Abstract
Diabetic nephropathy is a well-known complication of diabetes and is a leading cause of chronic renal failure in the Western world. It is characterized by the accumulation of extracellular matrix in the glomerular and tubulointerstitial compartments and by the thickening and hyalinization of intrarenal vasculature. The various cellular events and signaling pathways activated during diabetic nephropathy may be similar in different cell types. Such cellular events include excessive channeling of glucose intermediaries into various metabolic pathways with generation of advanced glycation products, activation of protein kinase C, increased expression of transforming growth factor β and GTP-binding proteins, and generation of reactive oxygen species. In addition to these metabolic and biochemical derangements, changes in the intraglomerular hemodynamics, modulated in part by local activation of the renin-angiotensin system, compound the hyperglycemia-induced injury. Events involving various intersecting pathways occur in most cell types of the kidney.
Keywords: hyperglycemia, advanced glycation products, protein kinase C, GTP-binding proteins, reactive oxygen species, hypertension, tubulointerstitial fibrosis
INTRODUCTION
Diabetic nephropathy is a known microvascular complication in patients with diabetes mellitus, a metabolic disorder in which chronic hyperglycemia induces dysfunction in various cell types of the kidney, ultimately leading to progressive renal failure (1–7). The annual incidence of this disease has more than doubled in the past decade, and at present it accounts for almost 50% of all end-stage renal diseases. Type 1 and type 2 diabetes are distinct in pathogenesis, but the changes these disorders induce in various kidney compartments— namely the glomerulus, tubulointerstitium, and vasculature—are virtually indistinguishable (7). Moreover, it seems that all the cell types of the kidney, including glomerular podocytes, mesangial and endothelial cells, tubular epithelia, interstitial fibroblasts, and vascular endothelia, are affected by hyperglycemic injury, although to varying degrees. Because these cells are derived from ectodermal, endodermal, and mesodermal progenitors, this susceptibility may imply that hyperglycemia can induce injury in cells of all three lineages; if so, the term microvascular complication may be a misnomer. Certainly, the initial stages of diabetic nephropathy can be ascribed to dysfunctions of glomerular capillaries, which clinically manifest as hyperfiltration and microalbuminuria (3, 8). Subsequent changes affect all the compartments and cell types of the kidney.
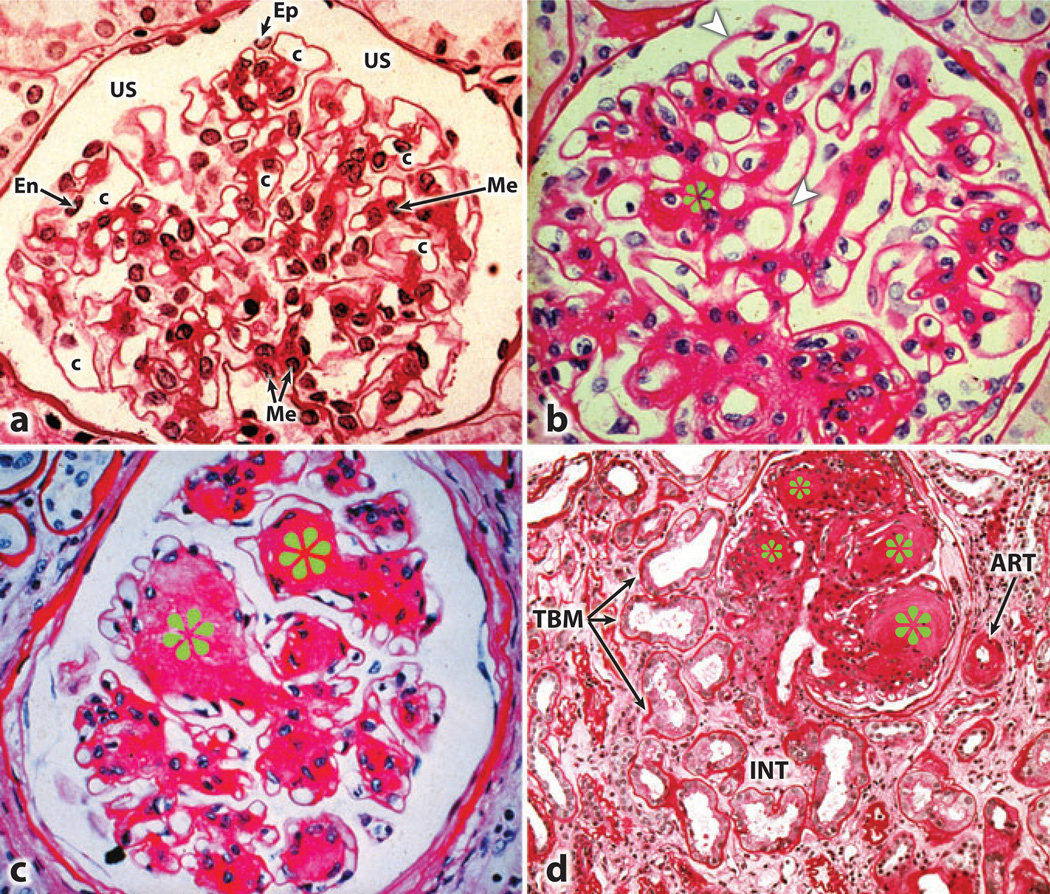
Well-described characteristic glomerular changes include mesangial cell proliferation and hypertrophy, excessive accumulation of extracellular matrix (ECM) proteins in the form of increased mesangial matrix, and thickening of the glomerular basement membrane (GBM), which eventually leads to nodular glomeru-losclerosis, also known as Kimmelstiel-Wilson lesions (Figure 1) (3, 9). Similar changes occur in the tubulointerstitial compartment, including tubular hypertrophy followed by thickening of the tubular basement membrane (TBM) and interstitial fibrosis (Figure 1d). The latter may be due to a unique process known as epithelialto-mesenchymal transformation (EMT), which transforms tubular cells into interstitial cells; this process causes excessive ECM to accumulate in the renal interstitium (10, 11). Vascular changes typically include thickening and hyalinization of the afferent arterioles and interlobular arteries with effacement of their endothelia.
Figure 1.
Light photomicrographs illustrating various stages of developing glomerular lesions and tubulointerstitial disease in diabetic nephropathy. (a) A normal glomerulus. (b) Thickened basement membranes (arrowheads) and expanded mesangial regions (asterisks). (c) The nodular appearance of the mesangial regions characteristic of Kimmelstiel-Wilson lesions (asterisks). (d) The tubulointerstitial lesions include thickened tubular basement membrane (TBM), hyalinization of afferent arteriole (ART), and fibrosis of the interstitium (INT). Abbreviations: c, capillary lumen; En, endothelial cell; Ep, visceral epithelial cell (podocyte); Me, mesangium; US, urinary space.
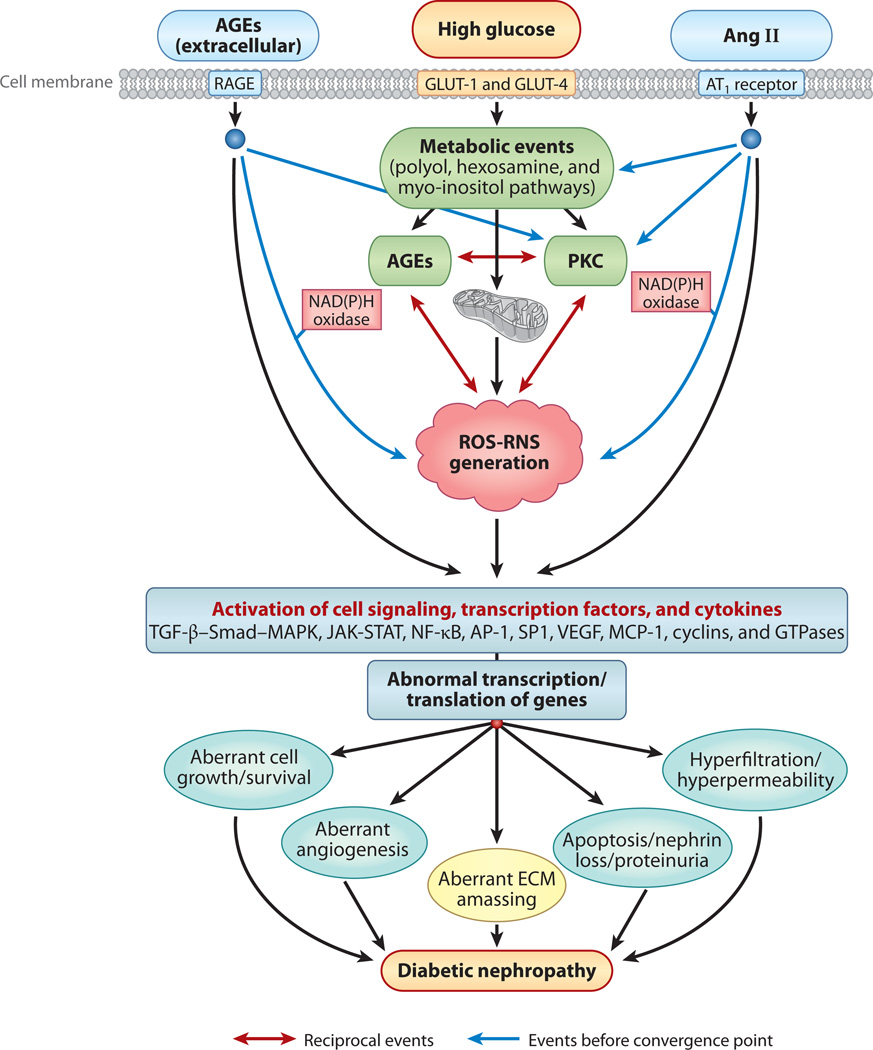
Several lines of evidence suggest that all the cellular elements of the kidney respond to hyperglycemic challenge by activating similar intracellular signaling events, albeit with some variation depending on the expression of a given molecule in a particular cell type, for instance, aldose reductase (AR) and myo-inositol oxygenase in the tubular cells and protein kinase C (PKC)-α and -β isoforms in the glomeruli (12–14). The intracellular events induced in the presence of high-glucose ambience include accentuated flux of polyols and hexosamines; generation of advanced glycation end products (AGEs) and reactive oxygen species (ROS); activation of the PKC, transforming growth factor β–Smad–mitogen-activated protein kinase (TGF-β–Smad–MAPK), and Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathways and of G protein signaling; aberrant expression of cyclin kinases and their inhibitors; and aberrant expression of ECM proteins, ECM-degrading enzymes, metalloproteinases, and their inhibitors (Figure 2) (9, 15–24).
Figure 2.
An overview of different signaling events induced by exposure of renal cells to high glucose concentrations, with resulting altered expression of various genes and cellular abnormalities leading to diabetic nephropathy. The schematic drawing also highlights the hypothetical cross talk between AGE-RAGE (advanced glycation end product–receptor for AGE) and the renin-angiotensin system (RAS) and the reciprocal-cyclical modulation of the interactions among AGEs, reactive oxygen species (ROS), and protein kinase C (PKC), with ROS as the central mediator. Abbreviations: Ang II, angiotensin II; AP-1, activator protein 1; AT1, Ang II receptor; ECM, extracellular matrix; GLUT, glucose transporter; JAK-STAT, Janus kinase–signal transducer and activator of transcription; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein 1; NF-κB; nuclear factor κB; RNS, reactive nitrogen species; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
Cross talk among these events occurs at various levels in different signaling pathways. Some of this cross talk, such as that between ROS and PKC, results in positive feedback signals (25) that accentuate the hyperglycemia-induced injury and initiate certain deleterious pathological processes, such as altered cell cycle and growth and excessive synthesis and accumulation of ECM, a hallmark of diabetic nephropathy in humans (Figure 2). Another important concept that is relevant to the pathogenesis of diabetic nephropathy is that hyperglycemia impairs autoregulation within the glomerulus by activating the local intrarenal renin-angiotensin aldosterone system (26, 27). This may increase the glomerular capillary pressure and mechanical stretch on the mesangial cell and then activate signaling molecules such as ROS, thereby amplifying various intracellular pathways and synergistically worsening the progression of diabetic nephropathy. Such an outcome may be regarded as a combination of metabolic and mechanical assaults on the kidneys within a hyperglycemic milieu (Figure 2).
In this review, we discuss pathogenetic mechanisms relevant to hyperglycemia-induced metabolic and mechanical renal injury. Overt diabetic nephropathy ensues following insidious accumulation of various by-products generated during early acute metabolic events, initiated by hyperglycemia, with disproportionate cellular intake of glucose.
CELLULAR UPTAKE OF GLUCOSE AND ITS PROCESSING INTO VARIOUS METABOLIC PATHWAYS
Translocation of glucose into the renal cells is regulated by various facilitative transporters such as glucose transporter (GLUT)-1 and −4, as well as by active energy-dependent transporters, such as sodium-glucose-linked transporter 1 and 2; the latter transport glucose via an electrochemical concentration gradient (28). The binding affinity/capacity kinetic characteristics of GLUT-1 and −4 reveal that they can be readily saturated under normal glucose concentrations, suggesting that their expression and/or activity may need to be upregulated for high glucose concentrations to result in increased glucose transport across the cell membrane; increased amounts of glucose would then be channeled into various metabolic pathways, which would ultimately lead to increased expression of ECM proteins such as type IV collagen and fibronectin. The hypothesis that increased expression of GLUT-1 may contribute to the pathobiology of diabetic complications stems from studies of GLUT-1-overexpressing mesangial cells; interestingly, these cells synthesize excessive ECM even under normal glucose ambience (29). Conversely, downregulation of GLUT-1 with antisense treatment reduces glucose-induced fibronectin expression in mesangial cells, which suggests that the very early steps of glucose transport can markedly influence downstream cellular events and finally very distal processes, such as ECM synthesis (28).
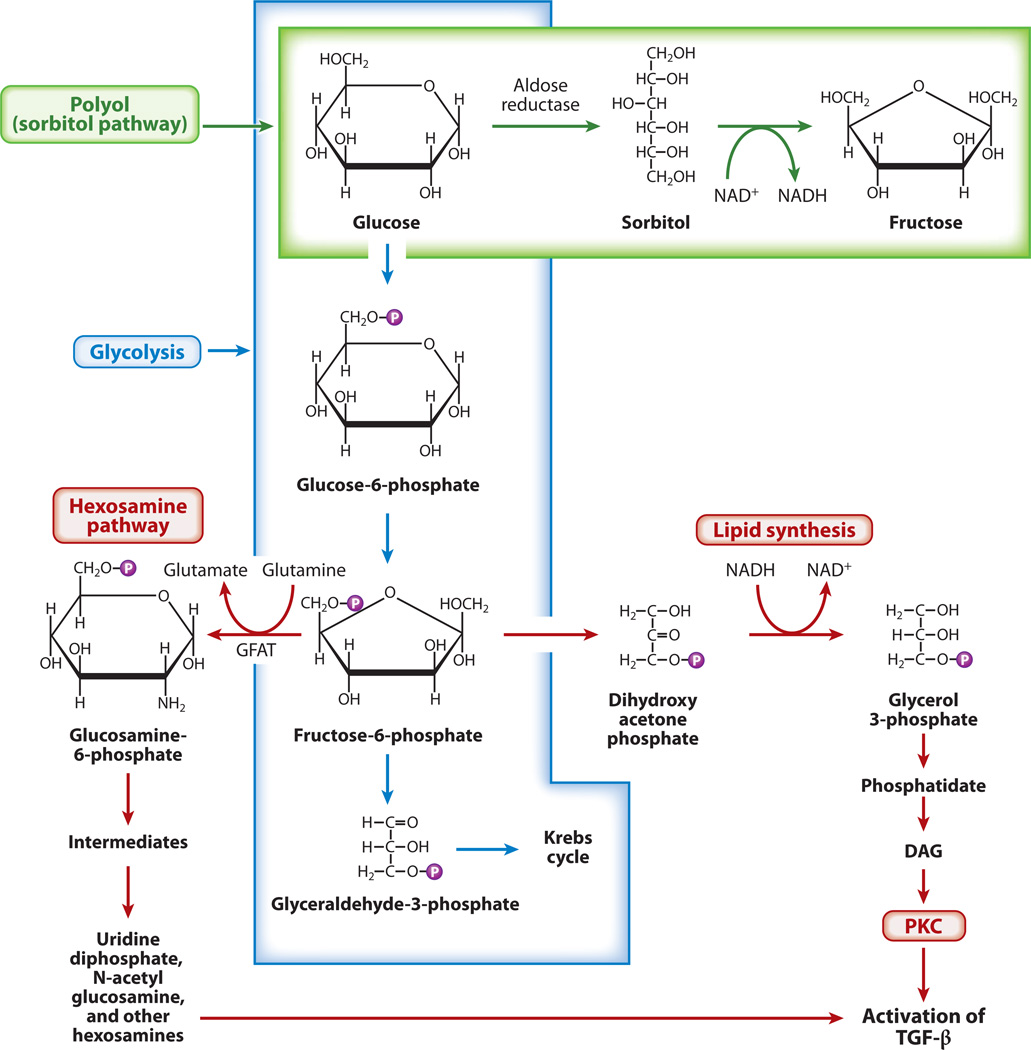
Following the cellular uptake of glucose, this molecule undergoes phosphorylation through conversion to glucose 6-phosphate (G6-P), which isomerizes to fructose 6-phosphate (F6-P); then, after a series of reactions, glyceraldehyde 3-phosphate (G3-P) is formed (Figure 3) (30). Through the actions of various transferases and phosphatases, G3-P forms glycerol phosphate, a precursor of diacylglycerol (DAG), which by virtue of being a second messenger is a well-known signaling molecule that is responsible for recruitment and activation of PKC (31). Under high-glucose ambience, F6-P is diverted into the hexosamine pathway, where it converts to glucosamine-6-phosphate by a rate-limiting enzyme, glutamine:fructose-6-phosphate-aminotransferase (GFAT), through the synthesis of UDP-N-acetylglucosamine, a precursor of ECM proteins such as the proteoglycans (32). The GFAT also modulates promoter activities of ECM, which modulates TGF-β1 and plasminogen activator inhibitor 1 (PAI-1) by phosphorylating the RNA polymerase–dependent transcription factor Sp1 (32).
Figure 3.
Schematics of different pathways for glucose metabolism that lead to the activation of protein kinase C (PKC) and transforming growth factor β(TGF-β). In addition to undergoing glycolysis, excess glucose is channeled into the polyol and hexosamine pathways, which results in increased lipid synthesis and the generation of diacylglycerol (DAG). This leads to the activation of PKC and TGF-β and, consequently, increased extracellular matrix (ECM) synthesis. Abbreviation: GFAT, glutamine:fructose-6-phosphate-aminotransferase.
Excess glucose is also channeled into the accessory polyol pathway, where it is reduced to polyalcohol sorbitol by AR, an NADPH-dependent enzyme (Figure 3) (16). The sorbitol is oxidized to fructose by sorbitol dehydrogenase, which uses NAD+ as a cofactor. Under basal conditions, minute amounts of glucose are handled via this route, but in a high-glucose milieu, as much as 30% of the glucose is channeled through this pathway (16). The accentuated activity of this pathway leads to relative depletion of NADPH and reduced glutathione (GSH), an increase in the NADH/NAD+ ratio, and decreased levels of nitric oxide (NO), which result in an altered cellular redox and therefore oxidant and osmotic stress. The relevance of the polyol pathway in diabetic lesions is highlighted by studies in which the eye lens of mice overexpressing AR exhibited reduced levels of GSH (16). However, mice deficient in AR had normal GSH levels and nerve conduction velocity (33). Also, the failure of AR inhibitors to ameliorate the cellular and matrix changes in the kidney of diabetic rodents argues against an important and nonredundant role of the polyol pathway in the pathogenesis of diabetic nephropathy (16).
Finally, glucose is channeled into another minor pathway, myo-inositol metabolism, that has received little attention despite having been implicated in the pathogenesis of diabetic nephropathy (34). L-myo-inositol,1-phosphate is generated from G6-P by the action of enzyme synthase. Upon dephosphorylation, it forms myo-inositol, which is oxidized to D-glucuronic acid by myo-inositol oxygenase (MIOX), which is expressed exclusively in the kidney (13). In diabetic nephropathy, the tissue concentration of myo-inositol decreases as it is increasingly excreted through the urine (13). At the same time, levels of Na+/K+-ATPase also decrease, partly because of the increased flux of polyols and synthesis of this enzyme’s inhibitors, namely arachidonic acid and prostaglandin E2, via PKC-induced activation of phospholipase A2 (35). These biochemical abnormalities can be rectified by myo-inositol supplementation (36). Likewise, addition of myo-inositol to cultures of proximal tubular epithelial cells normalizes glucose-induced proliferation and collagen synthesis (37). Myo-inositol deficiency reflects some combination of decreased synthesis and increased degradation by the renal-specific enzyme MIOX; interestingly, MIOX is upregulated in experimental diabetic nephropathy (13).
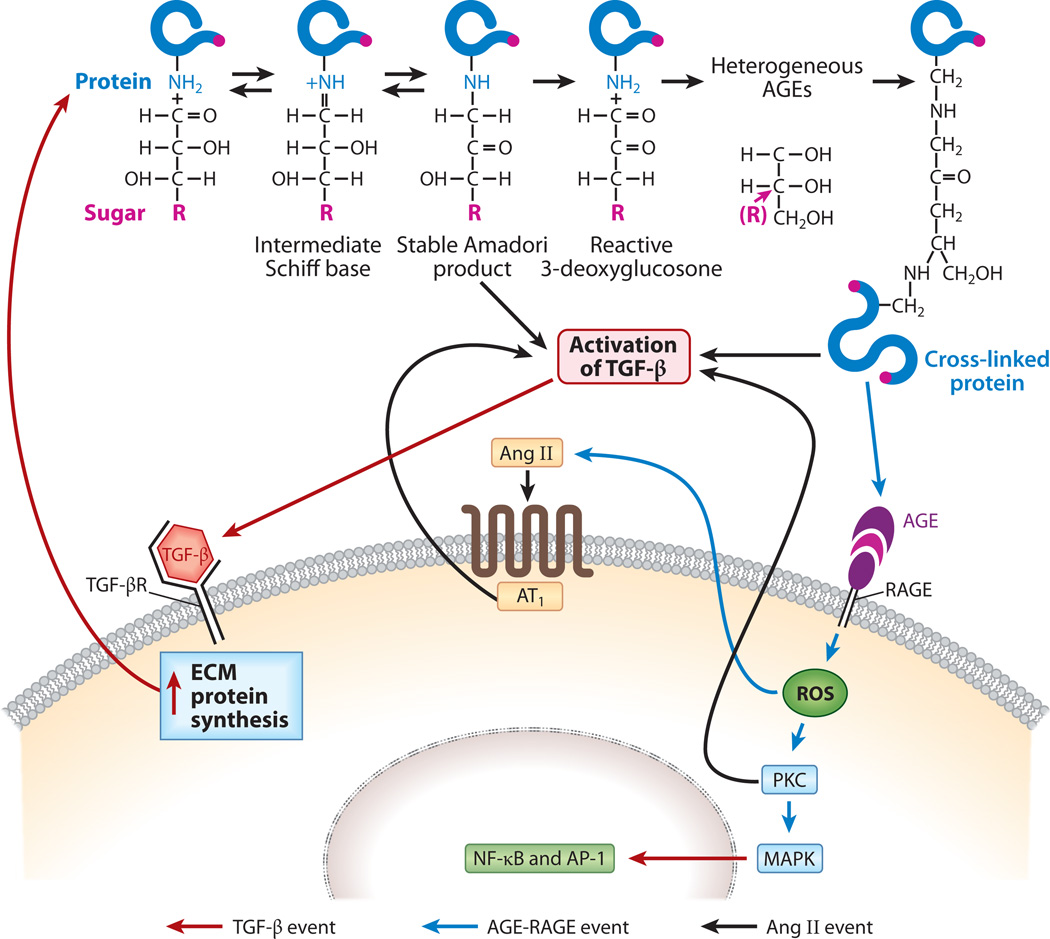
HYPERGLYCEMIA-INDUCED FORMATION OF ADVANCED GLYCATION END PRODUCTS AND ENSUING RENAL INJURY
Initial steps in the synthesis of AGEs include nonenzymatic condensation of sugar and a free amino group, which occurs through the formation of a labile Schiff base (Figure 4). The Schiff base undergoes an intramolecular Amadori rearrangement followed by a series of reactions and then dehydration and polymerization, which lead to the generation of macromolecular forms of AGEs (38). AGEs are produced in small amounts under normal physiological conditions, such as during aging, but their levels markedly increase in a chronic hyperglycemic milieu in both the cellular and extracellular compartments in various tissues, and more so in organs that are vascularized (39). Intracellularly, AGEs are derived from various dicarbonyls, primarily methylglyoxal, which is synthesized from G3-P or dihydroxyacetone following catalysis by G3-P dehydrogenase (GAPDH) (39). These AGEs can modulate various intracellular events, such as the activation of PKC, MAPK and transcription factors such as nuclear factor κB (NF-κB) (Figure 4) (40, 41). These events, in turn, regulate the expression of diverse growth factors and cytokines such as TGF-β, which inevitably influence the synthesis of various ECM proteins.
Figure 4.
Schematic drawing depicting the generation of advanced glycation end products (AGEs) and downstream events. AGEs are formed by condensation of a sugar (R) such as glucose with a reactive ε-amino (NH2) group of the protein; this process is followed by the formation of a Schiff base, Amadori rearrangement, and a complex series of reactions. AGE:RAGE (receptor for AGE) interactions lead to the generation of reactive oxygen species (ROS) and the activation of protein kinase C (PKC), transforming growth factor β (TGF-β), mitogenactivated protein kinase (MAPK), and transcription factors such as nuclear factor κB (NF-κB), leading to increased synthesis of the extracellular matrix (ECM). Diabetic injury is further amplified by the feedback loops of angiotensin II (Ang II) and cross-linking of de novo synthesized excess ECM proteins with sugars. Abbreviations: AT1, Ang II receptor; TGF-βR, TGF-β receptor.
Extracellular AGEs are formed by irreversible cross-linking of glucose with ECM structural proteins, such as type IV collagen, laminin, fibronectin, and proteoglycans (41). These modified proteins may have decreased susceptibility to enzymatic hydrolysis by matrix metalloproteinases (MMPs), which would allow them to accumulate in the extracellular space (24). Moreover, glycation of sulfated proteoglycans reduces their electronegativity and thus modifies the charge-selective filtration properties of the basement membrane, resulting in microalbuminuria (42, 43). In addition to adversely affecting ECM biology, extracellular AGEs can modulate cellular functions by interacting with their cognate receptor, RAGE, or with binding proteins, namely OST-48, 80K–H, galectin-3, and type II macrophage scavenger receptor, which may similarly alter cell and matrix functions (Figure 4) (39, 40). Such alterations may include interference with cell-matrix interactions as well as changes in adhesiveness, neurite growth, and the hyperpermeability of capillaries (17). Altered functions related to the vascular complications of diabetes mellitus can be partially reversed (a) by the administration of aminoguanidine, an AGE inhibitor and AGE cross-link breaker, or (b) by blocking RAGE, as has been highlighted in various experimental models (41).
Finally, other altered cellular events relating to high concentrations of intra- or extracellular AGEs in high-glucose ambience include the generation of ROS and quenching of NO; both ROS and NO are likely to modulate PKC and MAPK activities and to activate transcription factors such as NF-κB and activator protein 1 (AP-1), thereby promoting a further increase in the expression of ECM proteins (Figure 4) (39–41). Intriguingly, AGEs themselves can also covalently bind with ECM or cellular proteins, which further compound their deleterious effects in various tissues (39–41).
HYPERGLYCEMIA-INDUCED ACTIVATION OF PROTEIN KINASE C AND RELEVANT CELLULAR SIGNALING
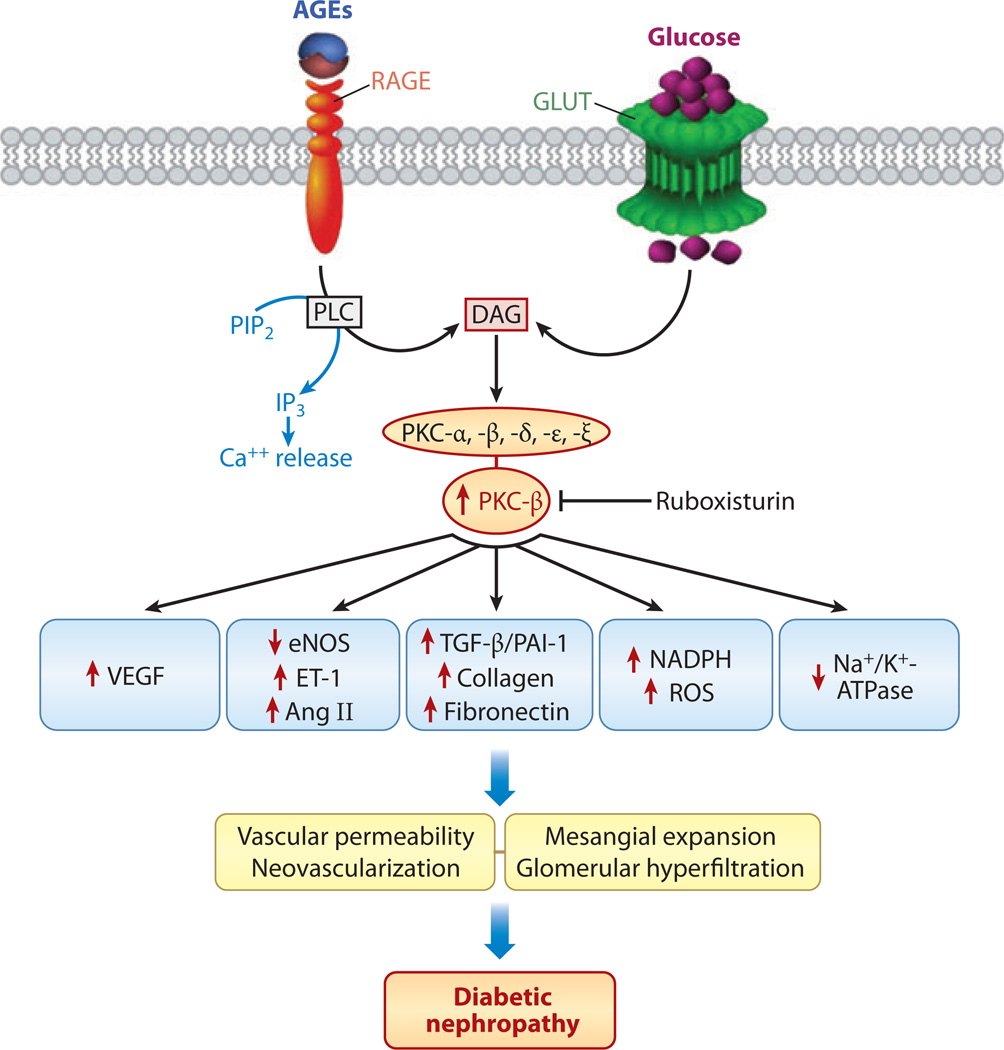
Among signaling kinases, PKC seems to be a centerpiece in the pathogenesis of diabetic nephropathy (Figure 5) (14, 44). A number of PKC isoforms, including PKC-α, -β, -δ, -ε, and -ξ, are expressed in the kidney and are activated in diabetic rodents and in glomerular cells studied in in vitro culture systems (45). PKC is activated by multiple routes, primarily DAG, which is formed by the channeling of glycolytic intermediate dihydroxyacetone phosphate to glycerol phosphate with subsequent removal of phosphate followed by acylation (45). PKC activation also occurs during the increased activity of the polyol pathway and following AGE:RAGE interaction (46). The latter interaction modulates the activity of membrane-bound phospholipase C, leading to an increase in cytosolic Ca2+ and DAG; these molecules may then act as second messengers, which could further boost PKC cellular signaling. This mechanism would imply a close relationship between the cellular levels of DAG and the extent of PKC activation. Indeed, such a correlation has been observed in various tissues, including renal mesangial cells subjected to high-glucose ambience (47).
Figure 5.
Sequence of events following AGE:RAGE (advanced glycation end products:receptor for advanced glycation end products) interactions and excess glucose entry into the cell via glucose transporter (GLUT). Diacylglycerol (DAG) and activated phospholipase C (PLC) increase the expression and activity of protein kinase C (PKC), which modulates the expression of a wide variety of genes that adversely affect glomerular pathophysiology, thereby leading to increased vascular permeability, mesangial expansion, hyperfiltration, and proteinuria. Abbreviations: Ang II, angiotensin II; e-NOS, endothelial nitric oxide synthase; ET-1, endothelin 1; IP3, inositol trisphosphate; PAI-1, plasminogen activator inhibitor 1; PIP2, phosphatidylinositol 4,5-bisphosphate; ROS, reactive oxygen species; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
PKC activation can influence a number of downstream pathophysiological changes, such as altered blood flow and capillary permeability, which may be due to (a) decreased expression of endothelial nitric oxide synthase (eNOS) and NO and (b) upregulation of endothelin 1 and vascular endothelial growth factor (VEGF), as exemplified by studies of eNOS knockout mice (Figure 5) (48). Vascular compromise may be accentuated by greater buildup of ECM proteins due to increased PKC-induced TGF-β1 expression and activation of plasmalemmal NADPH oxido-reductase; the latter may thereby induce cellular oxidant stress (18). The vascular injury may be further compounded by PKC-induced overexpression of PAI-1, which is an inhibitor of fibrinolysis, and concomitant activation of NF-κB, resulting in a severe inflammatory response and thrombotic angiopathy (25, 49). These cellular events suggest that PKC plays a pivotal role in the pathogenesis of microvascular complications of diabetes. That the administration of ruboxistaurin mesylate, a PKC inhibitor with affinity for the β1 and β2 isoforms, partially reduces renal damage in db/db mice (a model of type 2 diabetes) supports a central role for PKC in hyperglycemia-induced renovascular injury (50).
REACTIVE OXYGEN SPECIES MODULATION OF OTHER HYPERGLYCEMIA-INDUCED CELLULAR SIGNALING EVENTS
Recent studies suggest that the activities of AGEs, PKC, and ROS are interrelated and that the latter may serve as reciprocal inducers and amplifiers of the signaling cellular events that occur in high-glucose ambience (25, 42, 49, 51, 52). Normally, ROS are produced in minute amounts that are necessary to maintain cellular homeostasis, but in states of hyperglycemia their concentrations rise dramatically, damaging various target organs (49). The ROS that can induce renal injury include superoxide anion O2·−, H2O2, hydroxyl radical, and peroxynitrite (52). Enzymes that can scavenge ROS include cytoplasmic CuZn superoxide dismutase (CuZnSOD), mitochondrial manganese superoxide dismutase (MnSOD), and heme oxygenase 1 (53, 54). Interestingly, the latter undergoes a remarkable adaptive response (> 15-fold increase) in states of hyperglycemia, presumably to reduce ROS-mediated oxidant stress (54). ROS are generated via two systems: (a) predominantly via mitochondrial oxidative phosphorylation and (b) in small amounts via the NADPH-oxidase system (55–58).
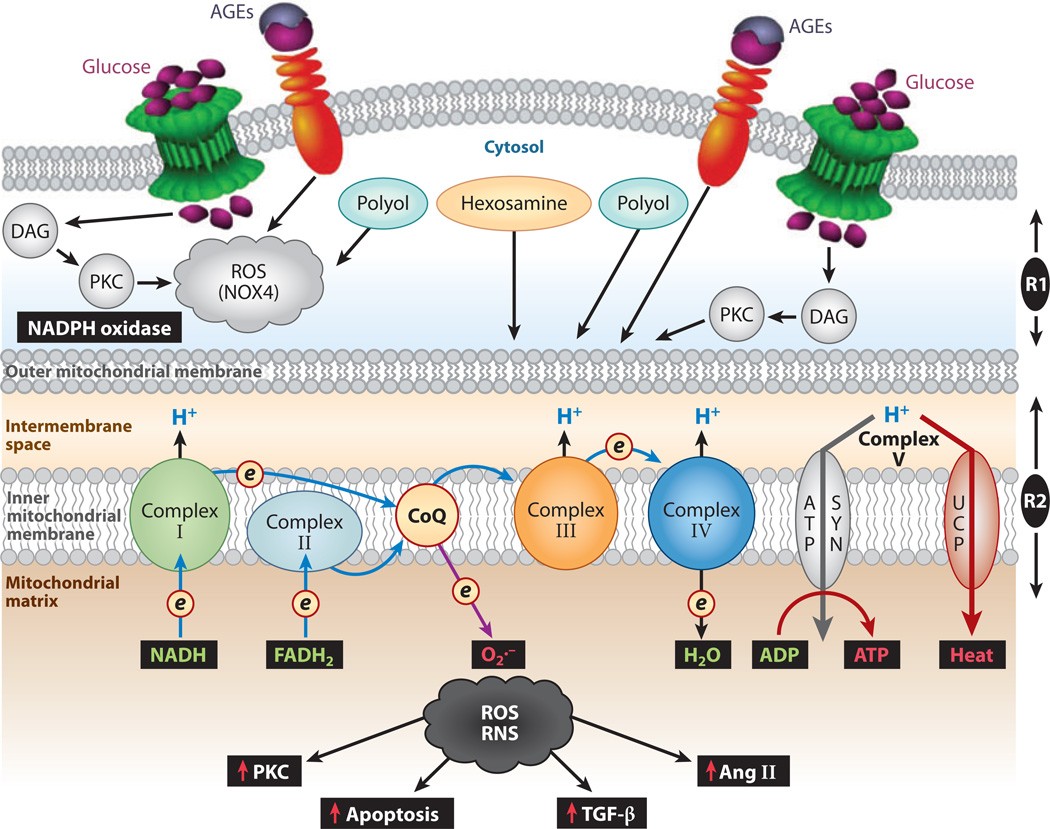
ROS are generated as a by-product of oxidative phosphorylation, in which electron donors such as NADH and FADH2 generate a high membrane potential by pumping protons across the mitochondrial inner membrane (Figure 6) (42, 59). As a result, electron transport is inhibited, the half-life of free-radical intermediates of coenzyme Q increases, and molecular O2 is reduced to O2·− with ensuing oxidant stress. In support of this hypothesis are results from studies in which (a) rotenone, an inhibitor of electron transfer chain complex I, and (b) m-cholorophenyl-hydrazone, an uncoupler of electron transport, blocked the generation of dichlorofluorescein-sensitive ROS (42). Similar results were observed following the overexpression of mitochondrial MnSOD or uncoupling protein 1 (42, 60). MnSOD or uncoupling protein 1 may reversibly modulate the four major cellular events induced by high glucose that can induce oxidant stress: polyol and hexosamine flux, AGE formation, and DAG-induced PKC activation (Figure 6). The O2·− that is generated initially inhibits GAPDH, which in turn amplifies the above-described critical cellular events through the cyclic generation of ROS (25). That gene disruption of GADPH also amplifies the above-named cellular events lends support to the hypothesis that GADPH plays a critical role in the generation of mitochondrial ROS (61).
Figure 6.
Summary of events leading to the generation of cytosolic reactive oxygen species (ROS) (R1) and mitochondrial ROS (R2). Extramitochondrial cytosolic ROS generation occurs following increased glucose flux, activation of the polyol pathway, and AGE:RAGE (advanced glycation end products:receptor for advanced glycation end products) interaction, as well as via the NADPH oxidase system, such as NOX4. Mitochondrial matrix ROS and reactive nitrogen species (RNS) are generated via the well-characterized electron-transport chain, Complex I–IV redox carriers, localized in the inner mitochondrial membrane. During hyperglycemia, there is an increased donation of electrons (e) by powerful NADH- and FADH2-reducing agents of Complex I and Complex II, respectively, characterized by pumping of protons (H+) into the intermembrane space, which gives rise to an increased mitochondrial membrane potential. As a result, the electron transport at complex III is inhibited; the system backs up, and the half-life of the free-radical intermediates of coenzyme Q (CoQ) is prolonged, which leads to an increase in the reduction of O2 to superoxide (O2.−) and in the production of ROS. The generated ROS and RNS release cytochrome c, activate caspases, and induce apoptosis. They also modulate the activity of angiotensin II (Ang II), protein kinase C (PKC), and transforming growth factor β(TGF-β), which ultimately affect the synthesis of the extracellular membrane. Abbreviation: DAG, diacylglycerol.
The relevance of extramitochondrial ROS to the pathogenesis of diabetic nephropathy is supported by studies in which overexpression of cytoplasmic CuZnSOD reduced glomerular pathophysiologic changes in db/db mice and in mice with streptozotocin (STZ)-induced diabetes (62, 63). Extramitochondrial ROS are generated via the NADPH-oxidase system, which is normally dormant but that, upon association of membrane-bound flavocytochrome b558 (heterodimer of gp91phox and p22phox) with various cytosolic proteins (p47phox; p67phos; p40phox; and a GTP-binding protein, p21rac), generates O2− and H2O2 (51, 56, 57). Among these cytosolic proteins, Nox4 is particularly interesting in renal pathobiology because it is a homolog of neutrophil gp91phox, which is expressed in the kidney (64).
Similar to the mitochondrial electrontransport system, the NADPH-oxidase system can be activated in renal cells by AGEs, PKC, DAG, and IP3 (inositol 1,4,5-triphosphate) in renal cells. In addition, the metabolites of the cyclo-oxygenase pathway can activate NADPH oxidase under high-glucose ambience (49, 65). The fact that fibronectin expression decreases in tubular or mesangial cells treated with inhibitors of oxidase, namely apocynin and diphenylene iodonium, suggests that NADPH oxidase may play a role in high glucose–mediated injury and supports its relevance in redox-sensitive processes, such as cell growth, apoptosis, migration, and ECM modeling (66, 68). ECM modeling can be markedly modulated by diverse signaling pathways, transcription factors, and growth factors (69–71). TGF-β, a potent cytokine, may adversely affect the biology of tubular cells via the induction of EMT and loss of epithelial cell adhesion, α–smooth muscle actin expression, cytoskeletal organization, and cell migration across the basement membrane, as well as by promoting tubulointerstitial fibrosis, which is an integral part of diabetic nephropathy (72, 73).
Finally, special forms of ROS, known as reactive nitrogen species (RNS), are produced following hyperglycemia-induced endothelial injury, in which a series of events leads to the disruption of the nitroso-redox balance (74). NO synthesis is modulated by a cofactor of eNOS known as tetrahydrobiopterin (BH4) (75, 76). Under high-glucose ambience, BH4 levels are reduced, with a proportionate decrease in the synthesis of NO by the endothelium and an altered ratio of BH4 to its oxidized form, BH2. These reductions eventually lead to increased generation of O2·− (77). That exposure of diabetic rat aortic-ring explants to BH4 ameliorates endothelial–smooth muscle dysfunction supports a role for RNS in the pathogenesis of hyperglycemic injury (77).
TRANSFORMING GROWTH FACTOR β CELLULAR SIGNALING IS CRITICAL TO THE INDUCTION OF GLOMERULAR AND TUBULOINTERSTITIAL FIBROSIS IN DIABETIC NEPHROPATHY
TGF-β1 is widely thought to be the most pertinent cytokine to the ECM glomerular pathology typically observed in patients with chronic progressive diabetic nephropathy. However, most of the consequences of TGF-β1-related signaling (Figure 7) have been identified in studies of cultured glomerular mesangial cells and podocytes in vitro (19, 23, 78, 79). TGF-β1, a prototype of the TGF-β superfamily, exerts pleiotropic effects—that is, it inhibits proliferation in certain cells and apoptosis in others—but it induces hyperplasia and hypertrophy of mesangial cells (19, 23, 78, 79). In the ECM, TGF-β1 exists as a latent, dormant form of propeptide complexed with TGF-β1-binding proteins, and both are cross-linked with matrix proteins by transglutaminase (68–71). Following cleavage by plasmin, MMP-2 and −9 or thrombospondin 1, an active free form of TGF-β, are generated.
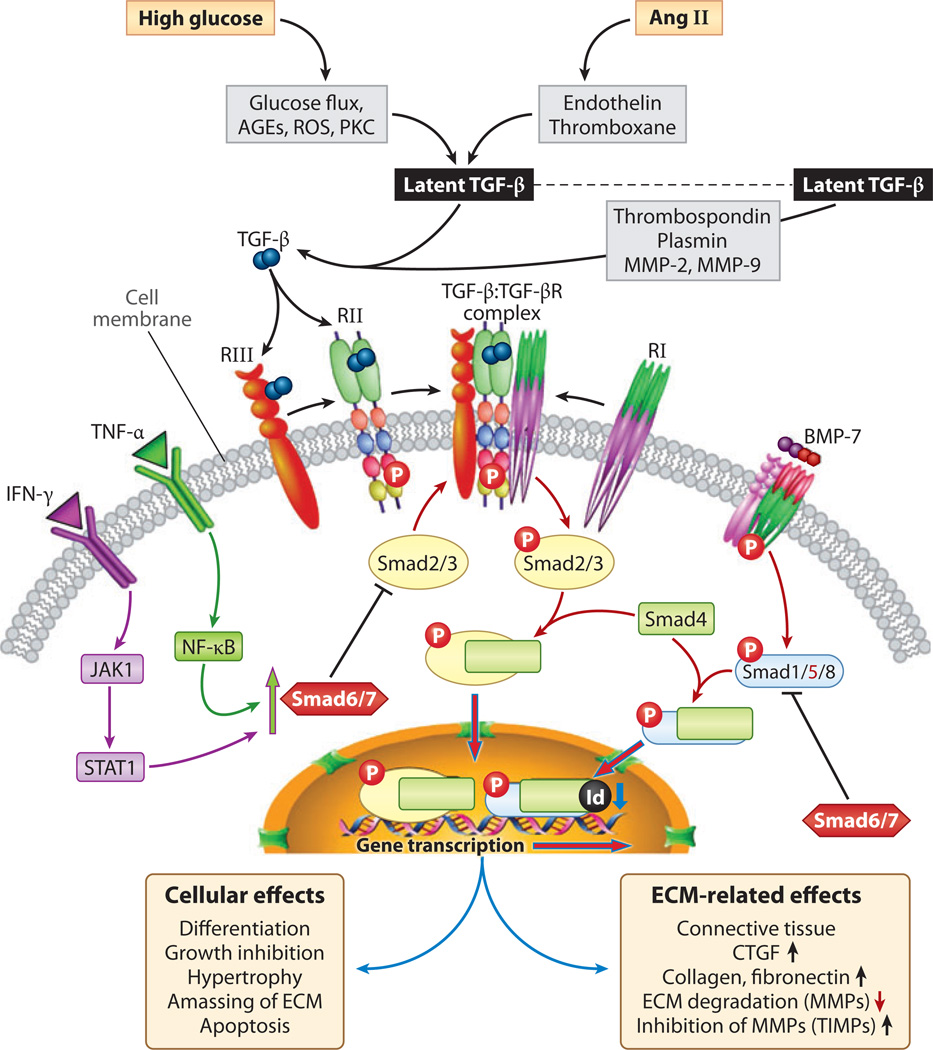
Figure 7.
Schematic depicting transforming growth factor β (TGF-β) and bone morphogenetic protein 7 (BMP-7) signaling. Activation of latent TGF-β by glucose, advanced glycation end products (AGEs), reactive oxygen species (ROS), and angiotensin II (Ang II) leads to the generation of TGF-p, which binds first to type II and III serine/threonine kinase receptors with recruitment and phosphorylation of type I. Activated heteromeric complex interacts with Smad2/3 and co-Smad4. Smad2/3 are inhibited by Smad6/7, which are induced by tumor necrosis factor α. (TNF-α) and interferon-γ (IFN-γ) signaling. The Smad2/3/4 complex translocates into the nucleus to initiate transcription of various extracellular matrix (ECM) genes and connective tissue growth factor (CTGF). BMP-7, however, activates Smad1/5/8, which bind to co-Smad4 and, upon translocation into the nucleus, induce Id proteins that inhibit differentiation and DNA binding of some of the transcription factors, thereby opposing the action of TGF-β. Abbreviations: JAK, Janus kinase; MMP, matrix metalloproteinase; NF-κB; nuclear factor κB; PKC, protein kinase C; STAT, signal transducer and activator of transcription; TIMP, tissue inhibitor of metalloproteinase.
The active TGF-β1 binds first to a type II serine/threonine kinase receptor, which transphosphorylates and thereby activates a type I receptor. This process is followed by modulation of the downstream-signaling Smad, MAPK, and perhaps protein kinase A cellular pathways and various nuclear events (Figure 7). The activated TGF-β1 receptor interacts with Smad2 and −3 to form a heterodimeric complex with common partner co-Smad4, which translocates into the nucleus and regulates transcription of TGF-β1 target genes such as collagen α1(I), PAI-1,Jun B, c-Jun, and fibronectin (68–71). TGF-β1-induced Smad2/3/4 signaling has a counterregulatory loop driven by Smad7; the latter is modulated by NF-κB or Jak1/Stat1, which are activated by inflammatory cytokines (20, 21, 67).
Along with the Smads, the extracellular signal-regulated kinases 1 and 2 (ERK1 and −2), the p44/p42 MAPKs, c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), and p38 MAPK modulate the TGF-β1 signaling cascade in mesangial cells (19). These kinases also regulate the transcriptional regulation of proα1(I) procollagen and fibronectin via AP-1, a heterodimer of the c-Fos and c-Jun family members (80). AP-1, regulated by ERK and JNK, can complex with Smad3, whereas the Smad3/4 complex can bind to AP-1 consensus sequences in various promoters of TGF-β target genes. These findings suggest that cross talk occurs between the Smad and MAPK pathways and that TGF-β signaling is central to ECM pathobiology in diabetic nephropathy (19, 81).
TGF-β1 signaling is initiated by numerous mediators generated under high-glucose ambience, such as the AGEs, ROS, DAG, PKC, and the hexosamines; other mediators that accelerate renal injury include vasoactive substances, such as angiotensin II, endothelin, and thromboxane, as well as the physical cyclical stretching and relaxation of mesangial cells (which mimic intraglomerular hypertension) (31, 82). The net effect is an increased synthesis of various matrix proteins and accumulation of ECM. The amassing of ECM may also be related to the inhibition of MMPs and the activation of tissue inhibitors of MMPs (TIMPs).
The expression of ECM genes is also modulated by another cytokine, connective tissue growth factor (CTGF), which is induced by TGF-β1 via consensus sequences of Smad and transcription enhancer factor elements that are present in the CTGF promoter (83, 84). CTGF promotes ECM production, cell adhesion, and collagen matrix contraction in various mesenchymal cells, such as cultured dermal fibroblasts and mesangial cells (83, 85). The in vitro effects of TGF-β1 have been well corroborated by in vivo studies, in which upregulation of TGF-β messenger RNA (mRNA) and protein expression, as well as its type II receptor and TGF-β1 bioactivity, were observed in the kidneys of various murine models of diabetes (86, 87). That administration of neutralizing anti-TGF-β1 antibodies prevents renal hypertrophy, mesangial matrix expansion, increase in α1(IV) collagen and fibronectin expression, and decline in renal function in mice with STZ-induced diabetes suggests a potential role for TGF-β1 in the pathogenesis of diabetic nephropathy (88). Similar observations have been made in db/db mice with genetic type 2 diabetes mellitus (89). Moreover, upregulated renal expression and increased urinary excretion of TGF-β1 have been reported in patients with diabetic nephropathy (90). Interestingly, angiotensin-converting enzyme (ACE) inhibitors that protect the kidney from end organ damage also reduce TGF-β1 production, thus leaving little doubt that this profi-brogenic cytokine is intricately involved in the renal pathobiology of diabetes (91).
Bone morphogenetic protein 7 (BMP-7), a member of the TGF-β superfamily, acts in opposition to the classical profibrogenic effects of TGF-β1 (92), perhaps because some of the BMPs induce Id proteins that inhibit differentiation or DNA binding via the Smad1/5 pathway, and these proteins may act as negative regulators of various basic helix-loop-helix transcription factors (93). BMP-7 is expressed early in embryonic life, when it plays a crucial role in eye and kidney development (92). In adult life, it is highly expressed in the tubular epithelia of the outer cortex and in glomerular podocytes (94, 95). The protective antifibrogenic role of BMP-7 in diabetic nephropathy has been demonstrated in transgenic mice, where its expression was driven by the phosphoenol–pyruvate carboxykinase promoter (96). These mice had reduced glomerulosclerosis and tubulointerstitial fibrosis when made diabetic by the administration of STZ. In addition to having reduced renal expression of collagen and fibronectin, the mice demonstrated reduced dropout of podocytes, reduced urinary protein (associated with partial restoration of nephrin expression), and decreased serum creatinine levels; these results suggested that there may be an imbalance in the activity among various members of the TGF-β superfamily in diabetic nephropathy.
The balance between ECM synthesis and degradation is also maintained by MMPs and TIMPs. Interestingly, glomerular mRNA levels of MMP-2 and MMP-3 are decreased in rats with STZ-induced diabetes, whereas TIMP mRNA levels are increased (24). These findings suggest that profibrogenic cytokines, such as TGF-β1, and MMPs and TIMPs play intertwined and complex roles in the pathogenesis of ECM accumulation during diabetes.
EMERGING RELEVANCE OF GTP-BINDING PROTEINS AND CELL-CYCLE PROTEINS IN VARIOUS PATHOGENETIC MECHANISMS OF DIABETIC NEPHROPATHY
To date, few reports in the literature have described the role of GTP-binding proteins in the pathogenesis of diabetic nephropathy. The major GTPases studied so far are the Ras, Ras-related, and Rho families of GTP-binding monomeric proteins, which range from 20 to 40 kDa in size and belong to a superfamily that comprises more than 100 small GTPases (97). They modulate a wide variety of cellular processes, including cell hypertrophy, morphogenesis, motility, axonal guidance, cytokinesis, and intracellular trafficking (51, 97, 98). By serving as molecular switches in these processes, they cycle between inactive (GDP-bound) and active (GTP-bound) states (97, 98). The state of activity is determined by the action of guanine exchange factors, whereas GTPase-activating proteins (GAPs) facilitate hydrolysis to GDP with a resulting loss of activity (Figure 8).
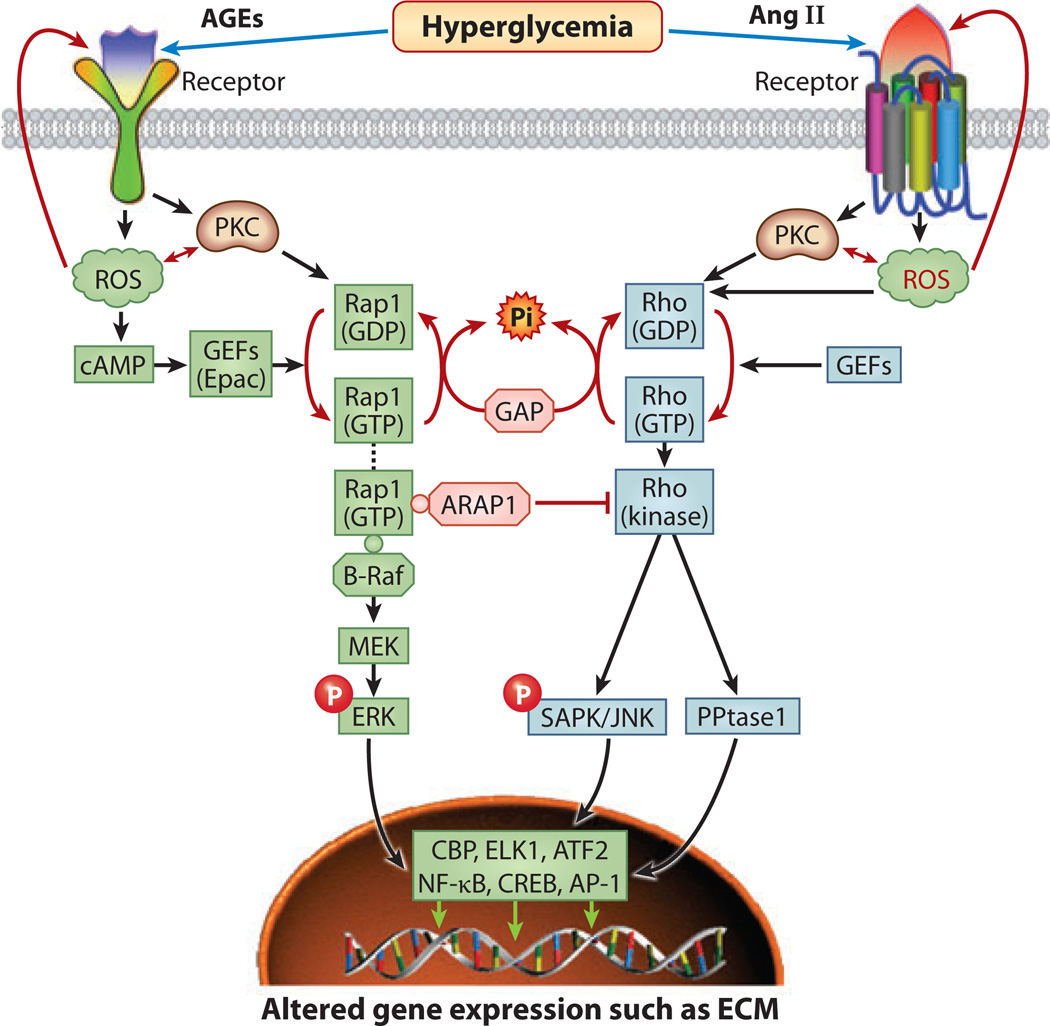
Figure 8.
Schematics depicting the sequence of events, following the interactions between advanced glycation end products (AGEs) and angiotensin II (Ang II) with their respective receptors, that lead to the generation of reactive oxygen species (ROS) and the activation of protein kinase C (PKC) and Rap1 and Rho GTPases. Both Rap1 and Rho, in association with guanine exchange factors (GEFs), are activated (GTP bound), whereas GTPase-activating proteins (GAPs) hydrolyze Rap1 and Rho into the inactive (GDP-bound) state. Thus, these small G proteins cycle between functional and nonfunctional states. Activated Rho GTP leads to the induction of Rho kinase and the phosphorylation of Janus kinase/stress-activated protein kinase (JNK/SAPK) kinase and phosphatase 1 (PPtase1), which then translocate into the nucleus to initiate transcription via various transcription factors. Similarly, Rap1 GTP, in association with another kinase known as B-Raf, causes the induction and phosphorylation of extracellular signal–related kinase (ERK) and mitogen-activated protein kinase/ERK (MEK). Upon translocation into the nucleus the transcription of target genes is initiated. Interestingly, the Rap1-ARAP1 (angiotensin II type 1 receptor–associated protein) complex inhibits the activity of Rho kinase. Abbreviations: AP-1, activator protein 1; ATF, activating transcription factor; CBP, cAMP response element–binding (CREB) protein; ELK1, ets-like gene 1; NF-κB, nuclear factor κB.
Among members of the GTPase superfamily, the Ras, Ras-proximate 1 (Rap1), and Rho families are of particular interest because they are pivotal to many transduction pathways. For instance, in the Ras/Raf/MEK (MAPK/ERK) signaling pathway, Ras serves as an intermediary between the activated/phosphorylated growth factor receptor and MEK, whereas the JNK/SAPK and P38/MAPK pathways may be directly activated by Rho and Rho-related Rac, respectively (22, 51, 99). Interestingly, Rap1 and Ras are in counterequilibrium, and their activity state depends on association with another serine/threonine kinase, Raf, which is activated by the ligand-induced phosphorylated form of the growth factor receptor. For instance, following insulin stimulation, Rap1 is inactivated by its dissociation from Raf, and the free Raf associates with GTP-bound activated Ras (100). However, insulin deficiency leads to activation of Rap1 through its association with Raf. Rap1 can also be activated by second messengers such as Ca2+, cAMP, and DAG. Activation of the Rap1/Raf/MAPK pathway can also occur through PKC and ROS that are generated following AGE:RAGE interaction (97, 98). Such signaling mechanisms have been elucidated both in vitro and in vivo through the upregulation of Rap1b in high-glucose ambience, which led to increased ECM-fibronectin synthesis (Figure 8) (101, 102). Posttranslational lipid modifications of Rap1b, such as farnesylation, may also be critical to Rap1 activation and translocation to the inner plasmalemmal leaflet in that they may ultimately have an effect on ECM protein synthesis. Similar modifications also occur in the Rho GTPases, but the latter undergo geranylgeranylation rather than farnesylation (100, 103).
The Rho family of proteins regulate cytoskeletal organization, actin polymerization, and cell migration and adhesion (51, 97, 98). However, recent findings suggest that the Rho GTPases play a pivotal role in the pathobiology of ECM proteins, such as fibronectin, that are modulated by TGF-β-induced upregulation of CTGF, a powerful profibrogenic cytokine expressed in renal glomerular and tubulointerstitial cells (100, 104). Similarly, Rho-dependent pathways are activated in the kidney by other profibrogenic molecules, including angiotensin II, platelet-derived growth factor, and endothelin 1 (Figure 8) (100, 105). These factors modulate the expression of various ECM proteins and thus are implicated in the pathogenesis of diabetic nephropathy. The relevance of Rho GTPases to diabetic nephropathy is further underscored by the fact that Rho GTPases activate JNK/SAPK, which can influence the activity of transcription factors such as Elk1, nuclear transcription factor, AP-1, NF-κB, Myc, and cAMP-responsive element–binding protein (CREB) (106, 107). The cis-acting cAMP-responsive elements, TGACGTCA, have been localized in the promoters of various ECM proteins, and therefore CREB could be regarded as the major transcription factor responsible for their increased expression in the diabetic state (Figure 8) (108).
GTPases have also been implicated in the modulation of other cellular processes, such as apoptosis, differentiation, and cellular proliferation, through the activation of JNK/SAPK and P38/MAPK and stimulation of serum response factor–dependent transcription, which is regulated by generic transcription factors such as AP-1 and NF-κB (51, 109). Such processes, especially apoptosis in tubular cells and the proliferation of mesangial cells, have been described as early features of diabetic nephropathy and evidently are modulated by various cell-cycle proteins and their inhibitors (23, 99, 103). Cell-cycle proteins are a complex of cyclins and cyclin-dependent kinases (CDKs); the latter are activated upon heterodimerization with cyclins (110, 111). Levels of these cyclins fluctuate during the cell cycle, and their expression may be regulated by growth factors, whereas CDKs are constitutively expressed, and their activity depends upon their state of phosphorylation and their binding to cyclins (112). The activity of the cyclin/CDK heterodimer complex is negatively regulated by another group of small proteins, known as CDK inhibitors (CDIs). CDIs comprise two main families, namely Cip/Kip (p21Cip1and p27Kip1) and 1NK4/ARF (p161NK4 and p14ARF) (110, 111). The role of cell-cycle proteins in diabetic nephropathy was first demonstrated in studies in db/db mice, a model of type 2 diabetes, in which investigators observed an increased expression of p27Kip1 relative to that of heterozygous db+ littermates (111). Likewise, kidneys of mice with an STZ-induced type 1 model of diabetes demonstrated a similar increased expression of CDIs (113). Mesangial cells exposed to high-glucose ambience also showed increased expression of p27Kip1, which may have been related to this protein’s phosphorylation by MAPKor PKC, both of which are activated in a diabetic milieu (111). Interestingly, exposure of mesangial cells to a gene-specific phosphorothioated antisense oligonucleotide resulted in decreased protein synthesis of p27Kip1, suggesting that a phenotypic conversion from the hypertrophic phase to a hyperplastic state takes place for cells exiting the G1 phase and entering the S phase of the cell cycle (113). Such a phenotypic change has also been reported in in vivo studies, in which induction of diabetes in p27Kip1−/− or p21Cip1−/− mice resulted in a hyperplastic rather than hypertrophic response in tubular cells (111, 114). The inability of STZ to induce overt renal lesions in p27Kip1−/− mice also underscores the significance of cell-cycle proteins in the pathogenesis of diabetic nephropathy (114). Further support for the role of cyclins in this setting is provided by studies in which decreased expression of p21Cip1 was associated with a reduction in kinase activities of CDK-2 and −4 in mesangial cells undergoing proliferation under high-glucose ambience (103). In such experiments, there was a concomitant increased expression of Ras and Rho, which were subsequently translocated to the inner leaflet of the plasma membrane. Such findings support the importance of cell-cycle proteins and the GTPase/p21 signaling pathway in the cellular response to hyperglycemic stress (103, 105).
ROLE OF INTRAGLOMERULAR HYPERTENSION IN THE AMPLIFICATION OF HYPERGLYCEMIA-INDUCED RENAL INJURY IN DIABETIC NEPHROPATHY
A well-recognized early pathophysiologic feature of diabetic nephropathy is hyperfiltration, which is reflected in a marked increase in the glomerular filtration rate originally described by Anderson & Brenner (115). This increase seems to result from intrarenal hypertension or, more specifically, from a rise in glomerular capillary pressure. The increased pressure may accelerate the renovascular complications, perhaps via cross talk between metabolic and hemodynamic factors in a diabetic milieu. This important hypothesis, which has evolved in recent years (116), arose from seminal micropuncture studies in which rats with experimental diabetes had intraglomerular hypertension despite having normal systemic blood pressure; this finding indicated that the autoregulation of local glomerular microcirculation was impaired because of the differential dilatation of arterioles (afferent versus efferent), which led to an increase in the transcapillary hydraulic pressure difference and plasma flow (117). Hyperglycemia may expose the kidney to a local increase in blood pressure, probably by activating the reninangiotensin system (RAS) with regional renal production of angiotensin II (82). That significant amelioration in the microalbuminuric response occurs upon reduction of the intraglomerular hypertension in states of hyperglycemia supports the above hypothesis (118).
Intriguingly, the intrinsic cells of the glomerulus, such as mesangial cells and podocytes, synthesize angiotensin II and express angiotensin II receptors, which may contribute to the regional activation of RAS, leading to mechanical pressure–induced damage and thereby an accentuation and perpetuation of hyperglycemia-mediated ROS or glycative stress injury to the kidney (Figure 9) (119–123). These proposed mechanisms favor the inhibition of ACE as an integral part of the therapy for amelioration of diabetic nephropathy. Lending support to this idea are ACE gene insertion/deletion polymorphism studies, which indicate that individuals with the ACE deletion/ deletion genotype respond poorly to ACE inhibition; this possibility suggests that in the setting of metabolically induced altered hemody-namics, genetics may be critical to determining the outcome of this disease (124). The relevance of hemodynamics is further suggested by animal studies in which a deficiency of ACE2, a negative regulator of ACE, is associated with a local increase of tubular angiotensin II and increased tubulointerstitial fibrosis in long-term experimental diabetes (125). That ACE2 knockout mice tend to develop glomerulosclerosis that is reminiscent of diabetic nephropathy also underscores the importance of ACE2 and intraglomerular hemodynamics in the pathogenesis of diabetic nephropathy (126).
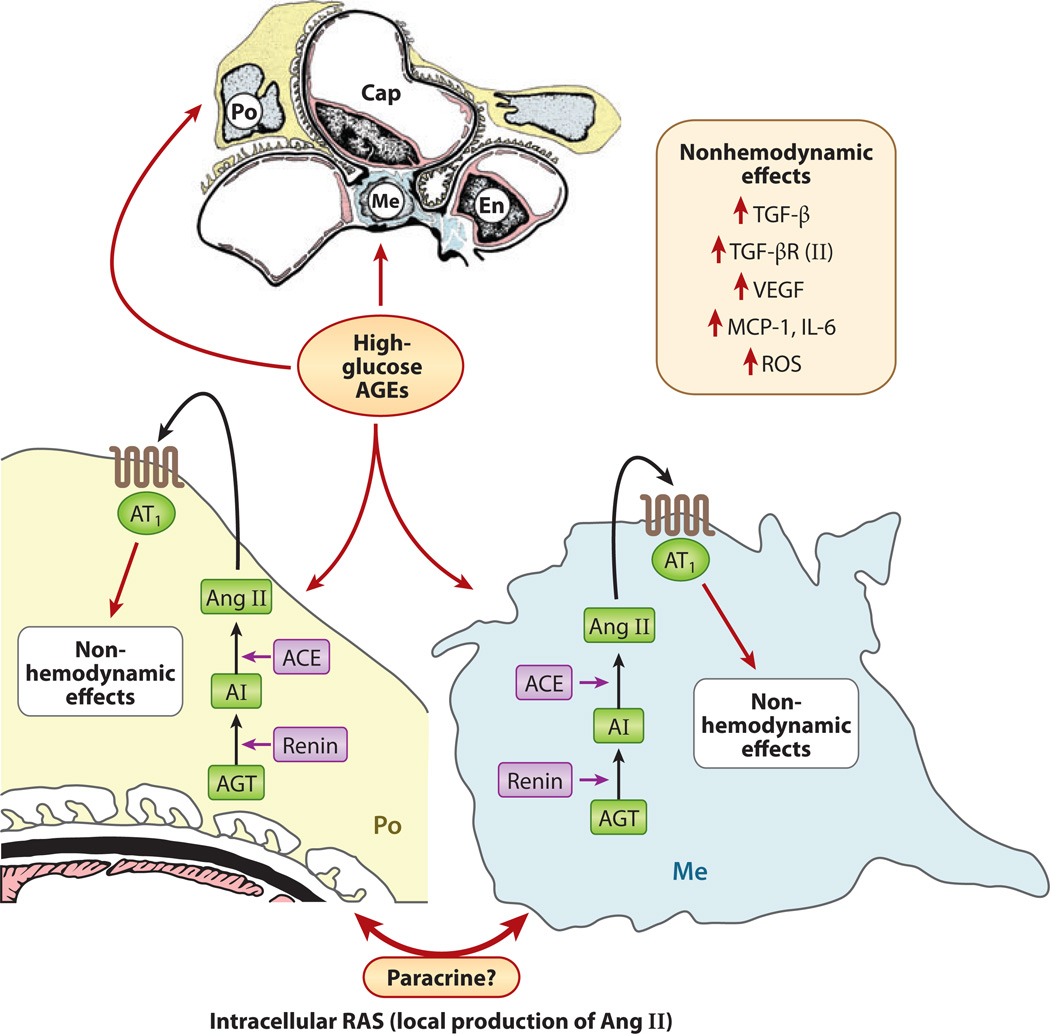
Figure 9.
Schematic drawing depicting local glomerular activation of the renin-angiotensin system (RAS) by glucose flux and advanced glycation end products (AGEs). Both podocytes (Po) and mesangial cells (Me) of the glomerulus express angiotensin II (Ang II) receptors (AT1). Angiotensin I (AI) is synthesized from angiotensinogen (AGT) by the action of renin. AI is converted into angiotensin II (Ang II) by angiotensin-converting enzyme (ACE). Ang II, upon binding to its AT1 receptor, induces various nonhemodynamic effects, including increased activity of transforming growth factor β (TGF-β) and expression of monocyte chemotactic protein 1 (MCP-1), vascular endothelial growth factor (VEGF), and reactive oxygen species (ROS). Abbreviations: Cap, capillary; En, endothelium; IL-6, interleukin-6; TGF-βR, TGF-β receptor.
As noted above, there are a multitude of pathways involved in the pathogenesis of diabetic nephropathy. However, a critical issue is identifying the mechanism(s) by which hyperglycemia induces the kidney to generate increased amounts of angiotensin II that exacerbate the hemodynamic injury. Increased production of angiotensin II is thought to be central to the early hyperplasia and late hypertrophy of the renal cells observed in diabetes, which occur through the upregulation of cytokines such as TGF-β, CTGF, interleukin-6, monocyte chemoattractant protein 1 (MCP-1), and VEGF-A, which modulate glomerular ECM pathobiology (Figure 9) (3, 82, 117). First, with respect to angiotensin II synthesis, high glucose upregulates the expression of renin and angiotensinogen in mesangial cells, which could increase intrarenal concentrations of angiotensin II and then, via various autocrine and paracrine pathways, lead to the generation of various cytokines and to ECM accumulation (127). Second, the ROS generated in various hyperglycemia-mediated cellular events, such as AGE:RAGE interactions, could also increase the expression of angiotensinogen and angiotensin II in renal cells (17, 128). Infusion of AGEs in vivo causes a remarkable increase in the expression of various components of the RAS (128). However, infusion of angiotensin II increases both serum and renal concentration of AGEs, thereby highlighting the complexity of the cellular events that occur in a high-glucose environment (128).
The angiotensin II–AGE juxtacrine loop may maintain a relatively constant increase in the glomerular capillary pressure by affecting the efferent arteriole, thereby transmitting a sustained-stretch stress on the glomerular cells, which may lead to parallel changes in the glomerular volume and to the activation of various signaling pathways and a rise in blood pressure (Figure 10) (124–126). Such an unrelenting mechanical stretch would adversely affect the biology of mesangial cells in several ways: First, it would induce the expression of GLUT-1 through an increase in cellular glucose concentration, despite a normal extracellular glucose ambience in the culture medium (123, 129). Second, recurring cyclic stretch and relaxation of mesangial cells in vitro enhance these cells’ proliferation and increase the synthesis of ECM while decreasing the expression of ECM-degrading enzymes (130). Third, the mechanical stretch could either directly induce the expression of TGF-β and its receptor or indirectly activate PKC and p38/MAPK with consequential increased synthesis of various ECM proteins (131). Another significant angiotensin II–mediated stretch stress response in mesangial cells includes the induction of MCP-1 (132), which upregulates intercellular adhesion molecule 1 and the generation of ROS, which may lead to amplification of the inflammatory response and renal injury. That the influx of monocytes, and their adherence to the glomerular mesangium, is often observed in clinical renal biopsies accords with the notion that inflammatory cytokines also play a role in the pathogenesis of diabetic nephropathy (133). In addition to mesangial cells, podocytes are adversely affected by the sustained glomerular capillary stretch, possibly via the induction of VEGF-A (79, 134). Such stress may lead to a reversible reorganization of the cytoskeletal assembly and to reduced expression of α3β1 integrin in podocytes. As a consequence, podocytes may detach from the underlying GBM and undergo apoptosis, leading to ensuing proteinuria (79, 135). VEGF signaling reduces the expression of an important glomerular slit-membrane protein known as nephrin (135, 136). Nephrin regulates the transcapillary passage of plasma proteins, and its loss may accentuate the proteinuric response during diabetes (137). Interestingly, decreased expression of nephrin with relative loss of podocytes has been observed in neonates of diabetic mothers, and such underdosing of nephrons with nephrin and the attendant podocytopenia during fetal development may predispose patients to developing hypertension later in adult life (138). Key metabolic and hemodynamic events are set in motion by hyperglycemia, and in the setting of a sufficient genetic predisposition, such events could cyclically amplify to give rise to the renovascular complications of diabetic kidney disease.
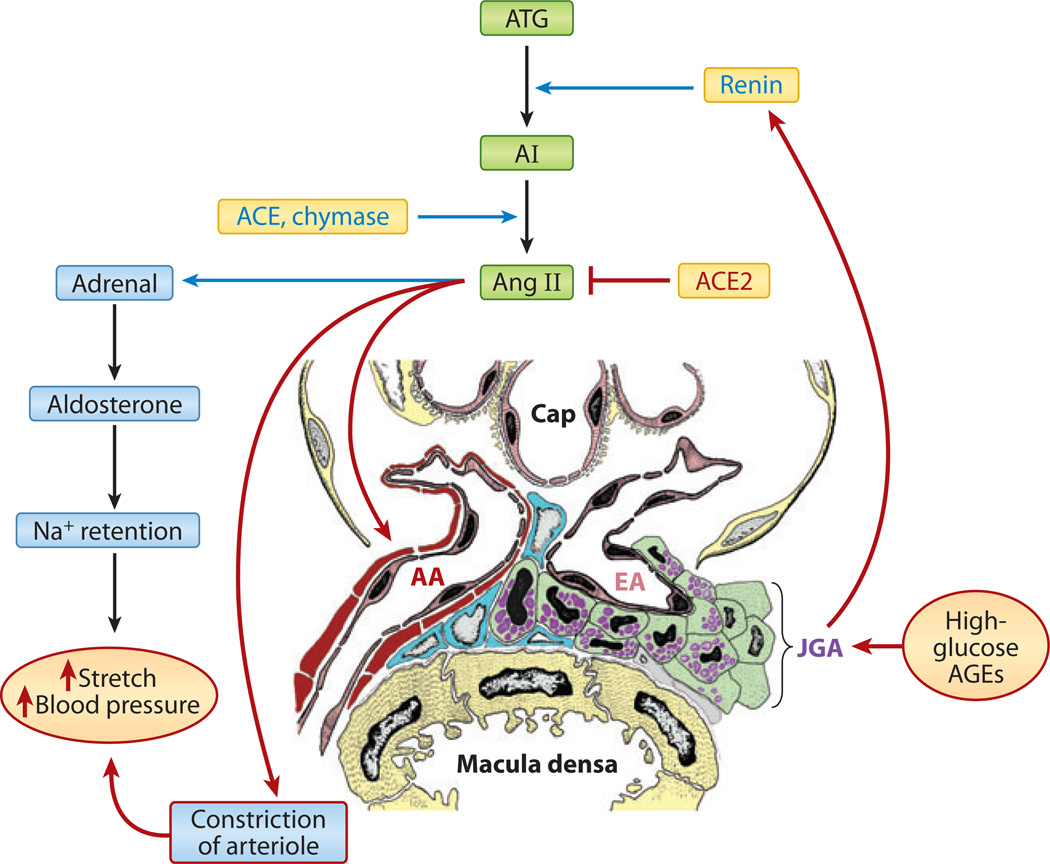
Figure 10.
Schematics depicting systemic activation of the renin-angiotensin system by glucose flux and advanced glycation end products (AGEs). High-glucose flux and AGEs may induce hypertrophy and hyperplasia of the juxtaglomerular apparatus (JGA), leading to increased secretion of renin from stored cellular granules. This process causes the generation of a potent vasoactive peptide known as angiotensin II (Ang II), which modulates the secretion of adrenal aldosterone with Na+ retention, and it also induces constriction of the efferent arteriole (EA). As a result of this mechanical stretch, systemic blood pressure rises, leading to worsening of the hyperglycemic injury in various target organs or cells. Abbreviations: AA, afferent arteriole; Cap, capillary; ACE, angiotensin-converting enzyme; AI, angiotensin I; ATG, angiotensinogen.
PATHOGENETIC MECHANISMS RELEVANT TO TUBULOINTERSTITIAL INJURY IN DIABETIC NEPHROPATHY
Until recently, studies to delineate the pathogenesis of diabetic nephropathy have focused on the renal glomerulus, and only a few reports have addressed the pathobiology of the tubulointerstitium (10, 73, 139–142). Although the tubulointerstitium is thought to be adversely affected at a later stage during the evolution of diabetic nephropathy than the glomerulus, the extent of the damage to this compartment correlates much better with renal dysfunction (e.g., decrease in the glomerular filtration rate) than does damage to the glomerular compartment (143). That the tubulointerstitial compartment represents the vast majority (∼90%) of the parenchymal mass of the kidney reinforces the importance of this compartment to the pathogenesis of diabetic nephropathy. For example, in the initial stages of diabetic nephropathy, when hyperfiltration and glomerular enlargement are prevalent, the renomegaly observed is related to the notable hypertrophy of the tubules (115, 142). Similarly, damage to this compartment in the later stages of diabetic nephropathy, albeit by varied pathogenetic mechanisms, may substantially impair renal function (142, 143).
Some mechanisms relevant to tubulointerstitial pathobiology may be similar to those described above for the renal glomerulus, but certain processes can selectively affect this compartment. The pathogenetic mechanisms that are common to both glomerular and tubulointerstitial compartments include increased channeling of glucose into the polyol pathway; generation of AGEs and ROS;and activation of various pathways pertaining to or influencing RAS, PKC-β, and TGF-β signaling. The actions of these mechanisms are supported by both in vivo and in vitro studies. For instance, direct exposure of tubular HK-2 cells to high-glucose ambience increases TGF-β activity and collagen production (37, 144). Also, in situ hybridization and immunohistochemical studies indicate that, with the inhibition of ACE or PKC-β, there is a significant decrease in TGF-β activity and in the expression of collagen and osteopontin, which is another ECM protein confined mainly to the tubulointerstitium (145, 146).
Tubulointerstitial injury may also occur secondary to adverse changes in the glomerulus, such as proteinuria observed in later stages of diabetic nephropathy (10, 140). Proteinuria, especially the nonselective type, can induce tubulointerstitial damage by several different mechanisms (147). First, a glomerular filtrate containing excessive amounts of profibrogenic cytokines would adversely affect the biology of tubular epithelium and interstitial cells (140, 148). Second, protein reabsorption overload in the tubular epithelium may cause lysosomal rupture, increased energy demand, and ultimately apoptosis. Third, tubular epithelial cell injury may be further augmented by increased concentrations of various activated components of the complement system and its endogenous regulatory proteins in tubular lumina, such as C3, C3a, C5a, C5b–9, and Crry (10, 149). Several of these components have chemotactic properties and therefore may generate an inflammatory response that leads to elaboration of MCP-1 and induction of an influx of monocytes, which consequently damage the tubulointerstitium. Support for this hypothesis comes from studies in which blockade of complement or MCP-1 activity ameliorated tubulointerstitial injury (150). Fourth, as in other chronic kidney diseases, proteinuria may be associated with transdifferentiation of tubular cells into myofibroblasts—a phenotypic change that is described in the literature as EMT (10, 151–153). Interestingly, the EMT is mediated by integrin-linked kinase and is stimulated by CTGF. The latter promotes fibrosis and sclerosis in the glomerular compartment, and its activity is modulated by TGF-β (83–85, 148, 154).
Another recently identified mechanism by which tubulointerstitial damage can occur in diabetic nephropathy relates to hypoxia-induced injury, irrespective of the presence or absence of proteinuria. Usually, hypoxia is attributed to chronic ischemia, which may occur by (a) intrarenal vasoconstriction secondary to local activation of RAS or loss of NO or (b) structural impairment of blood flow (10, 142, 155). The latter may occur secondary to interstitial fibrosis, which encases the peritubular capillaries in scarred tissue and thus restricts delivery of oxygen to the tubules (10, 156). Similarly, increased oxidant stress, along with a deficiency of NO, would further exacerbate capillary endothelial dysfunction, resulting in hypoxia of the tubulointerstitial compartment (157, 158).
Hypoxia is likely to induce functional impairment in the mitochondria of the tubular cells; the ensuing apoptosis is frequently observed in animal models of diabetic nephropathy as well as in humans (159). Also, hypoxia itself may induce activation of resident interstitial cells and EMT of the tubular cells that accelerate fibrosis with further compromise to the peritubular oxygen delivery (10, 156). In support of this idea, in vitro studies of renal interstitial fibroblasts subjected to oxygen deprivation demonstrated an increase in the transcription of collagen genes and TIMP-1 (160). The increase in TIMP-1 expression was related to the increased activity of its promoter, which is regulated by a transcription factor known as hypoxia-inducible factor 1 (HIF-1) (160, 161). Interestingly, HIF-1 can also bind to the promoter region of the profibrogenic cytokine CTGF, which could promote fibrosis (161). HIF-1 is a versatile transcription factor that modulates a number of pathways and the expression of several genes, includingVEGF and erythropoietin (EPO) (162–165). In the initial stages of hypoxia, the intact peritubular cells may produce sufficient EPO to maintain hemoglobin levels and O2 tension in the interstitium. However, with sustained damage by chronic hypoxia, hyperglycemia, oxidant stress, and endothelial dysfunction, loss of the EPO-producing fibroblasts may occur, along with anemia and progression of interstitial fibrosis, thereby initiating a vicious cycle of hypoxia and tubulointerstitial injury (10, 142, 156).
SUMMARY
Diabetic kidney injury begins with hyperglycemia, the sine qua non of diabetes mellitus. What happens as a result of hyperglycemia is a complex perturbation of cellular functioning and extracellular occurrences and hemodynamic effects that collectively alter the biology of virtually every type of kidney cell, whether in the vasculature, the nephron proper, or the surrounding interstitial parenchyma. The unassuming glucose molecule, when processed in greater flux through its diverse metabolic pathways, gives rise to DAG, GFAT, hexosamines, polyols, an altered redox environment, Amadori adducts, AGEs, and ROS. These molecules in turn trigger a cascade of signaling events that end up being maladaptive. Specifically, PKC, ERK, p38, JNK/SAPK, the small GTPases, AGE:RAGE signaling, angiotensin II, MCP-1, VEGF-A, and TGF-β1 are intertwined in a web of cross talk, impingements, activations, and repressions. As a result, the balance of cyclin kinases and transcription factors, such as AP-1, NF-κB, and Smads, tips in favor of a metabolic program that causes cellular hypertrophy, generates ECM, favors phenotypic switching, and selectively induces apoptosis. These processes give rise to the diabetic nephropathic manifestations of renomegaly, mesangial matrix expansion, Kimmelstiel-Wilson lesions, GBM thickening, podocytopenia, TBM thickening, interstitial fibrosis, and arteriolar hyalinization (Figure 1). However, these conditions represent only a fraction of the complexities of the renal cellular machinery. A complete description of the pathogenesis of diabetic nephropathy does not end here. Research challenges for the future may include the forging of new links between the metabolic and hemodynamic events and the elucidation of how all these myriad events interact to produce the clinical features of hypertension, proteinuria, and chronic kidney failure. Ongoing studies are beginning to elucidate the role of the GTP-binding proteins and are emphasizing the relevance of the tubulointerstitium, along with that of the mitochondrial-emanated oxidant stress, in diabetic nephropathy (166). Finally, other unresolved issues that are currently being investigated in many research laboratories and that are also important topics for future discussion include the role of macrophage, epigenetics, and microRNA in the pathogenesis of diabetic nephropathy (167–169).
ACKNOWLEDGMENTS
The authors’ work is supported by National Institutes of Health grants DK28492, DK44513, and DK60635.
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Yashpal S. Kanwar, Email: y-kanwar@northwestern.edu.
Lin Sun, Email: sunlinkid@163.com.
LITERATURE CITED
- 1.LeRoith D, Taylor SI, Olefsky JM. Diabetes Mellitus: A Fundamental and Clinical Text. 3rd ed. Philadelphia: Lippincott William & Wilkins; 2004. p. 1,540. [Google Scholar]
- 2.Reddy AS. Diabetic Nephropathy: Theory and Practice. East Hanover, NJ: College Book Publ; 2004. p. 563. [Google Scholar]
- 3.Wolf G. New insights into the pathophysiology of diabetic nephropathy: from hemodynamics to molecular pathology. Eur. J. Clin. Investig. 2004;34:785–796. doi: 10.1111/j.1365-2362.2004.01429.x. [DOI] [PubMed] [Google Scholar]
- 4.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J. Am. Soc. Nephrol. 2005;16:S30–S33. doi: 10.1681/asn.2004110970. [DOI] [PubMed] [Google Scholar]
- 5.Susztak K, Bottinger EP. Diabetic nephropathy: a frontier for personalized medicine. J. Am. Soc. Nephrol. 2006;17:361–367. doi: 10.1681/ASN.2005101109. [DOI] [PubMed] [Google Scholar]
- 6.Giunti S, Barit D, Cooper ME. Diabetic nephropathy: from mechanisms to rational therapies. Minerva Med. 2006;97:241–262. [PubMed] [Google Scholar]
- 7.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin. Nephrol. 2007;27:195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 9.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 10.Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern. Med. 2004;43:9–17. doi: 10.2169/internalmedicine.43.9. [DOI] [PubMed] [Google Scholar]
- 11.Zavadil J, Bottinger EP. TGF-βand epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 12.Dunlop M. Aldose reductase and the role of polyol pathway in diabetic nephropathy. Kidney Int. 2000;77:S3–S12. doi: 10.1046/j.1523-1755.2000.07702.x. [DOI] [PubMed] [Google Scholar]
- 13.Nayak B, Xie P, Akagi S, Yang Q, Sun L, et al. Modulation of renal-specific oxidoreductase/myoinositol oxygenase (MIOX) by high-glucose ambience. Proc. Natl. Acad. Sci. USA. 2005;102:17952–17957. doi: 10.1073/pnas.0509089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Gobe G. PKC activation and its role in kidney disease. Nephrology. 2006;11:428–434. doi: 10.1111/j.1440-1797.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2000;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 16.Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003;14:S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 17.Tan Al, Forbes JM, Cooper ME. AGE, RAGE and ROS in diabetic nephropathy. Semin. Nephrol. 2007;27:130–143. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, et al. PKC-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of Vascular NAD(P)H oxidase. J. Am. Soc. Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 19.Ziyadeh FN. Mediators of diabetic renal disease: the case for TGF-β as the major mediator. J. Am. Soc. Nephrol. 2004;15:S55–S57. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]
- 20.Schiffer M, von Gersdorf G, Bitzer M, Susztak K, Bottinger EP. Smad proteins and transforming growth factor βsignaling. Kidney Int. 2000;77:S45–S52. doi: 10.1046/j.1523-1755.2000.07708.x. [DOI] [PubMed] [Google Scholar]
- 21.Marrero MB, Barnes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephoropathy. Am. J. Physiol. Renal Physiol. 2006;290:F762–F768. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 22.Sharpe CC, Hendry BM. Signaling: focus on Rho in renal disease. J. Am. Soc. Nephrol. 2003;14:261–264. doi: 10.1097/01.asn.0000048223.05219.e4. [DOI] [PubMed] [Google Scholar]
- 23.Wolf G. Molecular mechanism of diabetic mesangial cell hypertrophy: a proliferation of novel factors. J. Am. Soc. Nephrol. 2002;13:2611–2613. doi: 10.1681/ASN.V13102611. [DOI] [PubMed] [Google Scholar]
- 24.Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Renal Physiol. 2007;292:F905–F911. doi: 10.1152/ajprenal.00421.2006. [DOI] [PubMed] [Google Scholar]
- 25.Ha H, Lee HB. Reactive oxygen species amplify glucose signaling in renal cells cultured under high glucose and in diabetic nephropathy. Nephrology. 2005;10:S7–S10. doi: 10.1111/j.1440-1797.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- 26.Hollenberg NK. Diabetes, nephropathy and the renin system. J. Hypertens. 2006;24:S81–S87. doi: 10.1097/01.hjh.0000220411.76740.bf. [DOI] [PubMed] [Google Scholar]
- 27.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the TGF-βpathway. Kidney Int. 2006;70:1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 28.Brosius FC, Heilig CW. Glucose transporters in diabetic nephropathy. Pediatr. Nephrol. 2005;20:445–451. doi: 10.1007/s00467-004-1748-x. [DOI] [PubMed] [Google Scholar]
- 29.Heilig CW, Concepcion LA, Riser BL, Freytag SO, Zhu M, Cortes P. Overexpression of glucose transporters in rat mesangial cells cultured in normal glucose milieu mimics the diabetic phenotype. J. Clin. Investig. 1995;96:1802–1814. doi: 10.1172/JCI118226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson DL, Cox MM. Glycolysis and catabolism of hexoses and the citric acid cycle. In: Nelson DL, Cox MM, editors. Lehninger Principles of Biochemistry. New York: Worth; 2000. pp. 527–597. [Google Scholar]
- 31.Quest AF, Ghosh S, Xie WQ, Bell RM. DAG second messengers: molecular switches and growth control. Adv. Exp. Med. Biol. 1997;400A:297–303. doi: 10.1007/978-1-4615-5325-0_42. [DOI] [PubMed] [Google Scholar]
- 32.Schleicher ED, Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int. 2000;77:S13–S18. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 33.Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, et al. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55:1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- 34.Arner RJ, Prabhu KS, Thompson JT, Hildenbrandt GR, Linken AD, Reddy CC. Myo-inositol oxygenase: molecular cloning and expression of a unique enzyme that oxidizes myo-inositol and D-chiroinositol. Biochem. J. 2001;300:313–320. doi: 10.1042/0264-6021:3600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greene DA, Lattimer SA, Sima AA. Sorbitol, phosphoinositides, and Na+/K+ATPase in the pathogenesis of diabetic complications. New Engl. J. Med. 1987;316:599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- 36.Greene DA, Lattimer SA, Sima AA. Are disturbances of sorbitol, phosphoinositide, and Na+/K+ATPase regulation involved in pathogenesis of diabetic neuropathy. Diabetes. 1988;37:688–693. doi: 10.2337/diab.37.6.688. [DOI] [PubMed] [Google Scholar]
- 37.Ziyadeh FN, Simmons DA, Snipes ER, Goldfarb S. Effect of myoinositol on cell proliferation and collagen transcription and secretion in proximal cells cultured in elevated glucose. J. Am. Soc. Nephrol. 1991;1:1220–1229. doi: 10.1681/ASN.V1111220. [DOI] [PubMed] [Google Scholar]
- 38.Schleicher ED, Bierhaus A, Haring HU, Nawroth PP, Lehmann R. Chemistry and pathobiology of advanced glycation end products. Contrib. Nephrol. 2001;131:1–9. doi: 10.1159/000060056. [DOI] [PubMed] [Google Scholar]
- 39.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol. Res. 2004;53:131–142. [PubMed] [Google Scholar]
- 40.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:S254–S258. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 41.Thallas-Bonke V, Lindschau C, Rizkalla B, Bach LA, Boner G, et al. Attenuation of extracellular matrix accumulation in diabetic nephropathy by advanced glycation end product cross-link breaker ALT-711 via a PKC-α-dependent pathway. Diabetes. 2004;53:2921–2930. doi: 10.2337/diabetes.53.11.2921. [DOI] [PubMed] [Google Scholar]
- 42.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 43.Wautier JL, Guillausseau PJ. Advanced glycation end products, their receptors and diabetic angiopathy. Diabetes Metab. 2001;27:535–542. [PubMed] [Google Scholar]
- 44.Noh H, King GL. The role of PKC activation in diabetic nephropathy. Kidney Int. 2007;106:S49–S53. doi: 10.1038/sj.ki.5002386. [DOI] [PubMed] [Google Scholar]
- 45.Parker PJ, Murray-Rust J. PKC at a glance. J. Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- 46.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, et al. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through PKC-α-dependent pathway. Diabetes. 2007;57:460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- 47.Whiteside CI, Dlugosz JA. Mesangial cell PKC isozyme activation in diabetic milieu. Am. J. Physiol. Renal Physiol. 2002;282:F975–F980. doi: 10.1152/ajprenal.00014.2002. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa T, Segal M, Croker B, Johnson RJ. A breakthrough in diabetic nephropathy: the role of endothelial dysfunction. Nephrol. Dial. Transplant. 2007;22:2775–2777. doi: 10.1093/ndt/gfm380. [DOI] [PubMed] [Google Scholar]
- 49.Lee HB, Yu MI-RA, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:S241–S245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 50.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC-β inhibitor in diabetic db/db mice, a rodent of type 2 diabetes. FASEB J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 51.Werner E. GTPases and reactive oxygen species: switches for killing and signaling. J. Cell Sci. 2004;117:143–153. doi: 10.1242/jcs.00937. [DOI] [PubMed] [Google Scholar]
- 52.Djordjevic VB. Free radicals in cell biology. Int. Rev. Cytol. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- 53.Catherwood MA, Powell LA, Anderson P, McMaster D, Sharpe PC, Trimble ER. Glucose-induced oxidative stress in mesangial cells. Kidney Int. 2002;61:599–608. doi: 10.1046/j.1523-1755.2002.00168.x. [DOI] [PubMed] [Google Scholar]
- 54.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effect of antioxidants in diabetes-induced oxidative stress in glomeruli of diabetic rats. J. Am. Soc. Nephrol. 2003;14:S250–S253. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 55.Kang D, Hamasaki N. Mitochondrial oxidative stress and mitochondrial DNA. Clin. Chem. Lab. Med. 2003;41:1281–1288. doi: 10.1515/CCLM.2003.195. [DOI] [PubMed] [Google Scholar]
- 56.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid. Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 57.Li J-M, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:S221–S226. doi: 10.1097/01.asn.0000077406.67663.e7. [DOI] [PubMed] [Google Scholar]
- 58.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity and mitochondrial damage in type 2 diabetes. Diabetes. 2004;53:1412–1417. doi: 10.2337/diabetes.53.6.1412. [DOI] [PubMed] [Google Scholar]
- 59.Maassen JA, Hart LM, Van Essen E, Heine RJ, Nijpels G, et al. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53:S103–S109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- 60.Hohmeier HE, Thigpen A, Tran VV, Davis R, Newgard CB. Stable overexpression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-β-induced cytotoxicity and reduces nitric oxide production. J. Clin. Investig. 1998;101:1811–1820. doi: 10.1172/JCI1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du X, Matsumura T, Edelstein D, Rossett L, Zsengeller Z, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003;112:1049–1457. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Craven PR, Melhem MF, Phillips SL, De Rubertis FR. Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes. 2001;50:2114–2125. doi: 10.2337/diabetes.50.9.2114. [DOI] [PubMed] [Google Scholar]
- 63.DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53:762–768. doi: 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- 64.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 65.Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, et al. Reactive oxygen species from mitochondria induce cyclooxygenase-2 (COX-2) gene expression in human mesangial cells. Diabetes. 2003;52:2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 66.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 67.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, et al. Role of reactive oxygen species in TGF-β-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 68.Leask A, Abraham TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 69.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 70.Padgett RW, Reiss M. TGF-βsuperfamily signaling: notes from the desert. Development. 2007;134:3565–3569. doi: 10.1242/dev.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahimi RA, Leof EB. TGF-β signaling: a tale of two responses. J. Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 72.Wahab NA, Mason RM. A critical look at growth factors and epithelial-to-mesenchymal transition in the adult kidney. Interrelationships between growth factors that regulate EMT in the adult kidney. Nephron Exp. Nephrol. 2006;104:e129–e134. doi: 10.1159/000094963. [DOI] [PubMed] [Google Scholar]
- 73.Phillips AO. The role of renal proximal tubular cells in diabetic nephropathy. Curr. Diabetes Rep. 2003;3:491–496. doi: 10.1007/s11892-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 74.Pacher P, Obrosova IG, Mablley JG, Szabo C. Role of nitrosative stress and peroxynitrite in pathogenesis of diabetic complications. Curr. Med. Chem. 2005;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic. Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 76.Consentino F, Katusic Z. Tetrahydrobiopterin and dysfunction of the endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- 77.Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J. Cardiovasc. Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999;56:393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 79.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: Podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 80.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 81.Mulder KM. Role of Ras and MAPKS in TGF-βsignaling. Cytokine Growth Factor Rev. 2000;11:23–25. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 82.Jandeleit-Dahm K, Cooper ME. Hypertension and diabetes: role of renin-angiotensin system. Endocrinol. Meab. Clin. N. Am. 2006;35:469–490. doi: 10.1016/j.ecl.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, et al. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor (CTGF) and other genes in human mesangial cells. J. Biol. Chem. 1999;274:5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt-Ott KM. Unraveling the role of connective tissue growth factor in diabetic nephropathy. Kidney Int. 2008;73:375–377. doi: 10.1038/sj.ki.5002661. [DOI] [PubMed] [Google Scholar]
- 85.Leask A. Transcriptional profiling of the scleroderma fibroblasts reveals a potential role for the connective tissue growth factor (CTGF) in pathological fibrosis. Keio J. Med. 2004;53:74–77. doi: 10.2302/kjm.53.74. [DOI] [PubMed] [Google Scholar]
- 86.Cohen MP, Sharma K, Guo J, Eltayeb BO, Ziyadeh FN. The renal TGF-β system in the db/db mouse model of diabetic nephropathy. Exp. Nephrol. 1998;6:226–233. doi: 10.1159/000020527. [DOI] [PubMed] [Google Scholar]
- 87.Isono M, Mogyorosi A, Han DC, Hoffman BB, Ziyadeh FN. Stimulation of TGF-β type II receptor by high glucose in mouse mesangial cells and in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2000;278:F830–F838. doi: 10.1152/ajprenal.2000.278.5.F830. [DOI] [PubMed] [Google Scholar]
- 88.Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-βby anti-TGF-βantibody attenuates kidney hypertrophy and enhanced extracellular expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 89.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal anti-TGF-βantibody in db/db mice. Proc. Natl. Acad. Sci. USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sato H, Iwano M, Akai Y, Kurioka H, Kubo A, et al. Increased excretion of urinary TGF-β in patients with diabetic nephropathy. Am. J. Nephrol. 1998;18:490–494. doi: 10.1159/000013415. [DOI] [PubMed] [Google Scholar]
- 91.Song JH, Cha SH, Lee HJ, Lee SW, Park GH, et al. Effect of low dose dual blockade of rennin-angiotensin system on urinary TGF-βin type-2 diabetic patients with advanced kidney disease. Nephrol. Dial. Transplant. 2006;21:683–689. doi: 10.1093/ndt/gfi310. [DOI] [PubMed] [Google Scholar]
- 92.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 93.Ying Q-L, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 94.Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002;61:51–60. doi: 10.1046/j.1523-1755.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 95.Mitu GM, Wang S, Hirschberg R. BMP-7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am. J. Physiol. Renal Physiol. 2007;293:F1641–F1648. doi: 10.1152/ajprenal.00179.2007. [DOI] [PubMed] [Google Scholar]
- 96.Wang S, de Caestecker M, Kopp J, Mitu G, LaPage J, Hirschberg R. Renal bone morphogenetic protein 7 protects against diabetic nephropathy. J. Am. Soc. Nephrol. 2006;17:2504–2512. doi: 10.1681/ASN.2006030278. [DOI] [PubMed] [Google Scholar]
- 97.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 98.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu. Rev. Cell Dev. Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 99.Kim SL, Kim HJ, Han DC, Lee HB. Effect of lovastatin on small GTP binding proteins and TGF-β1 and fibronectin expression. Kidney Int. 2000;58:S88–S92. doi: 10.1046/j.1523-1755.2000.07714.x. [DOI] [PubMed] [Google Scholar]
- 100.Katz ME, McCormik F. Signal transduction from multiple Ras effectors. Curr. Opin. Genet. Dev. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 101.Lin S, Chugh SS, Pan X, Wallner EI, Wada J, Kanwar YS. Identification of up-regulated Ras-like GTPase, Rap1b, by suppression subtractive hybridization. Kidney Int. 2001;60:2129–2141. doi: 10.1046/j.1523-1755.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- 102.Lin S, Sahai A, Chugh SS, Pan X, Wallner EI, et al. High glucose stimulates synthesis of fibronectin via a novel PKC, Rap1b and B- Raf signaling pathway. J. Biol. Chem. 2002;277:41725–4135. doi: 10.1074/jbc.M203957200. [DOI] [PubMed] [Google Scholar]
- 103.Danesh FR, Sadeghi MM, Amro N, Philips C, Zeng L, et al. HMG-CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase p21 signaling pathway: implications for diabetic nephropathy. Proc. Natl. Acad. Sci. USA. 2002;99:8301–8305. doi: 10.1073/pnas.122228799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furlong F, Crean J, Thornton L, O’Leary R, Murphy M, Martin F. Dysregulated intracellular signaling impairs CTGF-stimulated responses in human mesangial cells exposed to high extracellular glucose. Am. J. Physiol. Renal Physiol. 2007;292:F1691–F1700. doi: 10.1152/ajprenal.00342.2006. [DOI] [PubMed] [Google Scholar]
- 105.Zeng L, Xu H, Chew T-L, Chisholm R, Sadeghi MM, et al. Simvastatin modulates angiotensin II signaling pathway by preventing Rac1-mediated upregulation of p27 . J. Am. Soc. Nephrol. 2004;15:1711–1720. doi: 10.1097/01.asn.0000129839.91567.68. [DOI] [PubMed] [Google Scholar]
- 106.Makkinje A, Quinn Da, Chen A, Cadilla CL, Force T, et al. Gene 33/Mig-6, a transcriptionally inducible adapter protein that binds GTP-Cdc42 and activates SAPK/JNK. J. Biol. Chem. 2000;275:17838–17847. doi: 10.1074/jbc.M909735199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J, Lin A. Writing the cell signaling circuitry by NFκB and JNK1 crosstalk and its applications in human diseases. Oncogene. 2007;26:3267–3278. doi: 10.1038/sj.onc.1210417. [DOI] [PubMed] [Google Scholar]
- 108.Huha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- 109.Hall A. Rho GTPases and the control of cell behavior. Biochem. Soc. Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 110.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 111.Wolf G. Cell cycle regulation in diabetic nephropathy. Kidney Int. 2000;77:S59–S66. doi: 10.1046/j.1523-1755.2000.07710.x. [DOI] [PubMed] [Google Scholar]
- 112.Ekholm SV, Reed SI. Regulation of G1 cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 2000;12:676–684. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 113.Kuan CJ, Al-Douahji M, Shankland SJ. The cyclin kinase inhibitor p21WAF1.CIP1 is increased in experimental diabetic nephropathy. J. Am. Soc. Nephrol. 1998;9:986–1003. doi: 10.1681/ASN.V96986. [DOI] [PubMed] [Google Scholar]
- 114.Awaazu M, Omari S, Ishikura K, Hida M, Hiosayo F. The lack of cyclin kinase inhibitor pKip1 ameliorates progression of diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:699–703. doi: 10.1097/01.asn.0000051726.41601.c0. [DOI] [PubMed] [Google Scholar]
- 115.Anderson S, Brenner BM. Pathogenesis of diabetic glomerulopathy: hemodynamic considerations. Diabetes Metab. Rev. 1988;4:163–167. doi: 10.1002/dmr.5610040206. [DOI] [PubMed] [Google Scholar]
- 116.Guinti S, Barit D, Cooper ME. Mechanisms of diabetic nephropathy: role of hypertension. Hypertension. 2006;48:519–526. doi: 10.1161/01.HYP.0000240331.32352.0c. [DOI] [PubMed] [Google Scholar]
- 117.Hostetter TH, Rennke HG, Brenner BM. Case for intrarenal hypertension in initiation and progression of diabetic and other glomerulopathies. Am. J. Med. 1982;72:375–380. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- 118.Mogensen CE. Microalbuminuria and hypertension with focus on type I and type 2 diabetes. J. Intern. Med. 2003;254:45–66. doi: 10.1046/j.1365-2796.2003.01157.x. [DOI] [PubMed] [Google Scholar]
- 119.Singh R, Singh AK, Alavi N, Leehey DJ. Mechanism of in creased angiotensin II levels in mesangial cells cultured in high glucose. J. Am. Soc. Nephrol. 2003;14:873–880. doi: 10.1097/01.asn.0000060804.40201.6e. [DOI] [PubMed] [Google Scholar]
- 120.Vidotti DB, Casarini DE, Cristovam PC, Leite CE, Schor N, Boim MA. High glucose stimulates intracellular renin activity and angiotensin II generation in the mesangial cells. Am. J. Physiol. Renal. Physiol. 2004;286:F1039–F1045. doi: 10.1152/ajprenal.00371.2003. [DOI] [PubMed] [Google Scholar]
- 121.Liebau MC, Lang D, Bohm J, Endlich N, Bek MJ, et al. Functional expression of the renninangiotensin system in human podocytes. Am. J. Physiol. Renal. Physiol. 2004;290:F710–F719. doi: 10.1152/ajprenal.00475.2004. [DOI] [PubMed] [Google Scholar]
- 122.Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 123.Gnudi L, Thomas SM, Viberti G. Mechanical forces in diabetic kidney disease: a trigger for impaired glucose metabolism. J. Am. Soc. Nephrol. 2007;18:2226–2232. doi: 10.1681/ASN.2006121362. [DOI] [PubMed] [Google Scholar]
- 124.Jacobsen PK, Tarnow L, Parving H-H. Time to consider ACE insertion/deletion genotypes and individual renoprotective treatment in diabetic nephropathy. Kidney Int. 2006;69:1293–1295. doi: 10.1038/sj.ki.5000283. [DOI] [PubMed] [Google Scholar]
- 125.Tikellis C, Johnston CI, Forbes JM, Burns WC, et al. Characterization of renal angiotensinconverting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- 126.Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and kidney. Circ. Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 127.Singh R, Singh AK, Alavi N, Leehey DJ. Mechanism of increased angiotensin II levels in glomerular cells cultured in high glucose. J. Am. Soc. Nephrol. 2003;14:873–880. doi: 10.1097/01.asn.0000060804.40201.6e. [DOI] [PubMed] [Google Scholar]
- 128.Thomas MC, Tikellis C, Burns WM, Bialkowski K, Cao Z, et al. Interaction between renin angiotensin system and advanced glycation in the kidney. J. Am. Soc. Nephrol. 2005;16:2976–2984. doi: 10.1681/ASN.2005010013. [DOI] [PubMed] [Google Scholar]
- 129.Gnudi L, Viberti G, Raij L, Rodriguez V, Burt D, et al. GLUT-1 overexpression: link between hemodynamics and metabolic factors in glomerular injury? Hypertension. 2003;42:19–24. doi: 10.1161/01.HYP.0000075949.19968.EF. [DOI] [PubMed] [Google Scholar]
- 130.Yasuda T, Kondo S, Homma T, Harris RC. Regulation of extracellular matrix by mechanical stress in rat glomerular mesangial cells. J. Clin. Investig. 1996;98:1991–2000. doi: 10.1172/JCI119003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gruden G, Zonca S, Hayward A, Thomas S, Maestrini S, et al. Mechanical stretch-induced fibronectin and TGF-β1 production in human mesangial cells is p38 MAPK-dependent. Diabetes. 2000;49:655–661. doi: 10.2337/diabetes.49.4.655. [DOI] [PubMed] [Google Scholar]
- 132.Gruden G, Setti G, Hayward A, Sugden D, Duggan S, et al. Mechanical stretch induces monocyte chemoattractant activity via NF κB-dependent MCP-1-mediated pathway in human mesangial cells: inhibition by rosiglitazone. J. Am. Soc. Nephrol. 2005;16:688–696. doi: 10.1681/ASN.2004030251. [DOI] [PubMed] [Google Scholar]
- 133.Fututa T, Saito T, Ootaka T, Soma J, Obara K, et al. The role of macrophages in diabetic glomerulosclerosis. Am. J. Kidney Dis. 1993;21:480–485. doi: 10.1016/s0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- 134.Chen S, Kasama Y, Lee JS, Jim B, Marin M, Ziyadeh FN. Podocyte-derived vascular endothelial growth factor mediates the stimulation of α(IV) collagen production by transforming growth factor β in mouse podocytes. Diabetes. 2004;53:2939–2349. doi: 10.2337/diabetes.53.11.2939. [DOI] [PubMed] [Google Scholar]
- 135.White KE. Research into the glomerular podocyte—is it relevant to diabetic nephropathy? Diabet. Med. 2006;23:715–719. doi: 10.1111/j.1464-5491.2006.01790.x. [DOI] [PubMed] [Google Scholar]
- 136.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular growth factor signaling ameliorates diabetic albuminuria in mice. J. Am. Soc. Nephrol. 2006;17:3093–3104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 137.Cooper ME, Mundel P, Boner G. Role of nephrin in renal disease including diabetic nephropathy. Semin. Nephrol. 2002;22:393–398. doi: 10.1053/snep.2002.34724. [DOI] [PubMed] [Google Scholar]
- 138.Gross M-L, Dikow R, Ritz E. Diabetic nephropathy: Recent insights into the pathophysiology and progression of diabetic nephropathy. Kdney Int. 2005;67:S50–S53. doi: 10.1111/j.1523-1755.2005.09412.x. [DOI] [PubMed] [Google Scholar]
- 139.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kdney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 140.Wang SN, Hirschberg R. Growth factor ultrafiltration in experimental diabetic nephropathy contributes to interstitial fibrosis. Am. J. Physiol. Renal Physiol. 2000;278:F554–F560. doi: 10.1152/ajprenal.2000.278.4.F554. [DOI] [PubMed] [Google Scholar]
- 141.Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol. Histopathol. 2002;17:247–252. doi: 10.14670/HH-17.247. [DOI] [PubMed] [Google Scholar]
- 142.Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat. Clin. Pract. Nephrol. 2008;4:216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 143.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 144.Rocco MV, Chen Y, Goldfarb S, Ziyadeh FN. Elevated glucose stimulates TGF-βgene expression and bioactivity in proximal tubule. Kidney Int. 1992;41:107–114. doi: 10.1038/ki.1992.14. [DOI] [PubMed] [Google Scholar]
- 145.Gilbert RE, Cox A, Wu LL, Allen AJ, Hulthen UL, et al. Expression of TGF-β and type-IV collagen in the renal tubulo-interstitium in experimental diabetes. Diabetes. 1998;47:414–422. doi: 10.2337/diabetes.47.3.414. [DOI] [PubMed] [Google Scholar]
- 146.Kelly DJ, Chanty A, Gow RM, Zhang Y, Gilbert RE. PKC-β inhibition attenuates osteopontin expression, macrophage recruitment and tubulointerstitial injury in advanced experimental diabetic nephropathy. J. Am. Soc. Nephrol. 2005;16:1654–1660. doi: 10.1681/ASN.2004070578. [DOI] [PubMed] [Google Scholar]
- 147.Bazzi C, Petrini C, Rizza V, Arrigo G, D’Amico G. A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int. 2000;58:1732–1741. doi: 10.1046/j.1523-1755.2000.00334.x. [DOI] [PubMed] [Google Scholar]
- 148.Wang S, Denichilo M, Brubaker C, Hirschberg R. Connective tissue growth factor (CTGF) in tubulointerstitial injury of diabetic nephropathy. Kidney Int. 2001;60:96–105. doi: 10.1046/j.1523-1755.2001.00776.x. [DOI] [PubMed] [Google Scholar]
- 149.Hori Y, Yamada K, Hanafusa N, Okuda T, Okada N, et al. Crry, a complement regulatory protein, modulates renal interstitial disease induced by proteinuria. Kidney Int. 1999;56:2096–2106. doi: 10.1046/j.1523-1755.1999.00765.x. [DOI] [PubMed] [Google Scholar]
- 150.Shimizu H, Maruyama S, Yuzawa Y, Kato T, Miki Y, et al. Anti-MCP-1 gene therapy attenuates renal injury induced by protein-overload proteinuria. J. Am. Soc. Nephrol. 2003;14:1496–1505. doi: 10.1097/01.asn.0000069223.98703.8e. [DOI] [PubMed] [Google Scholar]
- 151.Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, et al. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998;54:864–876. doi: 10.1046/j.1523-1755.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- 152.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kalluri R. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu BC, Li MX, Zhang JD, Liu XC, Zhang XL, Phillips AO. Inhibition of integrin-linked kinase via a siRNA expression plasmid attenuates CTGF-induced human proximal tubular epithelial cells to mesenchymal transition. Am. J. Nephrol. 2008;28:143–151. doi: 10.1159/000110019. [DOI] [PubMed] [Google Scholar]
- 155.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front. Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 156.Choi YJ, Chakraborty S, Nguyen V, Nguyen C, Kim BK, et al. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of VEGF. Human Pathol. 2000;31:1491–1497. doi: 10.1053/hupa.2000.20373. [DOI] [PubMed] [Google Scholar]
- 157.Palm F, Buerk DG, Carlsson PO, Hansell P, Liss P. Reduced nitric oxide concentration in the renal cortex of streptozotocin-induced diabetic rats: effects on renal oxygenation and microcirculation. Diabetes. 2005;54:3282–3287. doi: 10.2337/diabetes.54.11.3282. [DOI] [PubMed] [Google Scholar]
- 158.Friederich M, Fasching A, Hansell P, Nordquist L, Palm F. Diabetes-induced up-regulation of uncoupling protein 2 results in increased mitochondrial uncoupling in kidney proximal tubular cells. Biochim. Biophys. Acta. 2008;1777:935–940. doi: 10.1016/j.bbabio.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 159.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic nephropathy. Mol. Cell Biochem. 2004;259:67–70. doi: 10.1023/b:mcbi.0000021346.03260.7e. [DOI] [PubMed] [Google Scholar]
- 160.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 161.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of CTGF is mediated by HIF-1. Am. J. Physiol. Renal Physiol. 2004;287:F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 162.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. Role of HIF-1αin hypoxiamediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 163.Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. VEGF modulation by targeting HIF-1α. Cancer Res. 2000;60:6248–6252. [PubMed] [Google Scholar]
- 164.Nangaku M, Eckardt KU. Hypoxia and HIF system in kidney disease. J. Mol. Med. 2007;85:1325–1330. doi: 10.1007/s00109-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 165.Maxwell P. HIF-1: an oxygen response system with a special relevance to the kidney. J. Am. Soc. Nephrol. 2003;14:2712–2722. doi: 10.1097/01.asn.0000092792.97122.e0. [DOI] [PubMed] [Google Scholar]
- 166.Sun L, Xie P, Wada J, Kashihara N, Liu F, et al. Rap1b GTPase ameliorates glucose-induced mitochondrial dysfunction. J. Am. Soc. Nephrol. 2008;19:2293–2301. doi: 10.1681/ASN.2008030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tesch GH. Macrophages and diabetic nephropathy. Semin. Nephrol. 2010;30:290–301. doi: 10.1016/j.semnephrol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 168.Schaeffer V, Abrass CK. Mechanisms and control of protein translation in the kidney. Am. J. Nephrol. 2010;31:189–201. doi: 10.1159/000268954. [DOI] [PubMed] [Google Scholar]
- 169.Villeneuve LM, Natarajan R. The role of epigenetics in the pathology of diabetic complications. Am. J. Physiol. Renal Physiol. 2010;299:F15–F25. doi: 10.1152/ajprenal.00200.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]