Abstract
We have developed a set of upper extremity functional tasks to guide the design and test the performance of rehabilitation technologies that restore arm motion in people with high tetraplegia. Our goal was to develop a short set of tasks that would be representative of a much larger set of activities of daily living while also being feasible for a unilateral user of an implanted Functional Electrical Stimulation (FES) system. To compile this list of tasks, we reviewed existing clinical outcome measures related to arm and hand function, and were further informed by surveys of patient desires. We ultimately selected a set of five tasks that captured the most common components of movement seen in these tasks, making them highly relevant for assessing FES-restored unilateral arm function in individuals with high cervical spinal cord injury (SCI). The tasks are intended to be used when setting design specifications and for evaluation and standardization of rehabilitation technologies under development. While not unique, this set of tasks will provide a common basis for comparing different interventions (e.g., FES, powered orthoses, robotic assistants) and testing different user command interfaces (e.g., sip-and-puff, head joysticks, brain-computer interfaces).
Keywords: Activities of Daily Living, Functional Assessment, Functional Electrical Stimulation, Functional Evaluation, Functional Neuromuscular Stimulation, Outcome Measures, Spinal Cord Injury, Tasks, Tetraplegia, Upper Extremity
2. Introduction
Functional Electrical Stimulation (FES) is a technology that allows individuals with paralysis to regain movement of their limbs by coordinated application of small amounts of electrical current to appropriate paralyzed muscles [1]. Currently, we are developing a FES system [2, 3] to restore movement to individuals with high-level (C1–C4) spinal cord injury, providing basic arm motions and thus increasing independence in simple yet critical activities of daily living. The performance of the various components of this FES system (e.g. user command interfaces, feedback control systems) must be assessed under functionally relevant conditions and in a reasonably short period.
We have therefore compiled a set of simple activities of daily living (ADL) that is fairly brief but that (1) can be used to extrapolate performance under a much wider range of movements and (2) are feasible for a unilateral FES recipient. This same set of activities will be equally relevant for evaluating other rehabilitation interventions, such as robotic assistants and mobile arm supports. The purpose of these tasks is to aid in the development, preclinical evaluation, and ultimately clinical assessment of these types of technologies.
Many clinical measures have been developed to quantify the functional abilities of individuals with motor deficits, some based on activities of daily living and some on more specific motor control tasks that translate into increased function. These measures have been used in rehabilitation to document changes in a patient’s status over time and to evaluate, empirically, the effectiveness of interventions. Several recent reviews have examined the literature related to these clinical measures [4, 5]. Although many of these measures are theoretically applicable to individuals with high tetraplegia, almost none of the tasks required by these assessment tools will be possible in these individuals – even if they have a state-of-the-art FES system. Further, two surveys of a high-level SCI population have identified arm and hand function as being crucial to improved quality of life, although no specific tasks are mentioned [6, 7].
To be more useful in providing a graded evaluation of interventions for high tetraplegia, a functional assessment tool will need to take into account the unique properties of this population and the nature of likely interventions (e.g., an FES system). These properties include the unilateral implementation of current FES systems, the complete paralysis of the lower extremities, paralysis of the torso that prevents whole-body movements typically synergistic with arm motions, the relative weakness of muscles under FES control, and the typical limitation of FES-restored hand function to lateral and palmar grasps only. In addition, common assessment tools do not accurately represent the realistic functional goals of FES users with high tetraplegia.
This study relied upon the published literature and the experience of the Cleveland FES Center to develop a list of five functional tasks that are representative of a larger set of important ADL and can be used to test novel upper-extremity FES systems and other rehabilitation interventions for this population. These tasks were also selected according to their ability to be programmed into a virtual reality environment that will be used in the future to test the performance and usability of novel user interfaces to upper extremity FES systems.
3. Methods
Our general approach for compiling a reasonably small but representative set of hand and arm tasks for evaluating functional interventions for high tetraplegia was (1) to compile a comprehensive list of ADL for the hand and arm from a variety of sources, (2) eliminate tasks that would not be feasible for an individual with a state-of-the-art interventions for high tetraplegia, (3) classify the feasible tasks into a common set of movement segments and count the various movement segment types in each given movement, and (4) select a small set of five simple tasks that encompass all of the important movement components.
Two different sources were consulted to draw conclusions about the relative importance of functional tasks for individuals with high tetraplegia: (1) the literature for studies on clinical measures and patient surveys, and (2) consultations with rehabilitation professionals and FES users.
3.1 Clinical Measures
We drew from seven published clinical measures that address upper extremity function, each described below. The basis of this list comes from the excellent review by van Tuijl, et al.[4], and includes The Barthel Index, The Rancho-Los Amigos Test, The FIM, The Quadriplegia Index of Function, The Spinal Cord Independence Measure, The ADL Abilities Test, and The Valutazione Funzionale Mielolesi.
No one measure was perfectly applicable. Rather, the final list of tasks for this study was formed from an amalgam of activities and tasks identified across these clinical measures. The fact that a specific task was included in many existing assessment tools does not necessarily imply that this task was a high priority for our target population. However, we do assume that—for clinical measures especially—inclusion in several studies indicates a task that may contribute greatly to an overall increase in independence.
The oldest applicable measure in the literature is the Barthel Index (BI) [8] and it’s revision, the Modified Barthel Index (MBI) [9]. They were designed to assess independent self-care in individuals with tetraplegia. For the purposes of this study, the relevant tasks of the Modified index are drinking from a cup, eating from a dish, and grooming. The Rancho Los Amigos Test Functional Activities Test (RLAT) is another test developed to analyze the ability of individuals with high SCI to perform self-care activities. The test covers eight categories: feeding, grooming, toileting and bathing, upper extremity dressing, lower extremity dressing, written communication, desk skills, and transfers [10]. The FIM test is the most cited measure in the rehabilitation literature [11, 12]. Appropriate items for high tetraplegia in the FIM test include eating and grooming, but there are no more specific, relevant tasks beyond these general measures. The Quadriplegia Index of Function (QIF) [13] was developed specifically to document improvement in individuals with tetraplegia. The feeding category of the QIF has been found to show more sensitivity than other measures such as the FIM [14]. A test specifically designed to address the needs of individuals with SCI was developed in 1997 and called the Spinal Cord Independence Measure (SCIM), which itself was revised in 2001 [15, 16], and 2007 [17], now called the SCIM-III. Applicable tasks listed in this test are: cutting food, opening containers, bringing food to mouth, drinking from a cup, washing hands, brushing teeth, combing hair, shaving, and applying makeup. The ADL Abilities Test (ADLAT) [18] was developed to analyze specific phases of tasks using a task analysis approach. The six core tasks of the ADLAT are eating with a fork, drinking from a glass, writing with a pen, dialing a phone, using a compact disc, and brushing teeth.
In a somewhat different approach, the Valutazione Funzionale Mielolesi (SCI Functional Assessment) was designed as a way to identify changes in a specific patient’s functional status over time, rather than as a measure to compare across patients for research purposes [19]. It includes 65 specific tasks, including the following which were deemed appropriate for this study: uses fork, uses spoon, uses knife, pours [a pitcher] out, uses cup or glass, washes hands, washes face, dries hand/face, brushes teeth, shaves/puts on makeup, comb hair, write in longhand, types, turns page, uses phone, uses remote control, open/closes door, uses keys, uses elevator.
A final set of well-cited activities of daily living also informed our conclusions. The Klein-Bell ADL Scale is a large set of activities from six domains [20]. Not specific for SCI, the test has been shown to document small changes in independence with various ADL. The many activities described in this measure were not specifically included in the analysis for this study, as they are highly specific.
3.2 Patient Surveys
Surveys directed at patient populations who have limited arm and hand function further informed the selection of the functional task list. Knowledge of the stated desires of those without arm and hand function enabled a choice of tasks that will be functionally relevant and of high priority to potential FES users.
Donnelly et al identified functional limitations in the SCI population associated with several broad areas [21]. The study surveyed 41 SCI patients in the early stage of recovery for their perceived level of satisfaction and performance in these areas. The top five identified issues were functional mobility including transfers and wheelchair use (19%), dressing (13%), grooming (11%), feeding (8%), and bathing (7%). Kilgore et al, 2001, interviewed nine SCI patients regarding priorities for research in FES and rehabilitation [22]. Each person was asked, “Can you prioritize the 2–3 activities that you would really like to be able to do that your injury prevents you from doing now?” Responses varied, but tended to include more specific activities such as walking, playing catch, dancing, changing an overhead light bulb, winding a clock, or cooking a meal. A common theme was frustration with the amount of time it took to complete ADL. Another strong theme was the desire for independence and not having to wait for caregivers or assistants. In 2004, Anderson performed a survey of 681 spinal-cord injured individuals to determine specific research activities that would most improve quality of life [6]. Nearly half of individuals with tetraplegia reported that regaining arm and hand function would most improve their quality of life, although there were no specific tasks listed. In the same year, Snoek published a similar survey of 565 subjects to determine the relative importance of hand function and seven other SCI impairment areas [7]. The study concluded that there is a high priority for hand function improvement compared to other impairments in tetraplegia, again without specific tasks cited.
Barreca et al, 2004, performed a literature search and survey to identify tasks for a new clinical measure for arm and hand activity in stroke patients [23]. This measure is called the Chedoke Arm and Hand Activity Inventory, or CAHAI, and is more relevant to the current study as a survey rather than as a clinical measure. The following items were listed as priority at least three times: combing hair, dressing using buttons, raising glass to mouth, pouring water from class, moving a can onto shelf and down again, preparing a hot drink, preparing a snack, cutting softer food, opening and closing a door, bringing a spoon to the mouth, writing, using/dialing the phone, reading books, picking up coins from a table, picking up a key and opening a lock, washing a floor, vacuuming, washing clothes by hand, opening and closing a jar, pulling on a shirt, and tying shoelaces.
To identify tasks for rehabilitation robots, Stanger et al, reviewed user task priorities generated by seven surveys conducted between 1966 and 1991 [24]. A sample of patients was interviewed representing SCI, Multiple Sclerosis, and other impairments. The top priorities of potential users were drinking from a glass, opening doors, washing/drying the face, using a vending machine, gardening, and manipulating printouts. Atkins et al performed a comprehensive survey of children and adults with upper limb loss [25]. The goal included identifying the “priorities identified by users as the most important areas for improvement in current prosthetic devices and future designs.” Users of many types of prostheses returned 1575 surveys. Patients ranked the top activities they would like to be able to perform with their prostheses. The top activities, combined across prosthesis types, were: type/use word processor, open door with knob, tie shoelaces, use spoon or fork, drink from glass, fasten a button, use a hammer and nail, and cut meat.
The patient surveys were used, qualitatively, to ensure tasks of high importance to potential users were included in the final selection. For instance, the high emphasis on grooming and feeding ensured that several tasks contained movements around the face, which would be essential to complete any of these high-value ADL. This helped ensure that the final selection of tasks for this study was not only representative of many other ADL, but that each task was important in itself.
3.3 Conversations with Rehabilitation Professionals and FES users
Our final selection of tasks was also influenced by the experience of rehabilitation professionals and our experience with FES systems in practice. We worked with one physician in Physical Medicine and Rehabilitation, one occupational therapist, one physical therapist, and two current users of FES systems. The conversations were intentionally informal, unstructured discussions that allowed exploration of each participant’s clinical and personal experience.
These discussions emphasized that many of the tasks deemed important by clinical measures and surveys are not feasible for individuals with severe impairments using existing or immediately foreseeable FES technology. These limitations include any task requiring bimanual manipulations, high levels of force, hand positions outside a non-impaired, central reaching workspace, or highly dexterous object manipulation. The results of these conversations are summarized largely by the “Feasibility Filter” illustrated in Table 1, which indicates which tasks were eliminated from further analysis only because of a consensus on their impracticality.
Table 1.
Summary of Clinical Measures. Each clinical assessment measure evaluated is listed across the top, with the specific tasks mentioned in that assessment tool indicated along the left side. The frequency is a count of how many times a specific task was used across all clinical measures. The feasibility filter marks tasks as possible (using “o”) or not possible (using “—”) with a foreseeable high-tetraplegia FES system.
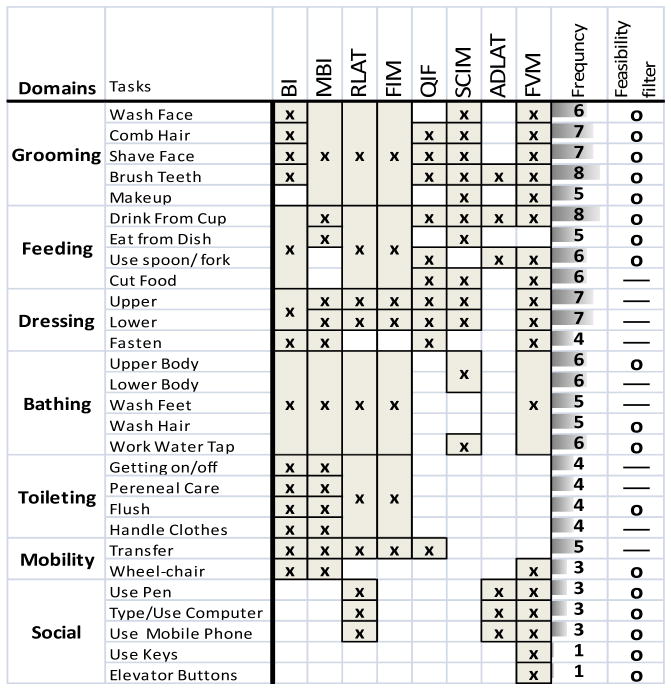
|
BI: Barthel Index. RLAT: Rancho Los Amigos. QIF: Quadriplegia Index of Function. SCIM: Spinal Cord Index of Function. FVM: Valutazione Funzionale Mielolesi. MBI: Modified Barthel Index.
4. Results
4.1 Common Themes for creation of the Functional Task List
As described in the Methods, we created a set of representative tasks by starting with a large list of tasks identified in literature, and culled that list using the experience of those in the field. We then identified the movement components used to complete each task, and finally created a new small set of functional tasks that included the movement components most commonly required for the larger, complete set. After reviewing the literature, we were able to create an initial list of 28 tasks in seven domains, as summarized in Table 1. The tasks and their domains are indicated in the first two columns. The remaining columns, one for each of the relevant clinical measures, indicate (by an “x”) whether a given clinical measure included each of the 28 tasks. The “frequency” column indicates how many of the various clinical measures included a specific task. The rightmost column indicates (by an “o”) whether each task would be a feasible goal for FES-restored movements in individuals with high tetraplegia (based on section 3.3). Overall, all tasks were represented in at least one clinical measure, and the feasibility filter reduced the initial task list to 18.
4.2 Movement Components
Table 2 lists the 18 feasible tasks selected from Table 1 in the columns labeled at the top. The rows of Table 2 (labeled “movement components”) list the different movement components—from a start position (with the arm resting on the armrest), to the completion of the task—that comprised the movements of interest in this study. An “x” across each of these rows indicates that a particular movement component was necessary for a particular functional activity. The rightmost column indicates how many times each of the movement components was included in the various feasible tasks.
Table 2.
Breaking tasks into movement components. The 18 feasible tasks from Figure 1 are listed across the top of this chart. Along the left side are components of movement that make up the feasible tasks, from the start of the movement to completion. The “component frequency” is the total number of times each component is performed in order to complete all the feasible tasks.
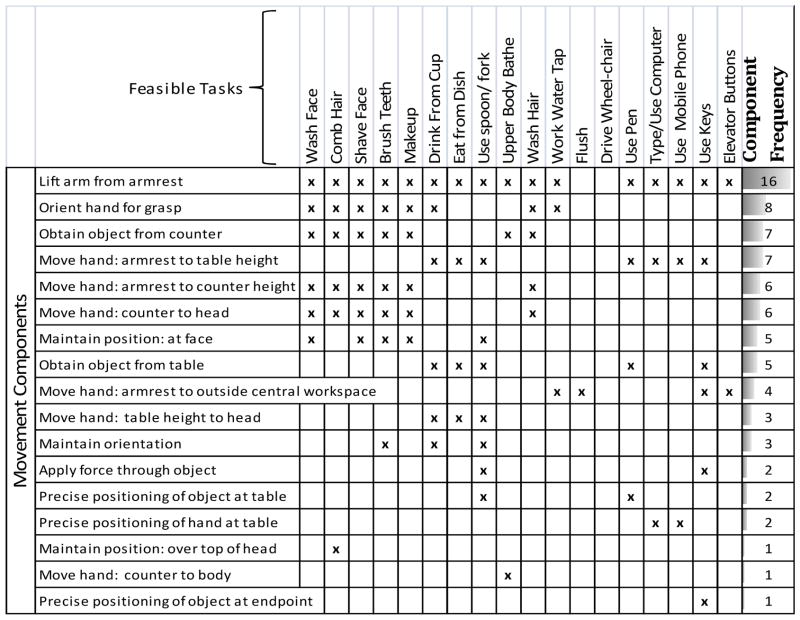
|
Tasks were broken into components in a method similar to that used in the study of Methods-Time Measurement (MTM), a method for predicting how long it should take a worker to complete a workplace task. This technique has been used in ADL analysis before, e.g. [26]. However, unlike typical MTM, no restrictions are placed on the precise sequence of movements. In this case, a movement component was marked only if the task could not be completed without that component. If a task was possible without that movement component then that component is not considered part of the task.
Note that the movement components are listed from top to bottom in order of their frequencies in the chosen functional tasks, i.e., “lift arm from armrest” occurred the most times (16) and is thus listed at the top. The frequencies of occurrence for the movement components are important, because the movement components in final list of functional tasks should approximate the relative frequency of components in the actual activities of daily living.
4.3 Functional Task List
Based on the movement components presented in Table 2, five composite tasks were chosen as representative activities that would be important and relevant to an individual with a high-level SCI and an FES neuroprosthesis. The tasks were chosen empirically, with the twin goals of choosing tasks in themselves important, and also representing the movement components from Table 2. Ideally, the representative tasks in the Functional Task List should represent all the movement components identified in the list of desired activities, and replicate the relative frequency of the movement components. These selected tasks are:
Touching the face.
Drinking from a mug with a straw
Eating with fingers from a plate.
Retrieving an object from a countertop.
Pressing an elevator button.
These tasks, along with their relationships to the 18 identified movement components, are indicated in Table 3. The columns in this Table mark (with an “x”) if a movement component is needed to complete the functional task. The rightmost column shows how often the movement component is used in all five functional tasks. Note that the relative frequency of motion segments in the functional task list is similar to the frequency of motion components identified by clinical tests, an important indicator of how completely the Functional Task List encompasses the entire list of desired functional activities.
Table 3.
Functional Task List. All the movement components are listed in rows, and the tasks of the functional task list are listed across the top. The frequency of each movement component is totaled in the rightmost column. Note that the frequency of each component in the functional task list roughly resembles the frequency of the components in the tasks from clinical measures.
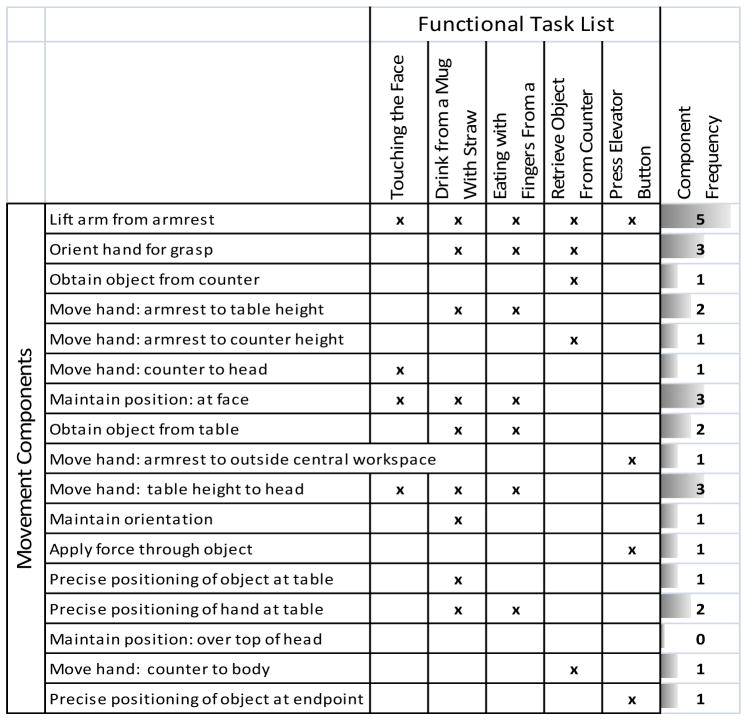
|
5. Discussion
We have developed a set of functional tasks to evaluate the performance of an FES neural prosthesis or any other intervention for restoring arm movements in high tetraplegia. The set of tasks is short, representative of a variety of movements, and feasible for a unilateral FES recipient. Our set of five tasks includes all of the movement components relevant to an individual with complete arm paralysis and an FES system, as well as replicating the relative frequencies of these movement components seen in a much larger set of “feasible tasks”. Thus, these five tasks represent a much broader range of movements, so performance in these five movements reflects likely performance in many relevant tasks. This should allow these tasks to be used as a functional benchmark while developing and deploying rehabilitation technologies.
While we believe that this list is most representative for the high tetraplegia population, it could easily be changed according to the movement impairment of a given patient population and/or the desires of a specific user. The framework identified here serves as a template for creating a new list meeting the desired criteria, for example by modifying the “feasibility filter”.
There is no relevant standard for evaluating movement restoration for individuals with high tetraplegia, since until recently there has been no possibility of movement-related rehabilitation. With the development of FES systems for high tetraplegia, the need for a functionally relevant method of evaluating the outcome of intervention has grown. For our purposes, the main limitation of existing surveys and clinical measures is that they focus on a loss of function specific to one condition. Since the options for high tetraplegia rehabilitation are very limited, there are no functional measures designed specifically to assess the motor performance of someone with paralysis to this extent. Understanding this, we examined the literature in a more general, thematic way to produce a simple list of tasks whose execution could represent the future functional ability of an arm enabled with FES. These tasks could be used to evaluate different types of FES systems, command interfaces (e.g., brain-computer interface, EMG, tongue pad, voice), control algorithms (e.g., with or without feedback), or sensor integrations (i.e., to compare sensor characteristics).
In addition, this set of tasks can provide a common basis for comparison of other interventions for high tetraplegia, including robots, powered exoskeletons, or different rehabilitation applications such as prosthetics for shoulder disarticulation amputees. We feel the utility of this method and these tasks is certainly not limited to FES applications in SCI. Finally, we anticipate that the tasks could be programmed in to a virtual reality (VR) game environment, to allow testing of any command and control scheme for the interventions or applications listed above.
6. Conclusions
We have created a set of functional tasks that will provide a common basis for evaluating interventions such as FES for restoring arm movements in individuals with high tetraplegia and other severe paralytic disorders. The five tasks chosen (touching the face, eating with fingers from a plate, drinking from a mug with a straw, retrieving an object from a countertop, pressing an elevator button) represent consistent themes in clinical measures and stated user desires, are important activities, and represent a larger set of similarly important motions. By selecting tasks that are important in their own right, but also contain movement components common across a broader range of motions, the simple set of five tasks form a basis for setting design specifications and evaluating the technical performance and efficacy of various upper extremity rehabilitation interventions in SCI and other upper extremity movement disorders.
Acknowledgments
Funding Sources:
NIH NICHD: N01-HD-5-3403, NIH T32: T32EB004314
Abbreviations
- FES
Functional Electrical Stimulation
- SCI
Spinal Cord Injury
- ADL
Activities of Daily Living
References
- 1.Peckham PH, Marsolais EB, Mortimer JT. Restoration of key grip and release in the C6 tetraplegic patient through functional electrical stimulation. J Hand Surg [Am] 1980;5(5):462–9. doi: 10.1016/s0363-5023(80)80076-1. [DOI] [PubMed] [Google Scholar]
- 2.Blana D, Kirsch RF, Chadwick EK. Combined feedforward and feedback control of a redundant, nonlinear, dynamic musculoskeletal system. Medical and Biological Engineering and Computing. 2009;47(5):533–542. doi: 10.1007/s11517-009-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polasek KH, et al. Stimulation Stability and Selectivity of Chronically Implanted Multicontact Nerve Cuff Electrodes in the Human Upper Extremity. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2009;17(5):428–437. doi: 10.1109/TNSRE.2009.2032603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Tuijl JH, Janssen-Potten YJM, Seelen HAM. Evaluation of upper extremity motor function tests in tetraplegics. Spinal Cord. 2002;40:51–64. doi: 10.1038/sj.sc.3101261. [DOI] [PubMed] [Google Scholar]
- 5.Miller WC, SB, Noonan VK, Tawashy AE, Aubut JL, Connolly SJ, Curt A, Elliott S, Hsieh JTC, Mortenson WB, Noreau L, Orenczuk SG, Sawatzky B, Steeves J, Wilkinson S, Wolfe DL. Outcome Measures. In: Eng TR, JJ, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Vancouver: 2010. Version 3.0. [Google Scholar]
- 6.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. Journal of neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 7.Snoek GJ, et al. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–532. doi: 10.1038/sj.sc.3101638. [DOI] [PubMed] [Google Scholar]
- 8.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Maryland state medical journal. 1965;14:61. [PubMed] [Google Scholar]
- 9.Yarkony GM, et al. Functional skills after spinal cord injury rehabilitation: threeyear longitudinal follow-up. Archives of physical medicine and rehabilitation. 1988;69(2):111. [PubMed] [Google Scholar]
- 10.Rogers JC, Figone JJ. Traumatic quadriplegia: follow-up study of self-care skills. Archives of physical medicine and rehabilitation. 1980;61(7):316. [PubMed] [Google Scholar]
- 11.Keith RA, Granger CV, Hamilton BB. Advances in Clinical Rehabilitation. Vol. 1. New York: Springer Publishing; 1987. The FIM: A new tool for rehabilitation; pp. 10–8. [PubMed] [Google Scholar]
- 12.Hamilton BB, et al. Interrater reliability of the 7-level functional independence measure (FIM) Scandinavian Journal of Rehabilitation Medicine. 1994;26(3):115. [PubMed] [Google Scholar]
- 13.Gresham GE, et al. The Quadriplegia Index of Function (QIF): sensitivity and reliability demonstrated in a study of thirty quadriplegic patients. Paraplegia. 1986;24(1):38. doi: 10.1038/sc.1986.7. [DOI] [PubMed] [Google Scholar]
- 14.Marino RJ, et al. Assessing selfcare status in quadriplegia: comparison of the quadriplegia index of function (QIF) and the functional independence measure (FIM) Paraplegia. 1993;31(4):225. doi: 10.1038/sc.1993.41. [DOI] [PubMed] [Google Scholar]
- 15.Catz A, et al. SCIM-spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35(12):850–856. doi: 10.1038/sj.sc.3100504. [DOI] [PubMed] [Google Scholar]
- 16.Catz A, et al. The spinal cord independence measure (SCIM): sensitivity to functional changes in subgroups of spinal cord lesion patients. Spinal Cord. 2001;39(2):97–100. doi: 10.1038/sj.sc.3101118. [DOI] [PubMed] [Google Scholar]
- 17.Itzkovich M, et al. The Spinal Cord Independence Measure (SCIM) version III: Reliability and validity in a multi-center international study. Disability & Rehabilitation. 2007;29(24):1926–1933. doi: 10.1080/09638280601046302. [DOI] [PubMed] [Google Scholar]
- 18.Bryden AM, et al. Assessing Activity of Daily Living Performance After Implantation of an Upper Extremity Neuroprosthesis. Topics in Spinal Cord Injury Rehabilitation. 2008;13(4):37–53. [Google Scholar]
- 19.Taricco M, et al. Functional status in patients with spinal cord injury: a new standardized measurement scale. Archives of physical medicine and rehabilitation. 2000;81(9):1173–1180. doi: 10.1053/apmr.2000.7161. [DOI] [PubMed] [Google Scholar]
- 20.Klein RM. The Klein-Bell ADL Scale Manual. Seattle: University of Washington Medical School; 1979. [Google Scholar]
- 21.Donnelly C, et al. Client-centred assessment and the identification of meaningful treatment goals for individuals with a spinal cord injury. Spinal Cord. 2004;42(5):302–307. doi: 10.1038/sj.sc.3101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilgore KL, et al. Neuroprosthesis consumers’ forum: consumer priorities for research directions. Development. 2001;38(6):655–660. [PubMed] [Google Scholar]
- 23.Barreca S, et al. Development of the Chedoke Arm and Hand Activity Inventory: theoretical constructs, item generation, and selection. Topics in Stroke Rehabilitation. 2004;11(4):31–42. doi: 10.1310/JU8P-UVK6-68VW-CF3W. [DOI] [PubMed] [Google Scholar]
- 24.Stanger CA, et al. Devices for assisting manipulation: a summary of user task priorities. Rehabilitation Engineering, IEEE Transactions on. 2002;2(4):256–265. [Google Scholar]
- 25.Atkins DJ, Heard DCY, Donovan WH. Epidemiologic overview of individuals with upper-limb loss and their reported research priorities. JPO: Journal of Prosthetics and Orthotics. 1996;8(1):2. [Google Scholar]
- 26.Burelbach JC, Crago PE. Instrumented assessment of FNS hand control during specific manipulation tasks. Rehabilitation Engineering, IEEE Transactions on. 2002;2(3):165–176. [Google Scholar]


