SUMMARY
To what extent dorsal horn interneurons contribute to the modality specific processing of pain and itch messages is not known. Here we report that loxp/cre-mediated CNS deletion of TR4, a testicular orphan nuclear receptor, results in loss of many excitatory interneurons in the superficial dorsal horn, but preservation of primary afferents and spinal projection neurons. The interneuron loss is associated with a near complete absence of supraspinally-integrated pain and itch behaviors, elevated mechanical withdrawal thresholds and loss of nerve injury-induced mechanical hypersensitivity, but reflex responsiveness to noxious heat, nerve injury-induced heat hypersensitivity and tissue injury-induced heat and mechanical hypersensitivity are intact. We conclude that different subsets of dorsal horn excitatory interneurons contribute to tissue and nerve injury-induced heat and mechanical pain and that the full expression of supraspinally-mediated pain and itch behaviors cannot be generated solely by nociceptor and pruritoceptor activation of projection neurons; concurrent activation of excitatory interneurons is essential.
INTRODUCTION
Pain and itch are unpleasant sensory experiences triggered by noxious and pruritic stimuli, respectively. Pain evokes a withdrawal reflex to avoid potentially harmful stimuli; itch provokes a scratch reflex to counteract the unpleasant sensation. Although there is evidence that pain and itch can arise from stimulation of a common set of unmyelinated afferent fibers (Imamachi et al., 2009), pain and itch are readily distinguished mechanistically (Ikoma et al., 2006). For example, pharmacologically, it is possible to selectively block itch without affecting pain and there is now considerable evidence that the spinal cord circuits that mediate pain and itch can be distinguished (Andrew and Craig, 2001; Han et al., 2012; Liu et al., 2011; Ross et al., 2010; Sun et al., 2009).
A subset of sensory neurons (nociceptors) transmits pain and itch-provoking stimuli from the skin, muscle and internal organs of the body to the dorsal horn of the spinal cord. Here information is conveyed to projection neurons that transmit the information to higher brain centers (Basbaum et al., 2009). Afferent input to the dorsal horn concurrently engages excitatory and inhibitory interneurons that regulate the output of the projection neurons. However, whether afferent-induced activation of projection neurons can drive and sustain behaviors indicative of pain and itch, independently of the regulation exerted by interneuronal circuits, is not known. Also unclear is the extent to which common or different populations of interneurons influence pain and itch. As these interneurons are potential targets for the development of novel therapeutics that can differentially control pain and itch, a better understanding of their contribution is clearly critical.
Orphan nuclear receptors, the endogenous ligands of which have yet to be identified, belong to the nuclear receptor superfamily, which contributes to development, cell differentiation and a host of physiological functions (Evans, 2005). In mammals, the testicular orphan nuclear receptor 4 (TR4) is widely expressed in central and peripheral organs, and its global deletion leads to significant developmental defects (Collins et al., 2004). With a view to specifying the contribution of TR4 in the nervous system, here we report that mice with a selective deletion of TR4 in the CNS using an inducible Cre-dependent deletion approach have a remarkable pain and itch phenotype, which is associated with loss of excitatory interneurons in the superficial dorsal horn of the spinal cord. The mice show dramatically reduced responses in a heat pain test, higher mechanical thresholds, and profound decreases in the pain behaviors produced by noxious chemical stimulation. The mice are also largely unresponsive to different pruritogens. Despite showing reduced pain behaviors that are organized at supraspinal levels, the mice have normal reflex responsiveness to noxious heat and normal tissue injury-induced heat and mechanical hypersensitivity. By contrast, nerve injury-induced mechanical hypersensitivity was lost. Our findings demonstrate not only that there are functionally distinct populations of excitatory interneurons of the superficial dorsal horn, which contribute to modality specificity in the processing of pain and itch messages, but also that activity of these interneurons is essential for the full expression of supraspinally-integrated pain and itch behaviors.
RESULTS
Conditional deletion of TR4 in the CNS
To explore the consequence of TR4 deletion from CNS neurons, we generated mice in which the translation start codon of exons 4 and 5 of the TR4 gene (Nr2c2) was floxed by loxP sites. This construct was linearized and introduced into embryonic stem cells to obtain TR4-floxed chimeric mice (Fig. 1A). Accurate targeting was confirmed by PCR (Fig. 1B). Next, we crossed Nestin-Cre mice (Bates et al., 1999) with the TR4-floxed mice to generate CNS specific conditional knockout (cKO) mice. PCR (Fig. 1C) and RT-PCR (Fig. 1D) in spinal cord and the loss of TR4 immunoreactivity in spinal cord tissue from the mutant mice, compared to its apparently ubiquitous neuronal expression in the spinal cord of wild type (WT) mice, confirmed deletion of the TR4 gene (Fig. 1E).
Fig. 1.
Consistent with findings after global TR4 deletion (Chen et al., 2007; Collins et al., 2004), we found that litters included equal numbers of male and female offspring, but both male and female TR4 cKO mice are ~ 20% smaller in size compared to their WT counterparts (Fig S1A). In contrast to the earlier report (Chen et al., 2005), we found that TR4 cKO mice had no difficulty negotiating an accelerating rotarod (Fig S1B). On the other hand, on average the cKO mice were impaired on the ledge test (Schaefer et al., 2000) (Fig S1C). Although some of the mutant mice remained on the ledge for the 60 sec test period, others did not. It is our impression that the mice did not fall from the ledge, but rather jumped. In fact, taken together with their clear ability to swim (Fig. S6A) and the fact that the number of ventral horn motoneurons marked by ChAT immunoreactivity in WT (Fig. S1D) and cKO (Fig. S1E) mice is comparable (Fig. S1F), we conclude that there are no significant motor abnormalities that could account for the behavioral abnormalities described below.
Profound alteration of pain behavior in the TR4 cKO mice
Next, we assessed the TR4 cKO mice in a battery of behavioral tests of acute and injury-associated persistent pain. The TR4 cKO mice did not differ from WT mice in the Hargreaves (Fig. 2A) and tail immersion reflex withdrawal tests of heat pain sensitivity (Fig. 2B). However, in the hot plate test, which triggers a behavioral response (licking of the paw) that involves both spinal cord and supraspinal processing of pain messages (Langerman et al., 1995; Le Bars et al., 2001), the cKO mice had significantly higher response latencies (Fig. 2C). Many of the animals went to cut-off, which is the maximal response permitted to avoid injury.
Fig. 2.
Compared to WT mice, the TR4 cKO mice have significantly increased reflex withdrawal thresholds in the von Frey test of mechanical pain (Fig. 2D). Also, capsaicin-induced licking/flinching (Fig. 2E) and pain behavior following hindpaw injection of formalin (5.0%) are profoundly reduced in the cKO mice (Fig. 2F). Formalin-evoked Fos expression in the superficial dorsal horn was also decreased, by 44.3% (Fig. 2I), mostly in lamina II, in cKO (Fig. 2H) compared to WT mice (Fig. 2G). Taken together, these results reveal a profound reduction of pain behaviors in response to a variety of noxious stimulus modalities, namely heat, mechanical and chemical, with preservation of the reflex responses provoked by noxious heat.
Nerve injury-induced persistent pain is altered in the TR4 cKO mice
Both tissue and nerve injury induce a prolonged state of mechanical and thermal hypersensitivity, largely due to changes (central sensitization) generated at the level of the spinal cord dorsal horn (Basbaum et al., 2009). These changes are usually manifest as a decreased mechanical withdrawal threshold and decreased withdrawal latency in response to a heat stimulus. Here we induced paw inflammation by injection of Complete Freund’s Adjuvant (CFA) and found that TR4 cKO and WT mice develop comparable heat hypersensitivity (Fig. 2J) in the Hargreaves (reflex) test. Furthermore, although mechanical thresholds at baseline are higher in the cKO mice, these animals did develop mechanical hypersensitivity. The magnitude of the mechanical hyperalgesia was somewhat less than in WT mice and the sensitized threshold in the cKO mice was considerably greater than in their WT littermates (Fig. 2K).
To model nerve injury-induced neuropathic pain, we used the chronic-constriction injury (CCI) as this results in both heat and mechanical hyperalgesia (Bennett and Xie, 1988; Urban et al., 2011). We found that cKO and WT mice developed comparable thermal hyperalgesia, however, the magnitude of the change in the cKO mice decreased slightly by 7 days, compared to WT mice (Fig. 2L). By contrast, although mechanical hyperalgesia was readily observed in the WT mice, this was absent in the TR4 cKO mice (Fig. 2M). In the spared nerve injury (SNI) model of neuropathic pain (Shields et al., 2003) we also found that cKO mice did not develop mechanical hyperalgesia following injury (Fig. 2N). On the other hand, despite the absence of nerve-injury induced mechanical hypersensitivity, there was comparable activation of microglia (assessed using Iba1 labeling) in the dorsal horn ipsilateral to the peripheral nerve injury, in WT (Fig. 2O) and cKO mice (Fig. 2P).
Pruritogen-induced itch is lost in TR4 cKO mice
As noted above, there is evidence that the spinal cord mechanisms underlying pain and itch differ. Here we evaluated the scratching provoked by nape of the neck injection of three different pruritogens, histamine, α-Me-5-HT and chloroquine. Figures 3A–C illustrate that in cKO mice there is an almost complete loss of scratching in response to the three pruritogens, even though these agents trigger itch by activating different populations of unmyelinated afferent (Imamachi et al., 2009; Liu et al., 2009).
Fig. 3.
Alteration of primary afferent terminations in the dorsal horn of TR4 cKO mice
Paralleling the profound behavioral pain deficits in the cKO mice, we observed an aberrant pattern of primary afferent terminations in the superficial dorsal horn. Thus, immunostaining for substance P (SP; Figs. 4A,D,G,H), CGRP (Figs. S2A,B), or TRPV1 (Figs. S2C,D) which marks the peptide population of unmyelinated nociceptors, revealed a significant reduction of the area occupied by nociceptor terminals in the superficial dorsal horn and an associated compaction of the afferent termination in lamina I in cKO compared to WT mice. To quantify these changes, we turned to a horseradish peroxidase-DAB immunocytochemical approach. For SP immunoreactivity (Fig. 4G,H) we recorded a decrease in the area, by 63.7% (Fig. 4I), and a corresponding increase in the immunostaining density (by 56.7%; Fig 4J). These values were calculated after correcting for the 9.4% reduced cross sectional area of the gray matter in the cKO mice. We presume that the compaction reflects a concentration of nociceptor terminals in lamina I, in association with a loss of their excitatory interneuron targets in lamina II (See below). Staining with the lectin IB4, which marks the nonpeptide subpopulation of nociceptors, revealed a comparable compaction (Figs. 4B,E). The abnormal staining is particularly notable at thoracic levels, where the unusually thin band of SP terminal staining in the cKO mice is almost obscured by the IB4 terminals (Fig. S2G,H). In addition, in segments of cervical and lumbar enlargement, there was a notable paucity of IB4 binding in the medial half of the dorsal horn (Figs. S2E,F and Figs. 4B,E).
Fig. 4.

As these anatomical phenotypes could reflect alterations in dorsal root ganglion (DRG) cell numbers, we also examined the DRG in WT and cKO mice. Figure S2I illustrates that there are, in fact, no changes in total number of DRG neurons (L4 and L5) or in the relative expression patterns of markers of subset of DRG neurons (SP, IB4, TRPV1) in cKO vs WT mice. Consistent with this finding, when we crossed the floxed TR4 mice with others that express peripherin-Cre, which is expressed in large numbers of unmyelinated and myelinated primary afferent fibers, we found no effect on pain processing (Figs. S3A–C) or on immunostaining patterns in the dorsal horn (data not shown). We conclude that deletion of TR4 from primary afferents is not responsible for the anatomical or for the functional phenotypes observed after Nestin-Cre mediated deletion. Of course, our finding of preserved nerve-injury induced activation of microglia, despite the loss of mechanical hypersensitivity, supports our contention that nociceptors convey injury inputs normally in the cKO mice. It follows that disruption of mechanically-relevant excitatory interneuron circuits downstream of the microglia must underlie the loss of nerve injury-induced mechanical hypersensitivity
Neuronal loss in the superficial dorsal horn of TR4 cKO mice
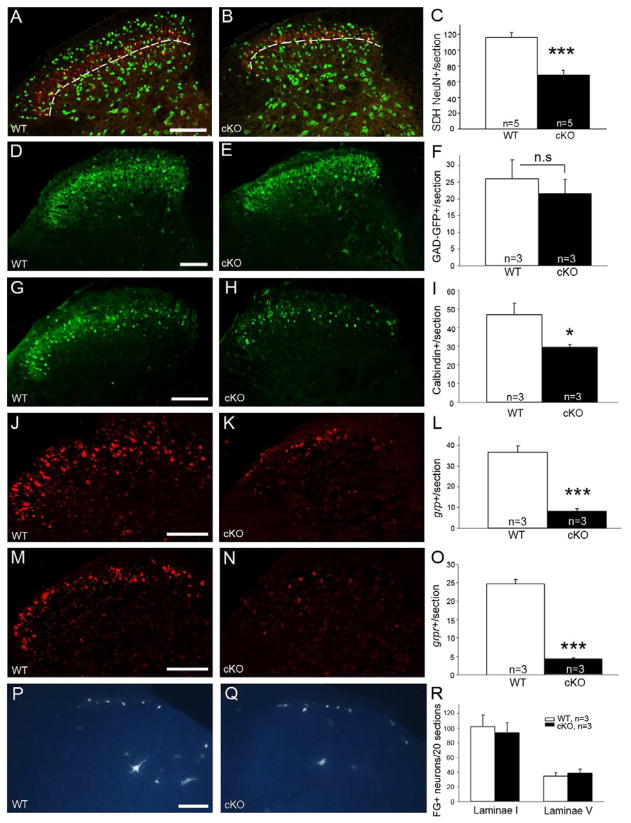
In addition to the altered pattern of SP termination in the superficial dorsal horn, we noted a profound decrease of SP staining in the lateral spinal nucleus (LSN) (Figs. 4A, D,G,H, arrow). As SP terminals in the LSN derive from neurons intrinsic to the spinal cord (Ahn and Basbaum, 2006; Cliffer et al., 1988), rather than from primary afferents, our attention turned to the possibility that the behavioral changes in the cKO resulted from loss of neurons in the superficial dorsal horn and LSN. As the smaller size of the spinal cord made it difficult to generate neuronal density measurements (i.e. numbers of cells per unit area), we developed an alternate strategy. Specifically, as counts of protein kinase C gamma (PKCγ) positive interneurons, which define a ventral border of the superficial dorsal horn (Neumann et al., 2008), did not differ in WT and mutant mice (Figs. S2J–L), we used these neurons to establish the ventral border of the counting region. We immunostained sections with NeuN a neuronal marker and counted all cells dorsal to the band of PKCγ interneurons. This analysis revealed a marked reduction, by 40.6%, in the number of neurons in the cKO mice (Figs. 5A–C). Subsequent counts of all neurons dorsal to the IB4 band revealed an even greater reduction (56.6%) (Figs, S4A–C), indicating that the consequence of TR4 deletion predominates in the most superficial dorsal horn (laminae I and/or outer II). That the defect is limited to the superficial dorsal horn is demonstrated by the fact that counts of neurons in the deep dorsal horn (from a line ventral to the band of PKCγ interneurons to the central canal) did not differ in WT and cKO mice (Fig. S4L).
Fig. 5.
Selective loss of excitatory neurons in the superficial dorsal horn of cKO mice
We next used a variety of markers to identify the missing neurons. To mark the terminals pf presumptive inhibitory interneurons, we immunostained spinal cord tissue for glutamic acid decarboxylase (GAD), the biosynthetic enzyme for GABA. Densitometric analysis revealed that there was no difference in the distribution of GAD-immunoreactive terminals (data not shown). Because cell bodies could not be counted using GAD antibodies, we also crossed the TR4 mice with mice that express GFP under the control of the GAD promoter. Counts of GAD-GFP+ neurons in these cKO:GAD-GFP mice confirmed that there is no change in the numbers of inhibitory interneurons (Figs. 5D–F). On the other hand, we recorded a significant reduction of calbindin-positive interneurons (Figs. 5G–I), which are almost exclusively excitatory in the spinal cord (Antal et al., 1991). Furthermore, using in situ hybridization, we localized mRNA for gastrin-releasing peptide (GRP) and gastrin-releasing peptide receptor (GRPR), which marks presumptive excitatory interneurons in circuits essential for triggering itch, and observed a 76.6% decrease of GRP-positive cells (Figs. 5J–L) and an 83% decrease of GRPR-positive cells in the cKO mice (Figs. 5M–O). Superficial dorsal horn reelin-expressing interneurons, which we implicated in nociceptive processing (Akopians et al., 2008; Villeda et al., 2006), were also affected. Specifically, reeler mRNA (Figs. S4D–F) and reelin-immunoreactive neurons (Figs. S4G–I) were decreased by 68.4 and 75.4%, respectively, in the superficial dorsal horn in cKO mice. Finally, Type 2 vesicular glutamate transporter (VGlut2) immunoreactivity, a general marker of excitatory terminals, was profoundly decreased in the superficial dorsal horn of cKO mice (Figs. S4J,K). There also appears to be decreased VGlut2 immunoreactivity ventral to lamina II, however, as we found no difference in the number of neurons in the deep dorsal horn (Fig S4L), we suggest that this difference reflects loss of the ventral arborization of some glutamatergic interneurons in the superficial dorsal horn (Todd et al., 2003), including the presumptive excitatory vertical cells (Grudt and Perl, 2002).
Projection neurons are spared in the cKO mice
To determine whether projection neurons are included among those missing in the cKO mice, we made injections of the retrograde tracer, Fluorogold (FG), into two major supraspinal targets of dorsal horn projection neurons, the parabrachial nucleus of the dorsolateral pons and the ventroposterolateral thalamus. We made large injections that encompassed these regions so as to reduce variability among animals. Counts of retrogradely labeled neurons revealed no differences in the number of FG-positive projection neurons, either in laminae I, V or the lateral spinal nucleus. Consistent with this finding, the number of neurokinin 1 receptor-immunoreactive (NK1R) neurons in lamina I in L4 and L5 segments, was comparable in WT and cKO mice (Figs. 5P–R and Fig. S5). As the NK1R-expressing neurons represent the great majority of projection neurons in lamina I (Todd et al., 2000), we conclude that deletion of the TR4 gene results in a significant decrease in the number of excitatory interneurons in the superficial dorsal horn, with preservation of inhibitory interneurons and projection neurons. The changes are concentrated dorsal to the band of PKCγ-expressing excitatory interneurons, which are not affected by TR4 deletion.
Decreased noxious stimulus-induced responsiveness of presumptive dorsal horn projection neurons in cKO mice
We can envision several consequences of the profound loss of dorsal horn excitatory interneurons. Noxious stimulus-evoked activity of the projection neurons and of the spared interneurons could be equivalent in the cKO and WT mice. This scenario seems unlikely, as it would provide sufficient noxious stimulus-evoked activity to engage the the projection neurons and their supraspinal targets that are required for the full expression of pain behaviors. Alternatively, activity of the surviving neurons could persist, but intensity coding of the projection neurons could be reduced to an extent that supraspinally-mediated pain behavior is profoundly diminished.
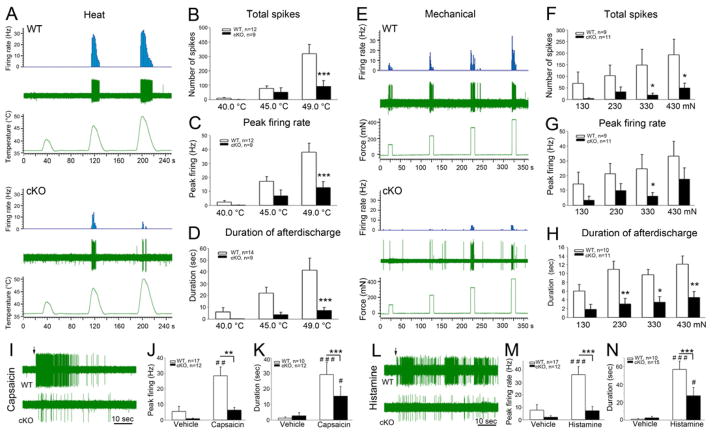
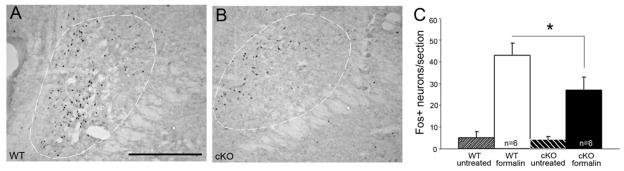
In Figs. 2G–I, we show that injection of formalin into the hindpaw evoked significantly less Fos-immunoreactivity in the cKO mice. However, as Fos only provides a global measure of the number of activated neurons, rather than a measure of the magnitude of the activity of individual neurons, we next made extracellular recording from neurons in the superficial dorsal horn, comparing the thermal and mechanical responsiveness in WT and cKO animals. Given the impedance of the electrodes used, we presume that these recordings are from the largest neurons, the majority of which are projection neurons in lamina I. Figure 6 shows that both the total number of spikes evoked during the stimulation period as well as peak firing in response to graded heat (Figs. 6A–C) and mechanical stimuli (Figs. 6E–G) were indeed significantly reduced in the cKO mice. The duration and magnitude of the afterdischarge, which presumably contributes to the sustained activity of the projection neurons, were also significantly reduced in neurons in the cKO mice (Figs. 6D, H). On the other hand, although intensity coding, with reduced response magnitude, was preserved for heat stimuli, coding of mechanical stimulus intensity was, in fact, lost in the cKO mice (Figs. 6E–G). The latter result is consistent with the more profound effect of TR4 deletion on the processing of noxious mechanical inputs.
Fig. 6.
As the cKO mice showed significantly reduced responsiveness to algogenic (capsaicin, formalin) and pruritogenic (histamine, chloroquine) stimulation, we also investigated the spinal cord responsiveness of superficial dorsal horn neurons following intraplantar injection of capsaicin, histamine, or their vehicles. As all of the neurons that responded to capsaicin or histamine were also activated by noxious heat, we presume that they receive a predominant, if not exclusive afferent drive from TRPV1-expressing nociceptors. Despite the profound decrease in the behavioral response to capsaicin and histamine, the relative abundance of the capsaicin- or histamine-responsive neurons did not differ between WT and cKO mice, Thus, 16 of 17 heat-sensitive units tested in WT and 10 of 12 in cKO were capsaicin-responsive, and 10 of 10 in WT and 13 of 15 in cKO mice responded to histamine. However, both peak firing rate and duration of the discharges in response to either capsaicin or histamine were significantly reduced in the cKO mice (Figs 6I–N).
Based on these findings, we hypothesized that the activity in the cKO mice of the lamina I projection neurons in response to algogenic and pruritogenic stimuli is not sufficient to drive the supraspinal sites that are required for the full expression of supraspinally-mediated pain behaviors. To test this hypothesis, we next evaluated noxious stimulus-evoked Fos induction in a major supraspinal target of NK1 receptor-expressing lamina I projection neurons, namely the lateral parabrachial nucleus of the dorsolateral pons (Al-Khater and Todd, 2009). Figure 7 illustrates that the number of formalin-induced Fos-immunoreactive neurons in the parabrachial nucleus is indeed significantly reduced in the cKO compared to WT mice. Taken together, we conclude that loss of a population of excitatory interneurons in the superficial dorsal horn underlies the reduced activity of supraspinal loci critical to the full expression of pain behaviors in response to noxious stimulation.
Fig. 7.
Cognitive function is minimally altered in the TR4 cKO mice and selective forebrain deletion of TR4 does not alter pain and itch functionality
Significant deficits in learning, memory and emotional processes unquestionably contribute to the experience of pain or itch. Thus, even though our findings indicate that a deficit in the transmission of pain and itch messages from the spinal cord to the brain is the critical contributor to the behavioral phenotype in the cKO mice, it was important to address a possible contribution of diminished higher cortical function in these mice. To this end, we assessed the mice in traditional tests of learning, memory and anxiety. Figure S6A shows that the TR4 cKO perform as well as their WT littermates in the Morris Water maze. The TR4 cKO and WT mice also performed comparably in the open field test (Fig. S6B), however, we did observe a small, but significant increase in the time spent in the open arms of the zero maze (Fig. S6C), which suggests that these mice are somewhat less anxious than the WT mice. It is unlikely, however, that this contributes significantly to the dramatically reduced pain and itch phenotypes observed in the cKO mice.
Consistent with this conclusion, when we crossed the floxed TR4 mice with an αCaMKII-Cre line, which restricted TR4 deletion to the forebrain (Silva et al., 1992; Tsien et al., 1996), or when we used a Cre-line that selectively targets the hypothalamus (SF1Cre) (Dhillon et al., 2006), we found that pain and itch behaviors were completely normal. Furthermore, and not surprisingly, we found no anatomical reorganization at the spinal cord level (data not shown). On the other hand, when we used a Pax3-Cre line, which is heavily expressed in the dorsal horn spinal cord ((Tsai et al., 2012) and Fig. S7A), but less so supraspinally, we completely recapitulated the behavioral and anatomical (pain and itch) findings observed after Nestin-Cre mediated TR4 deletion, including the loss of superficial dorsal horn interneurons (Figs. S7B–F, S8). Taken together the results from these different Cre-crosses strongly argue that the critical locus of the TR4 deletion to produce the anatomical and behavior phenotypes is in the superficial dorsal horn.
DISCUSSION
We report that neuronal deletion of TR4 results in a remarkably selective loss of a large complement of excitatory interneurons in the superficial dorsal horn and in these mice there is a profound decrease of pain behaviors that require processing of incoming messages by the brain. Pruritogen-induced itch, which also requires supraspinal processing of (afferent) pruritic stimuli, is also lost. These profound changes occurred despite preservation of the reflex responsiveness to noxious heat and of tissue injury-induced heat and mechanical hypersensitivity. On the other hand, nerve injury-induced mechanical hypersensitivity was severely compromised. Most importantly, these profound behavioral changes occurred in mice in which there was no change in the complement of primary afferents or of dorsal horn projection neurons. We conclude that primary afferent activation of projection neurons is not sufficient to generate fully the behaviors indicative of the experience of pain and itch; concurrent activation of excitatory interneurons is essential.
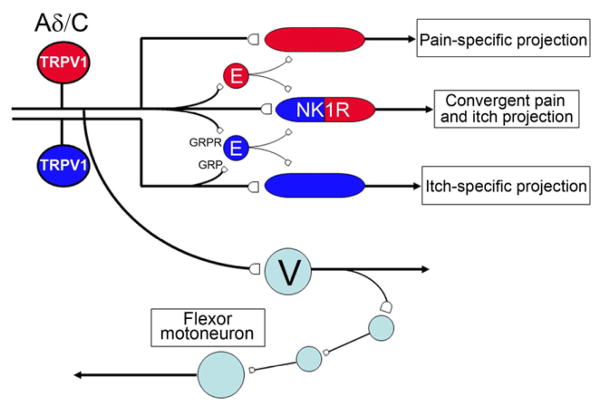
Figure 8 schematizes the feedforward networks that we envision engage the projection neurons. In the absence of the facilitatory drive provided by excitatory interneurons, the output of the projection neurons in response to nociceptive and pruritoceptive signals is significantly reduced. Because TRPV1-expressing afferents are both necessary and sufficient for the generation of noxious heat-evoked pain and withdrawal reflexes (Cavanaugh et al., 2009), as is the itch provoked by many pruritogens (Han et al., 2012; Imamachi et al., 2009), we highlighted these afferents to illustrate circuits implicated by our findings. Figure 8 also suggests how noxious heat-induced activation of TRPV1-expressing primary afferent nociceptors could trigger normal noxious heat-evoked withdrawal reflexes, despite the loss of more complex, supraspinally-mediated behaviors indicative of pain and itch. The critical difference arises from the presence of distinct populations of excitatory interneurons. One group in the superficial dorsal horn activates spinal cord projection neurons to engage pain and itch processing circuits in the brain; another group that we hypothesize is activated by TRPV1-expressing A delta nociceptors that arborize in the deep dorsal horn, engages spinal cord flexor reflex withdrawal circuits. Importantly, because both tissue and nerve-injury induced sensitization of heat-evoked withdrawal reflexes was preserved in the TR4 cKO mice, we presume that the excitatory interneurons that mediate this sensitization are also preserved. Whether they overlap with the population required for transmission of the pain message to the brain or whether they constitute a distinct population remains to be determined
Fig. 8.
Differential excitatory interneuron contributions to the processing of heat and mechanical pain messages
Despite the presence of polymodal primary afferent nociceptors that respond to both noxious heat and mechanical stimulation (Jankowski et al., 2012), there is now increasing evidence that different circuits underlie the pain produced by these different stimulus modalities. In a recent report we highlighted the differential contribution of subpopulations of primary afferent nociceptor to the transmission of heat and mechanical pain messages (Cavanaugh et al., 2009) and also demonstrated that heat and mechanical pain can be independently regulated by opioid agonists that target the mu and delta opioid receptors, respectively (Scherrer et al., 2009). In our new analysis, we found that loss of a subset of excitatory dorsal horn interneurons in the cKO mice is associated with a preservation of reflex responsiveness to noxious heat (using the Hargreaves and tail immersion tests), despite a profound increase in mechanical reflex withdrawal thresholds. Furthermore, partial nerve injury produced the expected heat hypersensitivity, but absolutely no mechanical hypersensitivity in the cKO mice. Taken together these observations indicate that the behaviorally relevant segregation of noxious stimulus modalities that we previously described for the primary afferent nociceptor is also manifest at the level of some circuits in the spinal cord. Conceivably loss of the same population of interneurons that contributes to the increased baseline mechanical threshold in the cKO mice accounts for the loss of mechanical hypersensitivity after nerve injury.
We have not attempted to schematize how a loss of excitatory interneurons could contribute to the mechanical phenotype recorded in the TR4 cKO mice. Particularly problematic is that we do not have a behavioral test of mechanical pain processing, comparable to the hot plate test, which is presumed to involve supraspinal processing of pain messages. Furthermore, in contrast to the selective contribution of TRPV1-expressing nociceptors to heat pain and injury-induced heat hypersensitivity (Cavanaugh et al., 2009), a plethora of afferents contribute to acute mechanical pain and to mechanical hypersensitivity: high threshold Aδ delta and C mechanoreceptors (Costigan et al., 2009); the MrgprD subset of nonpeptidergic afferents (Cavanaugh et al., 2009; Rau et al., 2009), low threshold C mechanoreceptors (Seal et al., 2009) and even A beta afferents (Costigan et al., 2009). Given the very different central projections of this heterogeneous population of mechanosensitive afferents, it is unlikely that a common set of excitatory interneurons underlies their contribution to mechanical pain and nerve-injury induced mechanical hypersensitivity.
The excitatory interneuron contribution to pain and itch: divergence or convergence of dorsal horn circuits?
Our observations also provide new insights into the circuitry through which pruritogen-induced itch is produced. Based on their finding that selective killing of GRPR-expressing neurons in the dorsal horn eliminated itch without affecting several measures of pain processing, Sun and Chen (2007) concluded that the GRPR neurons likely carry the itch message to the brain. However, those studies did not determine whether the GRPR neurons are interneurons or projection neurons. The dramatic loss of GRPR neurons in the TR4 cKO mice, in which projection neurons were preserved, suggests instead that the GRPR neuron are, in fact, excitatory interneurons that must trigger itch by engaging projection neurons. Figure 8 illustrates this circuit, but this schematic also includes a population of GRP-positive interneurons in the superficial dorsal horn, loss of which would also impact pruritogen-induced itch. Although most studies indicate that GRP is expressed by a subset of peptidergic and TRPV1-expressing primary afferents(Akiyama et al., 2013; Sun and Chen, 2007), our in situ analysis (and see also (Brohl et al., 2008; Mishra et al., 2012) suggests that pruritogen-responsive unmyelinated afferents may not only engage the GRPR circuits directly, but also indirectly, via GRP-expressing excitatory interneurons.
For pain and itch to be segregated, it follows that the GRPR-positive population of interneurons and a different (pain-provoking) population must engage different populations of projection neurons. In other words, there must be one labeled line for itch and another for pain. However, based on the concurrent reduction of pain and itch after deletion of the relatively large NK1 receptor-expressing subset of projection neurons Carstens et al (2010) concluded the opposite, namely that there is convergence of pain and itch circuits upon a common output system. Figure 8 illustrates both scenarios, one circuit in which there are labeled lines for pain and itch, and another in which “itch”- and “pain”-selective excitatory interneurons converge onto NK1R-expressing projection neurons. If the latter circuit indeed exists, then the differential perception of pain and itch must involve distinguishable firing codes generated by spinal cord projection neurons, codes that must be “read” by the brain. The fact that the great majority, if not all superficial dorsal horn neurons, responds to noxious heat and mechanical stimuli, as well as to pruritogens, certainly points to a convergent circuit. Clearly it is critical to determine the extent of convergence of GRPR-positive and negative interneurons upon projection neurons.
Figure 8 also highlights the fact that the extent of specificity in the processing of pain and itch messages must consider the contribution of the primary afferent. Consistent with several previous studies (Akiyama et al., 2009; Davidson et al., 2012), we found that all capsaicin and histamine responsive dorsal horn neurons are noxious heat-responsive. It follows that they all receive input from TRPV1-expressing primary afferents. However, that conclusion does not eliminate the possibility that there is physiological specificity in the central connections of these TRPV1 afferents. Indeed, there is very recent evidence that subsets of TRPV1 positive nociceptors and pruritoceptors respectively engage “pain” and “itch” relevant excitatory dorsal horn interneurons (Han et al., 2012).
Our findings parallel, albeit in a complementary manner, those of Ross et al (2010), who studied mice with a deletion of the Bhlhb5 homeobox gene. In the Bhlhb5 mutant mice, there is a selective loss of inhibitory interneurons in the superficial dorsal horn, which manifests in a condition of excessive scratching, i.e. exaggerated itch. In these mice acute pain was not altered. Whether projection neurons persisted after Bhlhb5 deletion was not determined, but given the preservation of pain behavior and excessive itch, the projection neurons likely survived. Importantly, however, just as there is no evidence that the Bhlhb5 deletion contributes directly to the itch phenotype, so the loss of TR4 does not contribute directly to any of the behavioral phenotypes that we observed. Rather, we presume that the loss of excitatory interneurons is a developmental manifestation of TR4 deletion, as is the loss of inhibitory interneurons a developmental consequence of Bhlhb5 deletion. These two gene deletions, however, fortuitously provided important insights into the superficial dorsal horn circuits and mechanisms through which pain and itch are generated.
Locus of the TR4 deletion-mediated defect
Since we did not study animals in which TR4 was deleted only from spinal cord, we cannot conclude unequivocally that the remarkable pain and itch phenotypes resulted only from loss of the excitatory interneuron population in the dorsal horn. On the other hand, selective deletion of TR4 from the forebrain, using an αCamKII-Cre line, did not reproduce any of the anatomical alterations or of the pain or itch-related defects. Furthermore, a more focused dorsal spinal cord deletion of TR4 using a Pax3-Cre line, recapitulated both the anatomical and behavioral pain phenotypes. The most parsimonious explanation of these results is that direct (i.e. monosynaptic) activation of projection neurons of the dorsal horn is not sufficient to trigger the full complement of behaviors indicative of pain and itch, both of which require integrated participation of supraspinal circuits. Rather, concurrent feedforward facilitation of projection neurons by excitatory interneurons in the superficial dorsal is absolutely required to achieve sufficient activity to generate fully the perception of pain and itch and their associated behaviors. Interestingly, the profound loss of interneuron-derived substance P immunoreactivity in the LSN suggests that concurrent facilitation of activity of projection neurons in both the superficial dorsal horn and LSN may be required for the full expression of these behaviors.
CONCLUSION
The very profound pain and itch processing defects after TR4 deletion reflects loss of functionally distinct, and possibly independent, excitatory interneuronal circuits in the dorsal horn. These different populations of excitatory interneurons contribute differentially to heat and mechanical modalities of tissue and nerve injury-induced acute and persistent pain. Our observations also support the view that the behaviorally relevant segregation of noxious heat and mechanical pain messages that is a feature of the nociceptor is also maintained and can be independently regulated at the level of dorsal horn interneuronal circuits. Most importantly, our findings demonstrate that transmission of pain and itch messages from sensory neurons to spinal cord projection neurons is not sufficient to sustain pain and itch behaviors. Feedforward facilitation from excitatory interneurons to spinal cord projection neurons is essential for noxious and pruritic stimuli to engage and fully activate the forebrain circuits that underlie the experience of pain and itch.
EXPERIMENTAL PROCEDURES
All animal experiments were approved by the Institutional Animal Care and Use Committee at UCSF and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory animals.
See Supplemental experimental procedures for details on the genotyping, RT-PCR, in situ hybridization, retrograde tracing, immunohistochemistry, quantification and behavioral assays.
Extracellular recording from the spinal cord dorsal horn
Extracellular single unit recording were made from nociresponsive neurons in the superficial dorsal horn of the lumbar spinal cord (Martin et al., 2004; Mazario and Basbaum, 2007). As for the behavioral analysis, true blinding is difficult because of the smaller size of the mutant. In these studies the mice were anesthetized by injection of 1.5 g/kg urethane (10% in saline, Sigma). A laminectomy was performed at vertebral levels T13 to L1, corresponding to spinal segments L4–L5. An agar pool was formed and then filled with 37 °C mineral oil. A fine-tipp ed tungsten microelectrode (6–8 MΩ at 1 kHz; FHC) was used to record unit activity. To search for neurons, we applied brief, moderate pressure with a blunt glass probe to different regions of the glabrous skin of the ipsilateral hindpaw. Average recording depths were 82.7±7.6 μm in WT, and 86.6±7.7 μm in cKO for neurons in the region of lamina I. Once a mechanical receptive field was identified, we characterized the unit with short (5 s) brush, pressure, and pinch stimuli or with a drop of 50 °C water. Next, we app lied graded mechanical and heat stimuli using a custom-built mechanical stimulator (ESTIMEC; Cibertec) or a contact Peltier device (kindly provided by Merck, Sharpe, and Dohme), respectively. Unit activity was amplified (CyberAmp380; Axon Instruments), digitized (Micro1401; CED), and discriminated (Spike2; CED). Changes in peak firing rates (Hz), number of spikes evoked during the stimulation period and length of the after discharge were compared (GraphPad).
To assay the responsiveness of superficial dorsal horn neurons to selective algogenic and pruritogenic stimuli, we examined the effects of intradermal microinjection of capsaicin (0.3 μg in 1 μl), or histamine (50 μg in 1 μl) or their vehicles into the receptive field with a 30.5-gauge needle connected to a 10 μl Hamilton microsyringe. The tested neurons were selected if they responded to noxious mechanical stimulation. Responses to the vehicles were investigated first. After the unit activity had recovered, we followed with capsaicin or histamine in the same receptive field, using a second 30.5 gauge needle. Differences in firing properties between WT and cKO were compared, including peak firing rates (Hz), response durations (sec), and numbers of spikes in 4 consecutive 20-sec periods after the injection. The number of spikes in a 20-sec period prior to the chemical administration (baseline activity) was subtracted from each of the 4 post-injection 20-sec periods to determine the activity induced by the chemicals.
Statistical analysis
Data are presented as mean ± standard error of mean (S.E.M). Student’s t test, one- and two-way repeated measures ANOVA (Bonferroni post test) were used to analyze the anatomical, behavioral and electrophysiological results; p<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This research was supported by: NIH grants: NS R37 NS014627 and R01AR059402. We thank members of the Neurobehavioral Core for Rehabilitation Research at UCSF for their assistance with many of the behavioral tests and members of the Basbaum lab for their comments on the manuscript. We are also grateful for the use of various Cre lines: αCamKII-Cre from John Rubenstein, Nestin-Cre from Nirao Shah, Pax3-Cre from David Rowitch, Peripherin-Cre from David Donovan and SF1-Cre from Holly Ingraham.
Footnotes
Supplemental information for this article includes eight figures and supplemental experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn AH, Basbaum AI. Tissue injury regulates serotonin 1D receptor expression: implications for the control of migraine and inflammatory pain. J Neurosci. 2006;26:8332–8338. doi: 10.1523/JNEUROSCI.1989-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol. 2009;102:2176–2183. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J Neurophysiol. 2013;109:742–748. doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopians AL, Babayan AH, Beffert U, Herz J, Basbaum AI, Phelps PE. Contribution of the Reelin signaling pathways to nociceptive processing. Eur J Neurosci. 2008;27:523–537. doi: 10.1111/j.1460-9568.2008.06056.x. [DOI] [PubMed] [Google Scholar]
- Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Antal M, Polgar E, Chalmers J, Minson JB, Llewellyn-Smith I, Heizmann CW, Somogyi P. Different populations of parvalbumin- and calbindin-D28k-immunoreactive neurons contain GABA and accumulate 3H–D-aspartate in the dorsal horn of the rat spinal cord. J Comp Neurol. 1991;314:114–124. doi: 10.1002/cne.903140111. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates B, Rios M, Trumpp A, Chen C, Fan G, Bishop JM, Jaenisch R. Neurotrophin-3 is required for proper cerebellar development. Nat Neurosci. 1999;2:115–117. doi: 10.1038/5669. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Brohl D, Strehle M, Wende H, Hori K, Bormuth I, Nave KA, Muller T, Birchmeier C. A transcriptional network coordinately determines transmitter and peptidergic fate in the dorsal spinal cord. Dev Biol. 2008;322:381–393. doi: 10.1016/j.ydbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Collins LL, Uno H, Chang C. Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4. Mol Cell Biol. 2005;25:2722–2732. doi: 10.1128/MCB.25.7.2722-2732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Collins LL, Uno H, Chou SM, Meshul CK, Chang SS, Chang C. Abnormal cerebellar cytoarchitecture and impaired inhibitory signaling in adult mice lacking TR4 orphan nuclear receptor. Brain Res. 2007;1168:72–82. doi: 10.1016/j.brainres.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffer KD, Urca G, Elde RP, Giesler GJ., Jr Studies of peptidergic input to the lateral spinal nucleus. Brain Res. 1988;460:356–360. doi: 10.1016/0006-8993(88)90381-2. [DOI] [PubMed] [Google Scholar]
- Collins LL, Lee YF, Heinlein CA, Liu NC, Chen YT, Shyr CR, Meshul CK, Uno H, Platt KA, Chang C. Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci U S A. 2004;101:15058–15063. doi: 10.1073/pnas.0405700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Evans RM. The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol. 2005;19:1429–1438. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2012;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Ekmann KM, Anderson CE, Molliver DC, Koerber HR. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain. 2012;153:410–419. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerman L, Zakowski MI, Piskoun B, Grant GJ. Hot plate versus tail flick: evaluation of acute tolerance to continuous morphine infusion in the rat model. J Pharmacol Toxicol Methods. 1995;34:23–27. doi: 10.1016/1056-8719(94)00077-h. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Cao Y, Basbaum AI. Characterization of wide dynamic range neurons in the deep dorsal horn of the spinal cord in preprotachykinin-a null mice in vivo. J Neurophysiol. 2004;91:1945–1954. doi: 10.1152/jn.00945.2003. [DOI] [PubMed] [Google Scholar]
- Mazario J, Basbaum AI. Contribution of substance P and neurokinin A to the differential injury-induced thermal and mechanical responsiveness of lamina I and V neurons. J Neurosci. 2007;27:762–770. doi: 10.1523/JNEUROSCI.2992-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Holzman S, Hoon MA. A nociceptive signaling role for neuromedin B. J Neurosci. 2012;32:8686–8695. doi: 10.1523/JNEUROSCI.1533-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, Anderson DJ, Koerber HR. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Wong ST, Wozniak DF, Muglia LM, Liauw JA, Zhuo M, Nardi A, Hartman RE, Vogt SK, Luedke CE, et al. Altered stress-induced anxiety in adenylyl cyclase type VIII-deficient mice. J Neurosci. 2000;20:4809–4820. doi: 10.1523/JNEUROSCI.20-13-04809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152:990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Akopians AL, Babayan AH, Basbaum AI, Phelps PE. Absence of Reelin results in altered nociception and aberrant neuronal positioning in the dorsal spinal cord. Neuroscience. 2006;139:1385–1396. doi: 10.1016/j.neuroscience.2006.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.