Abstract
BACKGROUND
Human cytomegalovirus (CMV) infection is associated with inferior survival in renal transplant patients, and ganciclovir (GCV) prophylaxis is associated with improved survival. In a murine CMV (MCMV) renal transplant model, GCV prophylaxis improved innate infiltrates and allograft damage during the period of prophylaxis. In this study, late effects were examined after discontinuation of prophylaxis.
METHODS
MCMV D+/R− and D−/R− allogeneic transplants were performed with cyclosporine immunosuppression. One D+/R− cohort received GCV prophylaxis for 14 days post-transplant, followed by 28 days without GCV. At 42 days post-transplant, grafts were analyzed for histologic tissue damage and immune infiltrates. Another D+/R− cohort was treated with anti-NK1.1 antibodies for 14 days post-transplant and compared to animals without NK depletion.
RESULTS
At day 42, MCMV infected transplants had higher damage scores (15.6+/−0.6) compared to uninfected transplants (8.3+/−0.9) (p<0.01), which improved in GCV treated allografts (9.5+/−1.4). MCMV infected grafts contained greater frequencies of natural killer (NK) cell and myeloid infiltrates compared to uninfected grafts (p<0.05), which decreased in the GCV treated grafts. NK depletion improved allograft histology of MCMV infected grafts.
CONCLUSIONS
MCMV infection exacerbates late renal allograft damage and is associated with NK and myeloid cell infiltrates. GCV prophylaxis reduces allograft injury, NK cell and myeloid infiltrates even after cessation of prophylaxis. NK depletion in MCMV infected transplants also improves histology. These results suggest that GCV prophylaxis may have a long-term beneficial effect upon CMV infected renal allografts, and suggest a potential role for NK cells in the pathogenesis of CMV associated allograft injury.
Keywords: cytomegalovirus, kidney, transplantation, ganciclovir
INTRODUCTION
Human cytomegalovirus (HCMV) infection is associated with adverse direct and indirect effects in renal transplantation ranging from acute rejection to inferior long-term allograft outcome (1–4). Ganciclovir (GCV) antiviral prophylaxis has been correlated with improved survival in clinical studies (5). In animal models, murine CMV (MCMV) and rat CMV (RCMV) accelerate both acute and late kidney transplant rejection (6–10).
Prior studies in animal models have demonstrated accelerated and intensified recruitment of leukocytes into CMV infected renal allografts compared to uninfected grafts. Early lymphocytic infiltration is described in both uninfected and CMV infected grafts, consistent with an allogeneic response, but is more abundant in the CMV infected animals, suggesting a potential role for antiviral lymphocyte activation. Mononuclear infiltrates are recruited preferentially to CMV infected grafts compared to uninfected grafts at both early and late times post-transplant, as well as NK cells, Gr-1+ myeloid cells, and antigen-presenting CD11c+ cells at early times post-transplant (6, 7, 9, 10). CMV infection is also associated with augmented induction of adhesion molecules, lymphoid activation markers, pro-inflammatory chemokine profiles, and fibrogenic molecules within infected allografts.
In cardiac and renal transplant models of CMV infection, treatment with GCV improves histopathologic manifestations of rejection (10, 11). In the cardiac allograft model, 30 days of GCV prophylaxis improves cardiac allograft vasculopathy at 90 days post-transplant. GCV prophylaxis in the renal allograft model also is associated with decreased graft infiltration by myeloid, antigen-presenting, mononuclear, and NK cells compared to untreated CMV infected grafts. Late effects of GCV prophylaxis upon renal allograft histology have not been previously examined in the animal model. We undertook the following study to investigate whether short-course (14 days) GCV prophylaxis affects late (42 day) histology and inflammation using the murine renal transplant model.
RESULTS
Late allograft histology is improved by GCV prophylaxis
MCMV infected BALB/c kidneys were transplanted into MCMV naïve C57BL/6 mice (D+/R− combination) with cyclosporine immunosuppression. Control transplants were performed between uninfected donors and recipients (D−/R−). For the experimental GCV prophylaxis group, D+/R− animals were treated with GCV for 14 days starting immediately post-transplant. Animals were sacrificed at day 42 and organs harvested for pathologic and flow cytometric analysis. Pathology was analyzed by a veterinary pathologist blinded to sample identity. Histology was scored according to a grading scale devised by the pathologist, based upon criteria used for grading of clinical renal transplant biopsies and histopathologic features of renal allograft rejection in rodents (9, 12–14).
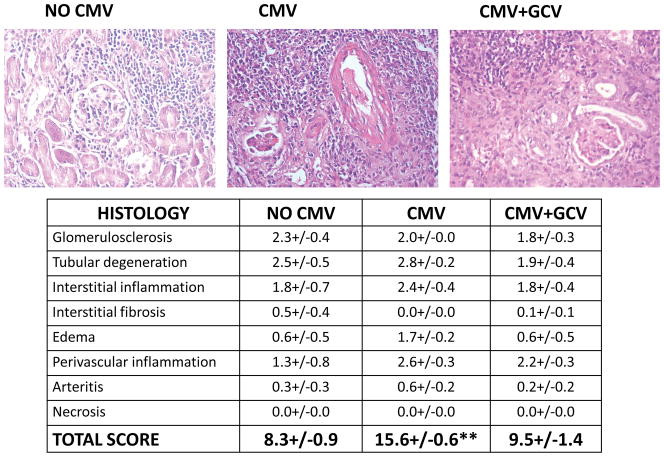
At day 42 post-transplant, MCMV infected allografts were more severely damaged than control uninfected grafts (Figure 1). The total histology score for uninfected grafts was 8.3+/−0.9, whereas the score for MCMV infected grafts was 15.6+/−0.6 (p<0.01). The greatest differences were seen in categories of tubular degeneration, interstitial inflammation, edema, and perivascular inflammation. Also notable was the global disruption of cytoarchitecture observed in MCMV infected grafts, compared to a patchy distribution of inflammation and tissue destruction in the uninfected grafts, in which areas of relatively preserved glomeruli and tubules were still observable. MCMV infected grafts receiving GCV prophylaxis showed significantly less severe histologic injury compared to MCMV infected grafts without prophylaxis, with a total histology score of 9.5+/−1.4 (p<0.01). GCV treated grafts had a histologic appearance more similar to uninfected grafts than infected grafts including the patchy distribution of leukocytic infiltrates in a peritubular distribution, and the focality of tissue damage with interspersed preservation of glomeruli and tubules; however, GCV treated grafts did contain areas of perivascular leukocytic infiltrates resembling those in MCMV infected grafts without GCV prophylaxis.
Figure 1. Improvement of histologic features in MCMV-infected allografts after GCVprophylaxis.
At day 42 post-transplant, uninfected renal allografts (No CMV), MCMV infected grafts (CMV) and MCMV infected grafts with GCV prophylaxis (CMV+GCV) were analyzed for histologic damage using a grading scale resembling that used for human renal transplant biopsies. Criteria were graded from 0 (none) to 3 (severe). Total score for CMV grafts was significantly different (** p<0.01) from scores of both uninfected and GCV treated grafts.
MCMV infection induces NK and myeloid infiltrates which are diminished with GCV prophylaxis
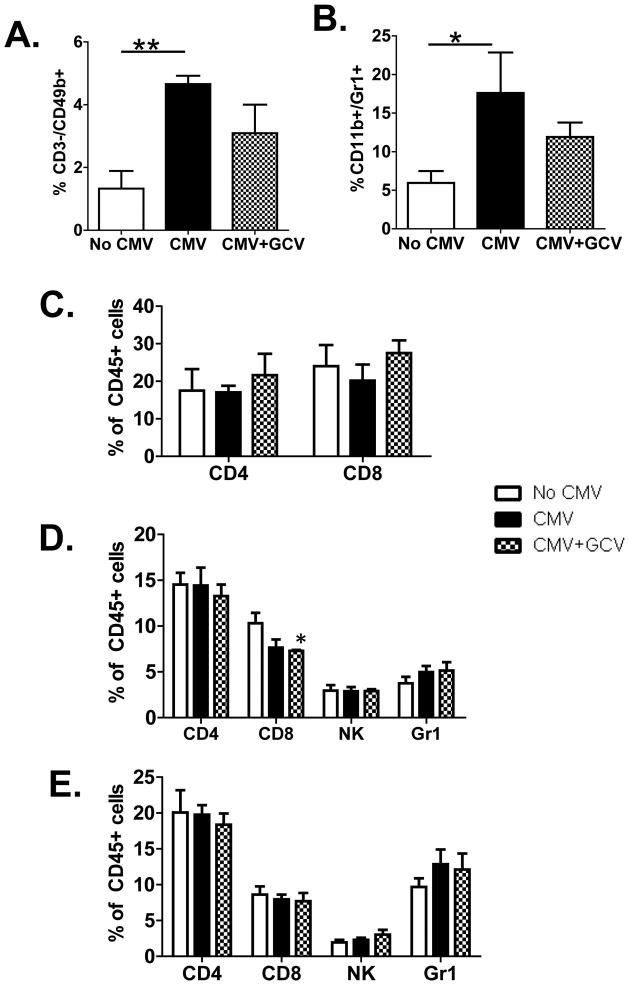
To define the nature of the leukocyte infiltrates, flow cytometric analysis of allograft infiltrating CD45+ leukocytes was performed. NK cells were quantitated as CD45+/CD3−/CD49b+ cells and compared between experimental groups (Figure 2A). MCMV infected grafts contained significantly greater frequencies of NK cells compared to uninfected grafts (p<0.01). GCV treated grafts exhibited a reduction in NK infiltrates towards levels displayed by uninfected grafts, such that the NK frequency became statistically similar to both the uninfected and MCMV infected grafts. In addition, the frequency of myeloid cells expressing the surface markers CD45, CD11b, and Gr-1 (Figure 2B) were also statistically greater in MCMV infected grafts compared to uninfected grafts, with diminution in GCV treated grafts toward frequencies statistically similar to uninfected grafts (p>0.05). In contrast, the frequencies of CD4+ and CD8+ T lymphocytes (Figure 2C) in the CD45+ leukocyte population did not show statistically significant differences between uninfected, MCMV infected, and GCV treated grafts at day 42 post-transplant. These results resemble those found in allografts at 14 days during GCV prophylaxis (10), with an induction of NK and myeloid infiltrates in MCMV infected grafts compared to uninfected grafts, and attenuation of these infiltrates (but not T cells) by GCV treatment. These results indicate that the reduction in NK and myeloid cells in the GCV treated grafts is durable beyond the period of antiviral prophylaxis.
Figure 2. MCMV-induced infiltration of NK and myeloid cells decreases after GCVprophylaxis.
(A–C) CD45+ cells from allografts at day 42 post-transplant were analyzed by flow cytometry for (A) NK cells (CD3−/CD49b+ cells), (B) myeloid cells (CD11b+/Gr-1+ cells), and (C) CD4+ and CD8+ T lymphocytes. CMV infected grafts showed significantly greater NK (** p<0.01) and myeloid (* p<0.05) infiltrates than uninfected grafts, and GCV prophylaxis decreased these infiltrates to levels statistically indistinguishable from uninfected grafts. The CD4+ and CD8+ lymphocyte frequencies were not significantly different between groups.
(D, E) The CD45+ cells in spleen (D) and liver (E) were analyzed for frequencies of CD4+,CD8+, CD3−/CD49b+, and Gr-1+ cells in transplant recipients receiving MCMV- grafts (No CMV), MCMV+ grafts (CMV), and MCMV+ grafts treated with ganciclovir (CMV+GCV). The frequencies of all cell types were similar between all groups, with the exception of splenic CD8+ cells which were statistically fewer in frequency in the spleens of the CMV+GCV group compared to the spleens from the No CMV group (* p<0.05).
Next, systemic immune responses were interrogated by analyzing leukocyte populations in the spleens (Figure 2D) and livers (Figure 2E) from transplant recipients receiving uninfected transplants (No CMV, white bars), MCMV infected transplants (CMV, black bars), and MCMV infected transplants with GCV prophylaxis (CMV+GCV, checkered bars). CD45+ populations were analyzed for frequencies of CD4+, CD8+, CD3−/CD49b+ (NK), and Gr-1+ cells. In the spleen (Figure 2D), only CD8+ cells showed any statistically significantly different results: GCV prophylaxis was associated with a statistically lower frequency of splenic CD8+ cells compared to uninfected transplants (asterisk), but was similar to the MCMV infected recipients without GCV prophylaxis. Frequencies of CD8+ cells in the liver were similar for all experimental groups. No differences in frequencies of CD4+, CD3−/CD49b+, and Gr-1+ cells were found in spleens or livers among the three experimental groups. These results indicate that the differences among the intragraft NK and Gr-1 responses were specific to the allograft and were not manifested in the systemic responses at day 42 post-transplant.
NK depletion improves MCMV associated allograft injury
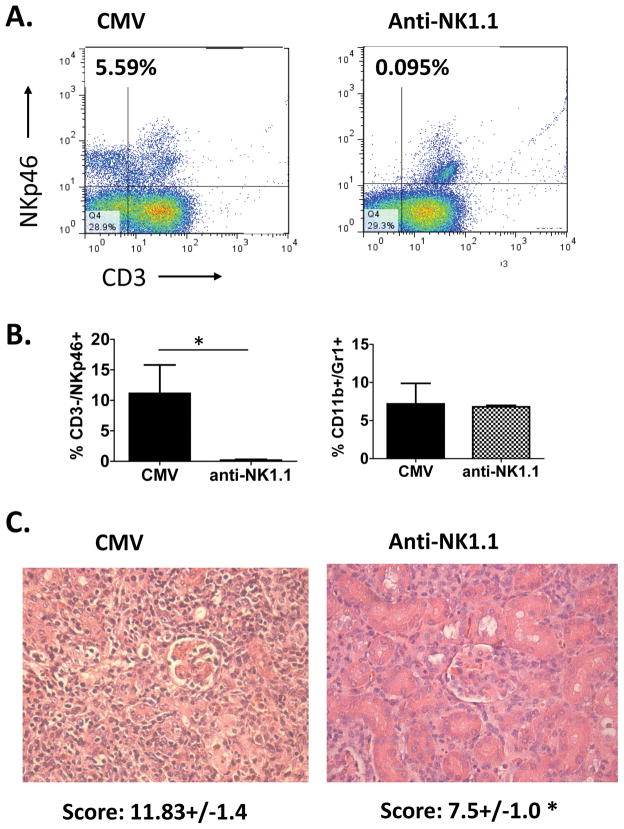
Next, to examine whether the quantity of NK infiltrates directly influences the degree of graft injury in MCMV infected allografts, NK cells were depleted from D+/R− MCMV infected transplants via treatment with anti-NK1.1 antibodies at days 0 and 7, and histology was analyzed at day 14 post-transplant. Animals undergoing NK depletion remained healthy, similar to animals not receiving anti-NK1.1 antibodies, and had no clinical symptoms of illness from viral infection. Efficacy of depletion was confirmed by flow cytometric analysis for CD45+/CD3−/NKp46+ cells (Figure 3A, B), which showed virtually complete absence of NK cells (Figure 3B, left panel, checkered bar) in anti-NK1.1 treated allografts at day 14 post-transplant (7 days after last antibody treatment) compared to untreated allografts which showed robust infiltration of NK cells (Figure 3B, left panel, black bar). Allograft histology showed improved damage scores in anti-NK1.1 treated grafts (total score 7.5+/− 1.0) compared to untreated grafts (total score 11.83+/−1.4) (p<0.05) (Figure 3C). The frequencies of myeloid cells were similar in untreated and NK depleted allografts (Figure 3B, right panel), indicating that NK depletion did not influence the myeloid infiltrates into MCMV infected allografts. These results indicate that direct depletion of NK cells can ameliorate virus associated allograft injury.
Figure 3. Depletion of NK cells results in improved allograft histologic scores.
Recipients of MCMV infected allografts were treated with anti-NK1.1 antibodies to deplete NK cells (anti-NK1.1) and compared to recipients without depletion (CMV) at day 14 post-transplant. (A) NK depletion was confirmed via flow cytometry of allograft infiltrates for CD3−/NKp46+ cells, gated on CD45+ cells. (B) In the left panel, frequencies of CD3−/NKp46+ cells in allograft were significantly lower (* p<0.05) in untreated grafts (CMV) compared to treated grafts (anti-NK1.1). In the right panel, frequencies of myeloid cells were similar in untreated and NK depleted allografts. (C) Histopathologic analysis of allografts showed improved damage scores in NK depleted animals (anti-NK1.1) compared to undepleted animals (CMV) (* p<0.05).
DISCUSSION
This study shows that short-term GCV prophylaxis improves late allograft tissue damage in the murine renal transplant model. MCMV infected grafts without antiviral treatment showed intense leukocytic infiltrates in the peritubular and perivascular areas of cortex and medulla, resulting in global tubular destruction with secondary loss of glomeruli by day 42 post-transplant. In contrast, uninfected grafts demonstrated patchy leukocyte infiltrates located in a peritubular and perivascular distribution, with relative preservation of glomeruli and interspersed with areas of normal tubules without significant peritubular leukocytic infiltration. Flow cytometric analysis suggested that these infiltrates in the uninfected grafts were largely CD4+ and CD8+ lymphocytes, which were also present in the MCMV infected grafts. The major difference in the cell types found in MCMV infected and uninfected grafts was found by flow cytometry to consist of NK cells and Gr-1+ myeloid cells. Analysis of grafts after GCV prophylaxis showed histologic findings resembling the uninfected grafts, with patchy leukocyte infiltrates and areas of relatively preserved tubules and glomeruli, and presence of CD4+ and CD8+ lymphocytes but continued modulation of NK and myeloid infiltrates after GCV prophylaxis. These results are consistent with findings previously described at day 14 post-transplant using this same model, in that GCV prophylaxis did not reduce early CD4+ and CD8+ infiltration into MCMV infected allografts (10), but was associated with reduced NK and Gr-1+ myeloid infiltrates during prophylaxis and a moderate increase in these cell types at 1 week after cessation of GCV prophylaxis (10). It is now shown in the current study that the infiltration of NK and myeloid cells into the infected allograft was still moderated in the grafts receiving GCV prophylaxis, even at late times after cessation of antiviral administration. Systemic splenic and liver responses did not reflect these intragraft differences in NK and myeloid cell recruitment, suggesting that local factors within the allografts might contribute to these differences in intragraft immune responses. The mechanism by which these differences in intragraft leukocyte recruitment are induced remains undefined.
Since GCV prophylaxis was associated with decreased NK infiltrates even after cessation of prophylaxis, the impact of NK cell depletion upon MCMV induced allograft injury was also interrogated. The NK-depleted MCMV infected allografts showed decreased allograft injury despite the concurrent presence of myeloid infiltrates. This result suggests that NK cells may contribute to virus-associated allograft injury by direct or indirect mechanisms. It remains undefined whether GCV prophylaxis directly influences NK cells, or whether the reduction in NK cells serves as a surrogate marker for other functions of antiviral therapy in ameliorating virus-associated graft injury. Further studies could elucidate mechanisms by which antivirals modulate tissue injury in CMV infected allografts, and define more clearly the significance of the NK cells in this pathogenesis.
In this animal model, MCMV infected allografts contained greater NK cell infiltrates compared to uninfected grafts at both early and late times post-transplant. CMV infection is well described to activate NK cells, which are critical for early control of CMV infections (15). In renal transplant patients, peripheral blood NK cells increase during HCMV infection and demonstrate an activated phenotype (16, 17). In clinical biopsies from renal transplant patients, NK cells are found by immunohistochemical staining and transcriptome analysis at early times post-transplant in kidneys which subsequently demonstrate late rejection, suggesting that NK infiltration into allografts may influence late graft outcomes (18, 19). To date, intragraft NK cells have not been examined in context of HCMV infection in clinical populations, and might provide relevant insight into mechanisms of indirect effects attributed to HCMV infection in renal transplant patients.
An alternative explanation for the described findings, not explored in these studies, includes the role of NKT cells. Although interest has been developing in this cell population in renal transplantation, NKT cells have not been well studied in context of CMV infections and transplantation but could constitute a population depleted by the anti-NK1.1 antibody. Future studies may dissect the respective roles of NKT cells from NK cells in MCMV associated graft injury by using this murine renal transplant model prior to analyzing these leukocyte populations in clinical transplantation.
In summary, studies in the murine model demonstrate that MCMV infected renal allografts experience more severe tissue injury at late times post-transplant in association with persistent NK infiltrates. GCV prophylaxis results in decreased NK infiltration and improved allograft histology, even at 28 days beyond cessation of prophylaxis. Direct NK depletion also ameliorates MCMV associated allograft damage. These results indicate that GCV exerts modulatory effects upon late tissue damage in MCMV infected allografts, and suggests a potential role of NK cells in the pathogenesis of MCMV infection in renal transplantation.
MATERIALS AND METHODS
Virus and animals
Murine cytomegalovirus strain Smith with an ORF m157 deletion (MCMVSmithΔm157) was propagated, prepared, and stored as previously described (10). Female BALB/cJ or C57BL/6J mice (Jackson Laboratory, Bar Harbor ME) were maintained in AAALAC-approved animal facilities maintained by the Animal Resources Program of the University of Alabama at Birmingham (Birmingham, AL) under specific pathogen-free (SPF) conditions (health surveillance protocol available at http://main.uab.edu/Sites/ComparativePathology/surveillance/). Mouse maintenance and experimental protocols were approved by the UAB Institutional Animal Care and Use Committee.
Renal transplantation surgery
For MCMV infected transplants, donor BALB/cJ (H-2d) mice were infected by intraperitoneal injection with 104 plaque forming units (pfu) of MCMV SmithΔ157 strain virus at least 12 weeks prior to renal transplantation (D+/R− transplants). Donors without MCMV infection (D−/R− transplant) were used as controls. Allogeneic orthotopic kidney transplantation from donor BALB/cJ mice into recipient C57BL/6J (H-2b) mice (R−) was performed as described (10, 20). The contralateral native kidney of the recipient was left intact, as life-sustaining transplantation was not required for these experiments. Recipients were treated with cyclosporine (Novartis Pharmaceuticals Corp., East Hanover NJ) at 10 mg/kg/day, subcutaneously once daily starting immediately postoperatively for 14 days (21). One experimental group of D+/R− animals was treated with GCV prophylaxis (Roche Laboratories, Nutley NJ) at 15 mg/kg/day, subcutaneously once daily starting immediately postoperatively for 14 days (22). A separate experimental group of D+/R− recipients was treated with anti-NK1.1 antibodies (clone PK136 [eBioscience, San Diego CA]) at 200 μg/dose intraperitoneally at day 0 and day 7 post-transplant (23). Results from 4–5 animals were analyzed per experimental group.
Flow cytometric analysis of allograft immune infiltrates
Organs were harvested at day 14 or day 42 post-transplant, and processed for flow cytometric analysis as described (10). After blocking Fc receptors with anti-mouse CD16/CD32 (clone 93, eBioscience), flow cytometric analysis of single cell suspensions was performed using a combination of the following monoclonal anti-mouse antibodies (eBioscience): FITC- or PE-conjugated CD45 (30-F11), FITC-conjugated Gr-1 (RB6-8C5), FITC- or PerCP-conjugated CD3e (145-2C11), PE-conjugated CD8α (53-6.7), APC-conjugated CD4 (GK1.5), APC-conjugated CD49b (DX5), and FITC-conjugated NKp46 (29A1.4). Flow cytometry studies were performed using a dual laser FACSCalibur and analyzed using FlowJo software (BD Biosciences, San Jose, CA). Results were quantitated as frequencies after gating on live cells expressing the pan leukocyte marker CD45.
Histology and scoring
Allografts were perfused with saline to organ pallor, and portions were fixed for 24 hours in 10% neutral buffered formalin (Sigma, St. Louis MO), processed routinely for paraffin embedding and sectioning, and stained with hematoxylin and eosin (HE). Sections were evaluated by a veterinary pathologist (T.R.S.) blinded to sample identity using a scale devised for this study, based upon the clinical Banff criteria for renal allograft histology scoring as well as scales published for grading of rodent renal allografts (9, 13, 14). The Banff criteria used for patient biopsy grading could not be used directly in this study as the kinetics of rejection in the animal model is more rapid compared to that observed in patients. Criteria in the grading scale included: glomerular changes (primarily sclerosis, and to a lesser extent, proliferation); tubular degeneration, including atrophy and epithelial cell necrosis; interstitial inflammatory cells; interstitial fibrosis; edema; perivascular inflammatory cell accumulation; arteritis (primarily endarteritis with occasional focally necrotizing arteritis); necrosis (foci of complete necrosis of multiple tubules); and capsulitis. Each was scored 0–3 for absent, mild, moderate, or severe, respectively, and individual scores were summed to yield an overall score with a maximum possible value of 27.
Statistical Analysis
All assays were analyzed using 4–5 animals for each experimental group. Groups were analyzed using ANOVA and pairs were compared using the Student’s t-test using Prism 5.0 software, accepting statistically significant differences at a p value of < 0.05 (GraphPad, San Diego CA).
Acknowledgments
This work was supported by NIH 5K08AI059428-02 (M.S.), the Children’s Center for Research and Innovation of the Alabama Children’s Hospital Foundation (M.S.), the Kaul Pediatric Research Initiative of The Children’s Hospital of Alabama (M.S.); the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research, NIH NIDDK 1P30 DK079337 (J.F.G.).; and the UAB Mucosal HIV and Immunobiology Center, NIH NIDDK DK64400.
ABREVIATIONS
- MCMV
murine cytomegalovirus
- GCV
ganciclovir
Footnotes
The authors declare no conflicts of interest.
M.S. conceived and designed the research; participated in the performance of the experiments and data analysis; wrote the manuscript. M.C.S. performed the majority of the experiments; participated in data analysis and manuscript preparation. L.G. performed renal transplant surgeries. U.S. performed experiments, participated in data analysis and manuscript preparation. T.R.S. devised the histologic grading scale and performed blinded analysis of allograft tissue, and critically reviewed the manuscript. J.F.G. and W.J.B participated in research design, data analysis, and manuscript review.
(1) Division of Pediatric Infectious Diseases, 1600 – 6th Avenue South, CHB 308, Birmingham AL 35233; (2) Division of Cardiothoracic Surgery, 1530 – 3rd Avenue South, LHRB 790, Birmingham AL 35294-0007; (3) Department of Genetics, KAUL 724, Birmingham AL 35294-0024; (5) Current address: Department of Internal Medicine D, Rheumatology and Clinical Immunology Unit, University Hospital Münster, Münster, Germany
This work was presented at the 24th International Congress of The Transplantation Society.
References
- 1.Freeman RB., Jr The ‘Indirect’ Effects of Cytomegalovirus Infection. American Journal of Transplantation. 2009;9 (11):2453. doi: 10.1111/j.1600-6143.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 2.Rubin RH, Tolkoff-Rubin NE, Oliver D, et al. Multicenter seroepidemiologic study of the impact of cytomegalovirus infection on renal transplantation. Transplantation. 1985;40 (3):243. doi: 10.1097/00007890-198509000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Gerstenkorn C, Balupuri S, Mohamed MA, et al. The impact of cytomegalovirus serology for 7-year graft survival in cadaveric kidney transplantation--the Newcastle experience. Transplant International. 2000a;13 (S1):S372. doi: 10.1007/s001470050364. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JT, Gallay B, Taranto SE, et al. Pretransplant recipient cytomegalovirus seropositivity and hemodialysis are associated with decreased renal allograft and patient survival. Transplantation. 2004;77 (9):1405. doi: 10.1097/01.tp.0000122184.97674.20. [DOI] [PubMed] [Google Scholar]
- 5.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in Long-Term Renal Graft Survival due to CMV Prophylaxis with Oral Ganciclovir: Results of a Randomized Clinical Trial. American Journal of Transplantation. 2008;8 (5):975. doi: 10.1111/j.1600-6143.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 6.Lautenschlager I, Soots A, Krogerus L, et al. Effect of cytomegalovirus on an experimental model of chronic renal allograft rejection under triple-drug treatment in the rat. Transplantation. 1997;64 (3):391. doi: 10.1097/00007890-199708150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Lautenschlager I, Soots A, Krogerus L, et al. Time-related effects of cytomegalovirus infection on the development of chronic renal allograft rejection in a rat model. Intervirology. 1999;42 (5–6):279. doi: 10.1159/000053961. [DOI] [PubMed] [Google Scholar]
- 8.Inkinen K, Holma K, Soots A, et al. Expression of TGF-beta and PDGF-AA antigens and corresponding mRNAs in cytomegalovirus-infected rat kidney allografts. Transplantation Proceedings. 2003;35 (2):804. doi: 10.1016/s0041-1345(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 9.Soule JL, Streblow DN, Andoh TF, Kreklywich CN, Orloff SL. Cytomegalovirus accelerates chronic allograft nephropathy in a rat renal transplant model with associated provocative chemokine profiles. Transplant Proc. 2006;38 (10):3214. doi: 10.1016/j.transproceed.2006.10.187. [DOI] [PubMed] [Google Scholar]
- 10.Shimamura M, Saunders U, Rha B, et al. Ganciclovir transiently attenuates murine cytomegalovirus-associated renal allograft inflammation. Transplantation. 2011;92 (7):759. doi: 10.1097/TP.0b013e31822c6e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemstrom K, Sihvola R, Bruggeman C, Hayry P, Koskinen P. Cytomegalovirus Infection–Enhanced Cardiac Allograft Vasculopathy Is Abolished by DHPG Prophylaxis in the Rat. Circulation. 1997;95 (12):2614. doi: 10.1161/01.cir.95.12.2614. [DOI] [PubMed] [Google Scholar]
- 12.Mannon RB, Kopp JB, Ruiz P, et al. Chronic rejection of mouse kidney allografts. 1999;55 (5):1935. doi: 10.1046/j.1523-1755.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 13.Solez K, Colvin RB, Racusen LC, et al. Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7 (3):518. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 14.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8 (4):753. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 15.Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52 (1):119. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venema H, Van Den Berg AP, Van Zanten C, Van Son WJ, Van Der Giessen M, Hauw T. Natural killer cell responses in renal transplant patients with cytomegalovirus infection. Journal of Medical Virology. 1994;42 (2):188. doi: 10.1002/jmv.1890420216. [DOI] [PubMed] [Google Scholar]
- 17.van Dam JG, Damoiseaux JG, Christiaans MH, Bruggeman CA. Acute primary infection with cytomegalovirus (CMV) in kidney transplant recipients results in the appearance of a phenotypically aberrant CD8+ T cell population. Microbiol Immunol. 2000;44 (12):1011. doi: 10.1111/j.1348-0421.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 18.Hancock WW, Gee D, De Moerloose P, Rickles FR, Ewan VA, Atkins RC. Immunohistological analysis of serial biopsies taken during human renal allograft rejection. Changing profile of infiltrating cells and activation of the coagulation system. Transplantation. 1985;39 (4):430. doi: 10.1097/00007890-198504000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Vitalone MJ, Naesens M, Sigdel T, Li L, Hseih S, Sarwal MM. The Dual Role of Epithelial-to-Mesenchymal Transition in Chronic Allograft Injury in Pediatric Renal Transplantation. Transplantation. 2011;92 (7):787. doi: 10.1097/TP.0b013e31822d092c. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16 (2):103. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 21.Andoh TF, Gardner MP, Bennett WM. Protective effects of dietary L-arginine supplementation on chronic cyclosporine nephrotoxicity. Transplantation. 1997;64 (9):1236. doi: 10.1097/00007890-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kern ER, Bidanset DJ, Hartline CB, Yan Z, Zemlicka J, Quenelle DC. Oral Activity of a Methylenecyclopropane Analog, Cyclopropavir, in Animal Models for Cytomegalovirus Infections. Antimicrob Agents Chemother. 2004;48 (12):4745. doi: 10.1128/AAC.48.12.4745-4753.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirohashi T, Chase CM, Della Pelle P, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2011;12 (2):313. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]