Abstract
Purpose
Copy number variations (duplications) of TANK binding kinase 1 (TBK1) have been associated with normal tension glaucoma (NTG), a common cause of blindness worldwide. Mutations in other genes involved in autophagy (TLR4 and OPTN) have been associated with NTG. Here we report searching for additional proteins involved in autophagy that may also have roles in NTG.
Materials and methods
HEK-293T cells were transfected to produce synthetic TBK1 protein with FLAG and S tags. Proteins that associate with TBK1 were isolated from HEK-293T lysates using tandem affinity purification (TAP) and polyacrylamide gel electrophoresis (PAGE). Isolated proteins were identified with mass spectrometry. A cohort of 148 NTG patients and 77 controls from Iowa were tested for glaucoma-causing mutations in genes that encode identified proteins that interact with TBK1 using high resolution melt (HRM) analysis and DNA sequencing.
Results
TAP studies show that three proteins expressed in HEK-293T cells (NAP1, TANK and TBKBP1) interact with TBK1. Testing cohorts of NTG and normal controls for disease-causing mutations in TANK, identified a total of nine unique variants including three non-synonymous changes, one synonymous changes and five intronic changes. When analyzed alone or as a group, the non-synonymous TBK1 coding sequence changes were not associated with either NTG or primary open angle glaucoma.
Conclusion
TAP showed that NAP1, TANK and TBKBP1 interact with TBK1 and are good candidates for contributing to NTG. A mutation screen of TANK detected three non-synonymous variants. Although, it remains possible that one or more of these TANK mutations may have a role in NTG, the data in this report do not provide statistical support for an association between TANK variants and NTG.
Keywords: Glaucoma, tandem affinity purification, TKB1
INTRODUCTION
One to two percent of Americans over the age of 40 are affected with glaucoma and every year 12,000 Americans are blinded by this disease.1,2 High intraocular pressure is a risk factor; however, glaucoma can occur at any pressure. Glaucoma that occurs below an intraocular pressure of 21 mm Hg is frequently termed normal tension glaucoma (NTG). Studies of twins, families and large populations of patients with glaucoma have shown that heredity has an important role in this condition. In the last fifteen years, six primary open angle glaucoma genes (MYOC, OPTN, WDR36, NTF4, TBK1 and ASB10) have been reported to cause Mendelian forms of glaucoma. Mutations in the MYOC gene are the most common known cause of glaucoma cases worldwide (~1 in 25 cases)3,4 and are associated with glaucoma that has high eye pressure. Mutations in OPTN, on the other hand, are associated with ~1–2% of NTG.5,6 The data to support a role for WDR36, NTF4 and ASB10 in the pathogenesis of glaucoma is controversial.6–14
Genes that are associated with more complex genetic forms of glaucoma have been identified with genome-wide association studies.6 Variants in toll-like receptor 4 (TLR4), S1 RNA binding domain 1 (SRBD1), elongation of long-chain fatty acid family member 5 (ELOVL5) and cyclin-dependent kinase inhibitor 2B antisense RNA 1 (CDKN2B-AS1) genes have been associated with NTG.15–17
The first copy number variation (duplication) associated with glaucoma was recently reported. Duplications of chromosome 12q14 that span the TANK binding kinase 1 (TBK1) gene located on chromosome 12 were shown to be associated with NTG.18 Duplications of TBK1 have been detected in an African American NTG pedigree,18 in 1–2% of Caucasian NTG cases,18 and in approximately 1% of Asian NTG cases.19
The TBK1 gene encodes a kinase protein that has a known function in autophagy and in NF-kB signaling. TBK1 and two other genes (TLR4 and OPTN) have key roles in autophagy, a process by which intracellular proteins and/or organelles are digested and which may in some cases lead to apoptosis.20 TBK1 has a key role in phosphorylating OPTN which then facilitates formation of the autophagosome and engulfment of proteins tagged with ubiquitin.21,22 The association of several NTG genes with autophagy suggests that this process may have a central role in the pathogenesis of glaucoma. Consequently, we sought to identify and characterize proteins that interact with TBK1 and participate in autophagy as candidates for causing NTG.
MATERIALS AND METHODS
Tandem Affinity Purification
Tandem affinity purification (TAP) of FS-TBK1 was conducted with HEK293T cells as previously described.23 Briefly, HEK293T cells were stably transfected with pSS-FS-TBK1 using FuGENE HD (Roche Applied Science, Indianapolis, IN). pSS-FS is derived from pEFGP-N1 (Clontech, Mountain View, CA) which has a CMV promoter and which has been modified to: (1) remove the GFP sequences and (2) add 3X FLAG tags, a TEV cleavage site, and 2X S-antigen tags to the amino terminus of TBK1 protein. Cell lysates from 20 15-cm dishes were loaded onto an anti-FLAG affinity gel (M2; Sigma, St. Louis, MO), and bound proteins were eluted with 3xFLAG peptide (100 μg/mL; Sigma, St. Louis, MO). Eluate was loaded onto an S-protein affinity gel (Novagen, Billerica, MA), and bound proteins were eluted in 2x SDS-PAGE sample loading buffer. Purified proteins were separated in 4–12% NuPAGE gels (Invitrogen, Grand Island, NY) and visualized with a SilverQuest Silver Staining Kit (Invitrogen, Grand Island, NY). Excised gel slices were submitted to the University of Iowa Proteomics Facility and protein identities were determined by mass spectrometry using a LTQ XL linear ion trap mass spectrometer (Thermo Scientific, Waltham, MA).
Human Subjects
All subjects enrolled in the study gave informed consent and the research was conducted with approval of the University of Iowa’s Internal Review Board. A cohort of 148 patients with NTG had excavation of their optic nerve head with resultant glaucomatous visual field loss in at least one eye. Glaucomatous optic nerves had cup-to-disc ratios of greater than 0.7 with thinning of the neural rim, asymmetry of the optic nerve cup-to-disc ratio of >0.2, or photographic documentation of progressive loss of the neural rim. Patients were 40 years of age or older at diagnosis and had open iridocorneal angles on gonioscopy (angle greater than Shaffer grade II). Patients were also required to have a maximum IOP of 21 mmHg or less. A cohort of 77 control subjects were a minimum of 50 years old and were examined and judged to have normal optic nerve head appearance and IOP ≤21 mm Hg by board-certified ophthalmologists. Corneal thickness data were available from 95 of 148 (64%) of NTG patients using pachymetry (average corneal thickness was 524 microns ±37 microns (SD)). Formal diurnal measurements of IOP (measured every three hours from 7AM to 10PM) were used to confirm that NTG patients had maximum IOP ≤21 mm Hg in 100 of 148 (68%) of subjects. All study subjects were examined by clinicians at the University of Iowa Hospitals and Clinics and ascertained in Iowa.
Testing Candidate Genes for Mutations
DNA samples were prepared from peripheral blood samples using standard procedures. The entire coding region of TANK (NM_001199135) was PCR amplified from patient and control DNA using overlapping primer pairs in standard PCR reactions (primers available on request). Amplified DNA was scanned for mutations with a combination of high resolution melt (HRM) analysis and bi-directional DNA sequencing with an Applied Biosystems model 3730 automated sequencer as previously described.18 Those mutations that result in amino acid substitutions were evaluated using the blosum62 matrix,24 SIFT25 and PolyPhen2.26
Statistical Analysis
The frequency of plausible disease-causing mutations (i.e. nonsynonymous coding sequence changes) were compared using Fisher’s exact test with p value<0.05 as the threshold for significance.
RESULTS
Tandem Affinity Purification
Proteins that interact with TBK1 are candidates for contributing to glaucoma pathogenesis. Consequently, we sought to identify TBK1 interacting proteins using TAP.
We transfected HEK-293T cells with the vector pSS-FS-TBK1 to generate a stable cell line that expresses TBK1 protein tagged with FLAG and S epitopes. Untransfected, parental cells were used as a negative control. Protein extracts from these cell lines were subjected to TAP. TBK1 and interacting proteins were isolated from the protein extracts first via co-immunoprecipitation using the FLAG epitope then via the S-antigen epitope. Eluted proteins were then separated based on size with SDS-PAGE and visualized with silver staining (Figure 1). Five prominent protein bands were observed with molecular weights of 44.9, 47.8, 67.7, 83.6 and 105 kDa. The four protein bands with lower molecular weights were further analyzed with mass spectrometry while the 105 kDa band corresponded to the predicted molecular weight of the epitope tagged TBK1 protein. Mass spectrometry indicated that the four protein bands each contained a unique protein previously reported to interact with TBK1 (Table 1): (1) NF-kB activating kinase associated protein 1 (NAP1), (2) TRAF family member-associated NFKB activator (TANK), (3) TANK binding kinase 1-binding protein 1 (TBKBP1) and (4) TBK1.
FIGURE 1.
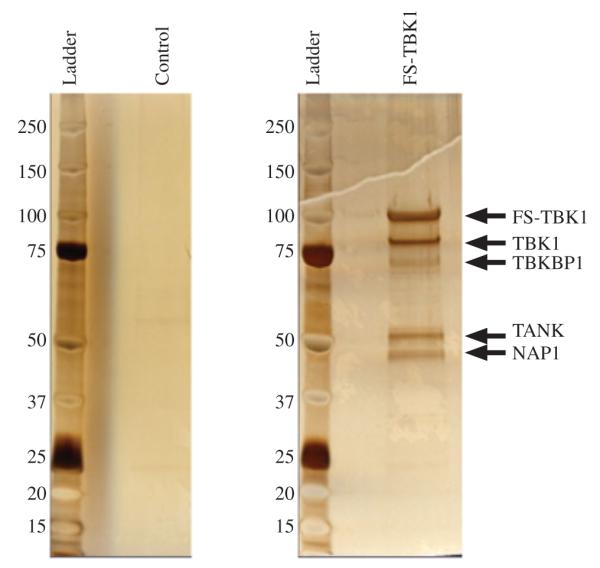
Purification of TBK1 containing protein complexes. Proteins were purified from control HEK-293T cells (left panel) and HEK-293T cells expressing TBK1 protein with amino-terminal FLAG and S tags (right panel) using TAP. Purified proteins were analyzed with SDS-PAGE and silver staining. Five prominent bands are indicated with arrows. The largest band has a molecular weight consistent with epitope tagged TBK1.
TABLE 1.
Mass spectrometry results.
| Protein name | Gene name | Molecular weight (kDa) |
Protein identification probability |
Number of unique peptides |
Number of unique spectra |
Number of total spectra |
Percentage sequence coverage |
|
|---|---|---|---|---|---|---|---|---|
| 1 | NF-kB activating kinase 1 (NAP1) |
NAP1 (AZI2) | 44.9 | 100.00% | 22 | 24 | 33 | 63.30% |
| 2 | TRAF family member- associated NFKB activator (TANK), transcript variant 1 |
TANK | 47.8 | 100.00% | 16 | 16 | 22 | 47.80% |
| 3 | TANK-binding kinase 1-binding protein 1 |
TBKBP1 | 67.7 | 100.00% | 27 | 29 | 38 | 58.20% |
| 4 | Serine/threonine-protein kinase TBK1 |
TBK1 | 83.6 | 100.00% | 42 | 53 | 98 | 64.50% |
Screening Glaucoma Patients for TANK Mutations
A cohort of 148 NTG patients and 77 control subjects were tested for glaucoma-causing mutations in the coding sequences of TANK using HRM analysis and DNA sequencing. A total of nine variants were detected (Table 2), of which three were non-synonymous changes (Gly292Arg, Arg394Gly and Arg394Gln). One synonymous change (Arg85Arg) and five intronic variants were also identified.
TABLE 2.
TANK variants detected in NTG patients and controls from Iowa.
| NTG | Normal controls |
Frequency in NHLBI exome sequencing Project |
|||||
|---|---|---|---|---|---|---|---|
| TANK non-synonymous coding sequence variants |
n=148 | n=77 | p Value | ~4300 Caucasians |
Blosum62 | SIFT | PolyPhen2 |
| Gly292Arg | 2 (1.4%) | 0 | 0.55 | 32 (0.74%) | −2 | Tolerated | Benign (0.007) |
| Arg394Gly | 3 (2.0%) | 2 (2.6%) | >0.99 | 54 (1.26%) | −2 | Tolerated | Possibly Damaging (0.719) |
| Arg394Gln | 1 (0.68%) | 0 | >0.99 | 5 (0.12%) | 1 | Tolerated | Benign (0.031) |
| Total | 6 (0.041%) | 2 (2.6%) | 0.72 | N/A | N/A | N/A | N/A |
| TANK synonymous coding sequence variants |
NTG n=148 |
Normal controls n=77 |
p Value | ||||
| Arg85Arg | 1 (0.68%) | 1 (1.3%) | >0.99 | ||||
| TANK intron variants | NTG n=148 |
Normal controls n=77 |
p Value | ||||
| IVS4 +43C>A | 1 (0.68%) | 0 | >0.99 | ||||
| IVS4 +44G>C | 1 (0.68%) | 0 | >0.99 | ||||
| IVS5 +90 ins A | 1 (0.68%) | 0 | >0.99 | ||||
| IVS7 −17T>C | 0.28 | ||||||
| CC | 76 (51.4%) | 38 (49.4%) | |||||
| CT | 37 (25.0%) | 14 (18.2%) | |||||
| TT | 35 (23.6%) | 25 (32.5%) | |||||
| IVS7 −88G>A | 13 (8.8%) | 4 (5.2%) | 0.43 |
Two of the non-synonymous variants, Gly292Arg and Arg394Gln, were detected only in the NTG patients. However, the frequency of the non-synonymous coding sequence variants was not statistically more common in NTG patients than control subjects (p value>0.05) when variants were compared individually or as a group (Table 2). When potential pathogenicity was assessed with the blosum62 matrix, SIFT and PolyPhen2, there was no consensus to suggest any of the variants are likely to cause disease (Table 2). Furthermore, each of the non-synonymous variants was detected at similar frequency in a large Caucasian control population from the National Heart Lung and Blood Institute Exome Sequencing Project (Table 2).27
DISCUSSION
A convergence of several types of data has suggested that autophagy has a key role in the pathogenesis of NTG. A series of human genetic studies have implicated autophagy in NTG. Two positional cloning studies of NTG pedigrees each identified glaucomacausing genes (OPTN5 and TBK118) that have key roles in autophagy and encode proteins that interact with each other. Another gene, TLR4, has an important role autophagy; interacts with other autophagy and NTG genes (TBK1 and OPTN); and has been identified as a genetic risk factor for complex genetic forms of NTG.16 Experimental animal models of glaucoma have also suggested that autophagy has a central role in glaucoma. Autophagy is activated in both optic nerve transection and ocular ischemia model systems.28,29 Consequently, we sought to identify additional NTG genes by investigating additional molecules that participate in autophagy. TAP was performed to identify proteins that might participate in autophagy by virtue of their association with TBK1. We detected three TBK1-interacting proteins, TANK,30 TBKBP131 and NAP1,32 that were previously known to associate with TBK1. A fourth identified protein was untagged, endogenous TBK1, suggesting that TBK1 may form a homo-oligomer.
TBK1 was first identified and named based on its kinase properties and its interaction with TANK.33 Our TAP assay also identified TANK as a protein that associates with TKB1. Moreover, TANK is known to stimulate NF-kB signaling in conjunction with adapter protein TNF receptor-associated receptor 2.30 Given its role in NF-kB signaling which potentially influences apoptosis, we considered TANK an excellent candidate gene for causing NTG. We tested a large cohort of patients with NTG for glaucoma-causing mutations in TANK. Although TANK variants were detected in some NTG patients from Iowa that were absent from Iowa control subjects (Table 1), all of these rare variants were also detected at a similar frequency in a large population database of exome sequences27 arguing against their pathogenicity. However, it is possible that a larger study might be able to show that some of these variants are present in glaucoma patients at a significantly higher frequency than in controls, i.e. statistically associated with disease.
In summary, we report identification of four proteins that interact with TBK1 using TAP in an HEK293T cell culture system. Three of the proteins (TANK, TBKBP1 and NAP1) were previously known to be associated with TBK1, while the fourth was TBK1 itself. TBKBP1 and NAP1 are currently being investigated for a role in glaucoma. TANK was evaluated as glaucoma candidate gene, however, no NTG-causing mutations were identified, suggesting that coding sequence variants are not a common cause of glaucoma. Nonetheless, it remains possible that copy number variants or other rare mutations in TANK that were not detected by our assay may have a role in glaucoma pathogenesis. Future TAP studies to identify molecules involved in glaucoma (an eye-specific disease) might benefit from using ocular tissue or cell lines that are more biologically relevant than HEK-293T cells.
Acknowledgments
The research was funded in part by NIH R01EY018825, NIH RO1EY022616, and The Knights Templar Eye Foundation.
Footnotes
DECLARATION OF INTEREST The authors report no conflict of interests. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Tielsch JM. Blindness and visual impairment in an american urban population. Arch Ophthalmol. 1990;108:286–290. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- 2.Kahn HA, Milton RC. Revised Framingham eye study prevalence of glaucoma and diabetic retinopathy. Am J Epidemiol. 1980;111:769–776. doi: 10.1093/oxfordjournals.aje.a112955. [DOI] [PubMed] [Google Scholar]
- 3.Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 4.Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- 5.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 6.Fingert JH. Primary open-angle glaucoma genes. Eye (Lond) 2011;25:587–595. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monemi S, Spaeth G, Dasilva A, Popinchalk S, Ilitchev E, Liebmann J, et al. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005;14:725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt AW, Dimasi DP, Mackey DA, Craig JE. A glaucoma case-control study of the WDR36 gene D658G sequence variant. Am J Ophthalmol. 2006;142:324–325. doi: 10.1016/j.ajo.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Fingert JH, Alward WL, Kwon YH, Shankar SP, Andorf JL, Mackey DA, et al. No association between variations in the WDR36 gene and primary open-angle glaucoma. Arch Ophthalmol. 2007;125:434–436. doi: 10.1001/archopht.125.3.434-b. [DOI] [PubMed] [Google Scholar]
- 10.Hauser MA, Allingham RR, Linkroum K, Wang J, LaRocque-Abramson K, Figueiredo D, et al. Distribution of WDR36 DNA sequence variants in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2542–2546. doi: 10.1167/iovs.05-1476. [DOI] [PubMed] [Google Scholar]
- 11.Pasutto F, Matsumoto T, Mardin CY, Sticht H, Brandstatter JH, Michels-Rautenstrauss K, et al. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. Am J Hum Genet. 2009;85:447–456. doi: 10.1016/j.ajhg.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Liu W, Crooks K, Schmidt S, Allingham RR, Hauser MA. No evidence of association of heterozygous NTF4 mutations in patients with primary open-angle glaucoma. Am J Hum Genet. 2010;86:498–499. doi: 10.1016/j.ajhg.2009.11.018. author reply 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasutto F, Keller KE, Weisschuh N, Sticht H, Samples JR, Yang YF, et al. Variants in ASB10 are associated with open-angle glaucoma. Hum Mol Genet. 2012;21:1336–1349. doi: 10.1093/hmg/ddr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fingert JH, Roos BR, Solivan-Timpe F, Miller KA, Oetting TA, Wang K, et al. Analysis of ASB10 variants in open angle glaucoma. Hum Mol Genet. 2012;21:4543–8. doi: 10.1093/hmg/dds288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meguro A, Inoko H, Ota M, Mizuki N, Bahram S. Genome-wide association study of normal tension glaucoma: common variants in SRBD1 and ELOVL5 contribute to disease susceptibility. Ophthalmology. 2010;117:1331–1338. e5. doi: 10.1016/j.ophtha.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Shibuya E, Meguro A, Ota M, Kashiwagi K, Mabuchi F, Iijima H, et al. Association of Toll-like receptor 4 gene polymorphisms with normal tension glaucoma. Invest Ophthalmol Vis Sci. 2008;49:4453–4457. doi: 10.1167/iovs.07-1575. [DOI] [PubMed] [Google Scholar]
- 17.Burdon KP, Crawford A, Casson RJ, Hewitt AW, Landers J, Danoy P, et al. Glaucoma risk alleles at CDKN2B-AS1 are associated with lower intraocular pressure, normal-tension glaucoma, and advanced glaucoma. Ophthalmology. 2012;119:1539–1545. doi: 10.1016/j.ophtha.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Fingert JH, Robin AL, Stone JL, Roos BR, Davis LK, Scheetz TE, et al. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet. 2011;20:2482–2494. doi: 10.1093/hmg/ddr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawase K, Allingham RR, Meguro A, Mizuki N, Roos B, Solivan-Timpe FM, et al. Confirmation of TBK1 duplication in normal tension glaucoma. Exp Eye Res. 2012;96:178–180. doi: 10.1016/j.exer.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidberg H, Elazar Z. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci Signal. 2011;4:pe39. doi: 10.1126/scisignal.2002355. [DOI] [PubMed] [Google Scholar]
- 22.Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, et al. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Exome Variant Server [last accessed Mar 2012];NHLBI Exome Sequencing Project (ESP) Available from: http://evs.gs.washington.edu/EVS/
- 28.Piras A, Gianetto D, Conte D, Bosone A, Vercelli A. Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure. PLoS One. 2011;6:e22514. doi: 10.1371/journal.pone.0022514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HY, Kim JH, Park CK. Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis. 2012;3:e290. doi: 10.1038/cddis.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD 40L-mediated NF-kappaB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 31.Ryzhakov G, Randow F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. EMBO J. 2007;26:3180–3190. doi: 10.1038/sj.emboj.7601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita F, Taniguchi Y, Kato T, Narita Y, Furuya A, Ogawa T, et al. Identification of NAP1, a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling. Mol Cell Biol. 2003;23:7780–7793. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]


