Aa increases the oligomerization of Cry11Aa in BBMV, in synthetic liposomes and in solution, indicating that the interaction with Cyt1Aa is sufficient to induce the oligomer formation of Cry11Aa. We also show that oligomerization correlates with pore-formation activity. These data are similar to the reported pore activity of Cry1A oligomeric structure (Gómez et al., 2002), indicating that the Cry11Aa oligomer is membrane insertion competent and induces the formation of ionic pores.
Finally, we demonstrated that the binding interaction between Cry11Aa and Cyt1Aa is necessary to induce the formation of the Cry11Aa oligomer and this interaction correlates with pore formation. A Cry11Aa mutant toxin (Cry11Aa-S259A) that bound Cyt1Aa with a 10-fold higher apparent dissociation constant (Kd) and is affected in synergism showed a severe reduction in oligomer formation and marginal ionic pore activity, suggesting that interaction of Cry11Aa with Cyt1Aa is important to trigger oligomer formation. Additionally, two different Cyt1Aa mutant toxins (Cyt1Aa-K225A and Cyt1Aa-K198A) with different binding affinities to Cry11Aa had a correlative effect on Cry11Aa oligomer formation and on pore formation. The Cyt1Aa-K225A mutant has a 10-fold higher Kd in its interaction with Cry11Aa and is also affected in synergism, while mutant Cyt1Aa-K198A has a slight lower Kd and enhanced synergistic activity (Pérez et al., 2005). The interaction of Cyt1Aa-K225A mutant with Cry11Aa or the interaction of the two mutant toxins, Cyt1Aa-K225A with Cry11Aa-S259A, resulted in lost of oligomer formation and no pore formation. It is important to note that the interaction of these two mutants showed a Kd value 75-fold higher than that of wild-type proteins (Pérez et al., 2005). In contrast, the activation of Cry11Aa with the Cyt1Aa-K198A resulted in the efficient production of Cry11Aa oligomer and high pore-formation activity, indicating that binding of Cry11Aa to Cyt1Aa is a necessary step for oligomer formation.
It has been shown that soon after the ingestion of Cyt1Aa toxin by susceptible larvae, Cyt1Aa binds throughout the midgut epithelium (Ravoahangimalala et al., 1993). This event will provide multiple binding sites for Cry11Aa toxin. The data presented here show that Cyt1Aa toxin induces the formation of Cry11Aa oligomeric structure just as the cadherin receptor in lepidopteran insects, as Manduca sexta, induces oligomerization of Cry1A toxins (Gómez et al., 2002). Therefore, Cyt1Aa synergizes or suppresses resistance to Cry11Aa in mosquitoes by providing a binding site for Cry11Aa that will result in an efficient formation of Cry11Aa pre-pore that inserts into membranes and induces pore formation.
Experimental procedures
Bacterial strains
Cry11Aa was produced in B. thuringiensis (Bt) CG6/pCG6 and Cyt1Aa in 4Q7/pWF45 (Chang et al., 1993; Wu et al., 1994). Bt strains were grown in nutrient broth sporulation medium supplemented with 10 µg ml−1 erythromycin (Lereclus et al., 1995) at 200 r.p.m. and 30°C.
Purification of Cry11Aa and Cyt1Aa proteins
Spores and crystal inclusions produced by the Bt strains were harvested and washed three times with 0.3 M NaCl, 0.01 M EDTA, pH 8.0. The pellet was suspended in 0.1% Triton X-100, 300 mM NaCl, 20 mM Tris-HCl pH 7.2, and inclusions were purified by sucrose gradient centrifugation (Thomas and Ellar, 1983). Purified Cry11Aa inclusions were solubilized in 100 mM NaOH. After solubilization Cry11Aa protoxin was dialysed for 12 h against 50 mM Na2CO3 pH 10.5. Purified Cyt1Aa inclusions were solubilized in 50 mM Na2CO3, 10 mM DTT, pH 10.5 and activated with 1:30 proteinase K (Sigma-Aldrich) w/w for 1 h at 30°C.
Preparation of BBM
Brush border membrane vesicles from dissected midguts of fourth-instar A. aegypti larvae were prepared as reported (Nielsen-LeRoux and Charles, 1992).
Preparation of SUV
Egg-yolk phosphatidyl choline (PC), cholesterol (Ch) (Avanti Polar Lipids, Alabaster, AL) and stearylamine (S) (Sigma, St Louis, MO) from a chloroform stocks were mixed in glass vials in a 10:3:1 proportion, respectively, at 2.6 µmol final concentration and dried by argon flow evaporation followed by overnight storage under vacuum to remove residual chloroform. The lipids were hydrated in 2.6 ml of 10 mM CHES, 150 mM KCl pH 9 (calcein assay) or with 10 mM Hepes 150 mM KCl pH 7 (membrane potential assay) by a 30 min incubation followed by vortex. To prepare SUV the lipid suspension was subjected to sonication twice for 2 min in a Branson-1200 bath sonicator (Danbury, CT). SUV were used within 2–3 days upon their preparation.
Activation of Cry11Aa
The oligomeric Cry11Aa structure was produced by activation of 10 µg of soluble protoxin with 1:50 trypsin (w/w) in the presence of 10 µg of BBMV of A. aegypti, in 100 µl of 50 mM Na2CO3 pH 10.5 for 1 h at 37°C. In some cases, this activation was also performed in the presence of 5 µg of Cyt1Aa solubilized protoxin or 5 µg of activated Cyt1Aa toxin. PMSF 1 mM was added to stop proteolysis. The membrane fraction was separated by centrifugation (1 h at 14 000 g) and the membrane pellet containing the oligomeric structure was separated using 10% acrylamide SDS-PAGE and visualized by Western blot using polyclonal anti-Cry11A antibody.
Alternatively, the activation of Cry11Aa was performed using synthetic SUV liposomes. For these assays, 2.5 µg of soluble Cry11Aa protoxin was incubated in 100 µl final volume with 200 µM SUV and 1:50 trypsin (w/w), in the absence or presence of 0.1 or 0.5 µg of Cyt1Aa activated toxin. The mixture was incubated for 2 h at 37°C and 1 mM PMSF was added to stop the reaction. The membrane fraction was separated by centrifugation (1 h at 100 000 g) and the pellet was suspended in two different buffer solutions depending on the pore formation assay, changes on membrane potential or the release of calcein. Oligomeric structures of Cry toxins are highly stable after boiling as well as after urea denaturation (Rausell et al., 2004b). The suspension was boiled for 4 min in order to denature the monomeric proteins that remain in the sample mixture. This sample was then used in the permeability assays or was analysed by SDS-PAGE and Western blot. Finally the oligomeric structure of Cry11Aa was also produced in solution by incubation of 3 µg of soluble Cry11Aa protoxin with 1:50 trypsin (w/w), in the presence of 0.5 µg activated Cyt1Aa.
Western Blot
Protein samples were boiled for 5 min in Laemmli sample loading buffer, separated in SDS-PAGE and electrotransferred onto PVDF membrane (Millipore, Bedford, MA). The Cry11Aa and Cyt1Aa proteins were detected using polyclonal anti-Cry11Aa or anti-Cyt1Aa antibodies (1/15000, 1 h) and a secondary antibody coupled with horseradish peroxidase (HRP) (Sigma, St Louis, MO) (1/5000, 1 h) followed by luminol (ECL; Amersham Pharmacia Biotech) as described by the manufacturers. Molecular weight markers used in all SDS-PAGE were precision pre-stained plus standards, all blue (Bio-Rad) or full-range rainbow (Amersham Biosciences).
Fluorescence measurements
The electrical potential differences were measured using the positively charged potential-sensitive dye, 3,3′-dipropylthiodicarbocyanine iodide [diS-C3-(5), Molecular Probes, Eugene, OR]. Fluorescence was recorded at the 620/670 nm excitation/emission wavelength pair using an Aminco Bowman Luminescence Spectrometer (Urbana, IL, USA) as described (Lorence et al., 1995). Stock dye solution (0.5 µl, 1 mM in DMSO) was added to 0.9 ml of buffer solution [150 mM MetilGlucamine Chloride (MeGluCl), 2 mM CaCl2, 10 mM Hepes pH 7] in a 1 cm path length cuvette. SUV (15 µM) previously loaded with 10 mM Hepes 150 mM KCl pH 7 were added. All determinations were made at 25°C with constant stirring. Dye calibration and the determination of resting membrane potentials were performed in the presence of valinomycin (0.1 µM) by successive additions of KCl (Fn1 = 1, Fn2 = 2.5, Fn3 = 6, Fn4 = 12.5, Fn5 = 26, Fn6 = 52, mM final concentration). The changes in fluorescence were normalized as percentage of changes in fluorescence arbitrary units (FAU). The percentage changes in FAU (%Δ FAU) versus external K+ concentration were determined and the slope (m) of this curve is directly correlated with the membrane permeability. Changes in fluorescence determinations were performed at least four times.
Calcein leakage experiments were performed as described (Rausell et al., 2004a). Calcein-containing vesicles were prepared by sonication (twice for 2 min) of the SUV in calcein 80 mM (Molecular probes, Eugene, OR) dissolved in 150 mM KCl, 10 mM CHES, pH 9. Non-entrapped calcein was removed by gel filtration on Sephadex G-50 (1 cm × 30 cm column) eluted with the same buffer. Calcein loaded SUV, 100 µl, were added to 900 µl of 150 mM KCl, CHES 10 mM, pH 9. Finally, activated samples of Cry11Aa and Cyt1Aa toxins were added and the release of calcein was analysed. The released calcein induced an increase in fluorescence due to the dequenching of the dye into the external medium. Calcein fluorescence was excited at 490 nm (10 nm slit) and monitored at 520 nm with an Aminco Bowman Luminescence Spectrometer (Urbana, IL, USA). Maximal leakage at the end of each experiment was assessed by lysis with 0.1% Triton X-100 (final concentration). All fluorescence experiments were performed in quadruplicate at 20°C.
Acknowledgements
We thank Lizbeth Cabrera for technical assistance. CONACyT U48631-Q and J44962Q; NIH 1R01 AI066014 and USDA 2207-35607-17780 supported this work
References
- Bravo A, Jansens S, Peferoen M. Immunocytochemical localization of Bacillus thuringiensis insecticidal crystal proteins in intoxicated insects. J Invertebr Pathol. 1992;60:237–246. [Google Scholar]
- Bravo A, Gómez I, Conde J, Muñoz-Garay C, Sánchez J, Miranda R, et al. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim Biophys Acta. 2004;1667:38–46. doi: 10.1016/j.bbamem.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Bravo A, Gill SS, Soberón M. Mode of action of Bacillus thuringiensis toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Yu YM, Dai SM, Law SK, Gill SS. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl Environ Microbiol. 1993;59:815–821. doi: 10.1128/aem.59.3.815-821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore N, Bone EJ, Williams JA, Ellar DJ. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp israelensis. FEMS Microbiol Lett. 1995;131:249–254. [Google Scholar]
- Fernandez LE, Aimanova KG, Gill SS, Bravo A, Soberón M. A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochem J. 2006;394:77–84. doi: 10.1042/BJ20051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez I, Sánchez J, Miranda R, Bravo A, Soberón M. Cadherin-like receptor binding facilitates proteolytic cleavage of helix α-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 2002;513:242–246. doi: 10.1016/s0014-5793(02)02321-9. [DOI] [PubMed] [Google Scholar]
- Herrero S, Gonzalez-Cabrera J, Ferré J, Bakker PL, de Maagd RA. Mutations in the Bacillus thuringiensis Cry1Ca toxin demonstrate the role of domain II and III in specificity towards Spodoptera exigua larvae. Biochem J. 2004;384:507–513. doi: 10.1042/BJ20041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurat-Fuentes JL, Adang MJ. Cry toxin mode of action in susceptible and resistant Heliothis virescens larvae. J Invertebr Pathol. 2006;92:166–171. doi: 10.1016/j.jip.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Knight P, Crickmore N, Ellar DJ. The receptor for Bacillus thuringiensis CryIA(c) delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase. Mol Microbiol. 1994;11:429–436. doi: 10.1111/j.1365-2958.1994.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles BH, Blatt MR, Tester M, Horsnell JM, Carroll J, Menestrina G, Ellar DJ. A cytolytic delta-endotoxin from Bacillus thuringiensis var. israelensis forms cation-selective channels in planar lipid bilayers. FEBS Lett. 1989;244:259–262. doi: 10.1016/0014-5793(89)80540-x. [DOI] [PubMed] [Google Scholar]
- Lereclus D, Agaisse H, Gominet M, Chaufaux J. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spoOA mutant. Biotechnology. 1995;13:67–71. doi: 10.1038/nbt0195-67. [DOI] [PubMed] [Google Scholar]
- Likitvivatanavong S, Katzenmeier G, Angsuthanasombat Ch. Asn183 in α5 is essential for oligomerization and toxicity of the Bacillus thuringiensis Cry4Ba toxin. Arch Biochem Biophys. 2006;445:46–55. doi: 10.1016/j.abb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Lorence A, Darszon A, Díaz C, Liévano A, Quintero R, Bravo A. δ-Endotoxins induce cation channels in Spodoptera frugiperda brush border membranes in suspension and in planar lipid bilayers. FEBS Lett. 1995;360:217–222. doi: 10.1016/0014-5793(95)00092-n. [DOI] [PubMed] [Google Scholar]
- de Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE. Structure, diversity and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu Rev Genet. 2003;37:409–433. doi: 10.1146/annurev.genet.37.110801.143042. [DOI] [PubMed] [Google Scholar]
- Muñoz-Garay C, Sánchez J, Darszon A, de Maagd RA, Bakker P, Soberón M, Bravo A. Permeability changes of Manduca sexta midgut brush border membranes induced by oligomeric structures of different cry toxins. J Membr Biol. 2006;212:61–68. doi: 10.1007/s00232-006-0003-8. [DOI] [PubMed] [Google Scholar]
- Nielsen-LeRoux C, Charles JF. Binding of Bacillus sphaericus binary toxin to a specific receptor on midgut brush-border membranes from mosquito larvae. Eur J Biochem. 1992;210:585–590. doi: 10.1111/j.1432-1033.1992.tb17458.x. [DOI] [PubMed] [Google Scholar]
- Pardo-López L, Gómez I, Rausell C, Sánchez J, Soberón M, Bravo A. Structural changes of the Cry1Ac oligomeric pre-pore from Bacillus thuringiensis induced by N-acetylgalactosamine facilitates toxin membrane insertion. Biochemistry. 2006;45:10329–10336. doi: 10.1021/bi060297z. [DOI] [PubMed] [Google Scholar]
- Parker MW, Feil SC. Pore-forming proteins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–124. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Pérez C, Fernandez LE, Sun J, Folch JL, Gill SS, Soberón M, Bravo A. Bti Cry11Aa and Cyt1Aa toxins interactions support the synergism-model that Cyt1Aa functions as membrane-bound receptor. Proc Natl Acad Sci USA. 2005;102:18303–18308. doi: 10.1073/pnas.0505494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell C, García-Robles I, Sánchez J, Muñoz-Garay C, Martínez-Ramírez AC, Real MD, Bravo A. Role of toxin activation on binding and pore formation activity of the Bacillus thuringiensis Cry3 toxins in membranes of Leptinotarsa decemlineata [Say] Biochem Biophys Acta. 2004a;1660:99–105. doi: 10.1016/j.bbamem.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Rausell C, Pardo-López L, Sánchez J, Muñoz-Garay C, Morera C, Soberón M, Bravo A. Unfolding events in the water-soluble monomeric Cry1Ab toxin during transition to oligomeric pre-pore and membrane inserted pore channel. J Biol Chem. 2004b;279:55168–55175. doi: 10.1074/jbc.M406279200. [DOI] [PubMed] [Google Scholar]
- Ravoahangimalala O, Charles JF, Schoeller-Raccaud J. Immunological localization of Bacillus thuringiensis serovar israelensis toxins in midgut cells of intoxicated Anopheles gambiae larvae (Diptera: Culicidae) Res Microbiol. 1993;144:271–278. doi: 10.1016/0923-2508(93)90011-p. [DOI] [PubMed] [Google Scholar]
- Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WE, Ellar DJ. Bacillus thuringiensis var. israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- Tigue NJ, Jacoby J, Ellar DJ. The alpha-helix 4 residue, Asn135, is involved in the oligomerization of Cry1Ac1 and Cry1Ab5 Bacillus thuringiensis toxins. Appl Environ Microbiol. 2001;67:5715–5720. doi: 10.1128/AEM.67.12.5715-5720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MC, Georghiou GP, Federici BA. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex quinquefasciatus. Proc Natl Acad Sci USA. 1997;94:10536–10540. doi: 10.1073/pnas.94.20.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MC, Walton WE, Federici BA. Cyt1Aa from Bacillus thuringiensis restores toxicity of Bacillus sphaericus against resistant Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2000;37:401–407. [PubMed] [Google Scholar]
- Wu D, Johnson JJ, Federici BA. Synergism of mosquitocidal toxicity between CytA and CryIV proteins using inclusions produced from cloned genes of Bacillus thuringiensis subsp. israelensis. Mol Microbiol. 1994;13:965–972. doi: 10.1111/j.1365-2958.1994.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Zhuang M, Oltean DI, Gómez I, Pullikuth AK, Soberón M, Bravo A, Gill SS. Heliothis virescens and Manduca sexta lipid rafts are involved in Cry1A toxin binding to the midgut epithelium and subsequent pore formation. J Biol Chem. 2002;277:13863–13872. doi: 10.1074/jbc.M110057200. [DOI] [PubMed] [Google Scholar]