Abstract
Aromatic boronic acids react rapidly with peroxynitrite (ONOO−) to yield phenols as major products. This reaction was used to monitor ONOO− formation in cellular systems. Previously, we proposed that the reaction between ONOO− and arylboronates (PhB(OH)2) yields a phenolic product (major pathway) and a radical pair PhB(OH)2O•−…•NO2 (minor pathway). [Sikora A. et al., Chem Res Toxicol 24, 687-97, 2011]. In this study, we investigated the influence of a bulky triphenylphosphonium (TPP) group on the reaction between ONOO− and mitochondria-targeted arylboronate isomers (o-, m-, and p-MitoPhB(OH)2). Results from the electron paramagnetic resonance (EPR) spin-trapping experiments unequivocally showed the presence of a phenyl radical intermediate from meta and para isomers, and not from the ortho isomer. The yield of o-MitoPhNO2 formed from the reaction between o-MitoPhB(OH)2 and ONOO− was not diminished by phenyl radical scavengers, suggesting a rapid fragmentation of the o-MitoPhB(OH)2O•− radical anion with subsequent reaction of the resulting phenyl radical with •NO2 in the solvent cage. The DFT quantum mechanical calculations showed that the energy barrier for the dissociation of o-MitoPhB(OH)2O•− radical anion is significantly lower than that of m-MitoPhB(OH)2O•− and p-MitoPhB(OH)2O•− radical anions. The nitrated product, o-MitoPhNO2, is not formed by nitrogen dioxide radical generated by myeloperoxidase in the presence of nitrite anion and hydrogen peroxide, indicating that this specific nitrated product may be used as a diagnostic marker product for ONOO−. Incubation of o-MitoPhB(OH)2 with RAW 264.7 macrophages activated to produce ONOO− yielded the corresponding phenol o-MitoPhOH as well as the diagnostic nitrated product, o-MitoPhNO2. We conclude that the ortho isomer probe reported here is most suitable for specific detection of ONOO− in biological systems.
Keywords: mitochondria-targeted probes, boronates, peroxynitrite, diagnostic product
INTRODUCTION
Recent research has focused on the development of targeted probes for detecting mitochondrial reactive oxygen and nitrogen species (ROS/RNS).(1;2) One of the ways in which a small molecule probe can be transported to mitochondria is through covalent attachment to a lipophilic, delocalized positivelycharged triphenylphosphonium (TPP) cation.(3) Due to both cationic and lipophilic character, chemical probes tagged with TPP cation accumulate preferentially within mitochondria.
Arylboronic acids react with hydrogen peroxide stoichiometrically to form the corresponding phenols.(4) Recently, boronate-based fluorogenic probes were synthesized for detecting cellular hydrogen peroxide.(5;6) Mitochondria-targeted TPP chemical probes were designed to detect mitochondrial ROS (MitoPY, MitoTEMPO-H).(7;8) Arylboronic-based TPP probe MitoB [m-MitoPhB(OH)2, (2-boronobenzyl)triphenylphosphonium salt] (Fig. 1) was used to detect H2O2 in living Drosophila.(9;10) In this study, we investigated the reaction between peroxynitrite (ONOO−) and the three isomers of MitoB.
Figure 1. Structures of MitoPhB(OH)2 isomers and the major, and minor products of their reactions with peroxynitrite.
Our previous studies have shown that arylboronates can be oxidized not only by H2O2, but also by other biologically relevant oxidants including hypochlorite and ONOO−.(11;12) Results showed that boronate-based fluorogenic probes are suitable for real time monitoring of ONOO− formed in cell-free systems and in activated macrophages.(13;14) Peroxynitrite (15;16) reacts with arylboronates rapidly (k ~ 106 M−1s−1 at pH 7.4) and stoichiometrically. The major oxidation products are phenols (yield ~ 85–90%).(11) The minor products (10–15%) are formed via a radical pathway of the reaction.(12) The proposed mechanism for the reaction between ONOO− and arylboronates is presented in Scheme 1. The initial step of the reaction involves the formation of an anionic adduct between ONOO− and the boronate. Further cleavage of O-O bond results in the formation of phenol and nitrite (major, heterolytic O-O cleavage pathway) or caged radical pair PhB(OH)2O•−····•NO2 (minor, homolytic O-O cleavage pathway). The subsequent fragmentation of PhB(OH)2O•− radical anion leads to the formation of phenyl radical Ph• that reacts with H-atom donors, nitrogen dioxide •NO2, or molecular oxygen.
Scheme 1.
Here we compared the reaction profiles of ONOO− with three isomeric TPP+-substituted phenylboronic acids (o-MitoPhB(OH)2, m-MitoPhB(OH)2, and p-MitoPhB(OH)2) (Figure 1). We determined that the yield of o-MitoPhNO2 formed from the reaction of o-MitoPhB(OH)2 with ONOO− is unaffected by the phenyl radical scavengers, suggesting fast fragmentation of the o-MitoPhB(OH)2O•− radical anion with subsequent reaction of the resulting phenyl radical with •NO2 in the solvent cage. Moreover, even in the presence of 2-propanol, o-MitoPhNO2 is formed in relatively high yield (9%). This is 9 times higher than that of m-MitoPhNO2 and p-MitoPhNO2 under the same conditions. We conclude that determination of both major and minor products from o-MitoPhB(OH)2 will serve as the unique tool providing a diagnostic marker for cellular ONOO− formation.
EXPERIMENTAL PROCEDURES
Chemicals
MitoPhB(OH)2 isomers, MitoPhNO2 and MitoPh were synthesized according to a published procedure of triphenylphosphine benzylation.(17) All solutions were prepared using deionized water (Millipore Milli-Q system). ONOO− was prepared according to the published procedure,(18) by reacting nitrite with H2O2. The concentration of ONOO− was determined by measuring the absorbance at 302 nm (ε = 1.7×103 M−1cm−1) in alkaline aqueous solutions (pH>12).(18) DPTA-NONOate(19;20) was from Cayman Chemicals. Xanthine oxidase (XO) and myeloperoxidase (MPO) were obtained from Sigma, and catalase was from Boehringer. All other reagents (of highest purity available) were from Sigma-Aldrich Corp.
Determination of O2•− and •NO Fluxes
•NO and O2•− fluxes were determined in phosphate buffer (100 mM, pH 7.4) containing dtpa (100 µM) as described previously.(14) Briefly, •NO fluxes were determined by measuring rate of the decomposition of DPTA-NONOate by following the decrease in the absorbance at 250 nm (ε = 8×103 M−1 cm−1).(19) Assuming that two molecules of •NO are released from nitric oxide donor, the measured rate of NONOate decomposition was multiplied by a factor of 2 to obtain the rate of •NO release. The superoxide was generated from XO catalyzed oxidation of hypoxanthine (HX). The flux of O2•− was determined by monitoring the superoxide dismutase-inhibitable reduction of cytochrome c following the increase in absorbance at 550 nm (Δε = 2.1×104 M−1cm−1).(21)
HPLC and UPLC/MS Analyses
HPLC analyses were performed using an Agilent 1100 HPLC system equipped with fluorescence and UV–Vis absorption detectors. In the studies on the reaction profile of peroxynitrite oxidation of MitoPhB(OH)2 isomers, 100 µl of sample was injected into the HPLC system equipped with a C18 column (Phenomenex, Kinetex™, 100×4.6 mm, 2.6 µm) equilibrated with 10% CH3CN [containing 0.1% (v/v) trifluoroacetic acid (TFA)] in 0.1% TFA aqueous solution. The compounds were separated by a linear increase in CH3CN phase concentration from 10 to 100% over 7 min, using a flow rate of 1.5 ml/min. Under these conditions, the following retention times (in min) were observed: o-MitoPhB(OH)2, 4.31; o-MitoPhOH, 4.50; o-MitoPhNO2, 4.61; MitoPh, 4.82; m-MitoPhB(OH)2, 4.05; m-MitoPhOH, 4.2; m-MitoPhNO2, 4.70; p-MitoPhB(OH)2, 3.98; p-MitoPhOH, 4.24; and p-MitoPhNO2, 4.71. The peak areas detected by monitoring the absorption at 268 nm were used for the quantitation.
To characterize the synthesized triphenylphosphonium compounds and to identify the products of the reaction of peroxynitrite with triphenylphosphonium-substituted arylboronic acids the UPLC/MS analyses were performed using an Acquity UPLC Waters Ltd. system coupled on-line to LCT Premier™ XE (Waters) mass spectrometer with ToF mass detector. 1 µl of sample was injected into the UPLC system equipped with a C18 column (Waters Acquity BEH C18, 100×2.1 mm, 1.7 µm) maintained at 40 °C and equilibrated with 30% CH3CN [containing 0.1% (v/v) trifluoroacetic acid (TFA)] in 0.1% TFA aqueous solution. The compounds were separated by a linear increase in CH3CN phase concentration from 30 to 60% from 0.8 min to 4 min, using a flow rate of 0.35 ml/min. Under these conditions, the following retention times (in min) were observed: o-MitoPhB(OH)2, 3.30; o-MitoPhOH, 3.52; o-MitoPhNO2, 3.59; MitoPh, 3.91; m-MitoPhB(OH)2, 2.86; m-MitoPhOH, 3.16; m-MitoPhNO2, 3.69; p-MitoPhB(OH)2, 2.76; p-MitoPhOH, 3.11; and p-MitoPhNO2, 3.72. The compounds were detected by monitoring the absorption at 268 ± 1.2 nm. Mass spectrometer was operated in W mode with lock mass correction. Lock mass solution (leucine-enkephalin, reference mass: [M+H]+C12 556.2771, [M+H]+C13 557.2801) was prepared at a concentration of 0.5 ng/µl in 50:50 (v/v), water:acetonitrile solution and stored in 4 °C until further use. Data acquisition and analyses were performed using the MassLynx™ 4.1 data system software (Waters) and OriginPro 8.6 (OriginLab).
To investigate the effect of MitoPhB(OH)2 isomers on the oxidation and nitration of tyrosine, 100 µl of sample was injected into the HPLC system equipped with a Phenomenex Synergi 4u Hydro-RP 80 Å column (250×4.6 mm, 4 µm) equilibrated with water containing 0.1% (v/v) TFA. The compounds were separated by a linear increase in CH3CN phase (containing 0.1% (v/v) TFA) concentration from 0 to 5% over 20 min, then further linear increase in CH3CN phase concentration from 5 to 100% over the next 30 min, using a flow rate of 1.0 ml/min. Under those conditions tyrosine eluted at 19.7 min, nitrotyrosine at 27.9 min, and dityrosine at 25.8 min. Formation of dityrosine was monitored with the use of a fluorescence detector (λex.= 284 nm, λem. = 410 nm), and nitrotyrosine was monitored at 350 nm with the use of absorption detector.
UV-Vis Absorption Measurements
The UV-Vis absorption spectra were collected using an Agilent 8453 spectrophotometer equipped with a photodiode array detector and thermostated cell holder.
EPR Experiments
All electron paramagnetic resonance (EPR) spectra were collected using a Bruker EMX spectrometer.
ABTS oxidation
Typically, incubation mixtures used in EPR experiments on ABTS oxidation consisted of 500 µM ABTS, 250 µM MitoPhB(OH)2 isomer in phosphate buffer (100 mM, pH 7.4) containing dtpa (100 µM) and were rapidly mixed with bolus ONOO− (250 µM). Samples were subsequently transferred to a 100 µl capillary tube, and EPR measurements were started within 30 s after the bolus addition of peroxynitrite. Spectrometer parameters were as follows: scan range, 60 G; field set, 3505 G; time constant, 1.28 ms; scan time, 42 s; modulation amplitude, 0.2 G; modulation frequency, 100 kHz; receiver gain, 2×105; and microwave power, 20 mW. The spectra shown are the averages of 10 scans.
EPR spin-trapping experiments
Typically, incubation mixtures used in spin-trapping experiments consisted of 250 µM MitoPhB(OH)2 isomer and MNP spin trap (40 mM) in phosphate buffer (100 mM, pH 7.4) containing dtpa (100 µM) and 5% CH3CN. Solutions were rapidly mixed with bolus ONOO− (200 µM). Samples were subsequently transferred to an EPR capillary tube, and spectra were recorded. Spectrometer parameters were as follows: scan range, 75 G; modulation amplitude, 1.0 G; receiver gain, 1×105. The spectra shown are the averages of 10 scans.
Theoretical Studies
All calculations were performed with the use of the Gaussian 09 rev.A.02 (G09) package.(22) The electronic structure calculations were carried out using the M06-2X function of Truhlar and co-workers (23;24) with the 6–31+G(d,p) basis set.(25;26) Initial geometry of the three isomeric MitoPhB(OH)2O•− radical anions was fully optimized and used for the relaxed potential energy scan performed along the reaction coordinate defined as an elongating boron-carbon bond length. All structures on the potential energy surfaces were fully optimized, and the stationary points were confirmed by performing harmonic vibrational analysis. Local minima were characterized by 3N-6 real normal modes of vibrations, whereas the transition states had exactly one imaginary frequency. The influence of the environment was modeled using the polarizable continuum solvent model (PCM)(27) with parameters for water as implemented in G09. Open shell species were treated using the unrestricted Hartree–Fock (UHF) method.(28) We have used the default convergence and optimization criteria in all calculations performed using the Gaussian package.
Cell Culture
RAW 264.7 macrophages were cultured in DMEM medium supplemented with 10% FBS. Details on the culturing conditions and protocol for stimulation of macrophages to produce ONOO− have been published elsewhere.(14) For stimulation of •NO production, the cells were incubated overnight (12–16 h) with LPS (1 µg/ml) and IFNγ (50 U/ml). For stimulation of O2•− production, cells were treated with 1 µM PMA. Co-stimulation of •NO and O2•− production leads to generation of ONOO−, as shown previously.(13;14)
RESULTS
Reaction of MitoPhB(OH)2 with ONOO−
The second-order rate constants for the reaction between peroxynitrite and mitochondria-targeted arylboronates for ortho, meta and para isomers were determined to be (3.5 ± 0.5)×105, (1 ± 0.1)×106, and (1 ± 0.1)×106 M−1s−1, respectively. The rate constants were determined by competition kinetics with resorufin boronate as a standard (k = (1 ± 0.1)×106 M−1s−1) using pulse radiolysis; the identity of the oxidizing species was confirmed by the inhibitory effect of superoxide dismutase on the yield of resorufin (unpublished data). Both para and meta isomers react with ONOO− with the rate constant close to the value of 1×106 M−1s−1, previously determined for phenylboronic acids.(11;13) On the other hand, the rate constant of the reaction of peroxynitrite with ortho isomer is three-fold lower, possibly due to the steric hindrance of the bulky triphenylphosphonium moiety. Nonetheless, the rate constants of the reaction of peroxynitrite with all three isomers of MitoPhB(OH)2 are several orders of magnitude higher than reported for the reaction of m-MitoPhB(OH)2 with hydrogen peroxide (k = 3.8 M−1s−1 at pH 8, T = 25 °C).(9)
Identification and Quantitation of Products Formed from Oxidation of MitoPhB(OH)2 Isomers
The structures of the oxidation products of MitoPhB(OH)2 were determined by mass spectral analysis and by comparison with synthesized authentic standards (Figure 2). Table S1 (see supplementary data) lists the mass spectral parameters for the isomers of MitoPhB(OH)2 and their major and minor oxidation products. As shown in Figure 2A oxidation of o-MitoPhB(OH)2 by peroxynitrite in phosphate buffer (pH 7.4) leads to the formation of two oxidation products: o-MitoPhOH (the major product) and o-MitoPhNO2, whereas the oxidation of two other MitoPhB(OH)2 isomers results mainly in the formation of corresponding phenols, with much lower yields of the nitrated products (Figures 2 B and C).The reaction between aryl boronic acids and ONOO− is stoichiometric, as the yield of boronate consumption is close to 100% with respect to the amount of peroxynitrite (Figure 3A).
Figure 2. UPLC analyses of products formed from the reaction between triphenylphosphonium-substituted arylboronic acids and peroxynitrite.
(A) o-MitoPhB(OH)2, (B) m-MitoPhB(OH)2 and (C) p-MitoPhB(OH)2. Incubation mixtures consistent of 200 µM o-, m-, p-MitoPhB(OH)2 in phosphate buffer (pH 7.4, 50 mM) containing dtpa (100 µM) and peroxynitrite at the indicated concentration. o-, m-, p-MitoPhB(OH)2 and their corresponding oxidation products were detected using the UV absorption detection at 268 ± 1.2 nm.
Figure 3. Relationship between substrate depletion and major/minor product formation during peroxynitrite reaction with MitoPhB(OH)2.
(A) Incubation mixtures consisted of 200 µM MitoPhB(OH)2 in phosphate buffer (pH 7.4, 100 mM) containing dtpa (100 µM), 10% 2-PrOH, catalase (100 U/ml). After bolus addition of peroxynitrite, the reaction mixtures were analyzed using HPLC/UV method. MitoPhB(OH)2, and the products were detected at 268 nm. Each point represents the average value of three samples. The concentrations were determined based on the calibration curves obtained for authentic standards. MitoPhOH standards were prepared by oxidation of MitoPhB(OH)2 in the presence of appropriate concentrations of H2O2 (incubation mixture contained MitoPhB(OH)2 in phosphate buffer (pH 7.4, 100 mM) and dtpa (100 µM), 10% 2-PrOH, and 10 mM H2O2). The standard deviations are smaller than the point’s size. (B) Analysis of the ratio of minor products (sum of MitoPh + MitoPhNO2) and major product (MitoPhOH). The ratio of the rate constants of homolytic (k2) and heterolytic (k1) cleavage pathways was estimated.
To characterize the reactions in more detail, the profiles of formation of the major and minor products (MitoPhOH, MitoPhNO2, and MitoPh, Figure 1) formed during the reaction between ONOO− and MitoPhB(OH)2 isomers were investigated. We used the HPLC technique to determine the amount of major and minor products formed during the reaction with peroxynitrite in the presence of 2-PrOH, an efficient phenyl radical scavenger. The consumption of substrates and the amount of products formed in the reaction with peroxynitrite were determined based on the calibration curves obtained for the boronate isomers MitoPhB(OH)2 and the oxidation products MitoPhOH, MitoPhNO2 isomers and MitoPh.
Figure 3A shows the substrate depletion and product formation during the reaction between MitoPhB(OH)2 isomers and ONOO−. Boronates (250 µM) in phosphate buffer (100 mM, pH 7.4) containing dtpa (100 µM), 10% 2-PrOH, and catalase (100 U/ml) were rapidly mixed with ONOO− (10–300 µM). Previously, we have shown that the addition of phenyl radical scavengers (e.g., 2-PrOH) to the reaction mixtures is a convenient way to estimate the yield of minor products formed during the reaction between arylboronates and peroxynitrite.(12) In the presence of 2-propanol, the phenyl radical formed in the radical pathway is almost quantitatively converted into the product in which boronate moiety is replaced by a hydrogen atom, with a smaller fraction undergoing a recombination reaction with •NO2. We have shown that the sum of MitoPh and MitoPhNO2 formed reflects the total amount of phenyl radicals produced. The enzyme, catalase, was used to inhibit the oxidation of boronates by H2O2 that can be formed after the hydrogen atom abstraction from 2-PrOH by phenyl radicals in the presence of oxygen.
As shown in Figure 3A, the extent of boronate consumption in the presence of ONOO− and the formation of major phenolic product, MitoPhOH, are very similar for all isomers. However, the relative yields of the minor product of the reaction between ONOO− and MitoPhB(OH)2 are different. With para and meta isomers, the dominant minor product formed from the radical pathway in the presence of 10% 2-PrOH (an effecient phenyl radical scavenger) is MitoPh, whereas with the ortho isomer, the only minor product detected was o-MitoPhNO2. More importantly, o-MitoPhNO2 formation was not observed in the MPO/H2O2/NO2− system generating •NO2 (Figure 4). In this system, o-MitoPhOH formed in the reaction between o-MitoPhB(OH)2 and H2O2 is efficiently nitrated by •NO2 produced by MPO/H2O2/NO2− system, as evidenced by the formation of two isomeric nitration products of o-MitoPhOH (e.g., C25H21NO3P) (see Figure 4 and Table S2 in Supplementary Materials).
Figure 4. UPLC analyses of products formed from o-MitoPhB(OH)2 in MPO/H2O2/nitrite system.
Incubation mixtures contained o-MitoPhB(OH)2 (100 µM), H2O2 (500 µM), MPO (5 U/ml) and NaNO2 (500 µM) in phosphate buffer (pH 7.4, 50 mM) with dtpa (100 µM). Reaction was initiated by the addition of H2O2. Products were determined 40 min after adding H2O2 at 268 ± 1.2 nm. A and B indicate two isomeric nitration products of o-MitoPhOH (general formula, C25H21NO3P) as determined with the use of MS detector (see Table S2 in Supplementary Materials).
Assuming that MitoPhOH, the major product of MitoPhB(OH)2 oxidation by peroxynitrite, is produced exclusively via the non-radical pathway and is not consumed under the experimental conditions, and that all the minor products (MitoPh and MitoPhNO2) are formed through the radical pathway (from the fragmentation of MitoPhB(OH)2O•−), the ratios of the rate constants of the radical (homolytic cleavage of the O-O bond) and non-radical (heterolytic cleavage of the O-O bond) pathways were determined from the plot of the sum of the minor products versus the amount of MitoPhOH formed (Fig. 3B).
The selective formation of o-MitoPhNO2 as the predominant minor product from the ONOO−/ o-MitoPhB(OH)2 reaction is unique and significant from a diagnostic perspective involving detection of ONOO−. Therefore, we also investigated the influence of some biologically relevant reductants . glutathione, NADH, and ascorbate – on minor oxidation products’ profiles, and found a similar effect to that seen with 2-PrOH. Glutathione and ascorbate are known to react with peroxynitrite (the reported rate constants are: k = 1.3×103 M−1s−1 for glutathione, at 37 °C and pH 7.5, (29), and k = 1×106 M−1s−1 for the formation of an adduct of ONOOH to monohydrogen ascorbate at pH 5.8 (30)). Therefore, although GSH decreased the consumption of o-MitoPhB(OH)2 by ca. 15% (Figure 5A), this cannot be explained by simple competition with boronate for ONOO−. Of note, the reported rate constant of the reaction of ONOOH with monohydroascorbate (ref. 30) seems to be overestimated, as one would expect much stronger inhibitory effects of ascorbate on the yield of o-MitoPhOH and o-MitoPhNO2, than observed. One can assume that all these reductants are also able to react with nitrogen dioxide radical (•NO2) and/or phenyl radicals. As shown in Figure 5 all the reductants had little or no effect on the product yield ratios of o-MitoPhOH and o-MitoPhNO2 formed from o-MitoPhB(OH)2 reaction with peroxynitrite.
Figure 5. Effects of ascorbate, glutathione, and NADH on substrate depletion and major/minor product formation.
Incubation mixtures consisted of 250 µM o-MitoPhB(OH)2 in phosphate buffer (pH 7.4, 100 mM) containing dtpa (100 µM), catalase (100 U/ml), and the reductant (as indicated). After a bolus addition of peroxynitrite (resulting in the 150 µM peroxynitrite concentration in the sample), the reaction mixtures were analyzed by HPLC/UV. Each bar represents the average value of three samples. The error bars represent standard deviations.
To fully understand the mechanistic basis for the differences in product formation from the reaction between ONOO− and MitoPhB(OH)2 isomers, we quantitated the amount of oxidizing radicals using ABTS, a potent radical scavenger. ABTS can be oxidized to its stable radical cation, ABTS•+, by radical species generated in the boronic acid/peroxynitrite system: phenylperoxyl, phenoxyl, and •NO2 radicals.(31–36) Formation of ABTS radical cation can be monitored by EPR or spectrophotometry (at 420 nm, ε = 3.6×104 M−1cm−1 and at 735 nm, ε = 1.6×104 M−1cm−1). As shown in Figures 6A and B, the oxidation of ABTS by ONOO−-derived radicals was decreased in the presence of para and meta isomers of MitoPhB(OH)2, and was almost completely suppressed by o-MitoPhB(OH)2. Of note, in the absence of boronates, the yield of ABTS radical cation decreased with increasing concentration of peroxynitrite (Figure 6B). This can be explained by the reaction between ONOO− and ONOOH (k ~ 3 × 104 M−1s−1). (37)
Figure 6. Oxidation of ABTS by oxidants formed from MitoPhB(OH)2 reaction with peroxynitrite.
(A) Incubation mixtures consisted of 500 µM ABTS, 250 µM MitoPhB(OH)2 (if indicated) in phosphate buffer (pH 7.4, 100 mM) containing dtpa (100 µM). The reaction mixture was transferred to an EPR capillary immediately after bolus addition of ONOO− (resulting in the 200 µM peroxynitrite concentration in the sample), and the spectra were recorded at room temperature. (B) Incubation mixtures consisted of 500 µM ABTS, 250 µM MitoPhB(OH)2 (as indicated) in phosphate buffer (pH 7.4, 100 mM) containing dtpa (100 µM). The absorption spectra were recorded immediately after bolus addition of ONOO− (resulting in the 0–200 µM peroxynitrite concentration in the sample). The concentration of ABTS radical cation was calculated based on the measured absorbance at 420 nm (ABTS radical cation molar absorption coefficient ε420 nm = 3.6×104 M−1cm−1). Each point represents the average value of three samples. The error bars represent standard deviations.
Elucidation of Radical Pathway: Spin-trapping of Phenyl Radical Intermediates
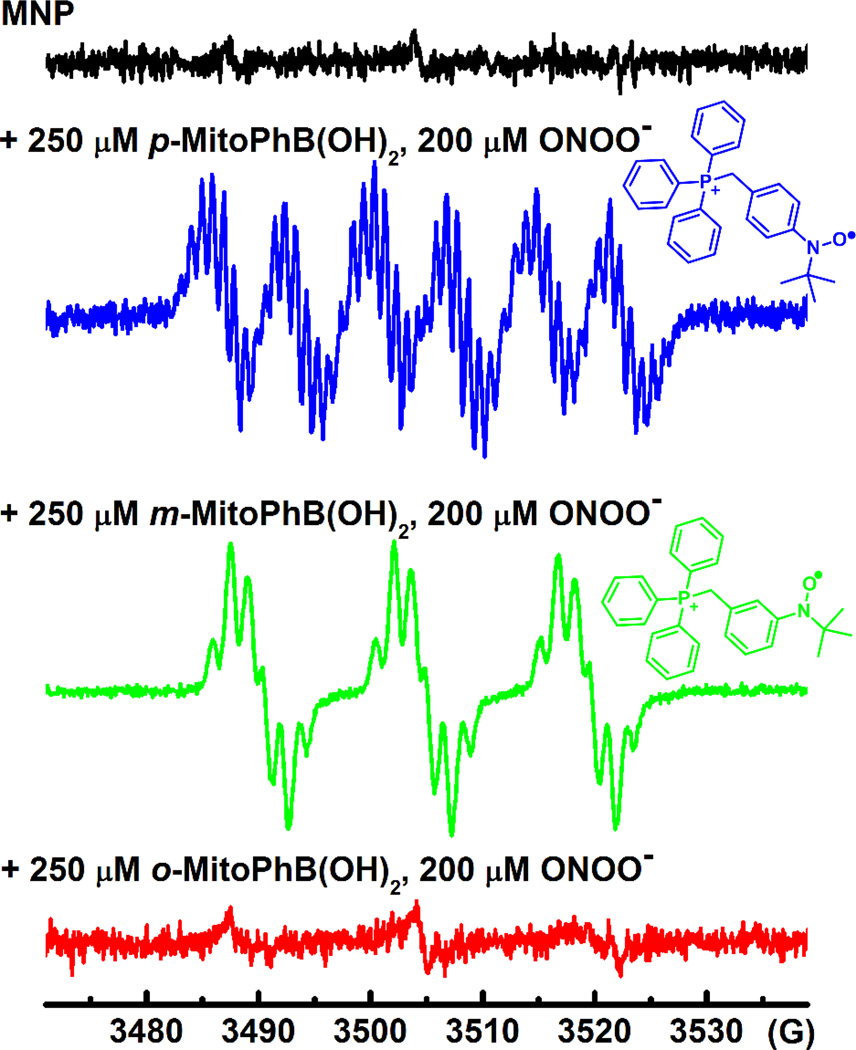
The EPR study using MNP spin-trap was performed primarily to detect and characterize radical intermediates formed during the reaction between peroxynitrite and isomers of MitoPhB(OH)2. Previously, we have shown that the phenyl radicals generated during ONOO−/arylboronate reaction can be trapped with MNP or DEPMPO spin-traps. The EPR spectrum of the MNP-phenyl adduct is more informative (i.e., reveals hyperfine couplings from the aromatic ring protons) than the EPR spectrum of the DEPMPO-phenyl adduct. The addition of a bolus amount of peroxynitrite to incubations containing p-MitoPhB(OH)2, or m-MitoPhB(OH)2, and MNP (pH 7.4) resulted in a multi-line spectrum that can be assigned to the corresponding MNP-phenyl radical adduct based on the hyperfine coupling values (Figure 7; Table 1).(12) The multi-line EPR spectrum of the adduct of p-MitoPh radical to MNP trap shows an additional hyperfine coupling (aP = 6.46 G) due to the presence of a phosphorus atom (I = ½) in the TPP cation, and a significant spin density at the para position (Figure 7). Incubation of peroxynitrite with o-MitoPhB(OH)2 did not result in the formation of a similar multi-line EPR spectrum. This further suggests a rapid radical-radical recombination mechanism between o-MitoPh•radicals and •NO2 inside the solvent cage.
Figure 7. Spin-trapping of phenyl radicals formed from the reaction between ONOO− and MitoPhB(OH)2.
Incubation mixtures contained the following compounds: MitoPhB(OH)2 (250 µM), MNP (40 mM) in phosphate buffer (100 mM, pH 7.4) containing dtpa (100 µM), and 5% CH3CN. The reaction mixture was transferred to an EPR capillary immediately after bolus addition of ONOO− (resulting in the 200 µM peroxynitrite concentration in the sample), and the spectra were recorded at room temperature.
Table 1.
Hyperfine coupling constants of MNP spin adducts
| Hyperfine Splitting Constants [G] | |
|---|---|
| m-MitoPhB(OH)2 | a(N) = 14.58; a(1H) = 1.82; a(1H) = 1.48; a(1H) =1.38 |
| p-MitoPhB(OH)2 | a(N) = 14.43; aortho(2H) = 1.86; ameta(2H) = 0.90; a(P) = 6.46 |
Arylboronate Isomers as Potential Antinitration Antioxidants
Previously, we showed that boronates are efficient scavengers of ONOO− and other oxidants.(11;13;38) Nitration of tyrosyl residues in proteins perturb their functional activity.(39–43) Thus, the ability to prevent ONOO−-mediated tyrosyl nitration is very significant. The finding that o-MitoPhB(OH)2 almost completely blocked ONOO−-dependent oxidation of ABTS implicates that this class of compounds could be used as potent antioxidants/antinitration agents. To this end, we investigated the effect of isomers of MitoPhB(OH)2 on ONOO−-induced nitration and oxidation of tyrosine. Tyrosine nitration was monitored spectroscopically at 440 nm while HPLC was used to follow both nitration and oxidation of tyrosine induced by ONOO−. Incubation mixtures contained 2.5 mM tyrosine and 500 µM MitoPhB(OH)2 in phosphate buffer (pH 7.4, 100 mM) containing dtpa (100 µM). The reaction was initiated with the rapid addition of ONOO−. The absorption spectra were recorded after a bolus addition of ONOO− (final concentration in the 0–400 µM range) and the absorbance at 440 nm was plotted as a function of ONOO− concentration (Fig. 8A). The HPLC analyses were performed using the same conditions. Both nitrotyrosine and dityrosine were quantified by HPLC analyses of the reaction mixtures (Fig. 8B). Figures 8A and B show the inhibitory effect of isomers of MitoPhB(OH)2 on tyrosine nitration and oxidation. Nitrotyrosine formation was attenuated by 87 ± 6%, 59 ± 5%, and 56 ± 5% in the presence of o-MitoPhB(OH)2, m-MitoPhB(OH)2 and p-MitoPhB(OH)2, respectively. The dityrosine formation was also inhibited by 89%, 85% and 76% in the presence of o-MitoPhB(OH)2, m-MitoPhB(OH)2, and p-MitoPhB(OH)2, respectively.
Figure 8. Inhibition of peroxynitrite-induced tyrosine nitration and oxidation by MitoPhB(OH)2.
(A) Formation of nitrotyrosine was monitored spectroscopically at 440 nm. Incubation mixtures consisted of 2.5 mM tyrosine, 500 µM MitoPhB(OH)2 (as indicated) in phosphate buffer (pH 7.4, 100 mM) containing dtpa (100 µM). The absorption spectra were recorded after bolus addition of ONOO− (resulting in the 0–400 µM peroxynitrite concentration in the sample). Each point represents the average value of three samples. The error bars represent standard deviations. (B) Formation of nitrotyrosine, and dityrosine was monitored by HPLC. Incubation mixtures consisted of 2.5 mM tyrosine, 500 µM MitoPhB(OH)2 (as indicated) in phosphate buffer (pH 7.4, 100 mM) containing dtpa (100 µM). The HPLC analyses were performed after bolus addition of ONOO− (resulting in the 400 µM peroxynitrite concentration in the sample). Each bar represents the average value of three samples. The error bars represent standard deviations. Student’s unpaired t test was used to determine the statistical significance of differences between the formation of nitrotyrosine (or dityrosine) in the absence and presence of appropiate MitoPhB(OH)2 isomer. P value of <0.01 and <0.001 was considered as significant, and highly significant (marked with ** and ***, respectively).
Theoretical Studies
DFT quantum mechanical calculations were performed to characterize the postulated key intermediates formed on the radical pathway of MitoPhB(OH)2 reaction with peroxynitrite, namely the MitoPhB(OH)2O•− radical anions. We assumed that the radical anions MitoPhB(OH)2O•− formed upon the homolytic O-O bond cleavage undergo further fragmentation resulting in the MitoPh• phenyl radicals formation. Starting from the optimized structures of the appropriate MitoPhB(OH)2O•− radical anion we computed potential energy curve based on which the respective transition state of the fragmentation process was found. According to the PCM/M06-2X/6-31+G(d,p) calculations the Gibbs energies of activation for the boron-carbon (B-C) bond cleavage in the MitoPhB(OH)2O•− radical anions are 16.3, 26.4, and 27.2 kcal/mol for the ortho-, meta-, and para- isomers, respectively. Table 2 contains the most important structure parameters of MitoPhB(OH)2O•− radical anions, and the transition states of their fragmentation reactions. Analysis of spin densities, clearly indicates that majority of radical character in MitoPhB(OH)2O•− radical anions is located on the O•− oxygen atom, whereas in the transition state it is partially carried by the carbon atom of the phenyl ring being attached to boron. Complete geometries and calculated energies for MitoPhB(OH)2O•− isomers and corresponding transition states are included in Supporting Information.
Table 2.
Structural parameters of MitoPhB(OH)2O•− radical anions, and the transition states (TS)of the fragmentation reactions.
| MitoPhB(OH)2O•− | TS | ΔE# [kJ/mol] | |||||
|---|---|---|---|---|---|---|---|
| B–C (Å) | O spin density | C spin density | B–C (Å) | O spin density | C spin density | ||
| para- | 1.65 | 0.87 | 0.12 | 2.21 | 0.25 | 0.80 | 27.2 |
| meta- | 1.65 | 0.87 | 0.11 | 2.19 | 0.27 | 0.79 | 26.4 |
| ortho- | 1.67 | 0.93 | 0.05 | 1.99 | 0.48 | 0.49 | 16.3 |
ΔE# represents Gibbs energy of activation of C-B bond cleavage, according to performed DFT calculations.
Oxidation of o-MitoPhB(OH)2 by in Situ Generated ONOO−
The oxidation of o-MitoPhB(OH)2 was investigated in the presence of O2•− and •NO generating systems. o-MitoPhB(OH)2 (100 µM) was incubated with HX (40 µM) and XO (1 mU/ml) in phosphate buffer (50 mM, pH 7.4) containing dtpa (100 µM) for 1 h at room temperature. Similar incubations were performed in the presence of nitric oxide donor, DPTA-NONOate (250 µM). The products were analyzed by HPLC (Fig. 9A). In the absence of DPTA-NONOate, there was a modest conversion of o-MitoPhB(OH)2 to o-MitoPhOH that was attributed to a slow oxidation of boronate by H2O2. In incubation mixtures containing DPTA-NONOate and HX/XO, the rate of o-MitoPhB(OH)2 oxidation was significantly enhanced along with the formation of o-MitoPhNO2. These results further confirm our notion that ONOO−, whether added as bolus or generated in situ, forms the specific nitrated product o-MitoPhNO2 in the presence of the ortho isomer.
Figure 9. HPLC analyses of products formed from oxidation of o-MitoPhB(OH)2 by ONOO− generated in situ in cell-free and cellular systems.
(A) Incubation mixtures contained 100 µM o-MitoPhB(OH)2 (MitoPBA), HX (40 µM) and XO (1 mU/ml) in phosphate buffer (pH 7.4, 50 mM) containing 100 µM dtpa, in the presence or absence of DPTA-NONOate (250 µM). After a 1 h incubation at room temperature, the products were analyzed as described in the Experimental Procedures section, (B) RAW 264.7 macrophages were activated using LPS (1 µg/ml), IFNγ (50 U/ml) and PMA (1 µM), and incubated for 1 h with o-MitoPhB(OH)2 (50 µM) in DPBS supplemented with glucose and pyruvate as described in the experimental section. After incubation the cells were lysed and the lysate deproteinized before HPLC analysis. (C) Same as in panel B, but cell media were analyzed.
Cell Culture Study
As a proof of concept, we used the macrophage-like RAW 264.7 cells as a model system. Activated RAW 264.7 cells produce ONOO−. We tested whether incubation of o-MitoPhB(OH)2 in activated RAW cells would form this nitrated product, o-MitoPhNO2 (Figs. 9B and C). We measured by HPLC both cell extracts and extracellular medium. Using boronate-based fluorogenic probes, we have shown previously that macrophages produce ONOO− after stimulation with lypopolysaccharide (LPS), interferon γ(IFNγ) and phorbol 12-myristate-13-acetate (PMA). As shown in Figure 9B, no oxidation of o-MitoPhB(OH)2 is observed in non-stimulated cells. However, when the macrophages are activated to produce peroxynitrite, both o-MitoPhOH and o-MitoPhNO2 can be detected. As o-MitoPhNO2 is not formed during the oxidation of o-MitoPhB(OH)2 by other oxidants (or by •NO2 formed by MPO), this means that ONOO− is actually produced in this system.
DISCUSSION
Here we report the study on the products formation profile for the reactions of isomeric mitochondria-targeted arylboronates with peroxynitrite. HPLC analyses showed that the major products of MitoPhB(OH)2 oxidation by peroxynitrite in the case of all isomers are the corresponding phenols (~90% yield), but the relative yields of the minor product of the reaction between ONOO− and MitoPhB(OH)2 are different. With para and meta isomers, the dominant minor product formed from the radical pathway in the presence of 10% 2-PrOH (an effective phenyl radical scavenger) is MitoPh, which is formed by a hydrogen atom abstraction from 2-PrOH by MitoPh• radicals. With the ortho isomer, the only minor product detected was o-MitoPhNO2, suggesting a rapid fragmentation of MitoPhB(OH)2O•− and subsequent recombination of the phenyl radical with •NO2 in the solvent cage. The ortho isomer forms a diagnostic marker product in the presence of ONOO−, what strongly suggests that there are significant differences in the decomposition pathway of the boronate-peroxynitrite intermediate in the radical cage. Support for phenyl radical intermediate came from EPR spin-trapping experiments.
Previously, it was reported that m-MitoPhB(OH)2 (MitoB) can be used for the detection and quantitation of mitochondria-derived hydrogen peroxide.(9;10) In this study, we show that m-MitoPhB(OH)2 and other isomers (o- and p-MitoPhB(OH)2) (11;13;38) also react with ONOO−. As with simple arylboronates, the peroxynitrite adduct to the boronate moiety decays via two pathways – the heterolysis of O-O bond (the major pathway) leading to formation of the corresponding phenols and nitrite, and the homolysis of the O-O bond (the minor reaction) that results in the formation of a radical anion, MitoPhB(OH)2O− and nitrogen dioxide. According to the DFT quantum mechanical calculations, the energy barrier for the decomposition of o-MitoPhB(OH)2O•− radical anion, leading to the formation of o-MitoPh•radicals, (~ 16 kJ/mol) is lower than the energy barrier for the fragmentation of meta and para isomeric MitoPhB(OH)2O•− radical anions (~27 kJ/mol). This strongly suggests the possibility of rapid and spontaneous o-MitoPhB(OH)2O•− fragmentation, resulting in the formation of o-MitoPh•radical, and its subsequent reaction with •NO2 radical within the solvent cage. It has to be noted that in case of meta and para isomers, the radical anion MitoPhB(OH)2O•− also undergoes spontaneous fragmentation, which is consistent with the low energy barrier. However, in the presence of phenyl radical scavenger (2-PrOH) the yield of MitoPhNO2 products is almost 10-fold lower than for ortho isomer, most probably due to less efficient radical recombination within the solvent cage. The overall mechanism can be summarized as shown in Scheme 2. The hydrogen atom donors (ABTS, GSH, NADH, and ascorbate) were unable to prevent formation of o-MitoPhNO2. The EPR spin-trapping experiments with o-MitoPhB(OH)2 and ONOO− did not provide major evidence for trappable radical intermediates, consistent with a rapid recombination of o-MitoPh•and •NO2 radicals in the solvent cage. In addition, the formation of other oxidizing and nitrating radicals formed from the reaction between ONOO− and o-MitoPhB(OH)2 was minimal, leading to an effective attenuation of ONOO−-mediated tyrosyl nitration. o-MitoPhNO2 formed in relatively high yields (η ≈ 9%) can be used as a diagnostic biomarker of ONOO− in cellular and biological systems. In contrast, ONOO−-dependent oxidation of meta and para isomers of MitoPhB(OH)2 results in the formation of free MitoPh• radicals that can be scavenged by oxygen, hydrogen atom donors, or trapped with MNP and DEPMPO spin traps to give characteristic EPR spectra of the corresponding phenyl radical adducts.
Scheme 2.
The mechanism of the reaction between boronates and peroxynitrite involving both non-radical and radical pathways appears to be quite general. However, it is not clear how other factors, including steric hindrance and electronic effects of substituents, influence the stability of the key reaction intermediates (e.g., boronate-ONOO− anionic adduct, and R-B(OH)2O•−radical anion) leading to specific product formation. Based on the present results obtained with o-MitoPhB(OH)2, we conclude that o-MitoPhNO2 formed can be used to detect unequivocally ONOO− formation in cell-free and cellular systems. Boronate probes containing bulky substituents at the ortho position may be used in cells to unequivocally confirm ONOO− formation, via the specific nitration of the boronate probe. Other oxidants (H2O2 and HOCl) that can oxidize boronates do not form nitrated products. The nitration of ortho- substituted boronates can also be used to distinguish the formation of ONOO− and •NO2 formed from MPO/H2O2/NO2•− reaction. o-MitoPhB(OH)2 or similar sterically-hindered boronates may be used as a potent anti-nitration agent for inhibiting tyrosyl nitration of cellular/mitochondrial proteins.
In this study, we report that substitution with a charged, bulky group at the ortho position (not meta or para position) of phenylboronates dramatically alters product formation (nitrobenzene derivative) in the presence of ONOO− via the minor radical pathway. All the isomers yielded nearly the same amount of the major product, the corresponding phenol. These differences in reaction pathways are attributed to the steric hindrance of the bulky group on radical reactions in the solvent cage of the boronateperoxynitrite intermediate. Results from the EPR spin-trapping experiments showed that MitoPh• radicals formed from the reaction between ONOO− and meta and para isomers (m-MitoPhB(OH)2 and p-MitoPhB(OH)2) and not from the ortho isomer (o-MitoPhB(OH)2) could be detected with 2-methyl-2-nitrosopropane (MNP) spin trap. Regardless of the presence of any biological reductants, ONOO−/o-MitoPhB(OH)2 reaction yields the same amount of o-MitoPhNO2. DFT quantum mechanical calculations suggest that the energy barrier for the dissociation of o-MitoPhB(OH)2O•− radical anion is lower than for m-MitoPhB(OH)2O•− and p-MitoPhB(OH)2O•− radical anions which explains the fast recombination of radicals formed within the solvent cage. Future studies will focus on the applicability of the ortho isomer probe described here or its long-chain analog for detection of mitochondrial ONOO− generation.
Supplementary Material
ACKNOWLEDGEMENT
Access to supercomputing facilities at Cyfronet (Poland) is gratefully acknowledged. The authors thank Mrs. Monika Zielonka for her assistance with cell culture experiments.
FUNDING SOURCES
This work was performed with the help of a grant (R01 HL063119) funded by the National Institutes of Health awarded to B.K. A.S. was supported the grant from the Foundation for Polish Science (FNP) within the “Homing Plus” program, and the grant coordinated by JCET, No. POIG.01.01.02-00-069/09 (both grants are supported by the European Union from the resources of the European Regional Development Fund under the Innovative Economy Programme).
ABBREVIATIONS
- 2-PrOH
2-propanol
- ABTS
2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonate)
- dtpa
diethylenetriaminepentaacetic acid
- DFT
Density Functional Theory
- diTyr
dityrosine
- DMEM
Dulbecco's modified Eagle's medium
- DPBS
Dulbecco's phosphate-buffered saline
- FBS
fetal bovine serum
- HX
hypoxanthine
- IFNγ
interferon γ
- LPS
lypopolysaccharide
- m-MitoPhB(OH)2
(3-boronobenzyl)triphenylphosphonium cation
- MitoPh
benzyltriphenylphosphonium cation
- m-MitoPhOH
(3-hydroxybenzyl)triphenylphosphonium cation
- m-MitoPhNO2
(3-nitrobenzyl)triphenylphosphonium cation
- MNP
2-methyl-2-nitrosopropane
- MPO
myeloperoxidase
- •NO
nitric oxide*
- •NO2
nitrogen dioxide
- o-MitoPhB(OH)2
(2-boronobenzyl)triphenylphosphonium cation
- o-MitoPhOH
(2-hydroxybenzyl)triphenylphosphonium cation
- o-MitoPhNO2
(2-nitrobenzyl)triphenylphosphonium cation
- O2•−
superoxide radical anion
- ONOO−/ONOOH
peroxynitrite*
- PMA
phorbol 12-myristate-13-acetate
- p-MitoPhB(OH)2
(4-boronobenzyl)triphenylphosphonium cation
- p-MitoPhOH
(4-hydroxybenzyl)triphenylphosphonium cation
- p-MitoPhNO2
(4-nitrobenzyl)triphenylphosphonium cation
- DPTA-NONOate
((Z)-1-[N-(3-aminopropyl)-N-(3-ammoniopropyl)amino]diazen-1-ium-1,2-diolate)
- Ph•
phenyl radical
- SOD
superoxide dismutase
- TFA
trifluoroacetic acid
- TyrNO2
nitrotyrosine
- X
xanthine
- XO
xanthine oxidase
* IUPAC-recommended names for peroxynitrite anion, peroxynitrous acid, and nitric oxide are oxidoperoxidonitrate(1-), (dioxidanido)oxidonitrogen, and nitrogen monoxide or oxidonitrogen(•), respectively.
Footnotes
SUPPORTING INFORMATION
This material includes the optimized geometries of all stationary points and the tables with mass spectrometry data identifying the products of peroxynitrite oxidation of o-, m-, and p-MitoPhB(OH)2, as well as the products formed from o-MitoPhB(OH)2 in MPO/H2O2/nitrite system. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular ROS. Antioxid. Redox. Signal. 2012 doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox. Signal. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 3.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Ainley AD, Challenger F. Studies of the boron-carbon linkage. Part I. The oxidation and nitration of phenyl-boric acid. J. Chem. Soc. 1930:2171–2180. [Google Scholar]
- 5.Lo LC, Chu CY. Development of highly selective and sensitive probes for hydrogen peroxide. Chem. Commun. 2003:2728–2729. doi: 10.1039/b309393j. [DOI] [PubMed] [Google Scholar]
- 6.Chang MC, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 2004;126:15392–15393. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dikalov SI, Kirilyuk IA, Voinov M, Grigor'ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic. Res. 2011;45:417–430. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson BC, Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008;130:9638–9639. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Saeed S, Carre JE, Singer M, Gems D, Hartley RC, Partridge L, Murphy MP. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocheme HM, Logan A, Prime TA, Abakumova I, Quin C, McQuaker SJ, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Hartley RC, Partridge L, Murphy MP. Using the mitochondria-targeted ratiometric mass spectrometry probe MitoB to measure H2O2 in living Drosophila. Nat. Protoc. 2012;7:946–958. doi: 10.1038/nprot.2012.035. [DOI] [PubMed] [Google Scholar]
- 11.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic. Biol. Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikora A, Zielonka J, Lopez M, Dybala-Defratyka A, Joseph J, Marcinek A, Kalyanaraman B. Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC Analyses, and quantum mechanical study of the free radical pathway. Chem. Res. Toxicol. 2011;24:687–697. doi: 10.1021/tx100439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J. Biol. Chem. 2012;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 17.Morrison DE, Issa F, Bhadbhade M, Groebler L, Witting PK, Kassiou M, Rutledge PJ, Rendina LM. Boronated phosphonium salts containing arylboronic acid, closo-carborane, or nido-carborane: synthesis, X-ray diffraction, in vitro cytotoxicity, and cellular uptake. J. Biol. Inorg. Chem. 2010;15:1305–1318. doi: 10.1007/s00775-010-0690-6. [DOI] [PubMed] [Google Scholar]
- 18.Bohle DS, Glassbrenner PA, Hansert B. Syntheses of pure tetramethylammonium peroxynitrite. Methods Enzymol. 1996;269:302–311. doi: 10.1016/s0076-6879(96)69031-4. [DOI] [PubMed] [Google Scholar]
- 19.Hrabie JA, Klose JR, Wink DA, Keefer LK. New nitric oxide-releasing zwitterions derived from polyamines. J. Org. Chem. 1993;58:1472–1476. [Google Scholar]
- 20.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate ("nitrosohydroxylamine") functional group and its oxygen-substituted derivatives. Chem. Rev. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 21.Van Gelder B, Slater EC. The extinction coefficient of cytochrome c. Biochim. Biophys. Acta. 1962;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]
- 22.Frisch MJ, et al. Gaussian 09, revision A.02. Wallingford CT: Gaussian, Inc; 2009. [Google Scholar]
- 23.Zhao Y, Schultz NE, Truhlar DG. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006;2:364–382. doi: 10.1021/ct0502763. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Truhlar DG. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008;41:157–167. doi: 10.1021/ar700111a. [DOI] [PubMed] [Google Scholar]
- 25.Hariharan PC, Pople JA. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta. 1973;28:213–222. [Google Scholar]
- 26.Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982;77:3654–3665. [Google Scholar]
- 27.Miertus S, Scrocco E, Tomasi J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981;55:117–129. [Google Scholar]
- 28.Pople JA, Nesbet RK. Self-consistent orbitals for radicals. J. Chem. Phys. 1954;22:571–572. [Google Scholar]
- 29.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 30.Kurz CR, Kissner R, Nauser T, Perrin D, Koppenol WH. Rapid scavenging of peroxynitrous acid by monohydroascorbate. Free Radic. Biol. Med. 2003;35:1529–1537. doi: 10.1016/j.freeradbiomed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Alfassi ZB, Marguet S, Neta P. Formation and reactivity of phenylperoxyl radicals in aqueous solutions. J. Phys. Chem. 1994;98:8019–8023. [Google Scholar]
- 32.Khaikin GI, Alfassi ZB, Neta P. Inter- and intramolecular redox reactions of substituted phenylperoxyl radicals in aqueous solutions. J. Phys. Chem. 1995;99:16722–16726. [Google Scholar]
- 33.Alfassi ZB, Khaikin GI, Neta P. Arylperoxyl radicals. Formation, absorption spectra, and reactivity in aqueous alcohol solutions. J. Phys. Chem. 1995;99:265–268. [Google Scholar]
- 34.Khaikin GI, Alfassi ZB, Neta P. Formation and reactions of halogenated phenylperoxyl radicals in aqueous alcohol solutions. J. Phys. Chem. 1995;99:11447–11451. [Google Scholar]
- 35.Neta P, Grodkowski J. Rate constants for reactions of phenoxyl radicals in solution. J. Phys. Chem. Ref. Data. 2005;34:109–200. [Google Scholar]
- 36.Forni LG, Mora-Arellano VO, Packer JE, Willson RL. Nitrogen dioxide and related free radicals: electron-transfer reactions with organic compounds in solutions containing nitrite or nitrate. J. Chem. Soc. , Perkin Trans. 1986;2:1–6. [Google Scholar]
- 37.Gupta D, Harish B, Kissner R, Koppenol WH. Peroxynitrate is formed rapidly during decomposition of peroxynitrite at neutral pH. Dalton Trans. 2009:5730–5736. doi: 10.1039/b905535e. [DOI] [PubMed] [Google Scholar]
- 38.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem. Res. Toxicol. 2012;25:1793–1799. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surmeli NB, Litterman NK, Miller AF, Groves JT. Peroxynitrite Mediates Active Site Tyrosine Nitration in Manganese Superoxide Dismutase. Evidence of a Role for the Carbonate Radical Anion. J. Am. Chem. Soc. 2010;132:17174–17185. doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchard-Fillion B, Souza JM, Friel T, Jiang GC, Vrana K, Sharov V, Barron L, Schöneich C, Quijano C, Alvarez B, Radi R, Przedborski S, Fernando GS, Horwitz J, Ischiropoulos H. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J. Biol. Chem. 2001;276:46017–46023. doi: 10.1074/jbc.M105564200. [DOI] [PubMed] [Google Scholar]
- 41.Castro L, Demicheli V, Tortora V, Radi R. Mitochondrial protein tyrosine nitration. Free Radic. Res. 2011;45:37–52. doi: 10.3109/10715762.2010.516254. [DOI] [PubMed] [Google Scholar]
- 42.Abriata LA, Cassina A, Tortora V, Marin M, Souza JM, Castro L, Vila AJ, Radi R. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J. Biol. Chem. 2009;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration--functional alteration or just a biomarker? Free Radic. Biol. Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.