Abstract
Immunoglobulin A nephropathy (IgAN) is a leading cause of chronic kidney disease, frequently associated with hypertension and renal inflammation. ω-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in fish oil (FO) improve kidney function in animal models, but have inconsistent metabolic effects in humans. Oxylipin profiles in serum from IgAN patients supplemented with either FO or corn oil (CO) placebo were analyzed by liquid chromatography coupled to tandem mass spectrometry. EPA cyclooxygenase and lipoxygenase metabolites, and EPA and DHA epoxides and diols were increased in response to FO supplementation, as were total epoxides and epoxide/diol ratios. Several of these metabolites were drivers of separation as assessed by multivariate analysis of FO patients pre- vs. post-supplementation, including 17,18-dihydroxyeicosatrienoic acid, prostaglandin D3, prostagalandin E3, Resolvin E1, 12-hydroxyeicosapentaenoic acid, and 10(11)-epoxydocosapentaenoic acid. In patients whose proteinuria improved, plasma total oxylipins as well as several hydroxyoctadecadienoic acids, hydroxyeicosatetraenoic acids, and leukotriene B4 metabolites were among the metabolites that were significantly lower than in patients whose proteinuria either did not improve or worsened. These data support the involvement of oxylipins in the inflammatory component of IgAN as well as the potential use of oxylipin profiles as biomarkers and for assessing responsiveness to ω-3 fatty acid supplementation in IgAN patients.
Keywords: Eicosanoids, EPA, DHA, Inflammation, Metabolomics, ω-3 fatty acid, Oxylipins, Signaling lipids, Kidney function
1 Introduction
IgAN is one of the most common forms of chronic kidney disease worldwide, and it is diagnosed in approximately 30% of patients who undergo renal biopsy in Asian countries such as Korea (Chang et al. 2009) and 20% in Western countries such as the United States (Swaminathan et al. 2006). This inflammatory glomerular disorder can progress to end-stage renal disease in 25–30% of IgAN patients over 20 years of age (Appel and Waldman 2006). Disease etiology has not yet been fully elucidated; however, the disease is initiated by an unknown defect in the mucosal immune system that results in the faulty glycosylation of IgA1 (Mestecky et al. 1995). The misglycosylated IgA1 forms complexes with IgG and IgA, and these complexes bind to mesangial cells in the kidney, leading to their accumulation in the glomerulus (Mestecky et al. 1995). Downstream glomerular inflammation (Roccatello et al. 1993), increased expression of cytokines (Libetta et al. 1997), proliferation of mesangial cells, and extracellular matrix formation result in kidney dysfunction. IgAN is associated with hypertension and markers of inflammation, especially at later stages of the disease (Myllymaki et al. 2006; J. V. Donadio, Jr. et al. 1999; J. V. Donadio et al. 1994; Syrjanen et al. 2000; Hogg 1995).
Current treatments for IgAN focus on blood pressure and proteinuria control—typically with angiotensin-converting enzyme inhibitors or angiotensin receptor blockade—and in selected cases supplementation with fish oil (FO), which is rich in ω-3 fatty acids (FA), whereas corticosteroids are generally reserved for rapidly progressing disease (Appel and Waldman 2006). However, while proteinuria reduction and corticosteroids are in general effective in proteinuric renal diseases, there have been no studies showing efficacy of specific treatments to IgAN. Animal studies show inhibition of cell growth and proliferation, inhibition of renal inflammation, reduction in serum lipids, reduction in blood pressure, and reductions in glomerular injury and proteinuria with FO supplementation (J. V. Donadio and Grande 2004; Imig et al. 2005; Zhao et al. 2004). However, human trials have shown mixed results, with 7 out of 13 trials finding beneficial effects from FO supplementation (J. V. Donadio, Jr. et al. 1994; Alexopoulos et al. 2004; Holman et al. 1994; J. V. Donadio, Jr. et al. 1999; J. V. Donadio, Jr. et al. 2001; Sulikowska et al. 2002; Sulikowska et al. 2004), 1 trial finding no benefit in certain outcomes (proteinuria and glomerular filtration rate) but improvements in other outcomes (tubular dysfunction, lipid profiles, and oxidative stress) (Parinyasiri et al. 2004), and the remaining 5 trials finding no beneficial effect from FO (Bennett et al. 1989; Branten et al. 2002; Cheng et al. 1990; Hogg 1995; Pettersson et al. 1994). Several potential reasons for these discrepancies include the use of different outcome measures, differences in ω-3 dosage, formulation, and composition (i.e. exact proportions of the key fatty acids eicosapentaenoic acid (EPA, 20:5n3), and docosahexaenoic acid (DHA, 22:6n3)), as well as underlying genetic and/or dietary- and lifestyle-induced differences in the intake or metabolism of the FA precursors and their oxylipin products among individuals.
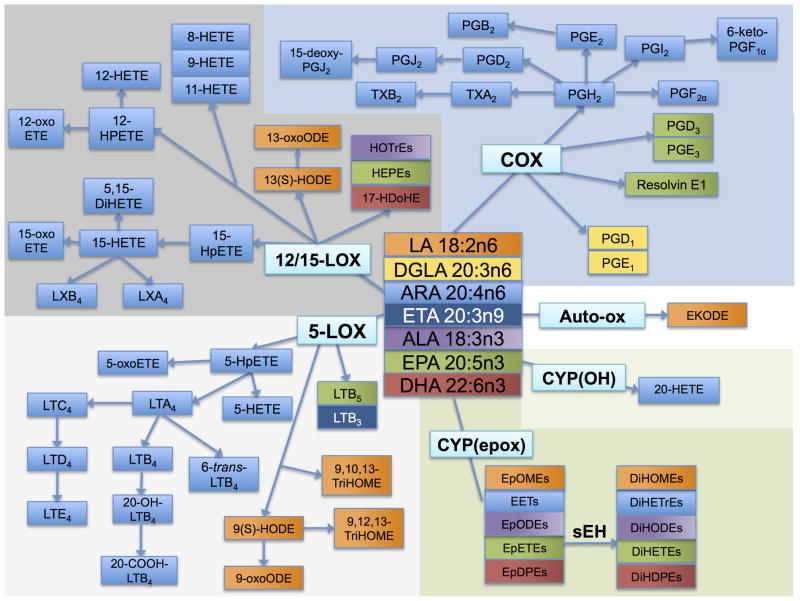
As shown in Figure 1, oxylipins are produced from a number of FA precursors—the ω-6 FA linoleic acid (LA, 18:2n6), dihomo-γ-linolenic acid (DGLA, 20:3n6), and arachidonic acid (ARA, 20:4n6), and the ω-3 FA α-linolenic acid (ALA, 18:3n3), EPA, and DHA (Funk 2001). Oxylipins are derived from the actions of a suite of enzymes, including those in the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) families, as well as by non-enzymatic oxidation. Virtually all cell types employ oxylipins as signals. In platelets oxylipins induce the blood clotting cascade (Catella-Lawson 2001), in smooth muscle cells oxylipins promote vasodilation (Larsen et al. 2006; Baxter 1995), and in immune cells, oxylipins signal the recruitment, activation, and adherence of these cells (Tager et al. 2003). Oxylipins have been implicated in a diverse array of autoimmune diseases including osteoarthritis, metabolic and inflammatory diseases including heart disease and neural degeneration, as well as cancer, and they have been used therapeutically in all of these conditions (Funk 2001; Clevers 2006; Baratelli et al. 2005b; Peeraully et al. 2006).
Fig. 1.
Fatty acid precursors and their oxylipin products. The fatty acids linoleic acid (LA; 18:2n6), α-linolenic acid (ALA, 18:3n3), arachidonic acid (ARA, 20:4n6), dihommo-γ-linolenic acid (DGLA; 20:3n6), eicosatrienoic acid (ETA; 20:3n9), eicosapentaenoic acid (EPA; 20:5n3), and docosahexaenoic acid (DHA; 22:6n3) are precursors to a number of oxylipin products produced via the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P 450 (CYP) enzymes. The oxylipin products of the COX pathway include prostaglandins (PGE1, PGD1, PGH2, PGF2α. PGE2, PGB2, PGD2, PGJ2, 15-deoxy-PGJ2, PGI2, 6-keto-PGF1α, PGE3, PGH3, and resolvin E1) and thromboxanes (TXA2, TXB2). The oxylipin products of the LOX pathway include hydroperoxyeicosatetraenoic acids (HpETEs) and dihydroxyeicosatetraenoic acid (DiHETE), (further converted to hydroxyeicosatetraenoic acids (HETEs)), hydroxyoctadecadienoic acids (HOTrEs), hydroxyeicosaptenaenoic acids (HEPEs), hydroxydocosahexaenoic acid (17-HDoHE), and leukotrienes (LTA4, LTB4, 20-OH-LTB4, 20-COOH-LTB4, 6-trans-LTB4, LTC4, LTD4, LTE4, LTB3, LTB5) as well as the hydroxyoctadienoic acids (HODEs), and trihydroxyoctamonoenoic acids (TriHOMEs). The products of the CYP hydroxy (OH) pathway include 20-HETE, and the products of the CYP epoxy pathway include the epoxyeicosatrienoic acids (EETs), epoxyoctadecadienoic acids (EpODEs), epoxyoctamonoenoic acids (EpOMEs), epoxyeicosatetreaenoic acids (EpETEs), and epoxydocosapentaenoic acids (EpDPEs), as well as the downstream soluble epoxide hydrolase (sEH) metabolites dihydroxyoctamonoenoic acids (DiHOMEs), dihydroxyeicosatrienoic acids (DiHETrEs), dihydroxyoctadecadienoic acids (DiHODEs), dihydroxyeicosatetraenoic acids (DiHETEs), and dihydroxydocosapentaenoic acids (DiHDPEs). Each fatty acid precursor and its oxylipin products are colored the same: LA, orange; DGLA, yellow; ETA, dark blue; ALA, purple; EPA, green; DHA, red; and ARA, light blue.
The COX pathway produces several pro-coagulant, proliferative metabolites from ARA including prostaglandin H2 (PGH2), PGF2α, thromboxane A2 (TXA2) and TXB2, as well as anti-inflammatory, anti-proliferative metabolites including prostaglandin E2 (PGE2), PGD2, and prostacyclin (PGI2). Other COX metabolites of ARA include PGJ2, PGB2, 15-deoxy-PGJ2, and 6-keto-PGF2α, DGLA can be metabolized by COX to PGD1 and PGE1, and EPA can be metabolized to PGD3 and PGE3. Resolvin E1 is produced from EPA by acetylated COX or by CYP followed by LOX activity (reviewed in (Serhan and Chiang 2004)).
The LOX pathway includes the 5-LOX, 12-LOX, and 15-LOX pathways. Among the pro-inflammatory 5-LOX products are 5-hydroxyeicosatetraenoic acids (5-HETE) and 5-oxo-ETE, as well as the leukotrienes LTA4, LTB4, LTC4, LTD4, and LTE4. Other ARA products of the 5-LOX pathway include 5-hydroperoxyeicosatetraenoic acids (HPETE), 20-OH-LTB4, 6-trans-LTB4, and 20-COOH-LTB4. 5-LOX also produces LA-derived products: 9(S)-hydroxyoctadecadienoic acid (HODE), 9-oxo-ODE, 9,10,13-trihydroxyoctadecamonoenoic acid (TriHOME), and 9,12,13-TriHOME; an EPA product LTB5; and an eicosatrienoic acid (ETA, 20:3n9) product LTB3. 9-HODE, as well as several other HODEs and HETEs, can also be produced by non-enzymatic auto-oxidation (Obinata et al. 2005) and CYPs. The 12- and 15-LOX pathways produce the pro-inflammatory 8-HETE, 9-HETE, and 11-HETE metabolites, as well as other ARA products—12-HPETE, 12-oxo-ETE, 12-HETE, 15-HPETE, 15-HETE, 15- oxoETE, 5,15-dihydroxyeicosatetraenoic acid (DiHETE), LXA4, and LXB4. LA products of the 12- and 15-LOX pathways include 13(S)-HODE and 13-oxo-ODE; EPA products are the hydroxyeicosapentaenoic acids (HEPE); ALA products are the hydroxyoctadecadienoic acids (HOTrE); and the DHA metabolite 17- hydroxydocosahexaenoic acid (17-HDoHE).
The CYP pathway produces the epoxyeicosatrienoic acids (EpETrE or EET) from ARA, formed by CYP epoxygenases, and 20-HETE, formed by CYP ω-oxidases. LA is metabolized by the CYP epoxygenases to epoxyoctadecamonoenoic acids (EpOME). The ω-3 FA ALA, EPA, and DHA are all metabolized by CYPs to yield the epoxyoctadecadienoic acids (EpODE), epoxyeicosateteaenoic acids (EpETE), and epoxydocosapentaenoic acids (EpDPE) respectively (Harmon et al. 2006; Viswanathan et al. 2003). These epoxides can be further metabolized to diols by the soluble epoxide hydrolase (sEH) enzyme, giving the diol products dihydroxyeicosatrienoic acids (DiHETrE), dihydroxyoctadecamonoenoic acids (DiHOME), dihydroxyoctadecadienoic acids (DiHODE), dihydroxyeicosatrienoic acids (DiHETE), and dihydroxydocosapentaenoic acids (DiHDPE), derived from ARA, ALA, EPA, and DHA, respectively.
A targeted metabolomic approach using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) capable of identifying and quantifying a total of 87 oxylipin metabolites was used to analyze oxylipin profiles of IgAN patients before and after supplementation with FO or corn oil (CO) (placebo). The aim of this study was to determine plasma oxylipin response to FO supplementation in IgAN patients in order to determine whether oxylipins are associated with responsiveness to treatment with ω-3 FA and kidney function in IgAN patients.
2 Study design and methods
2.1 IgAN patients and samples
Given the rarity of kidney biopsies performed in patients with presumed IgAN with stable renal function, large and well-controlled supplementation trials in IgAN patients are expensive, time-intensive, and difficult to perform. Therefore, the sample set used in this study is not likely to be repeated and represents a unique opportunity to evaluate response to ω-3 FA supplementation from the perspective of oxylipin metabolism that would otherwise not be possible. The subjects and study design were described previously in detail (Hogg et al. 2006). Briefly, the placebo-controlled, double-blind, prospective trial involved a network of 37 adult and pediatric nephrology centers.
A total of 96 patients were randomly assigned to one of three treatment groups: (1) alternate day prednisone (steroidal anti-inflammatory) (n = 33) (not analyzed in the present study), (2) 4 g/d concentrated FO containing ω-3 FA (1.9 g/d EPA + 1.5 g/d DHA) (n = 32), and (3) placebo (n = 31), half of whom were randomly assigned to either prednisone placebo (sugar pill) (not analyzed in the present study), and half of whom were randomly assigned to FO placebo in the form of CO. The outcome measure for the study was amelioration of kidney function as assessed by estimated glomerular filtration rate (eGFR) decreasing to <60% of baseline and as change in urine protein to creatinine ratio (UP/C). Subject inclusion criteria were: ≤40 years old at entry, biopsy confirmed IgAN, eGFR ≥50 mL/min per 1.73 m2, proteinuria or biopsy findings of either persistent severe proteinuria (first morning UP/C ≥1.0, or moderate proteinuria (UP/C ratio ≥0.5 plus renal biopsy changes indicating risk for progression—areas of glomerulosclerosis or proliferation). The subjects were supplemented for 24 months, and serum and first morning urine were collected at baseline as well as at regular intervals over the 24-month period, with time points at 6, 9, 12, 15, 18, and 24 months.
This study focused on a subset of samples from the original study (Hogg et al. 2006) and included subjects for whom matched Pre and Post samples were available, resulting in n=7 for the CO group and n=7 for the FO group. Baseline samples were used as Pre (pre-supplementation), and the 12-month time point was used for Post (post-supplementation) for as many patients as possible; however, in some cases, a 15- or 24-month time point was used as the Post time point when the 12-month time point was not available. In the CO group, all Post samples were from the 12-month time point, and in the FO group, two samples were from the 15-month time point, and one was from the 24-month time point. The resulting subset included paired Pre and Post samples for a total of 14 patients, 7 in the CO group and 7 in the FO group. The samples were stored at −80°C consistently, with no freeze-thaw cycles during the original study from collection to analysis, and the samples were shipped in dry ice and immediately placed into −80°C freezers upon receipt of the shipment.
2.2 Oxylipin profiling
Oxylipins were analyzed in accordance with protocols described elsewhere (Yang et al. 2009). Briefly, the plasma samples underwent solid phase extraction (SPE) on 60 mg Waters Oasis-HLB cartridges (Milford, MA). The elutions from the SPE cartridges were evaporated using a Speedvac (Jouan, St-Herblain, France) and reconstituted in a 200 nM 1-cyclohexyl ureido, 3-dodecanoic acid (CUDA) in a methanol solution. The LC system used for analysis was an Agilent 1200 SL (Agilent Corporation, Palo Alto, CA) equipped with a 2.1 × 150 mm Eclipse Plus C18 column with a 1.8 μm particle size (Agilent Corporation, Palo Alto, CA). The autosampler was kept at 4 °C. Mobile phase A was water with 0.1% glacial acetic acid. Mobile phase B consisted of acetonitrile/methanol (84:16) with 0.1% glacial acetic acid. Gradient elution was performed at a flow rate of 250 μL/min. Chromatography was optimized to separate all analytes in 21.5 min according to their polarity with the most polar analytes, prostaglandins and leukotrienes eluting first, followed by the hydroxy and epoxy fatty acids. The column was connected to a 4000 QTrap tandem mass spectrometer (Applied Biosystems Instrument Corporation, Foster City, CA) equipped with an electrospray source (Turbo V). The instrument was operated in negative multiple reaction monitor (MRM) mode. The optimized conditions and the MRM transitions, as well as extraction efficiencies were reported previously (Yang et al. 2009). Quality control samples were analyzed at a minimum frequency of 10 hours to ensure stability of the analytical calibration throughout the analysis. Analyst software 1.4.2 was used to quantify the peaks according to the standard curves.
2.3 Data processing and statistical analysis
Data were tested for normality and pre-processed to account for the high data density of the metabolomic approach used in this study (Zivkovic et al. 2009). Metabolites with >30% missing observations were excluded, observations that were >4 SD from the mean were excluded. Change-detection plots were used to determine whether the observed signal was greater than that which could be expected by chance (noise). All metabolite data were assessed for normality with histograms. When non-normality was detected, values were log transformed prior to analysis. Significance was set at α ≤0.05; no adjustments were made for multiple testing due to the exploratory nature of the analyses. Absolute concentrations of oxylipins are presented as nmol/L. Percentage change was calculated as ((Post-Pre)/Pre)*100. Student’s t-tests were performed using the software program JMP (SAS Institute Inc., Cary, NC).
Multivariate statistical procedures were employed to explore patterns in the oxylipin profiles of IgAN patients with paired samples before and after supplementation with CO (n = 7) or FO (n = 7). Oxylipin metabolite concentrations were log10-transformed and imported into SIMCA-P software (version 12.0.1; Umetrics, Umea, Sweden) for analysis. Data were mean centered and unit variance scaled. Unsupervised principal component analysis (PCA) was applied to all serum samples and scores plots were visually inspected for trends or outliers in the data. Partial least-squares discriminant analysis (PLS-DA) was then used to explore variations in metabolite concentrations between different classes within the data e.g. CO pre versus post, FO pre versus post and CO 12 mo versus FO 12 mo. A scores plot was created to visualize the PLS-DA model, and the corresponding loadings provided information on the contribution of metabolites to the separation of classes. The variable importance in the projection (VIP) value of each metabolite in the model was calculated to indicate its contribution to the classification of samples. Variables with a VIP value >1.5 were considered important in discriminating between groups. Differences in the metabolite concentrations between Pre and Post samples were validated using a paired t-test. The quality of all models was judged by the goodness-of-fit parameter (R2) and the predictive ability parameter (Q2), which is calculated by an internal cross-validation of the data and the predictability calculated on a leave-out basis. In addition, all PLS-DA models were validated externally by randomly selecting three-quarters of the samples to be used as a training data set, with the remaining samples serving as a test data set. The class of each sample in the test data set was predicted on the basis of the model built from the training set. This was repeated until all samples were predicted.
Paired Student’s t-tests were performed on paired samples before and after supplementation with CO (n = 7) or FO (n = 7) and % change for statistically significant metabolites presented in a heatmap. The IgAN patients as a group were further evaluated by the dichotomous variable of Improved (improved proteinuria as defined by ≤25% increase in UP/C ratio from baseline to 24 months) vs. Non-Improved (≥25% increase in UP/C ratio from baseline to 24 months), and Student’s t-test was used to evaluate significant differences in oxylipins between the two groups post-supplementation. Improved patients included 4 FO patients and 2 CO patients, and Non-Improved patients included 3 FO patients and 4 CO patients, with UP/C data missing for one of the CO patients.
3 Results
Out of a total of 87 metabolites measured, 2 metabolites (6-keto-PGF1α and 13-oxo-ODE) had >30% missing observations and were excluded from analysis. No individual observations were excluded on the basis of having concentrations >4 SD away from the mean.
3.1 Changes in oxylipins from pre- to post-supplementation within each group
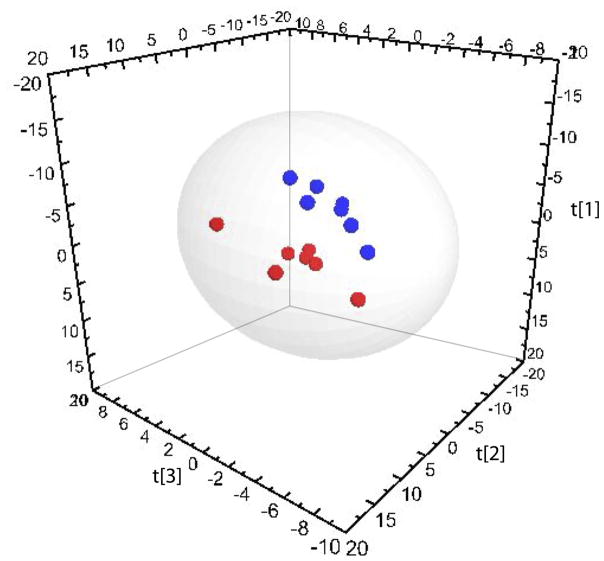
Multivariate statistical procedures were employed to compare oxylipin profiles before and after supplementation in paired Pre and Post samples from 7 IgAN patients consuming CO (placebo) and 7 IgAN patients consuming FO daily. PCA was applied to all CO samples and a scores plot was generated to examine potential outliers or apparent trends in the dataset. The first two components of the PCA accounted for 51% of the variation in the data. Visual inspection of the scores plot indicated no obvious separation of samples from before and after supplementation with the placebo. This was supported when supervised analysis failed to construct a robust model. PCA was repeated with all FO samples and the first three components accounted for 67% of the variation in the data. The PCA scores plot illustrated a clear separation of samples collected before and after FO supplementation. To investigate this further, PLS-DA was applied, resulting in a three-component model (Fig. 2: R2X = 0.60, R2Y = 0.97, Q2 = 0.65). External cross-validation of the model, as described in the methods section, indicated that 88 ± 14% of the samples were classified correctly. Analysis of VIP values revealed the oxylipin metabolites responsible for the separation of Pre and Post samples (Table 1). Paired samples t-tests confirmed that the concentrations of the oxylipins identified from multivariate analysis were significantly different in Pre and Post samples. The most significant discriminating oxylipins included (VIP >1.5) 17,18-DiHETE, PGD3, PGE3, Resolvin E1, 11,12-DiHETrE, 12-HEPE and 10(11)-EpDPE.
Fig. 2.
Partial least-squares discriminant analysis (PLS-DA) comparing serum oxylipin concentrations of IgA nephropathy patients before and after FO supplementation (R2X = 0.60, R2Y = 0.97, Q2 = 0.65). Red spheres (●) represent pre-supplementation samples; blue spheres (●) represent post-supplementatiom samples. Hotelling’s T2 95% confidence ellipse is indicated on the plot.
Table 1.
Oxylipin metabolites from IgA nephropathy patients pre and post supplementation with fish oil: variable importance in the projection (VIP) of partial least-squares discriminant analysis (PLS-DA)
| Oxylipin (nmol/L) | VIP | Pre-supplementationa,b | Post-supplementation | p-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SD | Mean | SD | |||
| 17,18-DiHETE | 1.9 | 0.28 | 0.26 | 1.7 | 1.0 | 0.003 |
| PGD3 | 1.8 | 27 | 27 | 270 | 200 | 0.001 |
| PGE3 | 1.6 | 2.2 | 1.4 | 8.3 | 5.1 | 0.04 |
| Resolvin E1 | 1.6 | 3.9 | 6.2 | 20 | 15 | 0.007 |
| 11,12-DiHETrE | 1.5 | 1.2 | 0.41 | 0.64 | 0.19 | 0.008 |
| 12-HEPE | 1.5 | 240 | 160 | 980 | 520 | 0.02 |
| 10(11)-EpDPE | 1.5 | 0.68 | 0.45 | 1.5 | 0.55 | 0.01 |
| 15-HEPE | 1.5 | 260 | 170 | 1,000 | 560 | 0.03 |
| 8(9)-EpETrE | 1.5 | 3.9 | 2.8 | 1.3 | 0.79 | 0.02 |
| 10,11-DiHDPE | 1.5 | 0.34 | 0.39 | 0.85 | 0.40 | 0.003 |
| 19(20)-EpDPE | 1.4 | 230 | 150 | 410 | 62 | 0.02 |
| 14(15)-EpETrE | 1.4 | 0.04 | 0.04 | 0.76 | 0.90 | 0.04 |
| 7,8-DiHDPE | 1.4 | 0.53 | 0.46 | 1.1 | 0.43 | 0.03 |
| 4,5-DiHDPE | 1.3 | 3.8 | 2.5 | 6.7 | 2.9 | 0.007 |
| 19,20-DiHDPE | 1.3 | 3.2 | 1.5 | 5.5 | 2.4 | 0.004 |
| 13(14)-EpDPE | 1.2 | 0.29 | 0.30 | 0.56 | 0.24 | 0.04 |
| 16(17)-EpDPE | 1.2 | 0.46 | 0.51 | 1.1 | 0.57 | 0.03 |
| 6-trans-LTB4 | 1.2 | 800 | 400 | 400 | 200 | 0.02 |
| 11,12-DiHETrE | 1.2 | 47 | 15 | 32 | 9.1 | 0.03 |
Shown are the metabolites with VIP values >1 as assessed by PLS-DA comparing serum oxylipin concentrations of IgA nephropathy patients before and after FO supplementation (R2X = 0.60, R2Y = 0.97, Q2 = 0.65); p-value of the paired t-test for all metabolites pre- vs. post-supplementation
Ep, epoxy; ODE, octadecadienoic acid; DiH, dihydroxy; ODE, octadecadienoic acid; EpTrE, eicosatrienoic acid; ETE, eicosatetraenoic acid; DPE, docosapentaenoic acid; PG, prostaglandin; LX, lipoxin; OME, octadecamonoenoic acid; LT, leukotriene; TX, thromboxane; TriH; trihydroxy; EPE, eicosapentaenoic acid; COX, cyclooxygenase; and LOX, lipoxygenase
In order to examine differences in the oxylipin profiles of CO- and FO-supplemented patients, post-supplementation samples from both groups were compared. The first two components of the PCA accounted for 52% of the variation in the data. Visual inspection of the PCA scores plot suggested that the oxylipin profiles from FO-supplemented patients were significantly different from the post samples collected from the CO group. PLS-DA was employed to probe the metabolic differences between these two groups of patients. A one-component model was constructed (R2X = 0.26, R2Y = 0.72, Q2 = 0.53) and analysis of VIP values identified several key metabolites. FO samples had higher concentrations of 17(18)-EpETE, 7,8-DiHDPE, 17,18-DiHETE, 19(20)-EpDPE, 4,5-DiHDPE, Resolvin E1, 10(11)-EpDPE, 16(17)-EpDPE, PGD3, 13(14)-EpDPE, 7(8)-EpDPE, 10,11-DiHDPE, and 19,20-DiHDPE, and lower concentrations of 6-trans-LTB4 and 5,6-DiHETrE. External validation of this model indicated that 81 ± 24% of samples were classified correctly.
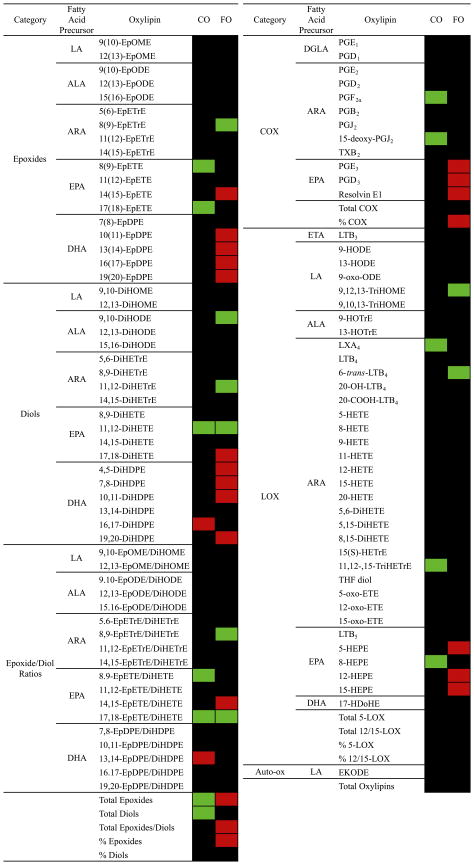
Metabolite changes from pre- to post-supplementation in FO- and CO-supplemented patients are shown in Fig. 3 as a heatmap. Metabolites were evaluated by paired t-test, and if significantly increased are shown in red and if significantly decreased are shown in green. In the CO group there were few changes; several EPA metabolites including two epoxides and their corresponding epoxide/diol ratios as well as total epoxides and total diols decreased. In the FO group, on the other hand, DHA epoxides and diols increased as did total epoxides, total epoxide/diol ratios, and percentage of epoxides. The EPA COX metabolites PGE3, PGD3, and Resolvin E1, the EPA epoxide 17,18-DiHETE, as well as the EPA LOX metabolites 5-, 12-, and 15-HEPE also increased. The ARA LOX metabolite 6-trans-LTB4 decreased as did the ARA epoxide 8,9-EpETrE.
Fig. 3.
Heatmap of changes in IgA nephropathy patients supplemented with FO (n = 7) and CO (n = 7) placebo. Data are percentage change from pre- to post-supplementation in each group for each metabolite. If the paired t-test was not significant, the squares are black, if the paired t-test was significant, the squares are either red (increased) or green (decreased). LA, linoleic acid; ALA, α-linolenic acid; ARA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DGLA, dihommo γ-linolenic acid; ETA, eicosatrienoic acid (20:3n9); Ep, epoxy; ODE, octadecadienoic acid; DiH, dihydroxy; ODE, octadecadienoic acid; ETrE, eicosatrienoic acid; ETE, eicosatetraenoic acid; DPE, docosapentaenoic acid; PG, prostaglandin; LX, lipoxin; OME, octadecamonoenoic acid; LT, leukotriene; TX, thromboxane; TriH; trihydroxy; EPE, eicosapentaenoic acid; COX, cyclooxygenase; and LOX, lipoxygenase.
3.2 Improved vs. Non-Improved patients
The IgAN patients as a group were further divided into patients whose proteinuria was Improved vs. Non-Improved based on the percentage increase in their UP/C ratios (an increase in the UP/C ratio is associated with decreased kidney function) from baseline to 24 months such that patients categorized as Improved had a >25% improvement in proteinuria as measured by UP/C ratios (i.e., ≤25% increase in UP/C from baseline to 24 months), and patients that were Non-Improved had ≤25% improvement (i.e., ≥25% increase in UP/C from baseline to 24 months). Differences between Improved and Non-Improved patients post supplementation were evaluated and results are presented in Table 2.
Table 2.
Oxylipin metabolite differences between IgA nephropathy patients whose kidney function improved compared with those whose kidney function did not improve
| Oxylipin (nmol/L) | Post-supplementationa,b
|
||||
|---|---|---|---|---|---|
| Non-Improved | Improved | p-value | |||
|
| |||||
| Mean | SE | Mean | SE | ||
| Total oxylipins | 96,000 | 19,000 | 50,000 | 6,200 | 0.01 |
| Total 12/15-LOX | 43,000 | 5,200 | 25,000 | 2,700 | 0.01 |
| Total 5-LOX | 42,000 | 8,400 | 24,000 | 3,800 | 0.04 |
| 9-HODE | 18,000 | 3,500 | 9,500 | 1,500 | 0.03 |
| 13-HODE | 17,000 | 4,000 | 8,100 | 1,500 | 0.03 |
| 9-HETE | 4,900 | 1,400 | 2,100 | 370 | 0.03 |
| 11-HETE | 4,600 | 1,000 | 1,900 | 360 | 0.02 |
| 8,15-DiHETE | 4,300 | 1,200 | 1,700 | 270 | 0.02 |
| 12-HETE | 3,100 | 830 | 1,200 | 230 | 0.01 |
| 8-HETE | 2,400 | 770 | 950 | 160 | 0.03 |
| 15-oxo-ETE | 1,600 | 470 | 690 | 100 | 0.03 |
| 6-trans-LTB4 | 660 | 75 | 420 | 84 | 0.04 |
| 9,12,13-TriHOME | 530 | 180 | 200 | 40 | 0.03 |
| 13-HOTrE | 500 | 120 | 130 | 31 | 0.01 |
| Total COX | 380 | 54 | 220 | 32 | 0.02 |
| 9-HOTrE | 320 | 82 | 92 | 13 | 0.01 |
| 11,12-,15-TriHETrE | 240 | 99 | 86 | 15 | 0.03 |
| LXA4 | 120 | 21 | 74 | 6.3 | 0.03 |
| PGD2 | 87 | 13 | 47 | 16 | 0.02 |
| PGB2 | 39 | 7.8 | 16 | 2.4 | 0.005 |
| PGF2α | 26 | 5.3 | 13 | 2.1 | 0.02 |
| EKODE | 25 | 6.6 | 10 | 1.8 | 0.04 |
| LTB4 | 19 | 2.7 | 11 | 1.6 | 0.02 |
| PGD1 | 17 | 1.7 | 11 | 3.0 | 0.04 |
| PGE3 | 8.3 | 2.0 | 3.4 | 1.1 | 0.04 |
| 11(12)-EpETrE/DiHETrE | 5.0 | 0.62 | 3.3 | 0.62 | 0.03 |
| 14(15)-EpETrE/DiHETrE | 4.2 | 0.79 | 2.5 | 0.42 | 0.03 |
| 20-OH-LTB4 | 3.4 | 0.89 | 1.2 | 0.37 | 0.03 |
| 14(15)-EpETrE | 2.6 | 0.40 | 1.7 | 0.18 | 0.04 |
| 8(9)-EpETrE | 1.9 | 0.31 | 1.0 | 0.21 | 0.02 |
| 8(9)-EpETrE/DiHETrE | 1.6 | 0.16 | 0.72 | 0.17 | 0.01 |
Metabolites significantly different by t-test comparison of patients whose kidney function was improved >25% as measured by UP/C ratios (i.e., ≤25% change in UP/C from baseline to 24 months) and patients whose kidney function did not improve (i.e., ≥25% change in UP/C from baseline to 24 months)
Ep, epoxy; ODE, octadecadienoic acid; DiH, dihydroxy; ODE, octadecadienoic acid; ETrE, eicosatrienoic acid; ETE, eicosatetraenoic acid; DPE, docosapentaenoic acid; PG, prostaglandin; LX, lipoxin; OME, octadecamonoenoic acid; LT, leukotriene; TX, thromboxane; TriH; trihydroxy; EPE, eicosapentaenoic acid; COX, cyclooxygenase; and LOX, lipoxygenase
Improved patients had lower concentrations of total oxylipins and a number of metabolites including the LA-derived HODEs, the ARA-derived HETEs and LTB4 and its metabolites 20-OH-LTB4 and 6-trans-LTB4. The HODEs and HETEs primarily accounted for the quantitative decrease in total oxylipins in Improved patients. Improved patients also had lower concentrations of several COX metabolites including PGB2 and PGF2α, and several ARA epoxide/diol ratios including 8(9)-, 11(12)-, and 14(15)-EpETrE/DiHETrE.
4 Discussion
In this study, changes in oxylipin profiles in response to supplementation with a high dose of ω-3 FA were evaluated in patients with IgAN. Multivariate analyses of FO patients pre vs. post supplementation showed that EPA- and DHA-derived oxylipins were the metabolites with the highest VIP values (>1.5), indicating that these metabolites primarily differentiated the pre and post samples. The EPA COX metabolites PGD3, PGE3, and Resolvin E1 increased as much as 10-fold post supplementation. Likewise, the EPA diol 17,18-DiHETE and the DHA epoxide 10(11)-EpDPE increased, as did the EPA LOX metabolite 12-HEPE, and these metabolites were all key drivers of the separation between pre and post samples. Several other EPA and DHA epoxides and diols and the EPA LOX metabolite 15-HEPE significantly increased and had VIP values >1. Several ARA metabolites also differentiated the pre and post samples, and included the diol 11,12-DiHETrE, the epoxide 8(9)-EpETrE, and the LOX metabolite 6-trans-LTB4, which all decreased post supplementation. These results suggest that EPA-derived COX metabolites, EPA-derived LOX metabolites, and EPA and DHA epoxides and diols all increased in response to supplementation with ω-3 FA, with concomitant decreases in a few key ARA-derived metabolites. These data confirm that at the ω-3 supplementation dosage in these IgAN patients, the levels of EPA and DHA provided a substrate concentration significant enough to increase the production of EPA and DHA oxylipins and that these particular oxylipins may be markers of ω-3 supplementation.
Multivariate analyses of data for FO vs. CO patients post supplementation also found that EPA and DHA epoxides and diols as well as the EPA-derived COX metabolites PGD3 and Resolvin E1, which were all higher in FO patients compared with CO patients, differentiated the two groups. The ARA LOX metabolite 6-trans-LTB4 was also a driver of the separation, and was lower in FO patients compared with CO patients post supplementation. These data support the observation that conversion of EPA and DHA to epoxides by CYPs and their subsequent conversion to diols by sEH as well as EPA conversion to COX and LOX metabolites occurred in response to supplementation with ω-3 FA in IgAN patients. It is interesting to note that all three branches of the oxylipin cascade (i.e., COX, LOX, and CYP) were affected by the supplementation by ω-3 FA.
As illustrated in the heatmap (Fig. 3), although total oxylipins did not significantly change in either the FO or the CO patients, total epoxides, total epoxide/diol ratios, and % epoxides increased in FO patients, whereas total epoxides and total diols decreased in CO patients. In FO patients, most of the DHA epoxides and diols increased post supplementation, whereas among the EPA epoxides and diols, only a few changed significantly. As observed in the multivariate analyses, the EPA COX and LOX metabolites—PGE3, PGD3, Resolvin E1, and 5-, 12-, 15-HEPE, respectively—increased significantly in FO-supplemented patients. These data suggest that in IgAN patients, the two ω-3 FA may have had different fates as oxylipin precursors, with EPA being diverted primarily toward the production of COX and LOX metabolites and DHA being diverted toward the production of epoxides and diols. Total oxylipins were not significantly decreased and only a few metabolite-selective changes in ARA oxylipin species were observed. This may be explained by the fact that some ARA-derived oxylipins are present at much higher concentrations compared with ω-3 FA-derived oxylipins. For example, the mean concentration of the ARA-derived LOX metabolite 5-HETE in FO patients pre supplementation was 14,000 nmol/L, whereas the mean concentration of the EPA-derived LOX metabolite 5-HEPE was 680 nmol/L, or nearly 20-fold lower. Even though 5-HETE decreased post supplementation to 11,000 nmol/L, this decrease was not statistically significant.
On the other hand, the lack of statistically significant decrease in total oxylipins and ARA-derived metabolites among FO patients may be related to the fact that kidney function improved in only 4 out of 7 of the patients. In fact, when the IgAN patients as a group (including both FO and CO patients) were subdivided into those whose kidney function (as measured by a decrease in proteinuria or UP/C ratios) improved regardless of whether they were supplemented with ω-3 FA, major differences among the ARA-derived oxylipins appeared. Notably, the HODEs including 9-HODE and 13-HODE, and HETEs including 8-, 9-, 11-, and 12-HETE were all about 2-fold lower in patients whose kidney function improved. Thus, the decreases in these LA- and ARA-derived metabolites may not be statistically significant among the FO patients because their decrease may be associated with improvement in kidney function, which only occurred in half of the FO patients.
Decreases in the HODEs and HETEs in a group of healthy subjects supplemented with similar amounts of ω-3 FA were reported (Shearer et al. 2009). Increases in the LA-derived 9-HODE and 13-HODE have been reported in patients with other inflammatory diseases including rheumatoid arthritis (Jira et al. 1997) and atherosclerosis (Jira et al. 1998). The HODEs are also known to induce inflammatory cytokine expression, including tumor necrosis factor- α and intercellular adhesion molecule, and reactive oxygen species production in vascular endothelial cells (Wang et al. 2009). Moreover, 9-HODE induces mitogen-activated protein kinase activation in macrophages found in atherosclerotic lesions (Obinata et al. 2005), induces collagen synthesis, cell proliferation, and extracellular matrix formation in mesangial cells (Negishi et al. 2004), and is the major component of oxidized LDL particles (Negishi et al. 2004). The HODEs as well as the HETEs were reported to be 4-fold and 20-fold higher in HDL and VLDL, respectively, of rats with proteinuria (Newman et al. 2007). Although the LA-derived HODEs and ARA-derived HETEs were not significantly different post ω-3 FA supplementation in the FO group, when both FO and CO patients whose kidney function improved were examined as a group, the HODEs and HETEs were significantly lower compared with those whose kidney function did not improve. These results suggest that the HODEs and HETEs may be indicative of or involved in kidney dysfunction.
The ARA LOX metabolites LTB4, 6-trans-LTB4, and 20-OH-LTB4 as well as the HETEs were all significantly lower in those patients whose kidney function improved, and 6-trans-LTB4 was a driver in the separation of FO and CO patients. Previous studies have shown that 5-LOX expression is correlated with impaired renal function in patients with nephrotic syndrome (Menegatti et al. 1999). Patients with glomerulonephritis (including IgAN patients) who expressed 5-LOX and the 5-LOX activating protein had lower glomerular filtration rates and higher proteinuria (Rifai et al. 1993). ω-3 FA inhibit 5-LOX activity without affecting gene expression (Taccone-Gallucci et al. 2006). The involvement of the 12- and 15-LOX pathways in IgAN is not known. The data suggest that in IgAN patients the production of inflammatory LA- and ARA-derived LOX metabolites may be decreased by ω-3 FA supplementation and that the ability to decrease the production of these metabolites may be associated with improved kidney function.
In this study, epoxide/diol ratios increased in IgAN patients, mostly due to increased DHA epoxides, after ω-3 FA supplementation. In a previous study in healthy subjects supplemented with a similar dose of ω-3, both EPA and DHA epoxides and diols increased in response to supplementation (Shearer et al. 2009). This suggests that in IgAN patients, the conversion of ω-3 epoxides to diols may be decreased following ω-3 FA supplementation. It has been postulated that the positive effects of ω-3 FA in IgAN patients may be the result of epoxides derived from EPA and DHA as these epoxides induce arteriolar vasodilation via large-conductance calcium-activated potassium channels in vitro (Ye et al. 2002). The epoxides of both EPA and DHA, formed by the action of CYP enzymes (Harmon et al. 2006), also inhibit NFkB inflammatory signaling via PPAR activation (Li et al. 2005). Interestingly, in this study no differences were observed in the EPA and DHA epoxides and diols in patients whose kidney function was Improved or Non-Improved, suggesting that although these metabolites increased in response to ω-3 FA supplementation, they may not be indicative of improvement in kidney function. It is also possible that changes in epoxide and diol concentrations occurred within the kidney but that these changes were not observable in the serum.
Limitations of the study include a low sample size and use of the UP/C ratios in lieu of 24-hr urine protein for measurement of proteinuria as a marker of kidney function. Despite these limitations, this study provides a unique perspective on signaling lipids and the effects of ω-3 FA supplementation in this inflammatory condition. The re-analysis of these stored samples from a large multi-center trial represented a unique opportunity that would be difficult, time consuming and expensive to repeat.
Many of the changes in oxylipins observed in this study are a reflection of divergent pathways in oxylipin metabolism and their divergence is the basis of further study. Resolving by accurate measurement how oxylipins are affected by different disease states has become a valuable aspect of diagnostic science, and successes in a variety of fields from eicosanoid production and inflammation (Funk 2001) to PGE2 and cancer (Baratelli et al. 2005a), demonstrate the value of oxylipin measurement as a clinical toolset. The current study showed significant divergent effects of ω-3 FA supplementation in IgAN patients compared with patients supplemented with CO and significant differences in oxylipin profiles among patients whose kidney function improved compared with those in whom kidney function did not improve or worsened. Yet, the biological activities of the ω-3-derived oxylipins are poorly understood, as is the nature of the net impact of the comprehensive oxylipin profile on assessing health and monitoring disease. More research is needed to elucidate the mechanisms by which the ω-3-derived oxylipins affect health status, disease risk, and treatment effectiveness so that more targeted treatments can be formulated.
Acknowledgments
The authors acknowledge Dr Danica Skibola for her support and help with clinical sample preparation, and C. J. Dillard with manuscript editing. This work was supported by The Center for Health and Nutrition Research (CHNR) Pilot Grant Program, National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK49368; The University of California Discovery Program (05GEB01NHB); the National Institute of Environmental Health Sciences (NIEHS) (P42ES004699), NIEHS R01 ES002710, and NIEHS Superfund Research Program P42 ES011269; the California Dairy Research Foundation; the CHARGE study (P01 ES11269); and partial support was provided by the American Asthma Association #09-0269. JY was supported by the Elizabeth Nash Memorial fellowship from the Cystic Fibrosis Research Inc. BDH is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Contributor Information
Angela M. Zivkovic, Email: amzivkovic@ucdavis.edu, Foods for Health Institute, Department of Entomology, Department of Food Science and Technology, University of California, Davis, CA, phone: 530-752-9211; Fax: 530-752-4759
Jun Yang, Department of Entomology, University of California, Davis, CA.
Katrin Georgi, Department of Entomology, University of California, Davis, CA.
Christine Hegedus, Department of Entomology, University of California, Davis, CA.
Malin L. Nording, Department of Entomology, University of California, Davis, CA
Aifric O’Sullivan, Foods for Health Institute, University of California, Davis, CA.
J. Bruce German, Foods for Health Institute, Department of Food Science and Technology, University of California, Davis, CA.
Ronald J. Hogg, Scott & White Clinic, Temple, TX
Robert H. Weiss, Nephrology Division, Department of Medicine, University of California at Davis, Davis, CA
Curt Bay, Department of Interdisciplinary Health Sciences, Arizona School of Health Sciences, A.T. Still University, Mesa, AZ.
Bruce D. Hammock, Foods for Health Institute, Department of Entomology, University of California, Davis, CA
References
- Alexopoulos E, Stangou M, Pantzaki A, Kirmizis D, Memmos D. Treatment of severe IgA nephropathy with omega-3 fatty acids: the effect of a “very low dose” regimen. Ren Fail. 2004;26(4):453–459. doi: 10.1081/jdi-200026763. [DOI] [PubMed] [Google Scholar]
- Appel GB, Waldman M. The IgA nephropathy treatment dilemma. Kidney Int. 2006;69(11):1939–1944. doi: 10.1038/sj.ki.5000434. [DOI] [PubMed] [Google Scholar]
- Baratelli F, Krysan K, Heuze-Vourc’h N, Zhu L, Escuadro B, Sharma S, et al. PGE2 confers survivin-dependent apoptosis resistance in human monocyte-derived dendritic cells. J Leukoc Biol. 2005a;78(2):555–564. doi: 10.1189/jlb.1004569. jlb.1004569 [pii] [DOI] [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005b;175(3):1483–1490. doi: 10.4049/jimmunol.175.3.1483. 175/3/1483 [pii]. [DOI] [PubMed] [Google Scholar]
- Baxter GM. Alterations of endothelium-dependent digital vascular responses in horses given low-dose endotoxin. Vet Surg. 1995;24(2):87–96. doi: 10.1111/j.1532-950x.1995.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Bennett WM, Walker RG, Kincaid-Smith P. Treatment of IgA nephropathy with eicosapentanoic acid (EPA): a two-year prospective trial. Clin Nephrol. 1989;31(3):128–131. [PubMed] [Google Scholar]
- Branten AJ, Klasen IS, Wetzels JF. Short-term effects of fish oil treatment on urinary excretion of high- and low-molecular weight proteins in patients with IgA nephropathy. Clin Nephrol. 2002;58(4):267–274. doi: 10.5414/cnp58267. [DOI] [PubMed] [Google Scholar]
- Catella-Lawson F. Vascular biology of thrombosis: platelet-vessel wall interactions and aspirin effects. Neurology. 2001;57(5 Suppl 2):S5–7. doi: 10.1212/wnl.57.suppl_2.s5. [DOI] [PubMed] [Google Scholar]
- Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant. 2009;24(8):2406–2410. doi: 10.1093/ndt/gfp091. gfp091 [pii] [DOI] [PubMed] [Google Scholar]
- Cheng IK, Chan PC, Chan MK. The effect of fish-oil dietary supplement on the progression of mesangial IgA glomerulonephritis. Nephrol Dial Transplant. 1990;5(4):241–246. doi: 10.1093/ndt/5.4.241. [DOI] [PubMed] [Google Scholar]
- Clevers H. Colon cancer--understanding how NSAIDs work. N Engl J Med. 2006;354(7):761–763. doi: 10.1056/NEJMcibr055457. 354/7/761 [pii] [DOI] [PubMed] [Google Scholar]
- Donadio JV, Bergstralh EJ, Offord KP, Holley KE, Spencer DC. Clinical and histopathologic associations with impaired renal function in IgA nephropathy. Mayo Nephrology Collaborative Group. Clin Nephrol. 1994;41(2):65–71. [PubMed] [Google Scholar]
- Donadio JV, Grande JP. The role of fish oil/omega-3 fatty acids in the treatment of IgA nephropathy. Semin Nephrol. 2004;24(3):225–243. doi: 10.1016/j.semnephrol.2004.01.004. S0270929504000063 [pii]. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N Engl J Med. 1994;331(18):1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Jr, Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am Soc Nephrol. 1999;10(8):1772–1777. doi: 10.1681/ASN.V1081772. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Jr, Larson TS, Bergstralh EJ, Grande JP. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol. 2001;12(4):791–799. doi: 10.1681/ASN.V124791. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Harmon SD, Fang X, Kaduce TL, Hu S, Raj Gopal V, Falck JR, et al. Oxygenation of [omega]-3 fatty acids by human cytochrome P450 4F3B: Effect on 20-hydroxyeicosatetraenoic acid production. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75(3):169–177. doi: 10.1016/j.plefa.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Hogg RJ. A randomized, placebo-controlled, multicenter trial evaluating alternate-day prednisone and fish oil supplements in young patients with immunoglobulin A nephropathy. Scientific Planning Committee of the IgA Nephropathy Study. Am J Kidney Dis. 1995;26(5):792–796. doi: 10.1016/0272-6386(95)90445-x. [DOI] [PubMed] [Google Scholar]
- Hogg RJ, Lee J, Nardelli N, Julian BA, Cattran D, Waldo B, et al. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol. 2006;1(3):467–474. doi: 10.2215/CJN.01020905. CJN.01020905 [pii] [DOI] [PubMed] [Google Scholar]
- Holman RT, Johnson SB, Bibus D, Spencer DC, Donadio JV., Jr Essential fatty acid deficiency profiles in idiopathic immunoglobulin A nephropathy. Am J Kidney Dis. 1994;23(5):648–654. doi: 10.1016/s0272-6386(12)70274-3. S0272638694000867 [pii]. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, et al. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46(4):975–981. doi: 10.1161/01.HYP.0000176237.74820.75. 01.HYP.0000176237.74820.75 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jira W, Spiteller G, Carson W, Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids. 1998;91(1):1–11. doi: 10.1016/s0009-3084(97)00095-9. S0009-3084(97)00095-9 [pii]. [DOI] [PubMed] [Google Scholar]
- Jira W, Spiteller G, Richter A. Increased levels of lipid oxidation products in low density lipoproteins of patients suffering from rheumatoid arthritis. Chem Phys Lipids. 1997;87(1):81–89. doi: 10.1016/s0009-3084(97)00030-3. S0009-3084(97)00030-3 [pii]. [DOI] [PubMed] [Google Scholar]
- Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290(2):H491–499. doi: 10.1152/ajpheart.00927.2005. 00927.2005 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-[gamma]-dependent mechanism. Kidney Int. 2005;67(3):867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- Libetta C, Rampino T, Palumbo G, Esposito C, Dal Canton A. Circulating serum lectins of patients with IgA nephropathy stimulate IL-6 release from mesangial cells. J Am Soc Nephrol. 1997;8(2):208–213. doi: 10.1681/ASN.V82208. [DOI] [PubMed] [Google Scholar]
- Menegatti E, Roccatello D, Fadden K, Piccoli G, De Rosa G, Sena LM, et al. Gene expression of 5-lipoxygenase and LTA4 hydrolase in renal tissue of nephrotic syndrome patients. Clin Exp Immunol. 1999;116(2):347–353. doi: 10.1046/j.1365-2249.1999.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J, Hashim OH, Tomana M. Alterations in the IgA carbohydrate chains influence the cellular distribution of IgA1. Contrib Nephrol. 1995;111:66–71. doi: 10.1159/000423879. discussion 71–62. [DOI] [PubMed] [Google Scholar]
- Myllymaki J, Syrjanen J, Helin H, Pasternack A, Kattainen A, Mustonen J. Vascular diseases and their risk factors in IgA nephropathy. Nephrol Dial Transplant. 2006;21(7):1876–1882. doi: 10.1093/ndt/gfl062. gfl062 [pii] [DOI] [PubMed] [Google Scholar]
- Negishi M, Shimizu H, Okada S, Kuwabara A, Okajima F, Mori M. 9HODE stimulates cell proliferation and extracellular matrix synthesis in human mesangial cells via PPARgamma. Exp Biol Med (Maywood) 2004;229(10):1053–1060. doi: 10.1177/153537020422901010. 229/10/1053 [pii]. [DOI] [PubMed] [Google Scholar]
- Newman JW, Kaysen GA, Hammock BD, Shearer GC. Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J Lipid Res. 2007;48(8):1792–1800. doi: 10.1194/jlr.M700146-JLR200. M700146-JLR200 [pii] [DOI] [PubMed] [Google Scholar]
- Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005;280(49):40676–40683. doi: 10.1074/jbc.M507787200. M507787200 [pii] [DOI] [PubMed] [Google Scholar]
- Parinyasiri U, Ong-Ajyooth L, Parichatikanond P, Ong-Ajyooth S, Liammongkolkul S, Kanyog S. Effect of fish oil on oxidative stress, lipid profile and renal function in IgA nephropathy. J Med Assoc Thai. 2004;87(2):143–149. [PubMed] [Google Scholar]
- Peeraully MR, Sievert H, Bullo M, Wang B, Trayhurn P. Prostaglandin D2 and J2-series (PGJ2, Delta12-PGJ2) prostaglandins stimulate IL-6 and MCP-1, but inhibit leptin, expression and secretion by 3T3-L1 adipocytes. Pflugers Arch. 2006;453(2):177–187. doi: 10.1007/s00424-006-0118-x. [DOI] [PubMed] [Google Scholar]
- Pettersson EE, Rekola S, Berglund L, Sundqvist KG, Angelin B, Diczfalusy U, et al. Treatment of IgA nephropathy with omega-3-polyunsaturated fatty acids: a prospective, double-blind, randomized study. Clin Nephrol. 1994;41(4):183–190. [PubMed] [Google Scholar]
- Rifai A, Sakai H, Yagame M. Expression of 5-lipoxygenase and 5-lipoxygenase activation protein in glomerulonephritis. Kidney Int Suppl. 1993;39:S95–99. [PubMed] [Google Scholar]
- Roccatello D, Picciotto G, Torchio M, Ropolo R, Ferro M, Franceschini R, et al. Removal systems of immunoglobulin A and immunoglobulin A containing complexes in IgA nephropathy and cirrhosis patients. The role of asialoglycoprotein receptors. Lab Invest. 1993;69(6):714–723. [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Novel endogenous small molecules as the checkpoint controllers in inflammation and resolution: entree for resoleomics. Rheum Dis Clin North Am. 2004;30(1):69–95. doi: 10.1016/S0889-857X(03)00117-0. S0889857X03001170 [pii]. [DOI] [PubMed] [Google Scholar]
- Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2009 doi: 10.1194/M900193-JLR200. M900193-JLR200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulikowska B, Manitius J, Nieweglowski T, Szydlowska-lysiak W, Rutkowski B. The effect of therapy with small doses of mega-3 polyunsaturated fatty acid on renal reserve and metabolic disturbances in patients with primary IGA glomerulopathy. Pol Arch Med Wewn. 2002;108(2):753–760. [PubMed] [Google Scholar]
- Sulikowska B, Nieweglowski T, Manitius J, Lysiak-Szydlowska W, Rutkowski B. Effect of 12-month therapy with omega-3 polyunsaturated acids on glomerular filtration response to dopamine in IgA nephropathy. Am J Nephrol. 2004;24(5):474–482. doi: 10.1159/000080670. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Leung N, Lager DJ, Melton LJ, 3rd, Bergstralh EJ, Rohlinger A, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1(3):483–487. doi: 10.2215/CJN.00710805. CJN.00710805 [pii] [DOI] [PubMed] [Google Scholar]
- Syrjanen J, Huang XH, Mustonen J, Koivula T, Lehtimaki T, Pasternack A. Angiotensin-converting enzyme insertion/deletion polymorphism and prognosis of IgA nephropathy. Nephron. 2000;86(2):115–121. doi: 10.1159/000045728. nef86115 [pii]. [DOI] [PubMed] [Google Scholar]
- Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M. N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006;69(8):1450–1454. doi: 10.1038/sj.ki.5000291. [DOI] [PubMed] [Google Scholar]
- Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4(10):982–990. doi: 10.1038/ni970. ni970 [pii]. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Hammock BD, Newman JW, Meerarani P, Toborek M, Hennig B. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J Am Coll Nutr. 2003;22(6):502–510. doi: 10.1080/07315724.2003.10719328. [DOI] [PubMed] [Google Scholar]
- Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50(2):204–213. doi: 10.1194/jlr.M700505-JLR200. M700505-JLR200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303(2):768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15(5):1244–1253. [PubMed] [Google Scholar]
- Zivkovic AM, Wiest MM, Nguyen UT, Davis R, Watkins SM, German JB. Effects of sample handling and storage on quantitative lipid analysis in human serum. Metabolomics. 2009;5(4):507–516. doi: 10.1007/s11306-009-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]