Abstract
Purpose
The aim of this work was to use metabolomics to evaluate sebum as a source of biomarkers for gamma-radiation exposure in the rat, and potentially in man. Proof of concept of radiation metabolomics was previously demonstrated in both mouse and rat urine, from the radiation dose- and time-dependent excretion of a set of urinary biomarkers.
Materials and methods
Rats were gamma-irradiated (3 Gy) or sham irradiated and groups of rats were euthanised at 1 h or 24 h post-irradiation. Sebum was collected by multiple washings of the carcasses with acetone. Nonpolar lipids were extracted, methylated, separated and quantitated using gas chromatography-mass spectrometry (GCMS). Metabolomic analysis of the GCMS data was performed using both orthogonal projection to latent structures-discriminant analysis and random forests machine learning algorithm.
Results
Irradiation did not alter sebum production. A total of 35 lipids were identified in rat sebum, 29 fatty acids, five fatty aldehydes, and cholesterol. Metabolomics showed that three fatty acids, palmitic, 2-hydroxypalmitic, and stearic acids were potential biomarkers. Sebaceous palmitic acid was marginally statistically significantly elevated (7.5–8.4%) at 24 h post-irradiation.
Conclusions
Rat sebaceous gland appears refractory to 3 Gy gamma-irradiation. Unfortunately, collection of sebum shortly after gamma-irradiation is unlikely to form the basis of high-throughput non-invasive radiation biodosimetry in man.
Keywords: metabolomics, sebum, gamma-radiation, fatty acids
Introduction
We have argued the obvious need for minimally-invasive high-throughput biomonitoring protocols for the mass screening of individuals in order to estimate the exposed radiation dose (Tyburski et al. 2008, 2009, Lanz et al. 2009) This would be a necessary part of the first response to a possible deployment of a terrorist nuclear or radiological device. As we have proposed previously (Tyburski et al. 2008, 2009, Lanz et al. 2009, Patterson et al. 2010), metabolomic approaches offer a realistic alternative to current procedures.
Radiation metabolomics seeks to define the radiation metabolomic fingerprint that is associated with exposure to radiation. Studies so far have been carried out in urine of irradiated and control animals (Tyburski et al. 2008, 2009, Lanz et al. 2009) but urine, while providing proof of concept, is not an ideal biofluid for the mass screening of populations. Sebum is an ideal biological material for mass screening as it is produced by the sebaceous glands of the skin where it serves to waterproof, moisturise and lubricate the skin. All parts of the skin of humans except the palms and soles contain sebaceous glands. However, the greatest density of these glands is found on the face and the scalp (Cheng and Russell 2004a). Sebum is a complex oily matrix, comprising monoesters, triglycerides, free fatty acids, free and esterified sterols, fatty alcohols, wax esters and squalene (Mackenna et al. 1950, Robosky et al. 2008). The occurrence of unusual branched-chain odd-numbered fatty acids in human sebum has been recognised for over five decades (Boughton and Wheatley 1959). The composition of sebum was found to be a unique property of the species. Differences between commonly used laboratory animals in terms of molecular identities and proportions of sebum components have been reported by Wheatley and James as well as remarkable differences between animals and man (Wheatley and James 1957). For instance, squalene that is present in human sebum up to 12% was reported to be missing in sebum of rodents (Wheatley and James 1957, Cheng and Russell 2004b). Sebum appears to be a matrix of first choice for radiation biodosimetry as a mass screening tool because of the ease of non-invasive and rapid collection using swabs soaked with an organic solvent. Furthermore, sebum contains a variety of unsaturated and polyunsaturated fatty acids which were believed to be susceptible to oxidation following high energy gamma irradiation with the formation of hydroxylated fatty acid derivatives. Therefore, the focus of this study was directed towards the investigation of the free fatty acid and triglyceride fractions that can be combined as one set of fatty acid methyl esters (FAME) in a solvent extraction and derivatisation protocol. Gas chromatography-mass spectrometry (GCMS) is the analytical technique of choice for the separation and determination of fatty acids (Eder 1995, Christie 2003). Some of the earliest applications of gas-liquid chromatography, the forerunner of modern GCMS, were studies on human (Mackenna et al. 1950, 1951, 1952, 1955, Boughton et al. 1955, 1957, 1959, James and Wheatley 1956, Boughton and Wheatley 1959) and animal (Wheatley 1953, Wheatley and James 1957) sebum.
In this report, a complete protocol for radiation metabolomics of rat sebum using low mass resolution GCMS is described. In addition, we also describe a protocol for the collection and analysis of fatty acids from human skin as a potential method of radiation biodosimetry. To our surprise, the production of sebum and lipid peroxidation within sebum was unaltered by gamma-irradiation. Moreover, there was only the slightest and barely statistically significant alteration in the composition of rat sebum after 3 Gy gamma-irradiation. Sebum is therefore unlikely to provide a resource for radiation biodosimetry despite being an information-rich biological matrix. The rat sebaceous gland appears to be acutely refractory to 3 Gy gamma-irradiation.
Materials and methods
Compounds
The following compounds were purchased from Sigma-Aldrich Chemie GmbH (Buchs, Switzerland): Butylated hydroxytoluene (BHT), FAME Mix C4-C24 Unsaturates, Linolenic Acid Methyl Ester Isomer Mix, Poly Unsaturated Fatty Acid (PUFA) No. 3 (from menhaden oil), and Bacterial Acid Methyl Ester (BAME) Mix. These four authentic standard mixes contained the methyl esters of the following fatty acids (lipid nomenclature): cis-13,16-docosadienoic acid (22:2 (n-6)), cis-4,7,10,13,16,19-docosahexaenoic acid (22:6 (n-3)), cis-7,10,13,16,19-docosapentaenoic acid (22:5 (n-3)), cis-11,14-eicosadienoic acid (20:2 (n-6)), cis-11-eicosenoic acid (20:1 (n-9)), cis-5,8,11,14, 17-eicosapentaenoic acid (20:5 (n-3)), cis-8,11, 14-eicosatrienoic acid (20:3 (n-6)), cis-11,14,17-eicosatrienoic acid (20:3 (n-3)), 9,12-hexadecadienoic acid (16:2 (n-4)), 6,9,12,15-octadecatetraenoic acid (18:4 (n-3)), cis-9,cis-12,trans-15-octadecatrienoic acid (18:3 (n-3)), cis-9,trans-12,cis-15-octadecatrienoic acid (18:3 (n-3)), cis-9,trans-12,trans-15-octadecatrienoic acid (18:3 (n-3)), trans-9,cis-12, cis-15-octadecatrienoic acid (18:3 (n-3)), trans-9,cis-12,trans-15-octadecatrienoic acid (18:3 (n-3)), trans-9,trans-12,cis-15-octadecatrienoic acid (18:3 (n-3)), cis-9,cis-12,cis-15-octadecatrienoic acid (18:3 (n-3)), trans-9,trans-12,trans-15-octadecatrienoic acid (18:3 (n-3)), arachidonic acid (20:4 (n-6)), behenic acid (22:0), butyric acid (4:0), caproic acid (6:0), decanoic acid (10:0), cis-13-docosenoic acid (22:1 (n-9), dodecanoic acid (12:0), eicosanoic acid (20:0), cis-11-eicosenoic acid (20:1 (n-9)), elaidic acid (18:1 (n-9)), heneicosanoic acid (21:0), heptadecanoic acid (17:0), cis-10-heptadecenoic acid (17:1 (n-7)), ( ± )-2-hydroxydecanoic acid (2-OH-10:0), 2-hydroxydodecanoic acid (2-OH-12:0), (±)-3-hydroxydodecanoic acid (3-OH-12:0), 2-hydroxyhexadecanoic acid (2-OH-16:0), 2-hydroxytetradecanoic acid (2-OH-14:0), 3-hydroxytetradecanoic acid (3-OH-14:0), linoleic acid (18:2 (n-6)), linolelaidic acid (18:2 (n-6)), α-linolenic acid (18:3 (n-3)), γ-linolenic acid (18:3 (n-6)), cis-9,10-methylene hexadecanoic acid (9,10-methylene-16:0), cis-9,10-methylene octadecanoic acid (9,10-methylene-18:0), 15-methylhexadecanoic acid (15-Me-16:0), 14-methylpentadecanoic acid (14-Me-15:0), 12-methyltetradecanoic acid (12-Me-14:0), 13-methyltetradecanoic acid (13-Me-14:0), myristic acid (14:0), myristoleic acid (14:1 (n-5)), octanoic acid (8:0), oleic acid (18:0 (n-9)), palmitic acid (16:0), palmitoleic acid (16:1 (n-7)), pentadecanoic acid (15:0), cis-10-pentadecenoic acid (15:1 (n-5)), stearic acid (18:0), tetracosanoic acid (24:0), cis-15-tetracosenoic acid (24:1 (n-9)), tricosanoic acid (23:0), tridecanoic acid (13:0), and undecanoic acid (11:0). Solvents and inorganic reagents were of the best available grade.
Animals
Male Fischer 344 rats (240–270g) were obtained from Charles River WIGA (Deutschland) GmbH (Sulzfeld, Germany) and were fed #3430 mouse and rat diet (Provimi Kliba AG, Kaiseraugst, Switzerland) ad libitum with free access to fresh drinking water and housed 3–4 per cage under a standard 12-h light, 12-h dark cycle. Animals were not selected for investigation until they had been housed at least one week in Bern. All animal handling and experimental protocols were designed for maximum possible well-being and were approved by the local ethics committee (Office for Agriculture and the Environment for Canton Bern).
Radiation exposure
Rats were γ-irradiated singly or in groups of 2 or 3 (as described below) with a nominal dose of 3.0 Gy using a Gammacell® 40 Exactor (Best Theratronics, Ottawa, Canada). This low dose-rate research irradiator was fitted with two 137Cs sources (above and below) with an activity of 1800 Ci/67 Tbq and delivering 1.0 Gy/min. The applied dose corresponds to a non-lethal dose of γ-radiation, which is similar to that reported during the Chernobyl accident in 1986 to cause Grade 1 (mild) acute radiation sickness (ARS) (0.1–3.3 Gy) (Belyi et al. 2010). Use of direct-reading dosimeters determined that the actual dose delivered to rats under the experimental conditions was 2.80–2.85 Gy. After irradiation, rats were immediately returned to their home cages (see below). Sham irradiation was performed by placing rats similarly into the irradiator but without exposure to the γ-emitting sources.
Sebum collection from rats
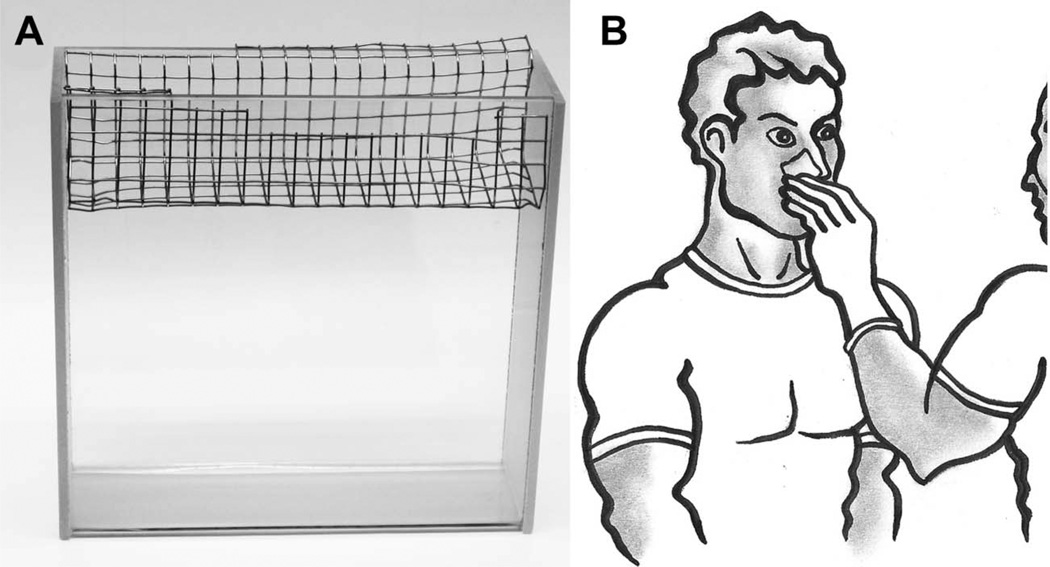
Twelve rats were randomly allocated into three groups of four animals each, as follows: Group 1, sham-irradiated controls; group 2, 3 Gy-irradiated, sebum collected after 1 h; group 3, 3 Gy-irradiated and sebum collected after 24 h. The study protocol was staged over three days, as follows: Day 1 morning and afternoon, one sham-irradiated and one irradiated rat, with euthanasia performed 1 h after irradiation/sham irradiation; day 2 morning and afternoon, one sham-irradiated and three irradiated rats, with euthanasia performed 1 h after irradiation for the sham control and one irradiated rat. The two remaining irradiated rats were housed in their home cages for 24 h with access to food and water; day 3 morning and afternoon, euthanasia for two rats 24 h post irradiation. Euthanasia of all rats was performed with pentobarbital. Immediately after euthanasia of each rat, sebum was harvested by the following protocol. The mouth and anal regions of freshly killed animals were cleaned with cotton swabs dipped in dichloromethane. The carcass was then suspended in a metal basket that had been constructed locally from stainless steel (Figure 1A) and fitted on top of a glass tank. Acetone (100 ml) was then poured over the back of the animal in such a way that as little as possible flowed over the ears, mouth and anal region. The acetone was then decanted into a glass beaker and poured back over the carcass a further five times in the same manner. Finally, the carcass was washed with fresh acetone (50 ml), which was combined with the main acetone fraction. Total acetone washings were then reduced to dryness in a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) under a light vacuum and at a maximum temperature of 40°C. The process described followed a published method (Wheatley and James 1957).
Figure 1.
Panel A: Rat sebum collection apparatus. The rat carcass is laid in the stainless steel tray and sebum eluted with acetone (see text); Panel B: Human sebum collection method. Sebum is collected from the alar wings of the nose using an acetone or isopropanol swab (see text).
Determination of thiobarbituric acid reactive substances in sebum
Thiobarbituric acid reactive substances (TBARS) were quantitated spectrophotometrically on the basis of malondialdehyde (MDA). MDA stock solutions (10 mM) were obtained by hydrolysing 1,1,3, 3-tetraethoxypropane (10 mM) in HCl (0.1 M) for 40 min at 40°C. Individual stock solutions of MDA were prepared for calibration and control samples including spiked sebum samples. Working solutions of MDA (500 µM) were made in HCl (0.1 M) from the stock solutions and for all consecutive dilutions water was used as the solvent. Acetone (10%) was added to the calibration and control samples. Calibration curves for MDA covered the range between 0.156 and 10.0 µM and two control samples containing MDA at concentrations of 0.4 and 4.0 µM were included to monitor the assay performance. Recovery was determined by spiking the sebum samples stemming from of a control rat and a rat euthanised 1 h after irradiation with MDA concentrations of 1.0 and 5.0 mM. For the analysis of rat sebum the back-up samples in acetone described below were thawed at 30°C in a water bath and tenfold diluted with water after brief centrifugation to remove insoluble precipitates. Spiked sebum samples were obtained by diluting the back-up samples 10-fold with an aqueous solution of MDA to obtain the desired concentration. To form the thiobarbituric acid (TBA) derivative an aliquot of each sample (400 µl) was combined with 400 µl of an aqueous solution containing TBA (1%) and trichloracetic acid (5%) and incubated for 1 h at 75°C. After cooling to room temperature, an aliquot of the samples (300 µl) was transferred to a 96-well microtiter plate and the ultraviolet (UV) absorbance immediately read at 530 nm on a Sunrise microplate reader from Tecan (Ma¨nnedorf, Switzerland) using the Magellan analysis software (version 4.00).
Sample preparation for GCMS
All solvents and solutions were degassed before use and the sample preparation performed under nitrogen. The volume of the total acetone washings from each animal was measured and fresh acetone added to a final volume of 130 ml. Two 10 ml aliquots were each combined with 0.1 ml of BHT solution (10 mg/ ml in acetone) as an antioxidant and stored under nitrogen at −80°C as back-up samples. Internal standard solution (0.5 ml) composed of perdeuterostearic acid (d35-octadecanoic acid; Sigma-Aldrich) and nonanoic acid (Sigma-Aldrich) (10 mM each in acetone) was added to the remaining 110 ml sebum solution, which was then reduced to 10–12 ml in a rotary evaporator under a light vacuum and at a maximum temperature of 40°C. The resultant solution was filtered to remove cellular debris and hairs through an Omnipore 0.45 µm Teflon-coated membrane (Millipore AG, Zug, Switzerland) into a weighed flask, the original flask rinsed with a small quantity of fresh acetone and the combined acetone portions were made up with acetone to a final volume of 15 ml. An aliquot of this solution (3 ml) was removed and stored under nitrogen at −80°C after the addition of 30 µl of BHT solution (10 mg/ ml in acetone). For the GCMS analysis of fatty acids in sebum, a further portion (1.875 ml) was reduced to dryness under nitrogen at room temperature in a 3 ml Reacti-Vial (Thermo Fisher Scientific (Schweiz) AG, Basel, Switzerland) and then methylated as described below. The remainder was reduced to dryness in a rotary evaporator under gentle vacuum and a temperature not exceeding 40°C. The amount of sebum collected from each animal was calculated after weighing of the flask. The viscous residue was dissolved in hexane (2.0 ml) containing BHT (100 mg/l) and the flask further rinsed with hexane (2.0 ml). The combined hexane fractions were stored under nitrogen at −80°C.
For methylation of the samples, toluene (0.5 ml) containing BHT (0.2 mg/ml) and freshly prepared 2% sulfuric acid in methanol (1 ml) was added to the dried residue. After overnight incubation (16 h) at 50°C the samples were cooled to room temperature in a water bath at 25°C, transferred to glass tubes and combined with 5% sodium chloride solution (3 ml) and hexane (3 ml) containing BHT (100 mg/l) added. After thorough vortex mixing for 1 min the samples were placed on a horizontal shaker for 15 min and then centrifuged at 780 g for 5 min. The organic supernatants were removed and further hexane (3 ml) containing BHT (100 mg/l) was added to the aqueous layer. The same extraction procedure as described above was repeated and the combined organic layers were washed with 2% potassium bicarbonate solution (2.5 ml) for 15 min on a horizontal shaker. After centrifugation at room temperature for 5 min at 780 g the organic layers were carefully removed and dried for 1 h at room temperature with 1.0–1.5 g of anhydrous sodium sulfate. After brief centrifugation to spin down the solid sodium sulphate, supernatant (200 ml) was transferred to autosampler vials and subjected to GCMS analysis. The remaining solutions were stored under nitrogen at −80°C.
Human sebum collection protocol
Human sebum was collected from both alar wings of the nose from five male volunteers (aged 33–65 years) who did not use soap, creams, aftershaves or any other cosmetics for the last 12 h prior to the sampling. The sebum was collected as depicted in Figure 1B with cotton swabs of 4.5 cm2, which had been weighed then soaked with acetone or isopropanol. After the sampling, the cotton swabs were dried in a fume hood and weighed to estimate the amount of sebum collected. The cotton swabs were then placed in 20 ml screw-top glass tubes and combined with acetone (4 ml) fortified with BHT (100 mg/l) and internal standard solution (0.1 ml), containing perdeuterostearic acid (d35-octadecanoic acid) and nonanoic acid (10 mM each in acetone). After repetitive vigorous shaking for 10 min, the acetone was transferred to clean glass tubes and another quantity of fresh acetone (2 ml) containing BHT (100 mg/l) was added to the cotton swab. The elution of the sebum from the cotton swab was completed in the same manner for another 10 min. The combined acetone fractions were filtered to remove cellular debris through an Omnipore 0.45 m m Teflon-coated membrane and dried under a gentle stream of nitrogen. Methylation of the samples was performed in toluene (0.5 ml) containing BHT (0.2 mg/ml) and freshly prepared 2% sulphuric acid in methanol (1 ml) as described above.
GCMS analysis of sebum
Samples were analysed using an Agilent 6890N gas chromatograph fitted with an Agilent 7683B series automatic liquid sampler and an Agilent 5975B series mass selective detector (MSD) from Agilent Technologies Inc. (Santa Clara, CA, USA). For the acquisition of data for multivariate data analysis, samples (1.0 µl) from rats and humans were injected in split mode (split ratio 3:1) and splitless, respectively, after 3 min column equilibration onto a Supelcowax 10 capillary column from Sigma-Aldrich (0.25 mm film thickness; 30 m ×.25 mm i.d.) subjected to a temperature program of 60°C for 4 min, 20°C/min to 220°C, 20 min at 220°C, 5°C/ min to 250°C, and finally 13 min at 250°C (total run time 51 min). The injector inlet was held at 250°C and the MSD transfer line at 280°C, and the carrier gas was helium (2.0 ml/min; 52 cm/sec). Mass spectral data were collected in electron impact mode (69.92 eV) from 3.2–20 min between 35.0 and 400.0 m/z, and from 20–51 min between 35.0 and 500.0 m/z. The mass spectrometer source was set at 230°C and the quadrupole at 150°C. The MSD tune parameters were all within normal ranges. MSD ChemStation software version E.01.01.335 from Agilent was employed for initial data analysis.
Bioinformatics
Raw mass spectrometry data were imported into MZmine (version 0.60; www.mzmine.sourceforge.net) for data pre-processing in terms of peak detection and peak alignment and the generation of a multivariate data matrix. The parameter settings were adjusted to include major masses of small chromatographic peaks while limiting the number of ions extracted from large peaks in the chromatogram. All peak tables created in MZmine covered the entire range of the chromatograms. Unless stated otherwise, calculations were based on peak height and the data for each ion were normalised by the height of the peak obtained for the molecular ion of the internal standard (perdeuterostearic acid; 333 m/z). All data were Pareto-scaled and further analysed by principal components analysis (PCA) and orthogonal projection to latent structures discriminant analysis (OPLS-DA) employing SIMCA-P+ software (version 12.0.0.0) from Umetrics AB (Umeå, Sweden). For OPLS-DA of the samples obtained from the animal experiment samples were classified according to their group membership as from control rats (class 1; y = 1), animals irradiated and killed after 1 h (class 2; y = 2) or rats irradiated and killed after 24 h (class 3; y =3). To determine which sebum component contribute most to the separation between sham control animals and irradiated animals, and thus elevated or decreased, separate OPLS-DA models were calculated comparing class 1 with class 2 and class 1 with class 3. Potential markers were selected by inspection of the scatter S-plots locating the variables according to their influence on the model (contribution) and their correlation (confidence), as previously published (Tyburski et al. 2008).
Identification and confirmation of sebum components
Identification of chromatographic peaks was obtained by comparing their mass spectra with the almost 575,000 spectra in the NIST Mass Spectral Database (NIST/EPA/NIH Mass Spectral Library version 2.0; U.S. Secretary of Commerce, USA). Confirmation was accomplished by analysing under the same analytical conditions mixtures of authentic standards of FAME and preparations of known composition of FAME and related compounds stemming from menhaden oil and bacteria. Retention times and mass spectra were compared to demonstrate the identities unambiguously.
Results
Animal performance
The body weight (bw) of the rats was recorded at the end of the study and did not differ between the three study groups. The mean values were found to be 248.5 ±5.2 g, 254.8 ±10.5 g and 247.8 ±4.2 for the sham control animals, the rats killed 1 h after irradiation, and those euthanised 24 h after gamma irradiation, respectively. The animals did not show outward signs of adverse health effects or distress during the study period of 24 h. During the collection of sebum, a mean value of 103 ±6 ml acetone was obtained from each animal. The amount of sebum collected was calculated on the basis of a fraction (57.1% of the initial sample) which was reduced to dryness in a weighed flask and found to be comparable for the three groups. Mean total sebum yield was calculated as 270±25 mg (1.09 ±0. 10 mg/g bw), 267 ±32 mg (1.04 ±0.15 mg/g bw) and 255 ±21 mg (1.03 ±0.10 mg/g bw) for the sham controls, the animals euthanised 1 h and 24 h after gamma irradiation, respectively.
GCMS of rat sebum
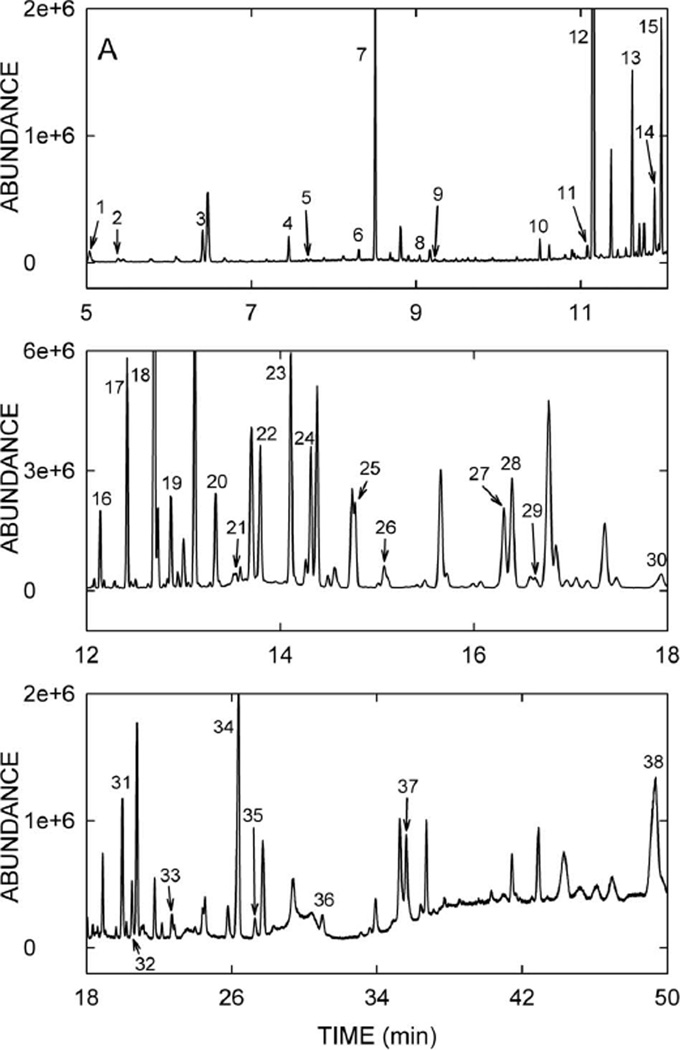
GCMS is the analytical technique of choice for the separation and determination of fatty acids after derivatisation to enhance the volatility of the components (Eder 1995, Christie 2003). A lipidomics approach based on the gas chromatographic separation and mass spectrometric detection of the maximum possible number of constituents together with multivariate data analysis was chosen to investigate simultaneously the fatty acid fraction of sebum. To reduce the risk of the introduction of artefacts and the loss of components of interest precautions were taken during the sample preparation, consisting of: (i) The avoidance of any material made of plastic during sample storage and sample preparation, (ii) the execution of the whole sample work-up under nitrogen and using degassed solvents, (iii) the restriction of the temperature used to reduce solvent volumes to 40°C, and (iv) the addition of BHT as an antioxidant to the samples and solvents. A standard protocol was used for the acid-catalysed esterification and transesterification in combination with liquid-liquid extraction of the FAME which allowed the formation of methyl esters of free fatty acids and in the same reaction of fatty acids esterified with glycerol. Variations in the efficiency of the methyl ester formation and transesterification as well as the extraction were compensated for adding two exogenous internal standards to the sebum samples. Nonanoic acid as an early eluting compound with a low molecular weight was used to survey the loss of volatile FAME during the sample preparation, whereas perdeuterostearic acid was used for the calculations of relative concentrations. Uniform peak area ratios of the two internal standards were considered to reflect stable conditions during the sample preparation, i.e., no discrimination between the recovery of volatile and less volatile components. Chromatographic parameters were chosen to maximise baseline separation of peaks to reduce coelution but at the expense of run time. Peak identification was based on comparison of mass spectra to spectra in mass spectral databases. Confirmation was achieved by comparing the mass spectra and retention times obtained for the peaks in the sebum samples to those recorded for authentic standard mixtures of FAME of various origins and analysed under the same instrumental conditions. For rat sebum samples, a total of about 120 chromatographic peaks could be separated within 48 min. A typical chromatogram of a sham control animal is depicted in Figure 2, divided into three panels for clarity. The identity of 38 sebum analytes together with the trivial names, the retention time, and an abbreviation indicating the number of carbon atoms and the degree of unsaturation are given in Table I. All of these lipids correspond to authentic standards and no assignment was made based purely on a library match. Peak #12 is the added antioxidant BHT and peak #38 is cholesterol. Therefore, 36 fatty acids could be identified in rat sebum. All saturated fatty acids from C12 to C24 were found, including all of the odd-numbered fatty acid methyl esters, with the exception of undecanoic acid (11:0). This is in stark contrast to plasma lipids where exclusively even-numbered fatty acids are present (Christie 2003). Monounsaturated fatty acids, specifically (9Z)-hexadecenoic acid, (10Z)-heptadecenoic acid, (9Z)-octadecenoic acid, (11Z)-eicosenoic acid, (13Z)-docosenoic acid, and (15Z)-tetracosanoic acid were identified as well as one polyunsaturated fatty acid, (9Z,12Z)-octadecadienoic acid. Furthermore, three branched chain fatty acids, one hydroxy fatty acid, and five short-chain fatty aldehydes were found. Palmitic acid was the most abundant sebum constituent followed by 14-methylheptadecanoic acid, stearic acid and oleic acid.
Figure 2.
A GCMS chromatogram of methylated rat sebum showing 38 annotated peaks. Peak numbers correspond to the analytes listed in Table I.
Table I.
GCMS detection of fatty acids and related compounds in rat sebum.
| Peak no. | Retention time (min) | Identity* | Trivial name | Lipid Nomenclature |
|---|---|---|---|---|
| 1 | 5.04 | hexanal | caproaldehyde | |
| 2 | 5.39 | hexanoic acid | caproic acid | 6:0 |
| 3 | 6.41 | heptanal | ||
| 4 | 7.45 | octanal | caprylaldehyde | |
| 5 | 8.31 | nonanal | ||
| 6 | 7.67 | octanoic acid | caprylic acid | 8:0 |
| 7 | 8.50 | nonanoic acid (internal standard #1) | pelargonic acid | 9:0 |
| 8 | 9.05 | decanal | ||
| 9 | 9.23 | decanoic acid | capric acid | 10:0 |
| 10 | 10.50 | dodecanoic acid | lauric acid | 12:0 |
| 11 | 11.09 | tridecanoic acid | tridecylic acid | 13:0 |
| 12 | 11.15 | 2,6-di-tert-butyl-4-methylphenol | butylated hydroxy toluene | BHT |
| 13 | 11.62 | tetradecanoic acid | myristic acid | 14:0 |
| 14 | 11.89 | 12-methyltetradecanoic acid | ||
| 15 | 11.98 | 13-methyltetradecanoic acid | ||
| 16 | 12.14 | pentadecanoic acid | pentadecylic acid | 15:0 |
| 17 | 12.42 | 14-methylpentadecanoic acid | ||
| 18 | 12.70 | hexadecanoic acid | palmitic acid | 16:0 |
| 19 | 12.87 | (9Z)-hexadecenoic acid | palmitoleic acid | (9Z) 16:1 |
| 20 | 13.33 | heptadecanoic acid | margaric acid | 17:0 |
| 21 | 13.55 | (10Z)-heptadecenoic acid | (10Z) 17:1 | |
| 22 | 13.80 | [2H35]octadecanoic acid (internal standard #2) | perdeuterostearic acid | d35−18:0 |
| 23 | 14.11 | octadecanoic acid | stearic acid | 18:0 |
| 24 | 14.32 | (9Z)-octadecenoic acid | (9Z) 18:1 | |
| 25 | 14.77 | (9Z,12Z)-octadecadienoic acid | linoleic acid | (9Z,12Z) 18:2 |
| 26 | 15.08 | nonadecanoic acid | nonadecylic acid | 19:0 |
| 27 | 16.31 | eicosanoic acid | arachidic acid | 20:0 |
| 28 | 16.40 | 2-hydroxyhexadecanoic acid | 2-hydroxypalmitic acid | |
| 29 | 16.63 | (11Z)-eicosenoic acid | (11Z) 20:1 | |
| 30 | 17.91 | heneicosanoic acid | heneicosylic acid | 21:0 |
| 31 | 19.98 | docosanoic acid | behenic acid | 22:0 |
| 32 | 20.50 | (13Z)-docosenoic acid | erucic acid | (13Z) 22:1 |
| 33 | 22.71 | tricosanoic acid | tricosylic acid | 23:0 |
| 34 | 26.37 | tetracosanoic acid | lignoceric acid | 24:0 |
| 35 | 27.28 | (15Z)-tetracosenoic acid | nervonic acid | (15Z) 24:1 |
| 36 | 31.00 | pentacosanoic acid | pentacosylic acid | 25:0 |
| 37 | 35.62 | hexacosanoic acid | cerotic acid | 26:0 |
| 38 | 49.33 | cholest-5-en-3β-ol | cholesterol |
Fatty acids detected as their methyl esters and aldehydes as their dimethyl acetals.
GCMS of human sebum
Approximately 110 peaks in sebum were resolved during a 48 min chromatogram. The chromatographic characteristics of derivatised human sebum extract were broadly similar to those of the rat. Like rat sebum, the most abundant fatty acid in human sebum was palmitic acid but, unlike rat sebum, squalene was also detected in human sebum.
Bioinformatics – PCA
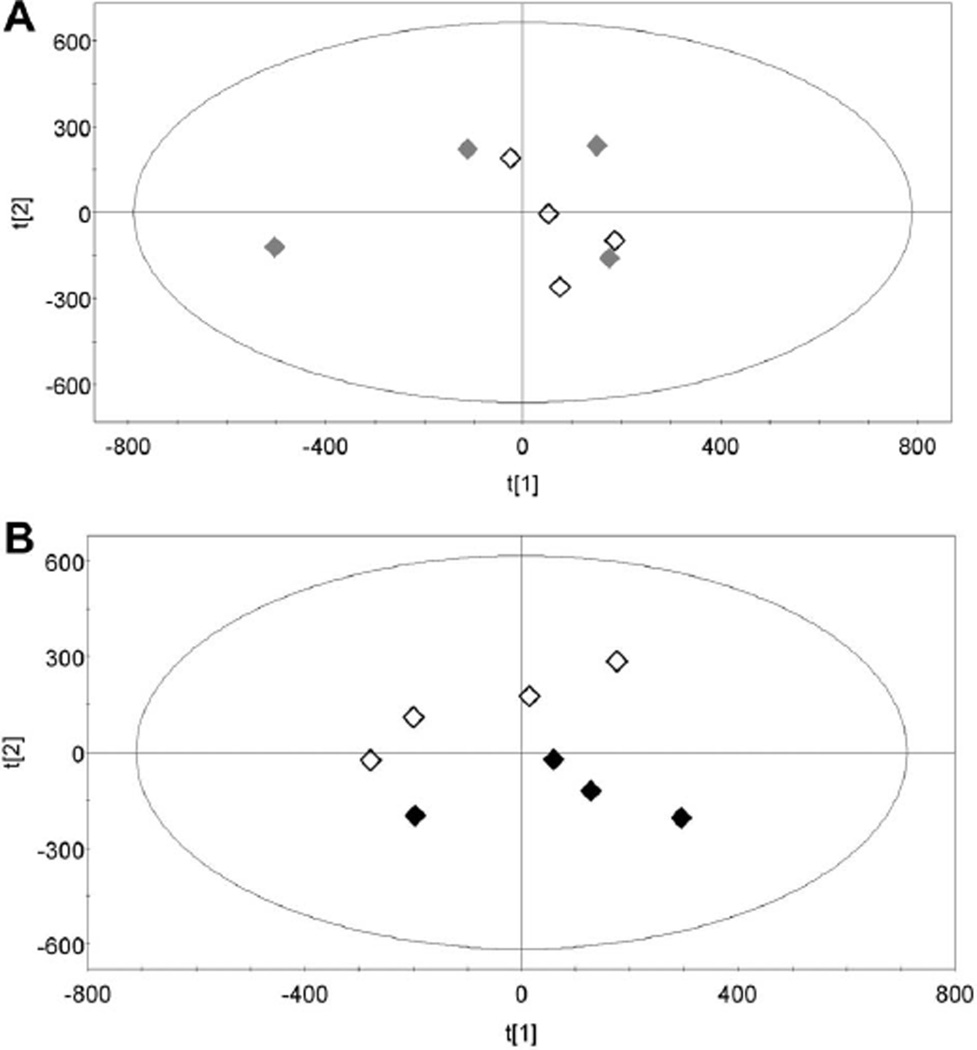
PCA is an unsupervised multivariate data analysis technique that best explains the variances in the data by reducing the number of variables into uncorrelated variables called principal components (Pearson 1901). It is important to recognise that PCA is an unsupervised method, that is, it does not employ class information for the variables, in this case, sham versus irradiated, and thus can be used as an exploratory technique to reveal the internal structure of the data. Figure 3A depicts a PCA scores plot where each point represents a sebum sample from an individual rat that has been analysed by GCMS. The data do not cluster into separate groups representing the sham and the 1 h post irradiation sebum samples. In contrast, the PCA scores plot in Figure 3B shows a clear separation of the sham and 24 h post irradiation sebum samples as analysed by GCMS. This finding suggests that there are ions detected by GCMS that differ in their abundance between the sham irradiated and 24 h post irradiation sebum samples. Therefore, these two specific data sets were further explored using supervised OPLS-DA.
Figure 3.
Panel A: Unsupervised principal components analysis (PCA) scores plot showing sebum samples from four sham-irradiated rats (♢) and from four 3 Gy-irradiated rats collected 1 h post irradiation (♦); Panel B: PCA scores plot showing sebum samples from four sham-irradiated rats (♢) and from four 3 Gy-irradiated rats collected 24 h post irradiation (♦).
Bioinformatics – OPLS-DA
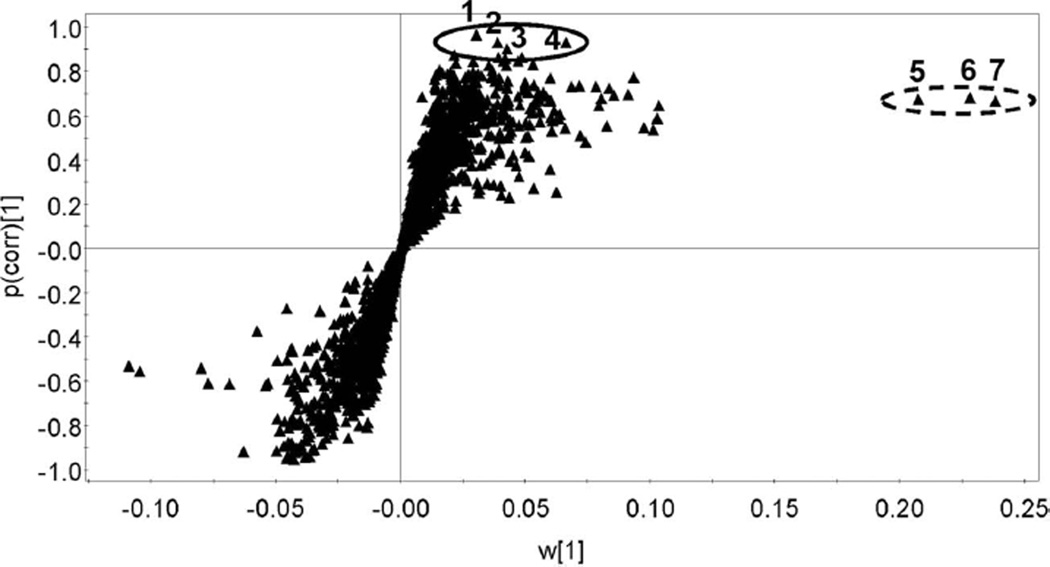
This supervised analysis was used to build a model of the data sets from the GCMS analysis of sebum samples from sham irradiated and 24 h post irradiation rats. An OPLS-DA loadings S-plot displays a measure of the relative abundance or weight (w[1]) of an ion versus its correlation to the model (p(corr)[1]) and thus its significance (Wiklund et al. 2008). Figure 4 shows such an S-plot for the sham versus 24 h post irradiation sebum samples. Two sets of ions were identified for further investigation, four that were highly correlated to the model with p(corr)[1] values from 0.90–0.97 (1–4 in Figure 4) and a second set of three more abundant ions that were less strongly correlated to the model with p(corr)[1] values between 0.67 and 0.68 (5–7 in Figure 4).
Figure 4.
Supervised orthogonal projection to latent structures-discriminant analysis (OPLS-DA) loadings S-plot representing the ions with best fit to the OPLS-DA model (p(corr)[1]) and abundance (w[1]). The solid ellipse encloses four ions (1–4) that are highly correlated to the model (p(corr)[1] > 0.9) and the dotted ellipse encloses three ions (5–7) that are highly abundant but correlate only weakly to the model (p(corr)[1] ≈ 0.7). These ions were further investigated as potential sebum markers of γ-irradiation.
Bioinformatics – Random forests analysis (RF)
RF analysis was also performed on these data according to our previously published procedures (Lanz et al. 2009). Five of the seven markers identified above (Figure 4) were also identified by random forests machine learning algorithm, as the #1 (marker #4), #2 (marker #1), #3 (marker #2), #12 (marker #3), and #31 (marker #5) most important ions contributing to the difference between sham irradiated and 24 h post sham sebum samples. Details of these seven markers are given in Table II.
Table II.
Identity of markers and the difference in their detection in sham and 24 h post irradiation rat sebum.
| Mean peak area ratio ± standard deviation |
||||||
|---|---|---|---|---|---|---|
| Marker # | RT | m/z | Identity | sham | 24 h | P† |
| 1 | 45.23 | 58 | noise | – | – | – |
| 2 | 36.67 | 87 | not assigned | 0.77±0.05 | 0.83 ±0.05 | 0.13 |
| 3 | 17.32 | 97 | not assigned | 0.79±0.06 | 0.81 ±0.03 | 0.31 |
| 4 | 16.38 | 227 | 2-hydroxypalmitic acid | 2.09±0.24 | 2.18 ±0.07 | 0.27 |
| 5 | 12.70 | 87 | palmitic acid | 27.4±1.8 | 29.7 ±1.1 | 0.03 |
| 7 | 12.70 | 74 | 39.8±2.5 | 42.8 ±1.6 | 0.05 | |
| 6 | 14.11 | 87 | stearic acid | 8.48±0.68 | 8.83 ±0.38 | 0.20 |
P values for one-sided Student’s t-test, as only elevations in biomarkers after irradiation were sought.
Identification of sebum markers of gamma-irradiation
Markers were identified on the basis of their retention times as they appear in Table I. Marker #1 was a late-running signal that on close inspection appeared to be due only to chromatographic noise. Markers #2 and #3 could not be assigned to authentic standards. Marker #4 corresponded to 2-hydroxypalmitic acid, markers #5 and #7 were ions deriving from palmitic acid, and marker #6 corresponded to stearic acid. Analysis of peak areas for these markers in sham and 24 h post irradiation sebum samples revealed small but not statistically significant rises in the sebum content of these fatty acids, although the two ions from palmitic acid (markers #5 and #7) approached statistical significance between the two groups (Table II). Two conclusions should be drawn from these findings. First, both OPLS-DA and RF were able to model noise, because marker #1 in the OPLS-DA analysis and marker #2 in the RF are based upon a nonexistent peak running at 45.23 min with an apparent m/z value of 58. Second, the highest abundance constituents of sebum, for example, palmitic and stearic acids (see Figure 2), showed the greatest statistical significant difference between sham and irradiated rats. Both overmodelling and emphasis on abundant signals are two of the characteristics of this type of analysis. It should be stated that the lack of a major difference in sebum yield and/or composition after gamma-irradiation of rats renders rat sebum not a useful matrix for further investigation in radiation biology. However, because sebum production, composition and function are different between rat and human, it is not possible to make this same judgement at this time regarding human sebum. Until data are produced to the contrary, human sebum remains a potential biological matrix within which to observe the effects of ionising radiation on the skin, especially as it is easy to collect and can be analysed using straightforward methods, as we have reported here.
TBARS in sebum
The calibration curve for MDA release from 1,1,3,3-tetraethoxypropane and determined as TBARS was linear from 0.1–10 mM (R2 =0.998). The accuracy of the assay was found to be good with mean deviations of duplicate analyses from the expected value being 10.4 and 8.2% for control samples of 0.4 and 4 mM, respectively. Mean recoveries determined with duplicate analyses of rat sebum samples spiked with 1.0 and 5.0 µM were 97–118% and 97– 105% for the higher and the lower concentrations, respectively. Duplicate analyses of sebum samples from sham irradiated rats (n=4) gave a TBARS concentration in the back-up sample of 3.87 ±0.76 µM MDA. For sebum samples from rats taken 1 h (n = 4) and 24 h (n = 4) after irradiation the TBARS concentrations in the back-up samples were 3.96 ± 0.59 and 3.51 ±0.71 µM MDA, respectively. Differences between sham and 1 h and sham and 24 h sebum samples were not statistically significant.
Discussion
The case has been made for minimally-invasive high-throughput biomonitoring of radiation exposure (Tyburski et al. 2008, 2009, Patterson et al. 2010, Garty et al. 2010). Proof of concept has already been demonstrated for a metabolomic approach in the mouse with the description of both dose- and time-dependent biomarkers of gamma radiation exposure in the range 1–13 Gy (Tyburski et al. 2008, 2009, Patterson et al. 2010). A device for the detection of metabolomic biomarkers of radiation exposure, employing differential mobility spectrometry-mass spectrometry (DMS-MS), that could be adapted to field use, is in advanced stages of development (Coy et al. 2010). In the field, it is unlikely that urinary biomarkers would have much utility. Urine gives an average of metabolic activity over several hours compared to blood, for example, which provides a snapshot in time. Nevertheless, harvesting of urine samples, under conditions where a terrorist attack has occurred, may not be ideal. Sebum, in contrast, is a readily accessible and collectable biofluid, with little or no invasion. For this and other reasons that will become apparent, we have investigated the potential of sebum as a source of metabolomic biomarkers of radiation exposure.
A sebum collection, extraction, methylation, and GCMS analysis protocol is described herein for both the rat and humans. The lipidomic analysis chosen focuses on the free fatty acid and glycerol ester fractions because these fractions were reported 60 years ago to be the major constituents of human sebum (Mackenna et al. 1950). Moreover, detailed analyses of fatty acid composition of human (Boughton and Wheatley 1959) and rodent (Wheatley and James 1957) sebum were also reported long ago. The quantitative pattern of fatty acids in rat sebum (Table I) was broadly similar to that already described in 1957 (Wheatley and James 1957), where the order of importance was 16:0 > branched-chain 18:0 = 18:1 >18:0. Cholesterol was also a major constituent of rat sebum in these early studies (Wheatley and James 1957). Both qualitatively and semi-quantitatively, the findings on sebum fatty acid composition reported here are in agreement with the published literature.
The principal aim of this investigation was to determine what constituents, if any, of rat sebum were elevated after a dose of 3 Gy of gamma radiation. The dose selected was in the dose range where adequate and rapid medical intervention makes survival of the subjects possible and is therefore the most important dose range to be identified in a nuclear event with the highest efficiency of intensive medical care. This dose range is reported to be 2.0–8.0 Gy for humans. Lower doses do not require medical treatment and with higher doses survival will not be probable even with intensive medical care (McFee 2009). For rats the dose of whole-body γ-irradiation needed to kill by 30 days 50% of the animals exposed is reported to be 3.0–7.8 Gy, depending on the susceptibility of the rat strain (Hayashi et al. 1993). For Wistar rats, a considerably higher dose was needed to kill 50% of the animals by 60 days and was reported to be 10.8 Gy (Sato et al. 1999). In addition, this dose corresponds to the mean estimated lethal dose of whole-body γ-irradiation needed to kill by 60 days 50% of the subjects exposed (3.25–4.0 Gy for humans) and is a reasonable assumption of the dose expected during a nuclear event. The dose correlated during the Chernobyl accident in 1986 to Grade 1 (mild) acute radiation sickness (ARS) was estimated to be 0.1–3.3 Gy (Belyi et al. 2010).
In order to address the principal aim question we employed bioinformatic methods to mine the GCMS chromatograms of extracted and methylated sebum samples that had been collected from sham irradiated and gamma-irradiated rats. An unsupervised PCA gave no resolution of the sham and 1 h post-irradiation samples, but gave a clear separation of the sham and 24 h post-irradiation sebum samples (Figure 3). Further refinement of the separation was made by modelling with OPLS-DA, where the loadings S-plot (Figure 4) clearly showed two groups of ions that were upregulated 24 h after gamma radiation with 3 Gy. Interestingly, three of the four ions that could be assigned were highly abundant ions deriving from palmitic acid (74 and 87 m/z, 12.70 min) and stearic acid (87 m/z, 14.11 min). It should be pointed out that 74 and 87 m/z are the two largest ions in the spectrum of methyl palmitate and 87 m/z is the second biggest ion in the spectrum of methyl stearate. The sebum response to gamma radiation appears to be barely statistically significant (P= 0.03, 0.05) elevation in the two most abundant ions (87 and 74 m/z, respectively) derived from the most abundant fatty acid found in sebum and reported elsewhere (Wheatley and James 1957). It is eminently possible that a more statistically significant result may have been obtained if more animals had been studied in each group, but the meagre elevation in signal for palmitic acid (+7.5% for 74 m/z, and +8.4% for 87 m/z) renders sebum fatty acids a ineffectual metabolomic biomonitor of radiation effect.
It could be suggested that changes in levels of free and esterified saturated, monounsaturated, polyunsaturated, branched-chain, or hydroxy fatty acids are not the most likely candidates for the effects of gamma radiation on sebaceous gland and skin biochemistry. Perhaps candidates such as isoprostanes and other lipid peroxidation products (Moore and Roberts 1998) would better represent the effects of ionising radiation on the skin lipidome of rodents and humans. With the exception of fatty aldehydes (see Table I), the determination of discrete lipid peroxidation products falls beyond the scope of this report. Our previous radiation metabolomics reports (Tyburski et al. 2008, 2009, Lanz et al. 2009) were predicated on the concept that ionising radiation causes radiolysis of cellular water molecules and thereby generates reactive oxygen species (ROS). There is empirical evidence that ionising radiation elicits major biological changes that damage the skin. In a 22-year follow-up of 190 persons who were exposed as a result of the Chernobyl accident in April 1986, 91 had confirmed acute radiation sickness (ARS), 38 with Grade 1 ARS (mild), 41 with Grade 2 ARS (moderate), and 12 with Grade 3 ARS (severe) (Belyi et al. 2010). Their corresponding exposures to gamma and beta radiation were estimated to be 0.1–3.3, 0.5–4.9, and 2.9–7.1 Gy, respectively (Belyi et al. 2010). Skin lesions were observed in 27 subjects, including three with severe acute radiation skin lesions involving keratosis and ulceration (Belyi et al. 2010). The mechanisms of these pathologies remain unknown, but it is reasonable to propose that ROS in the skin was in some way involved since ionising radiation increases mitochondria-dependent generation of ROS (Xiao and Whitnall 2009) which may be further aggravated by the accumulation of radiation-induced mitochondrial DNA mutations (Prithivirajsingh et al. 2004). Moreover, gamma irradiation of fibroblasts with up to 8 Gy, and skin microvascular endothelial cells with up to 10 Gy, generates high levels of ROS (Davis et al. 2009). Finally, 20 Gy gamma irradiation of human keratinocytes leads to a down-regulation after 24 h of enzymes involved in the control of oxidative stress, such as catalase, glutathione peroxidase, and superoxide dismutase activities (Isoir et al. 2006). Thus, for a multitude of reasons, one might expect ROS generation in the skin of rats irradiated with 3 Gy. Accordingly, the level of TBARS was determined in the sebum collected from sham-irradiated rats and from rats 1 h and 24 h post gamma irradiation. However, no statistically significant increase in sebum TBARS was observed at either time point after irradiation, suggesting that little or no lipid peroxidation occurred in or around the sebaceous glands.
It is possible that the failure to detect post-irradiation changes in neutral lipids and lipid peroxidation products is due to some flaw in our study design. We paid careful attention to the prevention of chemical degradation of reactive intermediates formed by the reaction of sebaceous lipids with ROS. Excess antioxidant BHT was present from the point of sebum collection through to storage of acetone extracts at −80°C. At no time were sebum solutions allowed to rise above body temperature. Clearly, our sebum collection and storage protocol preserved TBARS, but levels of these lipid peroxidation products in collected sebum did not alter after gamma irradiation of rats.
On the other hand, it cannot be excluded that higher radiation doses would lead to more pronounced and significant effects, which could be determined applying this protocol. The radiation dose needed to cause radiation damage is known to be highly tissue specific (McFee 2009). It can be speculated that the skin lipidome is more radio-resistant than the urinary metabolome of rats investigated in our previous study with the same dose (Lanz et al. 2009). Furthermore, the dose dependency of changes in the metabolic fingerprint has been clearly demonstrated in studies investigating the urinary mouse metabolome in the range 1–13 Gy (Tyburski et al. 2008, 2009, Patterson et al. 2010).
Despite the attractiveness of the concept that simple swabbing of the human nose might be used for radiation biomonitoring in a high-throughput context, sebum and/or superficial skin lipids would not appear to be a repository for biomarkers of radiation. Nevertheless, a protocol for the collection, extraction, derivatisation and GCMS analysis of both rat and human sebum is presented here.
The rat sebaceous gland appears to be largely refractory 1 h and 24 h after 3 Gy gamma-irradiation. This is clearly not the case for other structures in the skin, such as hair follicles, which are readily damaged by gamma-irradiation, as every radiotherapy patient knows. Additionally, the hind foot sweat glands of the mouse have been reported to display complete loss of function eight weeks after a single local dose of 13 Gy (Johns et al. 1995). Similarly, radiotherapy patients receiving 2 Gy fractions to the chest up to 46 Gy showed a decline in skin conductance, as a measure of sweat gland function, in relation to administered dose (Pigott et al. 2000). In a 3-D human skin model, micronuclei could be detected in single cells from doses as low as 0.1 Gy of 3.5 MeV protons (Schettino et al. 2010). What is clear is that the skin is an organ sensitive to ionising radiation. Both clinical and laboratory experience underscores this view. However, an important feature of the skin, in particular on the face and scalp, is the sebaceous glands that produce an oily secretion that lubricates, waterproofs and protects the skin. Perhaps due to their highly hydrophobic environment and thus little option for radiolysis of water molecules to yield ROS, these skin structures appear to be acutely resistant to gamma-irradiation in the rat.
Acknowledgements
The authors wish to thank Professor Bernhard Lauterburg in Bern for his support of this work and helpful discussions and Hans Sa¨gesser for producing the stainless steel cradle for collection of rat sebum. This work was performed as part of the Columbia University Center for Medical Countermeasures against Radiation (P.I. David Brenner) and funded by NIH (NIAID) grant U19 AI067773-05/-06. JRI is grateful to U.S. Smokeless Tobacco Company for a grant for collaborative research.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Belyi D, Kovalenko A, Bazyka D, Bebeshko V. Non-cancer effects in acute radiation syndrome survivors in Ukraine. Health Physics. 2010;98:876–884. doi: 10.1097/HP.0b013e3181d270e4. [DOI] [PubMed] [Google Scholar]
- Boughton B, Wheatley VR. Studies of sebum. Further studies of the composition of the unsaponifiable matter of human-forearm ‘sebum’. Biochemical Journal. 1959;73:144–149. doi: 10.1042/bj0730144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton B, Mackenna RM, Wheatley VR, Wormall A. Studies of sebum. VIII. Observations on the squalene and cholesterol content and the possible functions of squalene in human sebum. Biochemical Journal. 1957;66:32–38. doi: 10.1042/bj0660032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton B, Mackenna RM, Wheatley VR, Wormall A. The fatty acid composition of the surface skin fats (‘sebum’) in acne vulgaris and seborrheic dermatitis. Journal of Investigative Dermatology. 1959;33:57–64. doi: 10.1038/jid.1959.122. [DOI] [PubMed] [Google Scholar]
- Boughton B, Hodgson-Jones IS, Mackenna RM, Wheatley VR, Wormall A. Some observations of the nature, origin and possible function of the squalene and other hydrocarbons of human sebum. Journal of Investigative Dermatology. 1955;24:179–189. doi: 10.1038/jid.1955.30. [DOI] [PubMed] [Google Scholar]
- Cheng JB, Russell DW. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. Journal of Biological Chemistry. 2004a;279:37789–37797. doi: 10.1074/jbc.M406225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JB, Russell DW. Mammalian wax biosynthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. Journal of Biological Chemistry. 2004b;279:37798–37807. doi: 10.1074/jbc.M406226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie WW. 3rd ed. Bridgewater, UK: The Oily Press; 2003. Lipid analysis. Isolation, separation, identification and structural analysis of lipids. [Google Scholar]
- Coy SL, Krylov EV, Schneider BB, Covey TR, Brenner DJ, Tyburski JB, Patterson AD, Krausz KW, Fornace AJ, Nazarov EG. Detection of radiation-exposure biomarkers by Differential Mobility Prefiltered Mass Spectrometry (DMS-MS) International Journal of Mass Spectrometry. 2010;291:108–117. doi: 10.1016/j.ijms.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GD, Masilamoni JG, Arul V, Kumar MS, Baraneedharan U, Paul SF, Sakthivelu IV, Jesudason EP, Jayakumar R. Radioprotective effect of DL-alpha-lipoic acid on mice skin fibroblasts. Cell Biology and Toxicology. 2009;25:331–340. doi: 10.1007/s10565-008-9087-5. [DOI] [PubMed] [Google Scholar]
- Eder K. Gas chromatographic analysis of fatty acid methyl esters. Journal of Chromatography B Biomedical Applications. 1995;671:113–131. doi: 10.1016/0378-4347(95)00142-6. [DOI] [PubMed] [Google Scholar]
- Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, Bertucci A, Xu Y, Wang H, Simaan N, et al. The RABIT: A rapid automated biodosimetry tool for radiological triage. Health Physics. 2010;98:209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Endoh D, Kon Y, Yamashita T, Sato F, Kasai N, Namioka S. Hypersensitivity of LEC strain rats in radiation-induced acute bone-marrow death. Journal of Veterinary Medical Science. 1993;55:13–18. doi: 10.1292/jvms.55.13. [DOI] [PubMed] [Google Scholar]
- Isoir M, Buard V, Gasser P, Voisin P, Lati E, Benderitter M. Human keratinocyte radiosensitivity is linked to redox modulation. Journal of Dermatological Science. 2006;41:55–65. doi: 10.1016/j.jdermsci.2005.11.008. [DOI] [PubMed] [Google Scholar]
- James AT, Wheatley VR. Studies of sebum. 6. The determination of the component fatty acids of human forearm sebum by gas-liquid chromatography. Biochemical Journal. 1956;63:269–273. doi: 10.1042/bj0630269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns H, Morris WJ, Joiner MC. Radiation response of murine eccrine sweat glands. Radiotherapy and Oncology. 1995;36:56–64. doi: 10.1016/0167-8140(95)01560-4. [DOI] [PubMed] [Google Scholar]
- Lanz C, Patterson AD, Slavik J, Krausz KW, Ledermann M, Gonzalez FJ, Idle JR. Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography-mass spectrometry combined with random forests machine learning algorithm. Radiation Research. 2009;172:198–212. doi: 10.1667/RR1796.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenna RM, Wheatley VR, Wormall A. The composition of the surface skin fat (‘sebum’) from the human forearm. Journal of Investigative Dermatology. 1950;15:33–47. doi: 10.1038/jid.1950.69. [DOI] [PubMed] [Google Scholar]
- Mackenna RM, Wheatley VR, Wormall A. Some constituents of the unsaponifiable fraction of human sebum. Biochemical Journal. 1951;48:xxxviii. [PubMed] [Google Scholar]
- Mackenna RM, Wheatley VR, Wormall A. Studies of Sebum. II. Some constituents of the unsaponifiable matter of human sebum. Biochemical Journal. 1952;52:161–168. doi: 10.1042/bj0520161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenna RM, Wheatley VR, Wormall A. Squalene and other hydrocarbons in human sebum. Journal of Physiology. 1955;127:36P–37P. [PubMed] [Google Scholar]
- McFee RB. Radiation terrorism. In: Ballantyne B, Marrs T, Syversen T, editors. General and applied toxicology. 3rd ed. Chichester, UK: John Wiley and Sons Ltd; 2009. [Google Scholar]
- Moore K, Roberts LJ., 2nd Measurement of lipid peroxidation. Free Radical Research. 1998;28:659–671. doi: 10.3109/10715769809065821. [DOI] [PubMed] [Google Scholar]
- Patterson AD, Lanz C, Gonzalez FJ, Idle JR. The role of mass spectrometry-based metabolomics in medical countermeasures against radiation. Mass Spectrometry Reviews. 2010;29:503–521. doi: 10.1002/mas.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K. On lines and planes of closest fit to systems of points in space. Philosophical Magazine. 1901;2:559–572. [Google Scholar]
- Pigott KH, Dische S, Vojnovic B, Saunders MI. Sweat gland function as a measure of radiation change. Radiotherapy and Oncology. 2000;54:79–85. doi: 10.1016/s0167-8140(99)00157-7. [DOI] [PubMed] [Google Scholar]
- Prithivirajsingh S, Story MD, Bergh SA, Geara FB, Ang KK, Ismail SM, Stevens CW, Buchholz TA, Brock WA. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Letters. 2004;571:227–232. doi: 10.1016/j.febslet.2004.06.078. [DOI] [PubMed] [Google Scholar]
- Robosky LC, Wade K, Woolson D, Baker JD, Manning ML, Gage DA, Reily MD. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. Journal of Lipid Research. 2008;49:686–692. doi: 10.1194/jlr.D700035-JLR200. [DOI] [PubMed] [Google Scholar]
- Sato K, Ichimasa M, Miyahara K, Shiomi M, Nishimura Y, Ichimasa Y. J Radiation Research. Vol. 40. Tokyo: 1999. Radioprotective effects of sodium tungstate on hematopoietic injury by exposure to 60Co gamma-rays in Wistar rats; pp. 101–113. [DOI] [PubMed] [Google Scholar]
- Schettino G, Johnson GW, Marino SA, Brenner DJ. Development of a method for assessing non-targeted radiation damage in an artificial 3D human skin model. International Journal of Radiation Biology. 2010;86:593–601. doi: 10.3109/09553001003734535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavik J, Fornace AJ, Jr, Gonzalez FJ, Idle JR. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiation Research. 2008;170:1–14. doi: 10.1667/RR1265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavík J, Fornace AJ, Gonzalez FJ, Idle JR. Radiation metabolomics. 2. Dose-and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice. Radiation Research. 2009;172:42–57. doi: 10.1667/RR1703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley VR. Further studies on the composition of sebum; sebum-like materials of human origin and the sebum of certain animals. Biochemical Journal. 1953;53:xxi. [PubMed] [Google Scholar]
- Wheatley VR, James AT. Studies of sebum. 7. The composition of the sebum of some common rodents. Biochemical Journal. 1957;65:36–42. doi: 10.1042/bj0650036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Analytical Chemistry. 2008;80:115–122. doi: 10.1021/ac0713510. [DOI] [PubMed] [Google Scholar]
- Xiao M, Whitnall MH. Pharmacological countermeasures for the acute radiation syndrome. Current Molecular Pharmacology. 2009;2:122–133. doi: 10.2174/1874467210902010122. [DOI] [PubMed] [Google Scholar]