Abstract
Intercellular communication between γ-aminobutyric acid (GABA)ergic suprachiasmatic nucleus (SCN) neurons facilitates light-induced phase changes and synchronization of individual neural oscillators within the SCN network. We used ratiometric Ca2+ imaging techniques to record changes in the intracellular calcium concentration ([Ca2+]i) to study the role of GABA in interneuronal communication and the response of the SCN neuronal network to optic nerve stimulations that mimic entraining light signals. Stimulation of the retinohypothalamic tract (RHT) evoked divergent Ca2+ responses in neurons that varied regionally within the SCN with a pattern that correlated with those evoked by pharmacological GABA applications. GABAA and GABAB receptor agonists and antagonists were used to evaluate components of the GABA-induced changes in [Ca2+]i. Application of the GABAA receptor antagonist gabazine induced changes in baseline [Ca2+]i in a direction opposite to that evoked by GABA, and similarly altered the RHT stimulation-induced Ca2+ response. GABA application induced Ca2+ responses varied in time and region within the SCN network. The NKCC1 cotransporter blocker, bumetanide, and L-type calcium channel blocker, nimodipine, attenuated the GABA-induced rise of [Ca2+]i. These results suggest that physiological GABA induces opposing effects on [Ca2+]i based on the chloride equilibrium potential, and may play an important role in neuronal Ca2+ balance, synchronization and modulation of light input signaling in the SCN network.
Keywords: chloride cotransporter, circadian rhythm, retinohypothalamic tract, synaptic transmission
Introduction
Suprachiasmatic nucleus (SCN) neurons possess a molecular clock consisting of interconnected positive and negative gene transcription feedback loops that oscillate with periods ranging from 19 to 28 h (Welsh et al., 1995; Herzog et al., 1998). To function as a regulator of behavioral and physiological circadian rhythms, these neurons require cell-to-cell communication to synchronize individual neuronal oscillators to a common period close to 24 h (Quintero et al., 2003; Schaap et al., 2003; Yamaguchi et al., 2003; Aton et al., 2005; Aton & Herzog, 2005) and the ability to be dynamically reset by environmental signals, of which light is the most important (Ding et al., 1997; Gillette & Mitchell, 2002; Challet, 2007). Input signals from light-sensitive retinal ganglion cells (RGC) mediated via glutamatergic terminals of the retinohypothalamic tract (RHT) can reset the oscillators to a new phase (Hattar et al., 2002; Yan & Silver, 2002; Warren et al., 2003; Hirota & Fukada, 2004; Naito et al., 2008). Light evokes phase-advances during the late night and phase-delays during the early night, but has no effect on circadian timing during the day (Shirakawa & Moore, 1994; Ebling, 1996; Gillette & Mitchell, 2002; Challet, 2007). The magnitudes of these phase shifts depend on the timing and the intensity of light stimuli (Takahashi et al., 1984; Cui & Dyball, 1996; Gooley et al., 2001; Berson et al., 2002; Morin et al., 2003; Sollars et al., 2003; Warren et al., 2003). In addition, a light pulse or optic nerve stimulation can increase, decrease or not affect the action potential firing frequency of SCN neurons (Meijer et al., 1986, 1998; Cui & Dyball, 1996; Jiao & Rusak, 2003).
γ-Aminobutyric acid (GABA) can both excite and inhibit SCN neurons (Wagner et al., 1997). GABA can induce phase-advances (Smith et al., 1990) and GABAA antagonists can block light-induced phase-delays (Ralph & Menaker, 1989). Thus, within the SCN, neurons must integrate incoming glutamatergic and peptidergic (e.g. PACAP; Castel et al., 1993; Hannibal et al., 2000; Kopp et al., 2001) signaling from the RHT, and synchronize and stabilize to the new phase via GABAergic and peptidergic (e.g. VIP; Moore et al., 2002; Belenky et al., 2008) signaling from communicating neurons within the SCN network.
The activity of neurons in a network even in small brain areas receiving multiple changing inhibitory and excitatory synaptic inputs is very complex. Single-cell electrophysiological experiments generally do not take into account the complexity of neural circuits even in reduced preparations such as brain slices, and application of pharmacological agents alters the activity of all the neurons in the preparation not just in the neuron being recorded. Recently, several research groups have used imaging techniques together with calcium indicators to study the function of neuronal networks (Gobel & Helmchen, 2007; Gobel et al., 2007; Zhang et al., 2007). We used a similar experimental strategy to study the role of GABAergic neurotransmission in the response of the SCN network to optic nerve stimulations that mimic entraining light input signals.
Materials and methods
Tissue preparation
Male Spraque-Dawley rats (Charles River, Wilmington, MA, USA) were housed for at least 1 week on a 12 : 12 h light : dark schedule. During the light phase, 4-6-week-old rats were anesthetized with isofluorane (Novaplus, UK), their brains removed and coronal hypothalamic slices (250 μm) containing the SCN were cut with a vibrating blade microtome (Leica VT1000S, Nussloch, Germany) while the tissue was surrounded by ice-cold artificial cerebrospinal fluid (ACSF) containing (in mm): NaCl, 120; KCl, 2.5; NaH2PO4, 1.2; MgCl2, 5; CaCl2, 0.5; glucose, 10; NaHCO3, 26; adjusted to 300 mOsm with sucrose and saturated with 5% CO2 and 95% O2. The slices were maintained in a recording chamber (35°C) with a continuous laminar flow (1-2 mL/min) of an ACSF solution consisting of (in mm): NaCl, 120; KCl, 2.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.4; glucose, 10; HEPES, 10; NaHCO3, 26; adjusted to 300 mOsm and bubbled with 5% CO2 and 95% O2. The Institutional Animal Care and Use Committee of OHSU approved, in advance, all procedures involving animals.
Calcium imaging
Multiple SCN neurons maintained in hypothalamic slices were loaded with Ca2+-sensitive probes for the simultaneous recording of Ca2+ responses (Fig. 1A-C). We modified the method of Yuste (Yuste, 2000; Irwin & Allen, 2007), as CNS neurons in brain slices (older than 4 weeks) are more difficult to load with acetoxymethyl (AM) esters. Freshly prepared ice-cold SCN slices were treated with warm (30°C) ACSF containing fura-2 AM (50 μm) for 1-2 min (Invitrogen, CA, USA; from stock 3.3 mm in dimethylsulfoxide), followed by incubation with fura-2 AM (10-25 μm) in ACSF for 1-2 h. The slice was washed for at least 30 min prior to recording to allow for fura-2 AM deesterification.
Fig. 1. Measurement of intracellular Ca2+ in the suprachiasmatic nucleus (SCN).
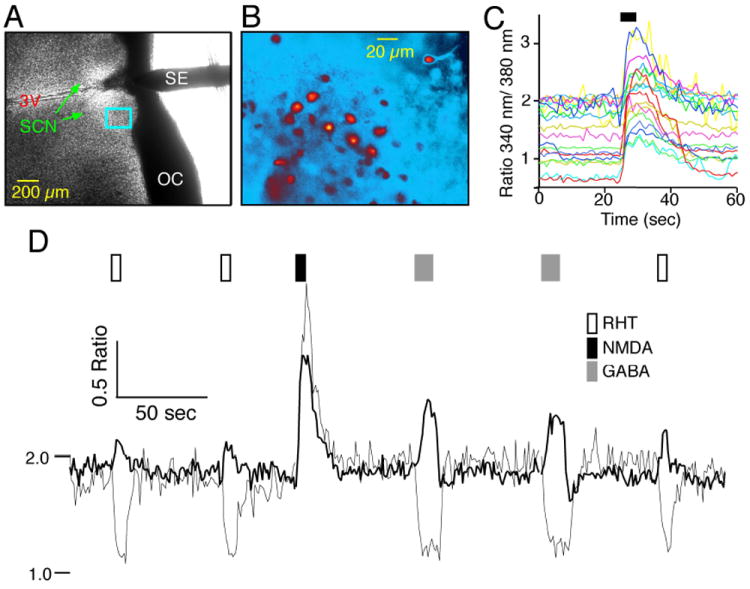
(A) Example of the recording setup showing a coronal hypothalamic slice containing the SCN and 3rd ventricle (3V) with a bipolar stimulating electrode (SE) used to stimulate the retinohypothalamic tract (RHT) positioned in the optic chiasm (OC). (B) Higher power fluorescent pseudo-color image of fura-2 AM-loaded SCN neurons located within the rectangle in (A). (C) N-methyl-d-aspartate (NMDA; 200 μm, 5 s) induced a transient increase in the Ca2+ ratio. Note the range of baseline Ca2+ ratios in these SCN neurons during the day (Zeitgeber time = 5 h). (D) Example showing postsynaptic Ca2+ in two SCN neurons with similar responses to NMDA (200 μm, 5 s), but divergent responses to stimulation of the RHT (100 pulses, 200 μs at 20 Hz) via a bipolar electrode placed in the OC (A) that paralleled the response to application of g-aminobutyric acid (GABA; 200 μm, 10 s).
Additional experiments were performed using microelectrodes that were filled with an internal solution to which bis-fura-2 hexapotassium salt (250 μm) was added (Invitrogen, Carlsbad, CA, USA; Irwin & Allen, 2007). Upon entering whole-cell mode, the SCN neuron including the dendrites rapidly filled with fluorescent probe.
Ca2+ measurements were obtained by recording a pair of images at excitation wavelengths of 340 and 380 nm, a 400-nm DCLP dichroic and an emission filter of 510 ± 40 nm (Chroma Technology, Rockingham, VT, USA). Excitation light was supplied via a monochronometer (Polychrome IV; Till Photonics, Martinsried, Germany) with a 10-nm bandwidth and passed through a UG11 optical filter to restrict harmonic wavelengths above 400 nm. Optical recordings were made with a cooled charge-coupled device camera (CCD, ORCA-ER 12 bit level, Hamamatsu, Japan) with acquisition time and binning adjusted to minimize photobleaching and maximize recording speed. Experiments were controlled via the digital imaging software Metafluor (Molecular Devices, Sunnyvale, CA, USA). Optical data were converted to relative fluorescence intensity units for each cell. The data were generally obtained from neurons located near the surface of the slice and intensely loaded with fura-2 AM. Data were presented as the emission ratio (R) at 510 ± 40 nm following excitation at 340 and 380 nm and background subtraction at each wavelength. Background fluorescence values were obtained from regions adjacent to each cell (including autofluorescence, residual probe in the interstitial space, and dendrites and cells out of the focal plane). Because of the background fluorescence associated with this slice preparation, the data are presented as measured ratios and not converted to [Ca2+]i units. However, an approximation for [Ca2+]i can be made utilizing the formula ~[Ca2+]i = (R - Rmin)/(Rmax - R) × Kd, where Kd is the disassociation constant for fura-2 AM. The observed Ca2+ ratios ranged from a minimum of 0.34 (Rmin) to a maximum of 3.7 (Rmax), and assuming a Kd of 224 nm, a ratio of 1 corresponds to ~55 nm and a ratio of 2 to ~219 nm. Neurons were identified by an increase in Ca2+ ratio in response to N-methyl-d-aspartate (NMDA; 200 μm for 5 s; Fig. 1C). Cells that did not respond to NMDA (200 μm, 5 s) or were dim were excluded. Ca2+ signals were initially sorted into three groups (increase, decrease or no response) by visual inspection. In a subset of the data where the Ca2+ signals were quantified, the response was defined as a change from baseline of more than 2 standard deviations. Neurons were considered to have a Ca2+ response to the GABAA antagonist gabazine by a sustained increase or decrease of the Ca2+ (340 nm/380 nm) ratio = 0.1 units. The localization of neurons to the dorsomedial (DM) and ventrolateral (VL) regions of the SCN were determined using the SCN coordinates for each neuron based on the calibrated 4 × brightfield image for each slice. The X, Y coordinate (0, 0) was the intersection of two perpendicular lines, one along the third ventricle and the other drawn along the ventral border of the SCN (Fig. 1A). A designation of VL was made when the coordinate X = Y for the neuron, and DM when the coordinate X < Y.
Test agents were applied using a perfusion device consisting of an eight-barrel array of tubes emptying into a common tip positioned approximately 500-1000 μm from the SCN and controlled electronically. The RHT was stimulated by 100 pulses (200 μs each) at 20 Hz via a bipolar electrode placed in the optic chiasm about 200 μm from the SCN (Fig. 1A).
Electrophysiology
Cell-attached and whole-cell patch-clamp recordings of SCN neurons were performed 0.5-8 h after slice preparation. Microelectrodes had an outside tip diameter of approximately 1 μm and resistances of 3-8 MΩ. For whole-cell recording experiments the microelectrode filling solution contained (in mm): K-gluconate, 140; KCl, 5; HEPES, 10; ATP, 4; GTP, 0.4; adjusted to pH 7.3 with KOH at (280–300 mOsm). Following microelectrode contact with a SCN neuron, negative pressure was applied to form seals with resistances of 1-5 GΩ. For whole-cell experiments additional negative pressure was used to rupture the cell membrane. For loose-seal recordings, the GABA-induced Ca2+ response was first determined followed by contacting the neuron with a microelectrode containing (in mm): NaCl, 140; KCl, 2.5; HEPES, 10; MgCl, 1.2; CaCl2, 2.4; adjusted to pH 7.3 with NaOH at (280–300 mOsm) and applying gentle negative pressure to form a loose seal (< 1 GΩ). Membrane voltage was measured in current-clamp mode with an Axoclamp 2A amplifier (Axon Instruments, Union City, CA, USA) or a HEKA EPC9 amplifier (HEKA, Lambrecht, Germany), and recorded using the data acquisition program Pulse (HEKA, Lambrecht, Germany), corrected for the liquid junction potential (-13 mV) and analysed using Igor (Wavemetrics, Lake Owsego, OR, USA).
Chemicals
GABA, NMDA, gabazine (SR 95531 hydrobromide), muscimol and CGP55845 were obtained from Tocris Bioscience (Ellisville, MO, USA). Fura-2 AM and bis-fura-2 were purchased from Invitrogen (Carlsbad, CA, USA). Other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA).
Data and statistical analysis
Igor Pro (Version 6.0.4, Wavemetrics, Lake Oswego, OR, USA) was used for curve-fitting and data analysis. Unless otherwise stated, data are presented as the mean ± SEM, and t-tests are two-tailed. t-Tests and Chi-square analysis were performed using Excel 11.5.1 (Microsoft, Redmond, WA, USA). anova, Kolmogorov-Smirnov and equality of variances F-test were performed using StatView 5.0.1 (SAS Institute, Cary, NC, USA). A P-level of = 0.05 was used to determine statistical significance.
Results
Time-dependence of the baseline Ca2+ concentration in SCN neurons
Simultaneous imaging of multiple neurons at different times of the circadian cycle and specific locations within the SCN (Fig 1A-C) was performed to examine the role of GABA in interneuronal communication and modulation of light-induced signaling to the SCN. We observed that stimulation of the RHT induced a range of postsynaptic Ca2+ responses that paralleled divergent responses seen with GABA applications (Fig. 1D).
To determine if endogenous GABA activity is modulating RHT signaling to the SCN, its importance in interneuronal communication, and if there are regional differences within the GABAergic SCN network, we first determined the variations of baseline Ca2+ at different times in the circadian phase, and time and regional variation in Ca2+ responses to exogenously applied and endogenous GABA, followed by the roles of GABAA and GABAB receptor subtypes, L-type Ca2+ channels, and the chloride cotransporter NKCC1 in the divergent postsynaptic responses to GABA.
During the day, SCN neurons had higher and more variable baseline Ca2+ ratios than during the night (Figs 1C and 2). The mean baseline Ca2+ ratio for each neuron was calculated based on the Zeitgeber time (total 1172 neurons) and demonstrated a diurnal change in the Ca2+ ratio (Fig. 2A; Colwell, 2000; Ikeda et al., 2003a). The variance of Ca2+ ratios during the day was significantly (P < 0.0001) different from that during the night (Fig. 2B and C). The distribution was narrower during the night-time and broader in the daytime. [Ca2+]i in SCN neurons varies with spontaneous action potential firing frequency (Irwin & Allen, 2007). The larger variance of the daytime baseline Ca2+ is consistent with the observations (Gillette, 1986; Jobst & Allen, 2002; Ikeda et al., 2003a) that during the day a greater number of SCN neurons are firing action potentials at higher frequencies. The change in variance of the Ca2+ ratio with time implies that there are quiescent and firing populations of SCN neurons.
Fig. 2. SCN neuronal baseline Ca2+ is dependent on the time of day.
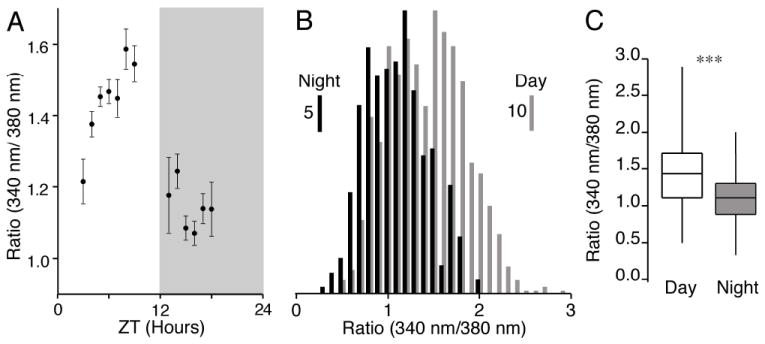
(A) The mean baseline Ca2+ ratio varied with the time of the day. Each point represents the mean ± SEM of the Ca2+ ratio (total 1172 neurons, mean n = 90 and range 8–236 neurons) at the indicated times. The darkened box indicates the night phase. (B) Distribution of the baseline Ca2+ ratios in SCN neurons recorded during the day (n = 861) and night (n = 311), respectively. The scale bars indicate the number of cells in each bin. (C) A box plot of the data in (B) showing the range (vertical line), 25th and 75th percentiles (box) and median (horizontal line) for day and night SCN neurons. The variance in Ca2+ ratios significantly differed between the day and night (equality of variances F860,310 = 1.65, ***P < 0.0001), and were shifted higher during the day compared with night (Kolmogorov-Smirnov test χ22 = 104.8, ***P < 0.0001). ZT, Zeitgeber time.
Whole-cell patch-clamp recordings with bis-fura-2 in the patch microelectrodes were used to further examine the relationship between membrane potential and [Ca2+]i (Fig. 3). Membrane potential hyperpolarization interrupted action potential firing and lowered the [Ca2+]i, while a further hyperpolarization of membrane potential did not further reduce [Ca2+]i. This indicates that there is a ‘floor’ to the voltage- [Ca2+]i relationship when voltage-dependent Ca2+ channels are closed and hyperpolarization below approximately -60 mV results in no further reduction in [Ca2+]i (Fig. 3B). Depolarizing the membrane potential resulted in a rapid increase in the spontaneous action potential firing frequency with a concomitant elevation of [Ca2+]i. We have previously shown that firing frequencies beyond 10-20 Hz did not yield a further increase in [Ca2+]i (Irwin & Allen, 2007). Taken together, these data suggest that there was a sigmoid relationship between membrane potential and the change in [Ca2+]i with a ‘ceiling’ associated with rapid firing of SCN action potentials (Fig 3B).
Fig. 3. The [Ca2+]i in SCN neurons varied with membrane potential and the spontaneous action potential firing frequency.
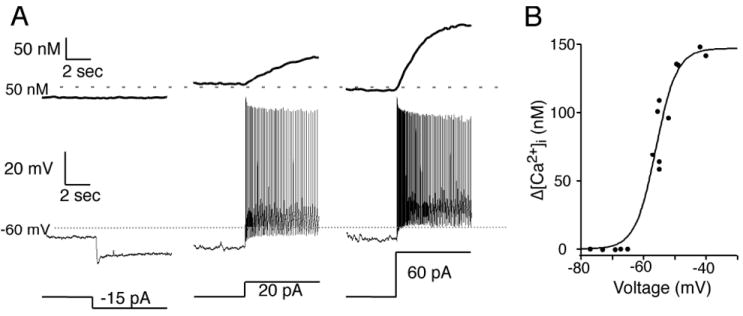
(A) A SCN neuron recorded during the day with a microelectrode filled with an internal solution containing the Ca2+-sensitive probe bis-fura-2 and external ACSF containing picrotoxin (50 μm) was hyperpolarized to inhibit spontaneous action potentials (not shown) followed by small current steps (bottom trace) to further hyperpolarize or depolarize the membrane potential (middle trace). A reduction of membrane potential did not lower the estimated [Ca2+]i, while depolarizing steps produced a rapid increase in spontaneous action potential firing with a concomitant elevation of [Ca2+]i (top trace). (B) Summary of the neuron in (A) showing a sigmoid relationship between the voltage reached from each hyperpolarizing and depolarizing current step and the corresponding change in somatic [Ca2+]i. During action potential firing membrane voltage was estimated by a line halfway between the action potential threshold and peak of the afterhyperpolarization. Estimated [Ca2+]i was calculated as previously described (Irwin & Allen, 2007).
We used tetrodotoxin (TTX) to evaluate the contribution of action potential firing to the [Ca2+]i of SCN neurons within the network. In fura-2 AM-loaded slices, application of TTX (0.5 μm), to inhibit Na+-dependent action potentials, reduced the Ca2+ ratio (= 0.1 ratio units) in significantly more neurons during the day (47% of 188 neurons) than during the night (26% of 62 neurons; χ21= 8.88, P = 0.0029). The baseline Ca2+ ratio of 1.59 ± 0.03 was reduced by -0.27 ± 0.02 ratio units during the day (t88= 13.69, P < 0.0001), and a baseline of 1.41 ± 0.06 was reduced by -0.23 ± 0.04 ratio units during the night (t15 = 6.47, P < 0.0001). Note that while during the day more neurons had a reduction of baseline Ca2+, the magnitude of the change was similar. These data were consistent with an interpretation that the higher amplitude and greater variance of the Ca2+ ratio observed during the day was due to a greater number of SCN neurons firing spontaneous action potentials during the day than at night (Colwell, 2000).
GABA has divergent effects on intracellular Ca2+ in SCN neurons that varies with time and region
GABA is generally viewed as an inhibitory neurotransmitter in the CNS (Roberts, 1984; Paul, 1995). However, several studies have observed excitatory GABA responses in neurons from several regions of the CNS including the SCN (Ben-Ari et al., 1989; Obrietan & van den Pol, 1996; Wagner et al., 1997, 2001; Staley & Smith, 2001; De Jeu & Pennartz, 2002; Ikeda et al., 2003b; Stein & Nicoll, 2003; Choi et al., 2008; Shulga et al., 2008; Zemkova et al., 2008). We tested the hypothesis that GABA has dual effects in SCN neurons that vary in time and location, where GABA can elevate [Ca2+]i in some SCN neurons while reducing the [Ca2+]i in others. We found that GABA (200 μm, 10 s) induced three types of Ca2+ responses in SCN neurons (Figs 1D and 4A): Ca2+ elevation [GABA(Ca+)]; Ca2+ reduction [GABA(Ca-)]; and those that did not respond [GABA(Ca0)]. We confirmed that the GABA-induced rise of Ca2+ was associated with membrane depolarization by performing loose-seal recordings (Jobst & Allen, 2002) from GABA(Ca+) neurons (n = 5; Fig. 4B). The Ca2+ response of SCN neurons to GABA application varied with the baseline Ca2+ ratio (Fig. 4C). In neurons with low baseline Ca2+ ratios there were fewer GABA(Ca-) neurons and more GABA(Ca0) neurons, while at higher Ca2+ baselines there were more GABA(Ca-) than GABA(Ca0) neurons, suggesting that when the baseline Ca2+ was low a GABA(Ca-) neuron was not able to further lower Ca2+ and may appear as a GABA(Ca0) neuron. The percentage of GABA(Ca+) neurons was reduced as the baseline Ca2+ increased. Testing 760 day and 292 night neurons, we found that a significantly lower percentage of GABA(Ca+) neurons was observed during the day (46.6%), than during the night (65.8%). Conversely, the percentage of GABA(Ca-) neurons decreased from 28.3% during the day to 8.2% at night (χ22= 52.41, P < 0.0001). The percentage of GABA(Ca0) neurons did not differ between the day (25.1%) and the night (26.0%; χ21 = 0.09, combined GABA(Ca+ and Ca-) vs. GABA(Ca0), P = 0.76; Fig. 4D). The percentage of GABA(Ca+) neurons was proportionally greater in the DM than the VL SCN (Figs 4E and 5). Conversely, a higher percentage of GABA(Ca-) and GABA(Ca0) neurons were observed in the VL than the DM SCN. Therefore we conclude that activation of GABA receptors induced divergent effects on [Ca2+]i that varied between the day and night, and location within the SCN.
Fig. 4. γ-Aminobutyric acid (GABA) induced divergent Ca2+ responses in SCN neurons.
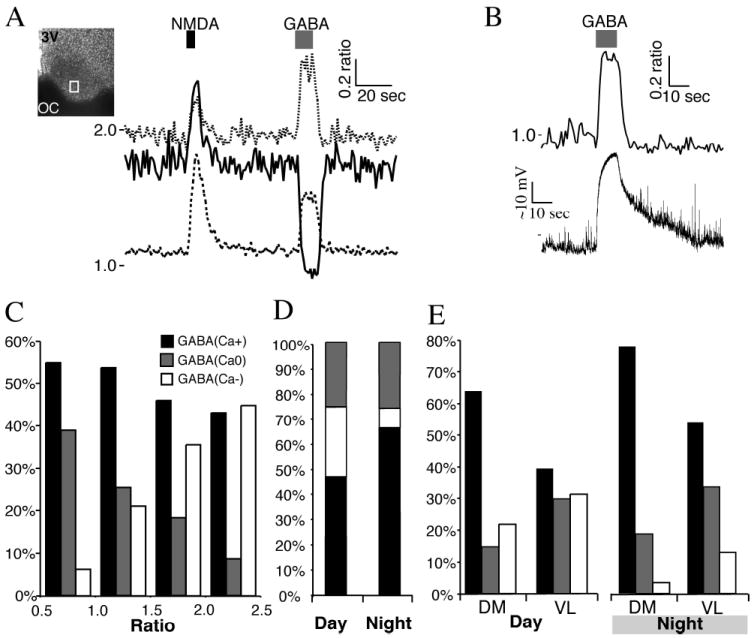
(A) SCN neurons imaged during the day, and treated with N-methyl-d-aspartate (NMDA; 200 μm, 5 s), which induced an increase in the Ca2+ ratio in all neurons. GABA (200 μm, 10 s) application induced divergent Ca2+ responses. The box within the SCN indicates the region recorded. Note the range of baseline Ca2+ concentrations. (B) Example of a GABA-induced rise in Ca2+ ratio and membrane depolarization in a SCN neuron recorded during the night using a loose-seal recording electrode. (C) The Ca2+ response to GABA (200 μm, 10 s) application varied with the baseline Ca2+ ratio. Ca2+ responses were separated into three groups: a transient elevation in the Ca2+ ratio [GABA(Ca+)]; a decrease in the Ca2+ ratio [GABA(Ca-)]; or no change in the Ca2+ ratio [GABA(Ca0)]. Data are the percentage of neurons in each response group based on the baseline Ca2+ ratio (range 0.5-1.0, n = 257 neurons; 1.0-1.5, n = 395; 1.5-2.0, n = 306; 2.0-2.5, n = 56). Note that the percentages of GABA(Ca-) and GABA(Ca0) neurons appeared reversed between low and high baseline Ca2+ ratios. (D) The percentage in the SCN neuronal response to GABA (200 μm, 10 s) varied between the day (n = 760) and night (n = 292). Note the change in the GABA(Ca+) and GABA(Ca-) groups, but not in the percentage of GABA(Ca0) neurons responding. (E) The percentage of SCN neurons with GABA-induced changes in the Ca2+ ratio varied between the dorsomedial (DM; n = 226 day and 145 night neurons) and ventrolateral (VL; n = 534 day and 147 night) regions of the SCN. The percentage of GABA(Ca+) neurons was higher in the DM during both the day and night. Conversely, the percentage of GABA(Ca-) and GABA(Ca0) neurons was higher in the VL in both day and night. 3V, 3rd ventricle; OC, optic chiasm.
Fig. 5. γ-Aminobutyric acid (GABA)-induced Ca2+ responses of SCN neurons varied regionally.
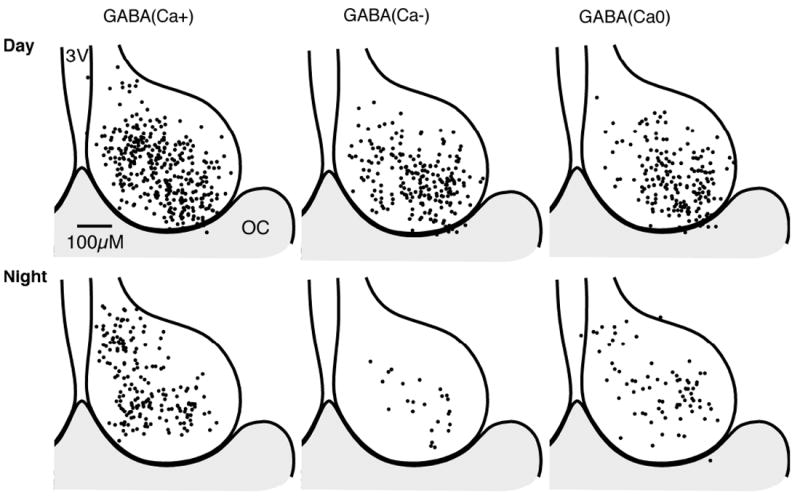
Regional and time of day variation of GABA-induced Ca2+ responses in SCN neurons (see Fig. 4D and E). The position for each neuron is superimposed on a representative drawing of the SCN, with the 3rd ventricle (3V) on the left and optic chiasm (OC) at the bottom. Note that while the number of cells in the day and night were not equal, the relative proportions varied between the day and the night.
Blocking the chloride cotransporter NKCC1 attenuated GABA-induced elevation of Ca2+
Inhibiting the sodium-potassium-chloride cotransporter NKCC1 activity reduced GABA-induced membrane depolarization (Khirug et al., 2008) in SCN neurons (Choi et al., 2008). SCN neurons were exposed to GABA before and after application of the NKCC1 blocker bumetanide (10 μm; Fig. 6A). The bumetanide effect on GABA-induced transients increased over time, reaching a maximal response after ~300 s (Fig. 6B). Bumetanide significantly attenuated daytime GABA-induced Ca2+ transients (65%) in GABA(Ca+) neurons and increased the magnitude of GABA-induced reductions of the Ca2+ ratio in GABA(Ca-) neurons (37%; Fig. 6C). These data show that the activity of the NKCC1 cotransporter is required for GABA-induced elevations of [Ca2+]i in SCN neurons.
Fig. 6. Blocking the chloride cotransporter NKCC1 attenuated the γ-aminobutyric acid (GABA)-induced elevation of Ca2+.
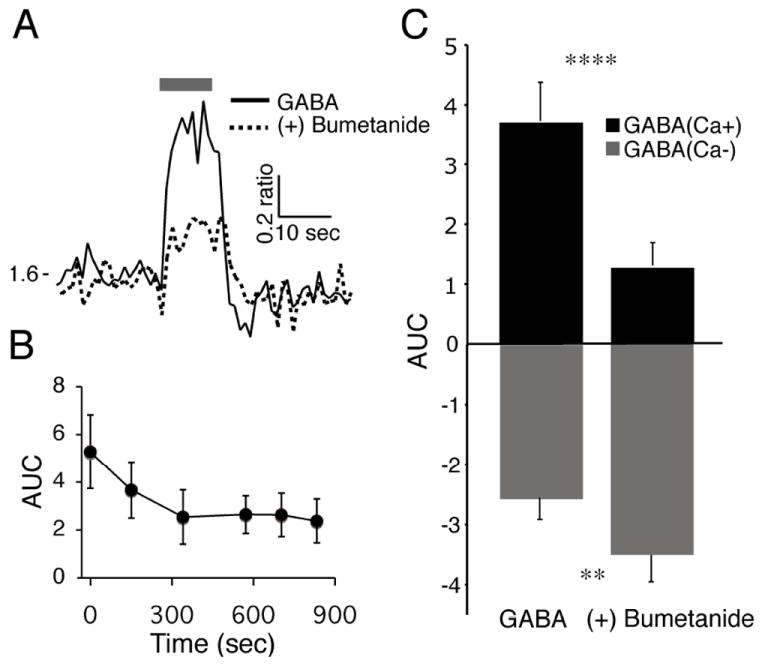
(A) Example of the Ca2+ response in a SCN neuron loaded with fura-2 AM and treated with GABA (200 μm, 10 s) during the day before and after bumetanide (10 μm, > 300 s) application. (B) The maximal bumetanide attenuation of the GABA-induced Ca2+ transient required > 300 s of treatment. The y-axis represents the area under the Ca2+ ratio curve [AUC (Ca2+ ratio/s), mean ± SEM] during the first 10 s of GABA treatment in 13 GABA(Ca+) neurons. (C) Bumetanide attenuated GABA-induced Ca2+ transients in GABA(Ca+) neurons and further reduced the Ca2+ ratio in GABA(Ca-) neurons. Data are the mean ± SEM of the AUC (0-10 s) of three different SCN slices for GABA(Ca+) (n = 39, t38 = 5.80, ****P < 0.00001) and GABA(Ca-) (n = 29, t28 = 2.89, **P = 0.007) SCN neurons before and after treatment with bumetanide (> 300 s).
Blocking L-type voltage-dependent Ca2+ channels attenuated GABA(Ca+)-induced Ca2+ transients
L-type voltage-dependent Ca2+ channels mediate a significant proportion of the depolarization-induced Ca2+ transients in SCN neurons (Irwin & Allen, 2007). Therefore, we examined whether the L-type Ca2+ channels contributed to GABA(Ca+) responses in SCN neurons. SCN neurons were treated during the day, with GABA (200 μm, 10 s) or the selective GABAA agonist muscimol (50 μm, 10 s) before and after the application of the L-type Ca2+ blocker nimodipine (20 μm for = 60 s; Fig. 7A). Nimodipine attenuated GABA- and muscimol-induced increases of Ca2+ in GABA(Ca+) neurons (n = 30, P < 0.00001; Fig. 7B). Further, nimodipine application was associated with a -0.16 ± 0.02 ratio unit reduction of the baseline Ca2+ ratio (1.40 ± 0.05, n = 40, t39 = 7.49, P < 0.0001 daytime neurons), suggesting that L-type Ca2+ channels play a role in setting the baseline Ca2+ in SCN neurons during the day. These data suggest that GABA increases the [Ca2+]i of SCN neurons in part by depolarizing the membrane potential and activating L-type voltage-dependent Ca2+ channels.
Fig. 7. Blocking L-type voltage-dependent Ca2+ channels attenuated γ-aminobutyric acid (GABA)-induced increases of [Ca2+]i.
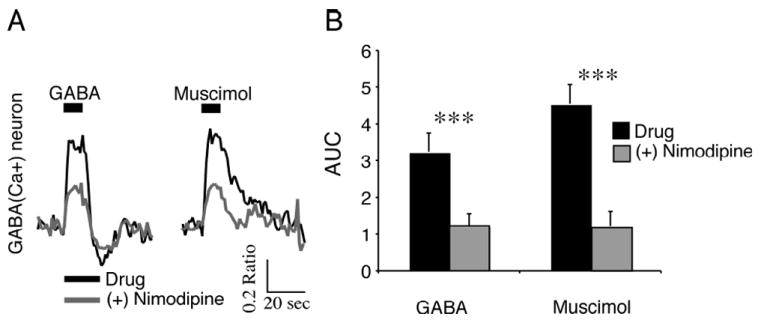
(A) Ca2+ responses induced by GABA (200 μm, 10 s) or muscimol (50 μm, 10 s) in a GABA(Ca+) neuron during the day before and after nimodipine (20 μM) application. Both GABA- and muscimol-induced Ca2+ transients were attenuated. (B) Nimodipine attenuated GABA- and muscimol-induced Ca2+ transients in GABA(Ca+) neurons. Data are the mean ± SEM of the area under the curve (AUC; Ca2+ ratio/s over 0-10 s of GABA treatment) for GABA(Ca+) (n = 30, t29 = 6.03 GABA, t29 = 10.6 muscimol, ***P < 0.00001) SCN neurons.
The contributions of postsynaptic GABAA and GABAB receptors as mediators of the GABA-induced Ca2+ response
To evaluate the contributions of GABAA and GABAB receptors in the GABA-induced Ca2+ response, SCN slices were treated with GABA in the absence and presence of the GABAA antagonist gabazine with or without the GABAB antagonist CGP55485 (Fig. 8A). Simultaneous inhibition of GABAA and GABAB receptors eliminated the GABA-induced Ca2+ changes. Inhibiting GABAA receptors eliminated the GABA-induced Ca2+ elevations, but not all reductions (Fig. 8A and B). However, inhibiting the GABAB receptors first did not alter the GABA-induced Ca2+ elevations but attenuated a delayed Ca2+ reduction that occurred in some neurons (Fig. 8C). We evaluated the selective GABAB agonists, baclofen and muscimol, on both GABA(Ca+) and GABA(Ca-) neurons. Baclofen lowered the [Ca2+]i in both GABA(Ca+) (Fig. 8D) and GABA(Ca-) (Fig. 8E) neurons; while muscimol-induced both increases and decreases of [Ca2+]i corresponding to the GABA responses. Baclofen induced a similar reduction of [Ca2+]i in both GABA(Ca+) and GABA(Ca-) neurons that persisted for some time after the application was discontinued.
Fig. 8. γ-Aminobutyric acid (GABA)A and GABAB receptor-mediated components of the GABA-induced Ca2+ response.
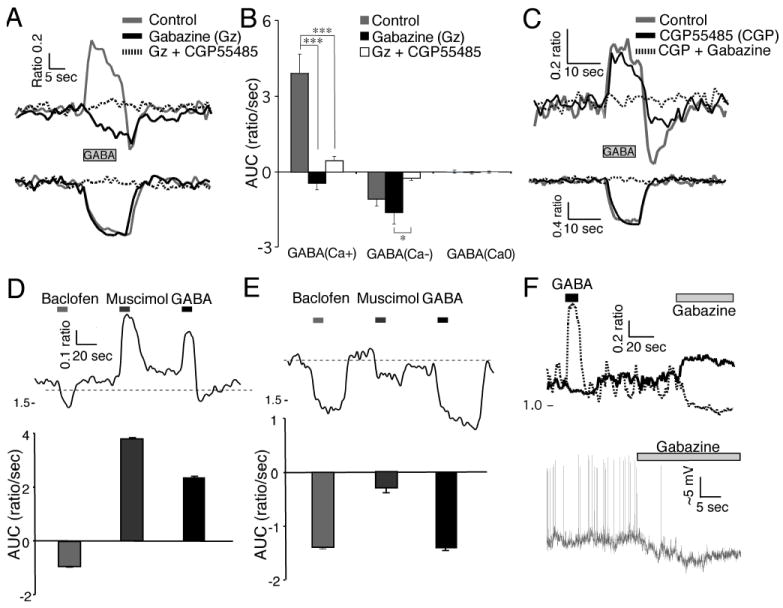
(A) GABA (200 μm, 10 s) induced Ca2+ responses before and during treatment with gabazine (10 μm) followed by gabazine together with CGP55485 (3 μm) in a GABA(Ca+) (top) and GABA(Ca-) (bottom) neuron. Note that the addition of gabazine in this GABA(Ca-) neuron did not attenuate the GABA-induced reduction in Ca2+. (B) Gabazine inhibited the GABA-induced rise of Ca2+ but did not alter the GABA-induced reduction of Ca2+. Simultaneous inhibition of GABAA and GABAB receptors eliminated the GABA-induced Ca2+ changes. Data are the mean ± SEM of the area under the curve (AUC; Ca2+ ratio/s over 0-10 s of GABA treatment) of neurons (GABA(Ca+) (n = 19, F2,36 = 28.91, P < 0.0001, power = 1.00); GABA(Ca-) (n = 12, F2,22 = 6.56, P = 0.0058, power 0.875); GABA(Ca0) (n = 4, F2,6 = 0.198, P = 0.826, power = 0.069) from three different SCN slices with repeated-measures anova analysis and post hoc Bonferroni t-test adjusted for three comparisons [(GABA(Ca+) ***P < 0.0001, Gz vs. Gz + CGP55485 P = 0.46; GABA(Ca-) control vs. Gz P = 0.49, control vs. Gz + CGP55485 P = 0.13, *P = 0.0048). (C) Example showing GABA-induced Ca2+ response during CGP55485 application followed by CGP55485 together with gabazine in GABA(Ca+) (top) and GABA(Ca-) (bottom) neurons. Note that the addition of gabazine in this GABA(Ca-) neuron eliminated the GABA-induced reduction in Ca2+. (D) Effects of baclofen (10 μm), muscimol (50 μm) and GABA (200 μM) on GABA(Ca+) neurons. The top trace is an example of Ca2+ responses in a GABA(Ca+) neuron. Below is the mean ± SEM of the AUC in 69 GABA(Ca+) neurons. (E) Effects of baclofen, muscimol and GABA as in (D), but with GABA(Ca-) neurons. Below is the mean ± SEM of the Ca2+ change in 21 GABA(Ca-) neurons. (F) The top traces show GABA- and gabazine-induced changes in the baseline Ca2+ ratio in two SCN neurons during the night. Gabazine induced a reduction in the baseline Ca2+ ratio in the GABA(Ca+) neuron (dashes) and an elevation of the baseline Ca2+ ratio in the GABA(Ca-) neuron (solid). The bottom trace shows an example of gabazine-induced hyperpolarization in a GABA(Ca+) SCN neuron during the night recorded using a loose-seal recording electrode.
The effect of endogenous GABA in the SCN network on Ca2+ homeostasis
As the effect of pharmacological application of GABA on [Ca2+]i in SCN neurons appeared to vary with regional location and activity phenotype, we evaluated whether endogenous GABA has a role in regulating Ca2+ homeostasis. SCN neurons were characterized as to the Ca2+ response induced by blocking GABAA receptors and contrasted with the pharmacological Ca2+ response to GABA. If endogenous GABA has a role in setting the level of intracellular Ca2+ in SCN neurons then an application of a GABAA blocker would be expected to reduce the baseline [Ca2+]i in GABA(Ca+) neurons and elevate the baseline [Ca2+]i in GABA(Ca-) neurons (Fig. 8F). SCN neurons were separated into three groups based on the baseline Ca2+ ratio response to gabazine (10 μm; Fig. 9). Similarly, bicuculline, a GABAA antagonist, has been shown to increase or decrease spontaneous firing of SCN neurons (Wagner et al., 1997; Shirakawa et al., 2000; De Jeu & Pennartz, 2002; Choi et al., 2008). In neurons responding to both GABA and gabazine, 75% of GABA(Ca+) neurons (n = 125) were gabazine(Ca-), and 68% of GABA(Ca-) neurons (n = 77) were gabazine(Ca+) (χ21 = 38.03, P < 0.0001). Differences between the pharmacological GABA response and the expected gabazine response may be attributed in part to a residual GABAB effect on [Ca2+]i. The Ca2+ responses to gabazine application demonstrated a distribution within the SCN similar to that predicted using GABA agonists, such that the mean change in Ca2+ induced by gabazine was in the opposite direction to that elicited by GABA. GABA(Ca-) and GABA(Ca0) neurons responding to gabazine had a similar change in the Ca2+ ratio, suggesting that pharmacological GABA(Ca0) neurons have physiologically inhibitory GABA responses (Fig. 9, bottom right). Collectively, these data suggest that physiological levels of GABA in the SCN network have a role in modulating the [Ca2+]i.
Fig. 9. Inhibiting endogenous γ-aminobutyric acid (GABA)A alters [Ca2+]i in SCN neurons.
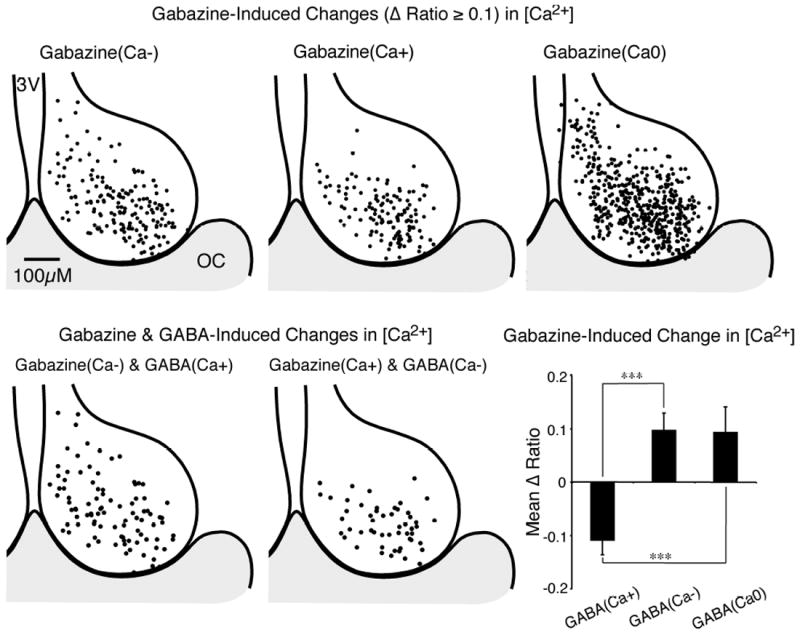
(Top) Regional variation of gabazine-induced (10 μm) changes in baseline Ca2+ ratio in SCN neurons. Responses were separated into three groups: a decrease of the Ca2+ ratio [gabazine(Ca-), top-left]; an increase of the Ca2+ ratio [gabazine(Ca+), top-middle]; and a non-responsive group Ca2+ [gabazine(Ca0), top-right]. The position for each neuron was superimposed on a representative drawing of the SCN, with the 3rd ventricle (3V) on the left and optic chiasm (OC) at the bottom. (Bottom left) Regional localization of individual SCN neurons with a GABA (200 μm)-induced increase in Ca2+ and a gabazine-induced decrease in Ca2+ (see Figs 5 and 8F). (Bottom middle) Regional variation of individual SCN neurons demonstrating both a GABA-induced decease and a gabazine-induced increase of [Ca2+]i. (Bottom right) The mean change in the baseline Ca2+ ratio produced by gabazine was in an opposite direction to the Ca2+ response evoked by GABA. Data are the mean ± SEM of the Ca2+ ratio change in gabazine-responsive neurons for GABA(Ca+) (n = 125), GABA(Ca-) (n = 77) and GABA(Ca0) (n = 65) neurons (F2,264 = 14.74, P < 0.0001, power = 1.00) by anova with Bonferroni t-tests adjusted for three comparisons, ***P < 0.0001. Note that GABA(Ca-) and GABA(Ca0) respond similarly (P = 1).
RHT signal modulation by GABA in the SCN network
Because GABA may have a role modulating light signaling to the SCN network, we determined if endogenous GABA alters the SCN neuronal response to RHT stimulation-induced transient changes in Ca2+. We have previously demonstrated that electrical stimulation of the RHT evokes an elevation of [Ca2+]i in SCN neurons by inducing action potentials (Irwin & Allen, 2007). When the spontaneous firing frequency was low, as occurs at night, RHT stimulation evoked large Ca2+ transients, while small Ca2+ changes occurred during high-frequency firing. These studies were performed using a GABAA blocker to eliminate interneuronal GABAergic neurotransmission. In the absence of GABAA receptor antagonists, stimulation of the RHT induced a range of postsynaptic Ca2+ responses comprising basic three types: a transient elevation in Ca2+ [RHT(Ca+)]; a transient reduction in Ca2+ [RHT(Ca-)]; or no alteration in the Ca2+ ratio [RHT(Ca0)] (Figs 1D and 10). GABA was applied to assess the pharmacological Ca2+ response ‘phenotype’ for each neuron. Most of the RHT(Ca+)-responding neurons also were GABA(Ca+) (~90%), and RHT(Ca-)-responding neurons also were GABA(Ca-) (~70%), with little day and night differences (Fig. 11, bottom right).
Fig. 10. Retinohypothalamic tract (RHT) stimulation induces a range of postsynaptic Ca2+ responses.
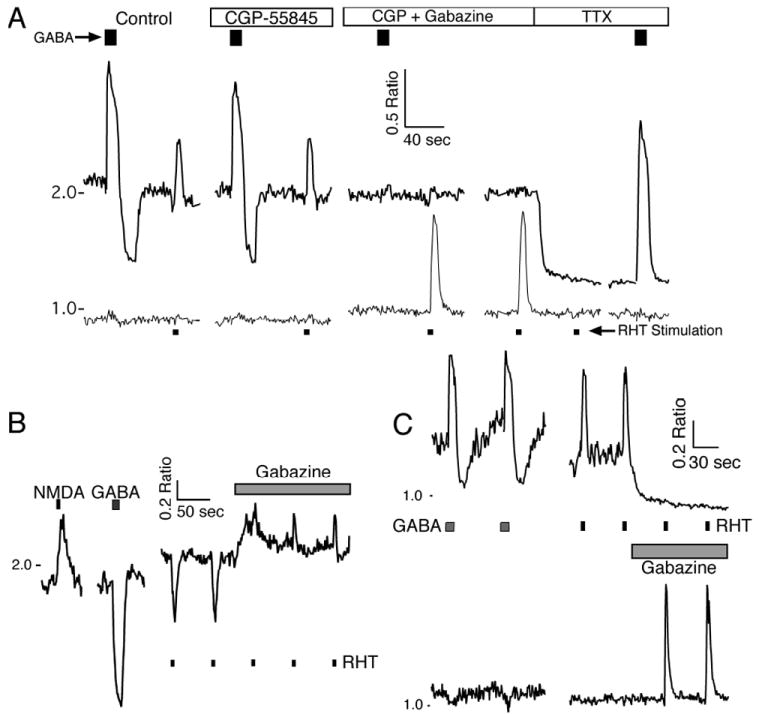
Stimulation of the RHT (100 pulses, 200 μs at 20 Hz) via a bipolar electrode placed in the optic chiasm (Fig. 1A) induced a transient elevation in Ca2+ [RHT(Ca+)], a transient reduction in Ca2+ [RHT(Ca-)] or no alteration in the Ca2+ ratio [RHT(Ca0)]. (A) The Ca2+ response to RHT stimulation and g-aminobutyric acid (GABA; 200 μm, 10 s) in two SCN neurons recorded during the day. CGP55845 (3 μm) application did not alter the RHT-induced Ca2+ signal in either cell, but the addition of gabazine (10 μm) eliminated the RHT-induced Ca2+ transient in the GABA(Ca+) neuron and induced a RHT-evoked Ca2+ response and a small elevation in the baseline Ca2+ ratio in the GABA(Ca0) neuron. Tetrodotoxin (TTX; 0.5 μm) eliminated the RHT-induced Ca2+ transients in both neurons. Note that GABA induced a biphasic Ca2+ response in the neuron with the higher baseline Ca2+ ratio (top trace), and the GABAB blocker reduced the duration of the later phase. Blocking both GABAA and GABAB eliminated both phases, and TTX reduced the baseline Ca2+ ratio but did not eliminate the rise in Ca2+ induced by GABA. (B) Example of a RHT(Ca-) response in a GABA(Ca-) SCN neuron during the day. Gabazine elevated the baseline Ca2+ and changed the direction of the RHT-induced Ca2+ response [RHT-Gz(Ca+)]. (C) Example showing the Ca2+ response following RHT stimulation in GABA(Ca+) and GABA(Ca-) neurons recorded during the night. Gabazine lowered the baseline Ca2+ in the GABA(Ca+) neuron and eliminated the RHT-induced transient elevation of Ca2+ [RHT-Gz(Ca-)]. In contrast, gabazine increased the RHT-induced Ca2+ elevation in a GABA(Ca-) neuron. NMDA, N-methyl-d-aspartate.
Fig. 11. Regional variation of retinohypothalamic tract (RHT) stimulation induced Ca2+ transients in the SCN.
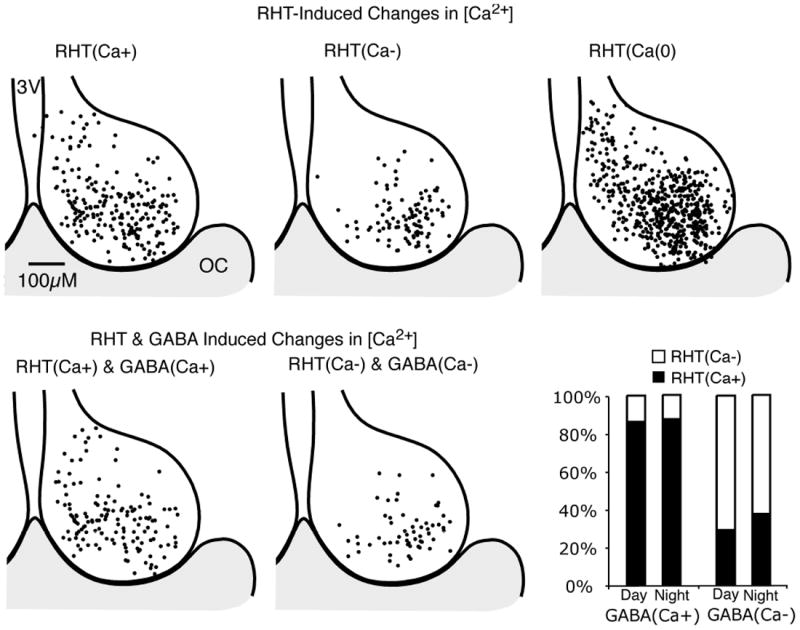
(Top) Regional variation in Ca2+ responses following optic chiasm (OC) stimulation (100 pulses at 20 Hz). Responses were separated into three groups: a transient elevation in Ca2+ [RHT(Ca+)]; a reduction [RHT(Ca-)]; and no alteration in the Ca2+ ratio [RHT(Ca0)]. The position for each neuron was superimposed on a representative drawing of the SCN, with the 3rd ventricle (3V) on the left and OC at the bottom. The distribution appears similar to that of the Ca2+ response to GABA (Fig. 5). (Bottom left) Regional variation of individual SCN neurons that showed both a RHT stimulation-induced and GABA-induced (200 μm) increase in Ca2+. (Bottom middle) Regional variation of individual SCN neurons with a RHT- and GABA-induced decease of Ca2+. (Bottom right) The percentage of GABA(Ca+) (day n = 94, night n = 71) and GABA(Ca-) (day n = 76, night n = 8) SCN neurons also responding to RHT stimulation.
Regional variation of RHT stimulation induced Ca2+ transients in the SCN network
The RHT stimulation-induced postsynaptic Ca2+ transients in the SCN network demonstrated a regional variation similar to the Ca2+ response pattern observed from application of pharmacological GABA (Figs 5 and 11). GABA application was used to assess the pharmacological phenotype of each neuron (Fig. 10), while gabazine was used to assess the effect of physiological GABA following RHT signaling within the SCN network. The use of GABAB antagonists was limited, as presynaptic GABAB receptors reduce the glutamate release from RHT axon terminals (Jiang et al., 1995; Moldavan et al., 2006). Gabazine application altered the RHT stimulation-induced Ca2+ responses in 77% of 247 neurons. An increase by gabazine [RHT-Gz(Ca+)] was defined as an increase in the RHT(Ca+) Ca2+ transient or a reduction in RHT(Ca-) response, and a decrease [RHT-Gz(Ca-)] in Ca2+ was defined as a reduction of the RHT(Ca+) Ca2+ transient or an increase in RHT(Ca-) response, and little or no change [RHT-Gz(Ca0)]. The effect of gabazine on the RHT stimulation-induced Ca2+ transients resulted in a decrease of the Ca2+ response in 53%, an increase in 21% and no change in 25% of 135 GABA(Ca+) neurons, and an increased Ca2+ response in 57%, decreased in 23% and did not change in 20% of 74 GABA(Ca-) neurons (χ22= 28.35, P < 0.0001). The gabazine response on Ca2+ transients was opposite in 59%, paralleled in 16% and did not change in 25% of 171 SCN neurons with corresponding combined pharmacological GABA and RHT Ca2+ response phenotypes (χ22 = 40.89, P < 0.0001). In some neurons without an initial Ca2+ response to RHT stimulation, application of gabazine unmasked a Ca2+ elevation in 15% and a decrease in 3% of 412 neurons.
These data suggest that GABA plays a role in modulating postsynaptic responses to RHT signaling. Because most SCN neurons are GABAergic, the ability of GABA release within the SCN network to modulate intracellular Ca2+ in neighboring neurons may emerge as a major level of control within the SCN.
Discussion
Activation of GABA receptors by endogenous GABA or application of pharmacological GABA agonists produced changes of the [Ca2+]i in SCN neurons that was dependent on the time of day, type of GABA receptors activated and the regional location of the neuron. RHT stimulation, mimicking light entraining input, induced elevations and reductions in [Ca2+]i with a localization pattern that frequently paralleled the GABA ‘pharmacological phenotype’. GABA-induced Ca2+ increases in the SCN were associated with membrane depolarization and activation of L-type Ca2+ channels, and had a higher proportion during the night than the day. These data are consistent with previous studies demonstrating that activation of GABAA receptors excites SCN neurons, based on the values of the chloride equilibrium potential and the membrane potential (Wagner et al., 2001), with a subsequent elevation of [Ca2+]i (Obrietan & van den Pol, 1996; Ikeda et al., 2003b; Choi et al., 2008; Shulga et al., 2008). Activation of GABAA and GABAB receptors produced a reduction in [Ca2+]i. GABAA receptors can induce hyperpolarization due to an inward Cl- current, that may reduce [Ca2+]i via a reduction or elimination of action potential firing (Irwin & Allen, 2007) and by closing voltage-dependent calcium channels. Additionally, it is conceivable that the Ca2+ reduction may also be augmented by other mechanisms, such as intracellular sequestration, extrusion or closing of other Ca2+ channels. The GABAB receptor-mediated effect is likely secondary to activation of a hyperpolarizing K+ current (Jiang et al., 1995). In a third group of SCN neurons the Ca2+ was not altered by GABA application [GABA(Ca0)] or RHT stimulation [RHT(Ca0)]. Because the physiological firing state of an individual neuron influences the Ca2+ response from RHT stimulation (Irwin & Allen, 2007), it is conceivable that a GABA(Ca-) quiescent neuron with a low [Ca2+]i during the night or a GABA(Ca+) high [Ca2+]i neuron firing action potentials during the day may not be able to further lower or raise [Ca2+]i, respectively. In most neurons with similar responses to RHT stimulation and GABA, application of gabazine to block GABAA receptor activity altered the RHT stimulation Ca2+ response in an opposite manner to the original response. Similar experiments could not be performed with GABAB receptor antagonists, as presynaptic GABAB receptors are present on presynaptic RHT terminals (Jiang et al., 1995; Moldavan et al., 2006). An increase of [Ca2+]i within SCN neurons during the night phase can shift the circadian clock (Gillette & Mitchell, 2002; Challet, 2007) and is thought to be required to initiate the transduction events leading to photic entrainment (Ding et al., 1994, 1998). Therefore, these data suggest that endogenous GABA activity within the SCN network can modulate the neuronal response to RGC light input signaling and may also play an important role in integrating the incoming light information to synchronize SCN neurons to produce a unified circadian rhythm.
GABAA receptors are located postsynaptically on SCN neurons and presynaptically as autoreceptors (Gao et al., 1995; Belenky et al., 2003), while GABAB receptors are present postsynaptically (Jiang et al., 1995; Belenky et al., 2008) with high expression in dorsal SCN neurons (Belenky et al., 2008) and presynaptically on RHT terminals (Moldavan et al., 2006). To evaluate the contributions of postsynaptic GABAA and GABAB receptors as mediators of the GABA-induced Ca2+ response, we applied gabazine to block GABAA receptors and eliminated the GABA-induced rise in [Ca2+]i, whereas blocking both GABAA and GABAB receptors eliminated both GABA-induced reductions and elevations of [Ca2+]i. The GABAA agonist muscimol produced divergent effects on [Ca2+]i that paralleled the GABA responses; whereas the GABAB agonist baclofen reduced [Ca2+]i often with a longer duration, consistent with activation of a G-protein-coupled receptor and K+ current.
Results from exogenous application of neurotransmitters can be difficult to interpret, as these agents are often applied at possible supraphysiological levels, the concentration may vary secondary to uptake mechanisms, and the levels may not accurately reflect the duration and location associated with either synaptic or tonic concentrations (Walker & Semyanov, 2008). Therefore, we applied gabazine to block endogenous GABAA receptor activity within the SCN network. Gabazine generally altered the baseline [Ca2+]i in a direction opposite to that of the pharmacological GABA phenotype and localization pattern within the SCN network. This suggests that the heterogeneous pharmacological GABA phenotype reflects the ‘physiological GABA phenotype’. Correspondingly, the GABAA blocker bicuculline inhibits multiunit electrical activity in the dorsal SCN but increases firing in the ventral SCN (Albus et al., 2005).
Recent evidence suggested that the functional organization of the SCN has broad divisions, such as core and shell (Moore et al., 2002), DM and VL (Masumoto et al., 2006; Belenky et al., 2008), or more complex divisions (Morin & Allen, 2006; Morin, 2007) based on physiological characterizations and localizations of peptides, receptors and gene products. Similar to the GABA-induced excitation and inhibition SCN localization observed by Choi et al. (2008), we found that the percentage of GABA(Ca+) neurons was proportionally greater in the DM than the VL half, while the percentage of GABA(Ca-) and GABA(Ca0) neurons was greater in the VL than the DM SCN. The excitatory and inhibitory pattern of physiological GABA modulation of [Ca2+]i within the SCN suggests a network of neighboring SCN neurons with divergent responses to GABA but grouped by regional localization with a preponderance of phenotypes.
We confirmed that the [Ca2+]i of SCN neurons demonstrates rhythmicity, with intracellular Ca2+ levels having a larger mean and variance during the day than the night (Colwell, 2000; Ikeda et al., 2003a). Application of TTX to inhibit firing of Na+-dependent action potentials reduced the baseline Ca2+ ratio in more neurons during the day than at night, consistent with a model in which [Ca2+]i varied with the frequency of spontaneous action potential firing. These data were in agreement with our previous whole-cell current-clamp recordings of SCN neurons, establishing that the [Ca2+]i is higher in SCN neurons with depolarized membrane potentials and faster action potential firing frequencies (Irwin & Allen, 2007). The greater amplitude and variance of the Ca2+ ratio observed during the day is, therefore, likely due to the greater number of SCN neurons firing spontaneous action potentials during the day than at night. Similarly, Colwell demonstrated a TTX-sensitive day-night difference in [Ca2+]i (Colwell, 2000). However, our previous observations using the Ca2+ indicator cameleon demonstrated a TTX-insensitive rhythm of Ca2+ released from intracellular stores (Ikeda et al., 2003a). Collectively, these data suggest that cameleon and fura-2 AM are measuring two different pools of Ca2+ regulated by different signaling mechanisms.
For a neuronal network to function there needs to be integration of both excitatory and inhibitory inputs. Within the SCN neuronal network, GABA signaling may serve both functions, as the equilibrium potential for the GABAA chloride channel is near the resting membrane potential in SCN neurons (Wagner et al., 1997, 2001; De Jeu & Pennartz, 2002; Choi et al., 2008). The transmembrane chloride gradient is regulated by two chloride transporters, the sodium-potassium-chloride cotransporter (NKCC1) that increases and the potassium-chloride cotransporter (KCC2) that decreases the intracellular chloride concentration ([Cl-]i; Payne et al., 2003; Stein & Nicoll, 2003). Modification of the expression or activity of these transporters will alter the chloride equilibrium potential and the magnitude and direction of GABAA-activated currents. We used the NKCC1 cotransporter blocker bumetanide (Kahle et al., 2008) to lower the [Cl-]i and significantly attenuated the GABA-induced rise of Ca2+ in GABA(Ca+) neurons and further reduced Ca2+ in GABA(Ca-) neurons. NKCC1 expression is higher in the dorsal SCN during the night than the day (Choi et al., 2008), and KCC2, responsible for chloride efflux, is predominately expressed in the ventral SCN (Belenky et al., 2008), and is consistent with our observation of a higher percentage of GABA(Ca+) neurons in the DM SCN at night (Fig. 4D). These observations demonstrate that modulation of [Cl-]i leads to GABA producing both excitatory and inhibitory effects on [Ca2+]i in SCN neurons.
Data from several studies indicate that GABA mediates both excitatory and inhibitory neurotransmission in the SCN (Wagner 1997, 2001; De Jeu & Pennartz, 2002; Ikeda et al, 2003; Choi et al. 2008). However, the results of these studies differ in critical ways, for reasons that remain unknown. Wagner et al. (1997, 2001) reported that excitatory responses predominated during the day, while De Jeu & Pennartz (2002) found that GABA primarily inhibits SCN neurons during the day but excites approximately half of the neurons at night. Recently, Choi et al. (2008) found GABA inhibitory and excitatory effects occurring during both the day and night, but found excitatory responses were more common at night especially in the dorsal SCN. We demonstrated a greater number of SCN neurons with GABA(Ca+) responses during the night than the day, similar to the data reported by De Jeu & Pennartz (2002) and Choi et al. (2008). At night, more GABA(Ca+) neurons were present in the DM SCN, and GABA(Ca-) responses predominated in the VL SCN, not dissimilar to the dorsal localization of excitatory responses observed by Choi et al. (2008). These studies indicate that the response of a SCN neuron to GABA depends on many factors, including the time of day, regional localization and its activity state.
Our observations suggest a model where physiological GABA release within the SCN network divergently modulates the response to RHT input signaling. In neurons with high NKCC1 activity (high [Cl-]i), opening GABAA receptors removes Cl- and facilitates depolarization, increasing firing frequency and increasing [Ca2+]i (Fig. 12A). In neurons with high KCC2 activity (low [Cl-]i), GABAA activity moves Cl- into the neuron hyperpolarizing the cell beyond the depolarizing effect of RHT signaling, slowing the firing rate and decreasing [Ca2+]i (Fig. 12B). Lastly, it is likely that many if not most neurons are in a balance that varies over time where the chloride level, set by cotransporters, may serve a gating role in the SCN network, and may neutralize the effects of RHT input without altering the membrane potential or firing frequency resulting in no change in [Ca2+]i. Further work is needed to further test this model and explore the role of internal calcium stores, co-released peptides and the reduction of [Ca2+]i by the GABAB receptor. In conclusion, these data suggest that GABA has both excitatory and inhibitory communication based on the chloride equilibrium potential and may play an important role in neuronal [Ca2+]i balance, synchronization and modulation of light input signaling within the SCN neuronal network.
Fig. 12. Theoretical model of glutamatergic light input signaling to the g-aminobutyric acid (GABA)ergic suprachiasmatic nucleus (SCN) network.
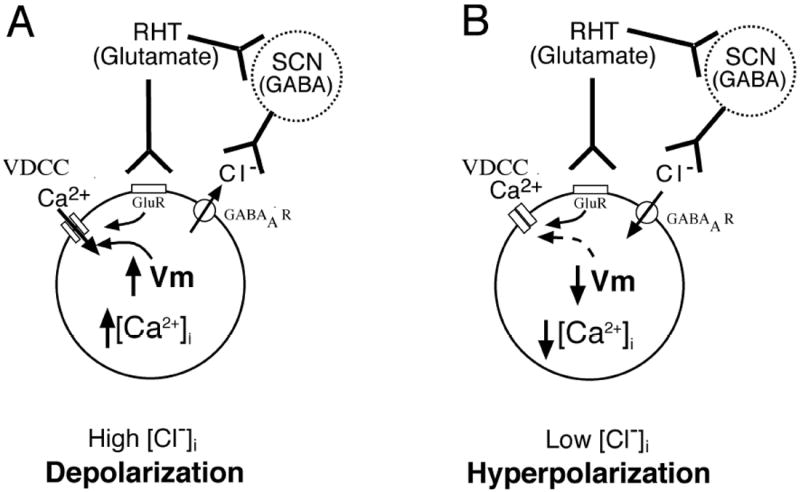
The effect of GABA on membrane potential and [Ca2+]i is a balance, with excitatory and inhibitory effects of GABA neurotransmission within the network driven by the chloride gradient that is under control by the cotransporters, NKCC1 and KCC2. (A) Depolarization induced by external light input signaling to glutamate receptors (GluR) via the retinohypothalamic tract (RHT) is facilitated by GABA release from other SCN neurons (dashed circle) within the network to a SCN neuron (solid circle) with a high intercellular chloride concentration ([Cl-]i), where opening of GABAA receptors (GABAAR) moves Cl- out of the neuron, further depolarizing the membrane potential (Vm), opening voltage-dependent calcium channels (VDCC) and increasing [Ca2+]i. (B) In neurons with low baseline [Cl-]i, GABA released from the SCN network opens GABAAR and moves Cl- into the neuron hyperpolarizing the cell beyond the depolarizing effect of RHT signaling and decreasing [Ca2+]i. A balance may also occur between the depolarizing action of RHT light input signaling with a hyperpolarizing effect from GABA release within the network, and no change in membrane potential, firing frequency or [Ca2+]i occurs.
Acknowledgments
This study was supported by Grant MH 70922 (C.N.A.).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AM
acetoxymethyl
- DM
dorsomedial
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-d-aspartate
- RGC
retinal ganglion cells
- RHT
retinohypothalamic tract
- SCN
suprachiasmatic nucleus
- TTX
tetrodotoxin
- VL
ventrolateral
References
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature neuroscience. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Herzog ED. Come together, right…now: synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Sagiv N, Fritschy JM, Yarom Y. Presynaptic and postsynaptic GABAA receptors in rat suprachiasmatic nucleus. Neuroscience. 2003;118:909–923. doi: 10.1016/s0306-4522(03)00062-9. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. The Journal of comparative neurology. 2008;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. The Journal of physiology. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. The European journal of neuroscience. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim do Y, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. The European journal of neuroscience. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui LN, Dyball RE. Synaptic input from the retina to the suprachiasmatic nucleus changes with the light-dark cycle in the Syrian hamster. The Journal of physiology. 1996;497(Pt 2):483–493. doi: 10.1113/jphysiol.1996.sp021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. Journal of neurophysiology. 2002;87:834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling FJ. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Progress in neurobiology. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Moore RY. GABAA-receptor subunit composition in the circadian timing system. Brain research. 1995;700:142–156. doi: 10.1016/0006-8993(95)00944-l. [DOI] [PubMed] [Google Scholar]
- Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain research. 1986;379:176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell and tissue research. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- Gobel W, Helmchen F. In vivo calcium imaging of neural network function. Physiology (Bethesda, Md) 2007;22:358–365. doi: 10.1152/physiol.00032.2007. [DOI] [PubMed] [Google Scholar]
- Gobel W, Kampa BM, Helmchen F. Imaging cellular network dynamics in three dimensions using fast 3D laser scanning. Nature methods. 2007;4:73–79. doi: 10.1038/nmeth989. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature neuroscience. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. The Journal of comparative neurology. 2000;418:147–155. [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nature neuroscience. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoological science. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003a;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Yoshioka T, Allen CN. Developmental and circadian changes in Ca2+ mobilization mediated by GABAA and NMDA receptors in the suprachiasmatic nucleus. The European journal of neuroscience. 2003b;17:58–70. doi: 10.1046/j.1460-9568.2003.02427.x. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci. 2007;27:11748–11757. doi: 10.1523/JNEUROSCI.1840-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Allen CN, North RA. Presynaptic inhibition by baclofen of retinohypothalamic excitatory synaptic transmission in rat suprachiasmatic nucleus. Neuroscience. 1995;64:813–819. doi: 10.1016/0306-4522(94)00429-9. [DOI] [PubMed] [Google Scholar]
- Jiao YY, Rusak B. Electrophysiology of optic nerve input to suprachiasmatic nucleus neurons in rats and degus. Brain research. 2003;960:142–151. doi: 10.1016/s0006-8993(02)03804-0. [DOI] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. The European journal of neuroscience. 2002;16:2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nature clinical practice. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28:4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp MD, Meissl H, Dehghani F, Korf HW. The pituitary adenylate cyclase-activating polypeptide modulates glutamatergic calcium signalling: investigations on rat suprachiasmatic nucleus neurons. Journal of neurochemistry. 2001;79:161–171. doi: 10.1046/j.1471-4159.2001.00553.x. [DOI] [PubMed] [Google Scholar]
- Masumoto KH, Nagano M, Takashima N, Hayasaka N, Hiyama H, Matsumoto S, Inouye ST, Shigeyoshi Y. Distinct localization of prokineticin 2 and prokineticin receptor 2 mRNAs in the rat suprachiasmatic nucleus. The European journal of neuroscience. 2006;23:2959–2970. doi: 10.1111/j.1460-9568.2006.04834.x. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Groos GA, Rusak B. Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain research. 1986;382:109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan MG, Irwin RP, Allen CN. Presynaptic GABA(B) receptors regulate retinohypothalamic tract synaptic transmission by inhibiting voltage-gated Ca2+ channels. Journal of neurophysiology. 2006;95:3727–3741. doi: 10.1152/jn.00909.2005. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell and tissue research. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- Morin LP. SCN organization reconsidered. Journal of biological rhythms. 2007;22:3–13. doi: 10.1177/0748730406296749. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. The Journal of comparative neurology. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Naito E, Watanabe T, Tei H, Yoshimura T, Ebihara S. Reorganization of the suprachiasmatic nucleus coding for day length. Journal of biological rhythms. 2008;23:140–149. doi: 10.1177/0748730408314572. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. Neuropeptide Y depresses GABA-mediated calcium transients in developing suprachiasmatic nucleus neurons: a novel form of calcium long-term depression. J Neurosci. 1996;16:3521–3533. doi: 10.1523/JNEUROSCI.16-10-03521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM. GABA and glycine. In: Bloom FE, K D, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 87–94. [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends in neurosciences. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J Neurosci. 1989;9:2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. GABA neurons in the mammalian central nervous system: model for a minimal basic neural unit. Neuroscience letters. 1984;47:195–200. doi: 10.1016/0304-3940(84)90513-5. [DOI] [PubMed] [Google Scholar]
- Schaap J, Albus H, VanderLeest HT, Eilers PH, Detari L, Meijer JH. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: Implications for circadian waveform and photoperiodic encoding. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15994–15999. doi: 10.1073/pnas.2436298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T, Honma S, Katsuno Y, Oguchi H, Honma KI. Synchronization of circadian firing rhythms in cultured rat suprachiasmatic neurons. The European journal of neuroscience. 2000;12:2833–2838. doi: 10.1046/j.1460-9568.2000.00170.x. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Moore RY. Glutamate shifts the phase of the circadian neuronal firing rhythm in the rat suprachiasmatic nucleus in vitro. Neuroscience letters. 1994;178:47–50. doi: 10.1016/0304-3940(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Shulga A, Thomas-Crusells J, Sigl T, Blaesse A, Mestres P, Meyer M, Yan Q, Kaila K, Saarma M, Rivera C, Giehl KM. Posttraumatic GABA(A)-mediated [Ca2+]i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. J Neurosci. 2008;28:6996–7005. doi: 10.1523/JNEUROSCI.5268-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Turek FW, Slater NT. Bicuculline and picrotoxin block phase advances induced by GABA agonists in the circadian rhythm of locomotor activity in the golden hamster by a phaclofen-insensitive mechanism. Brain research. 1990;530:275–282. doi: 10.1016/0006-8993(90)91295-r. [DOI] [PubMed] [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Visual neuroscience. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- Staley K, Smith R. A new form of feedback at the GABA(A) receptor. Nature neuroscience. 2001;4:674–676. doi: 10.1038/89439. [DOI] [PubMed] [Google Scholar]
- Stein V, Nicoll RA. GABA generates excitement. Neuron. 2003;37:375–378. doi: 10.1016/s0896-6273(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- Wagner S, Sagiv N, Yarom Y. GABA-induced current and circadian regulation of chloride in neurones of the rat suprachiasmatic nucleus. The Journal of physiology. 2001;537:853–869. doi: 10.1111/j.1469-7793.2001.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABA(A) receptors. Results and problems in cell differentiation. 2008;44:29–48. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. The European journal of neuroscience. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. The European journal of neuroscience. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Loading brain slices with AM esters of calcium indicators. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2000. pp. 34.31–34.39. [Google Scholar]
- Zemkova HW, Bjelobaba I, Tomic M, Zemkova H, Stojilkovic SS. Molecular, pharmacological and functional properties of GABA(A) receptors in anterior pituitary cells. The Journal of physiology. 2008;586:3097–3111. doi: 10.1113/jphysiol.2008.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]


