Abstract
Background
The immune system has been shown to play an important role in gastrointestinal stromal tumor (GIST). The neutrophil-to-lymphocyte ratio (NLR) in blood is an easily assessable parameter of systemic inflammatory response. The aim of this study was to determine whether the NLR is prognostic in GIST.
Methods
339 previously untreated patients with primary, localized GIST operated at our institution between 1995 and 2010 were identified from a prospectively collected sarcoma database. NLR was assessed preoperatively. Patients who received adjuvant imatinib treatment were excluded from the analysis (n=64). Cox regression models were calculated and correlation analyses were performed.
Results
On univariate analysis, NLR was associated with recurrence-free survival (RFS) (P=0.003, hazard ratio = 3.3, 95% confidence interval 1.5-7.4). Patients with a low NLR had a 1- and 5- year RFS of 98% and 91%, compared with 89% and 76% in those with a high NLR. The median RFS was not reached. Positive correlations were found between NLR and mitotic rate (Pearson correlation coefficient (r)=0.15, P=0.03), and NLR and tumor size (r=0.36, P=0.0001). RFS in patients with a GIST > 5 cm with low NLR was significantly longer compared to patients with high NLR (P=0.002). Flow cytometry analysis of freshly obtained GISTs revealed that neutrophils constituted a minimal percentage of intratumoral immune cells.
Conclusion
NLR is a surrogate for high risk tumor features. Elevated blood NLR appears to represent systemic inflammation in patients with high risk GIST.
Keywords: gastrointestinal stromal tumor, neutrophil-to-lymphocyte ratio, immune response, prognosis
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is the most common subtype of intestinal sarcoma1 with an estimated annual incidence of 4,000 cases in the U.S. While GIST can occur throughout the GI tract, the most common location is the stomach, accounting for 60% to 70% of tumors.2 The molecular alterations leading to the development of GIST have been well defined.3, 4 Novel small molecule tyrosine kinase inhibitors (TKI) were introduced in the treatment of GIST and have revolutionized the oncologic outcome of patients. The progress made in the management of this disease in the last 10 years may have been greater than in any other type of cancer.5 In the era of adjuvant imatinib treatment, determining the risk for tumor recurrence has become increasingly important. Tumor specific parameters such as mitotic rate, size, and anatomical location of the primary lesion help in assessment of the biology of the disease.6 More accurate risk stratification is possible by using nomograms, which permit quantifying the risk for recurrence after resection.7, 8
The immune response is also an important factor in regard to outcome in GIST. Low secretion of interferon-γ by stimulated peripheral natural killer (NK) cells from patients treated with imatinib9 and NK cell expression of the NKp30c isoform10 both correlate with worse outcome. Recently, we showed that imatinib inhibits tumor cell production of the immunosuppressive protein indoleamine 2,3-dioxygenase and subsequently increases the intratumoral ratio of CD8 T cells to T regulatory cells.11 In addition, the profile of tumor infiltrating T lymphocytes correlates with imatinib sensitivity in human GISTs. Furthermore, in a mouse model of GIST, CTLA-4 inhibition was synergistic with imatinib therapy.11
While intratumoral immune cells in GIST are prognostic, peripheral biomarkers would be a more useful clinical tool. The blood neutrophil-to-lymphocyte ratio (NLR) is an easily measured, reproducible, and inexpensive marker of systemic inflammation. High NLR has been associated with poor prognosis in several tumors, including renal cell,12 lung,13 ovarian,14 colorectal,15 and gastric cancer.16 Elevated neutrophil counts in peripheral blood of GIST patients undergoing imatinib treatment were found to be predictive of poor treatment response and outcome.17-20 In this study, we sought to determine whether the blood NLR correlates with outcome in untreated, primary GIST.
METHODS
Patients
With approval of the Institutional Review Board and in accordance with Health Insurance Portability and Accountability Act regulations, a prospectively maintained sarcoma database was used. We identified 339 consecutive, previously untreated patients with a primary, localized GIST operated at Memorial Sloan-Kettering Cancer Center between 1995 and 2010. Patients who received adjuvant imatinib treatment (n=64) were excluded from the survival analysis. Four patients had an incomplete set of blood values and were excluded as well. Extent of disease was preoperatively assessed by an abdominal/pelvic computed tomography (CT) or magnetic resonance imaging (MRI) and chest X-ray. All patients underwent surgical resection with curative intent. Recurrence was defined as evidence of disease relapse on CT or MRI and/or positron emission tomography (PET).
Peripheral blood counts
Preoperative peripheral blood samples were collected within 10 days before surgery. No patient had clinical signs of infection at the time of blood sampling. Blood NLR was calculated as neutrophil count (number of neutrophils/μL) divided by lymphocyte count (number of lymphocytes/μL). High and low NLR were defined with respect to the sample median, which was 2.7. Similarly, the median values for the neutrophil and lymphocyte counts, the NLR, as well as the total white blood cell (WBC) count were entered into a univariate and multivariate Cox regression model. Their role as markers for recurrence- free survival (RFS) was assessed and a potential correlation with established risk criteria6 was analyzed.
Pathology
Tumor histology was established by a pathologist with expertise in sarcoma (C.R.A.). Immunohistochemical stains to determine the diagnosis of GIST were done on formalin-fixed, paraffin-embedded tumors using standard protocols, as previously described.21 Tumor size, site, and mitotic rate (number of mitoses per 50 high-power fields (HPFs)) were recorded. Mitotic rate was available in 261 (96%) cases as a categorical value (<5/HPF or ≥5/HPF) and in 200 (74%) patients as a continuous variable. Correlation analyses were performed with continuous variables. Survival analyses including mitotic rate, and tumor size and location were computed with categorical data.
Analysis of neutrophils in fresh tumor specimens
Twenty-two untreated, primary gastric GIST specimens were obtained freshly from the operating room from consenting patients under an IRB-approved protocol. Single cell suspensions were prepared by mincing tumor specimens and then digesting them in 12 mg/mL collagenase type II (Worthington Biochemicals) and 0.5 mg/mL DNAse I (Roche Diagnostics) for 30 min in a shaker at 37°. Tumor suspensions were then filtered through a 100 μm nylon mesh filter and then a 40 μm filter. Matched peripheral blood was collected in heparinized tubes in 11 patients prior to the induction of anesthesia. Peripheral blood mononuclear cells (PBMCs) were obtained using density centrifugation over a Ficoll-Paque gradient (GE Healthcare). Cell suspensions from tumors and PBMCs were then stained with fluorochrome-conjugated antibodies for CD45 (clone 2D1; APC-Cy7), CD3 (clone SK7; PE-Cy7), CD19 (clone SJ25C1; PE-Cy7), CD56 (clone B159; PE-Cy7), CD11b (clone ICRF44; PE), CD14 (clone MϕP9; APC), and CD16 (clone 3G8; AF-700), all from BD Biosciences. Fluorescence-activated cell sorting was performed using a FACSAria (BD Biosciences). Tumor neutrophils were defined as live CD45+lineage-(CD3,19,56)CD11b+CD14-CD16hi cells.
Statistical analysis
Categorical data were compared by the chi-square test. Continuous values were analyzed by the Wilcoxon-Mann-Whitney U-test. Pairwise correlations were evaluated by the two-tailed Pearson-test. RFS was defined as the time from surgical resection to radiologic evidence of recurrence. Survival curves were computed by the Kaplan-Meier product limit method and compared by the log-rank test. Median values are reported with corresponding interquartile range (IQR). Univariate and multivariate Cox proportional hazard regression models were performed to identify associations with outcome variables. ANOVA test was used to compare differences between groups. Values are shown as hazard ratio (HR) with 95% confidence interval (CI) and corresponding P value. P<0.05 was considered statistically significant. The statistical analysis was performed with IBM SPSS 19 software (SPSS Inc., Chicago, IL).
RESULTS
Demographic and tumor profiles
The median age of the entire population at diagnosis was 65 years and half of the patients were female. The most common location was stomach (70%), followed by small bowel (18%), rectum (6%), and other (5%). The median mitotic rate was 3 mitoses/50 HPF (IQR 1-10) and the median tumor size was 5 cm (IQR 3–8.5). The median 2- and 5- year nomogram-predicted RFS19 in this series was 88% and 79%, respectively. Table 1 shows the baseline demographic, tumor, and treatment characteristics stratified by NLR in 335 GIST patients. Significantly more male (58%) than female (42%) patients had a high NLR.
Table 1.
Patient, tumor, and treatment characteristics stratified by preoperative neutrophil-to-lymphocyte ratio.
| Low NLR (N = 170) | High NLR (N = 165) | P value* | |
|---|---|---|---|
| Age, years (range) | 66 (56-74) | 66 (55-75) | 0.35 |
| Sex, female (%) | 100 (59) | 70 (42) | 0.003 |
| Tumor location | |||
| • Stomach (%) | 119 (70) | 117 (71) | 0.86 |
| • Small bowel (%) | 30 (18) | 31 (19) | 0.53 |
| • Rectum (%) | 5 (3) | 15 (9) | 0.03 |
| • Other (%) | 6 (3) | 12 (7) | 0.15 |
| Imatinib treatment | |||
| • Adjuvant (%) † | 33 (19) | 31 (19) | 0.89 |
| Nomogram prediction, % | |||
| • 2- year RFS (range) | 89 (36-92) | 87 (40-96) | 0.17 |
| • 5- year RFS (range) | 81 (36-92) | 78 (17-93) | 0.22 |
NLR: neutrophil-to-lymphocyte ratio. Median NLR value = 2.7. Low NLR: ≤2.7, high NLR: >2.7. Values are shown as median and interquartile range or number of patients with corresponding percentage. RFS: recurrence-free survival.
Wilcoxon-Mann-Whitney U-test or Pearson chi-square test as appropriate.
These patients (n= 64) were excluded from recurrence-free survival analysis.
NLR and established prognostic criteria
A significant correlation was observed between blood NLR and mitotic rate (Pearson correlation coefficient (r)=0.15, P=0.03). An even stronger correlation was found between NLR and tumor size (r=0.36, P=0.0001; Figure 1a). Similarly, the blood neutrophil count correlated with tumor size (r=0.34, P=0.0001; Figure 1b). There was a tendency for reduced blood lymphocyte values in large GIST (r=-0.11, P=0.08; Figure 1c). Blood neutrophil (r=0.05, P=0.45) and blood lymphocyte count (r=-0.07, P=0.30) alone did not correlate with mitotic rate. There was a higher likelihood of having a high NLR in rectal GIST (Table 1). Patients were classified into no risk of recurrence (n=57), low (n=127), moderate (n=70), or high (n=81) according to established criteria.22 The median NLR in the high risk group (4.7) was significantly different (P=0.006) from the moderate risk group (3.3) and the low risk group (3.1).
Figure 1.
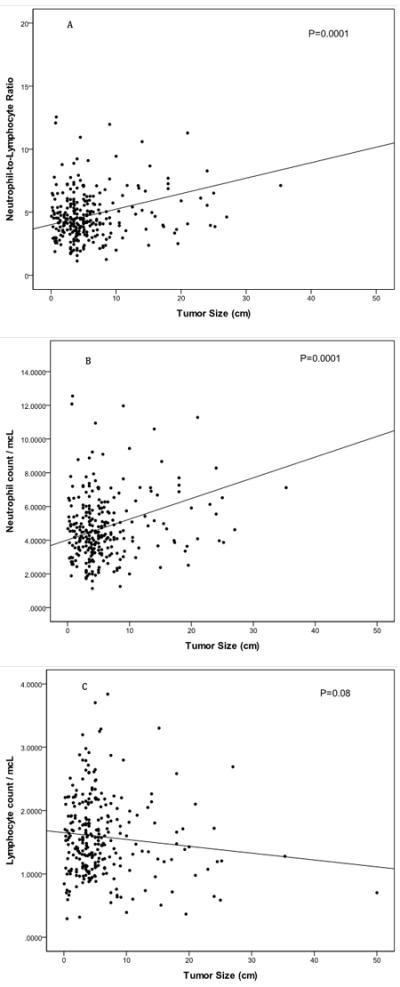
a: Tumor size is significantly (P < 0.01, Pearson correlation coefficient +0.36) associated with blood neutrophil-to-lymphocyte ratio (n=271). Patients with large tumors have high neutrophil-to-lymphocyte ratio.
b: Tumor size is significantly (P < 0.01, Pearson correlation coefficient +0.34) associated with blood neutrophil counts (n=271). Patients with large tumors have high numbers of blood neutrophils.
c: Tumor size is not significantly (P = 0.08, Pearson correlation coefficient -0.11) associated with blood lymphocyte counts (n=271). However, patients with large tumors tend to have low numbers of blood lymphocytes.
Recurrence-free survival analysis
With a median follow-up of 27 months (IQR 6-59), there were 8 (24%) recurrences in the low NLR group and 24 (76%) recurrences in the high NLR group. On univariate analysis, NLR (HR 3.3) and neutrophil count (HR 2.1), but not lymphocyte count and WBC, were associated with recurrence-free survival (Table 2). NLR was not independent from the nomogram-predicted outcome7 (P=0.12) or Miettinen risk category22 (P=0.11) in a multivariate model. Patients with a high NLR had a shorter RFS (Figure 2, P=0.002). The 1- and 5-year RFS in the high NLR group was 89% and 76%, compared to 98% and 91% in the low group, and the medians were not reached. Since we found a strong correlation between NLR and tumor size, we analyzed RFS by these 2 variables (Figure 3). Patients with tumors greater than 5 cm in size had a 1- and 5-year RFS of 94% and 81% if the NLR was low, but 80% and 57% if the NLR was high (P=0.02).
Table 2.
Univariate Cox regression analysis for recurrence-free survival.
| Variable | Univariate analysis | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| NLR | 3.3 | 1.5-7.4 | 0.003 |
| Neutrophils | 2.1 | 1.9-4.3 | 0.037 |
| Lymphocytes | 0.7 | 0.3-1.4 | 0.308 |
| WBC | 1.9 | 0.9-4.0 | 0.062 |
| Tumor size | 6.0 | 2.6-13.7 | <0.001 |
| Mitotic rate | 7.7 | 3.6-16.6 | <0.001 |
| Location* | 3.9 | 2.0-7.7 | <0.001 |
95% CI: 95% confidence interval. HR: hazard ratio. Patients after adjuvant imatinib treatment (n=64) were excluded from this analysis.
Analyzed as categorical variable for patients with non-gastric or gastric GIST.
Figure 2.
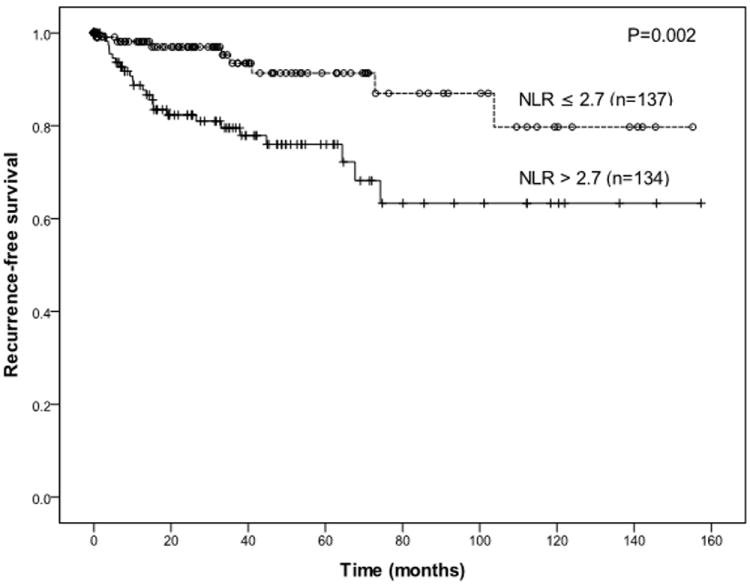
Long recurrence-free survival is significantly associated with low neutrophil-to-lymphocyte ratio (NLR) in patients with a primary GIST after curative resection.
Figure 3.
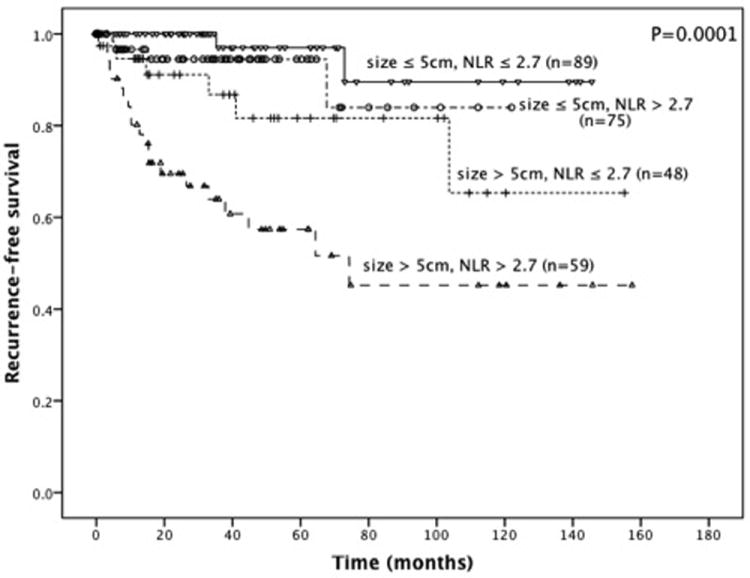
Recurrence-free survival stratified by the median neutrophil-to-lymphocyte ratio and median tumor size.
Intratumoral neutrophils
We first analyzed intratumoral neutrophils from 14 freshly obtained GIST specimens and matched blood samples by using flow cytometry. Neutrophils were defined as live CD45+lineage-(CD3,19, 56)CD11b+CD14-CD16hi cells. While there was the expected number of neutrophils in peripheral blood, the neutrophils comprised a low percentage of the immune (CD45+) cells within the tumor (Figure 4). These results were confirmed in 8 other untreated primary GISTs (data not shown).
Figure 4.
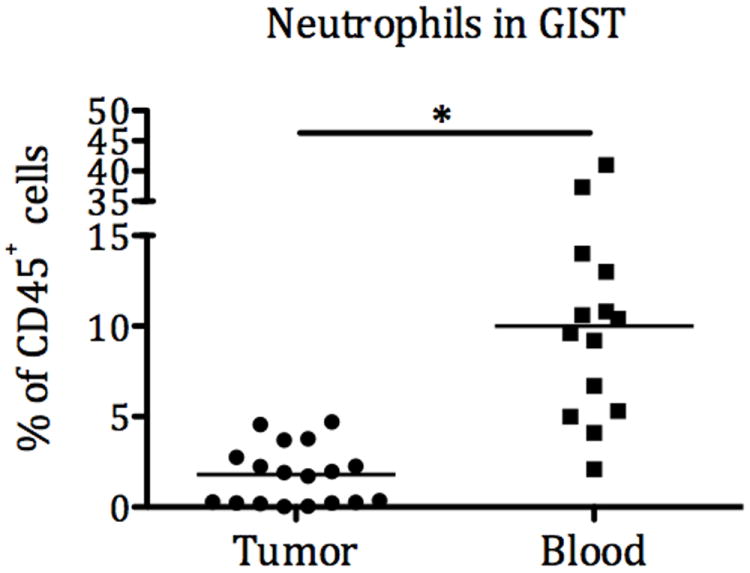
Intratumoral and peripheral blood neutrophils in 14 patients.
DISCUSSION
There is growing evidence that the immune system is important in untreated, primary GIST. We previously reported that GISTs induce an intratumoral T cell immune response.11 However, the baseline ratio of CD8 T cells to T regulatory cells was relatively unfavorable and the tumor cells expressed an immunosuppressive enzyme (indoleamine 2,3-dioxygenase), the inhibition of which played a key role in the efficacy of imatinib treatment.11 Thus, there appears to be local immune suppression in GIST. Nevertheless, tumor-restricted antigens in GIST have been identified as potential immunological targets and were shown to correlate with prognosis.23, 24 We now have discovered that an increased level of peripheral blood neutrophil cells correlates with tumor biology. High NLR was associated with high risk tumor features. Thus, high risk GISTs induce a systemic inflammatory response.
Despite decades of investigation, the interaction between cancer cells and neutrophils is poorly understood. High neutrophil counts have been associated with poor survival in patients with metastatic melanoma, metastatic renal cell, breast, and advanced non-small cell lung cancer among others.25-30 On the other hand, multiple older studies have advocated a role of direct or antibody-dependent killing of tumor cells by neutrophils,31 with one of the oldest reports originating from our institution, by William Coley.32 He observed that patients suffering from a febrile streptococcal infection within an ulcerated tumor had a better prognosis than patients with uninfected tumors. Since Coley’s initial report, knowledge about regulatory mechanisms in cellular immunity has increased vastly. However, cancer therapy utilizing a neutrophil-mediated approach has not yet been established.
In this study, the number of circulating blood lymphocytes was not associated with either RFS, or any of the established prognostic criteria (size, location, and mitotic rate). However, a non-significant correlation for reduced numbers of blood lymphocytes was seen in patients with large GISTs. In solid tumors, the pre-treatment blood lymphocyte value has not been associated with prognosis. Thus, the NLR in untreated, primary GIST seems to be mainly determined by the neutrophil count. In contrast to tumor infiltrating lymphocytes, we found that tumor infiltrating neutrophils are unlikely to be important in untreated GIST since analysis by flow cytometry indicated there were few of them. It remains unclear why neutrophils are high in peripheral blood of patients with high risk tumors but are virtually absent in tumor tissue.
There are several limitations of the present study. We excluded patients treated with adjuvant imatinib and therefore eliminated many high risk patients, which reduced the number of recurrences. Since adjuvant imatinib significantly prolongs RFS and reduces the risk of recurrence,33 we could not include such patients. However, our data in primary GIST are consistent with the fact that the baseline granulocyte count correlated to poor outcome in the European17 and North American18 Phase III trials of imatinib in advanced GIST.
In conclusion, we observed a systemic inflammatory response in untreated patients with a high risk GIST. NLR is a surrogate for poor prognosis in GIST and may represent a valuable parameter for predicting tumor biology from peripheral blood. The mechanism of neutrophilia in high risk GIST remains unclear and requires further study.
Acknowledgments
This work was supported by NIH grant CA102613, the Geoffrey Beene Cancer Research Center, Mr. J.H.L. Pit and Mrs. Pit-van Karnebeek and the Dutch GIST Foundation, GIST Cancer Research Fund, and Swim Across America (RPD).
Footnotes
Presented in part at the 2012 Society of Surgical Oncology meeting in Orlando, Florida
References
- 1.Cassier PA, Ducimetiere F, Lurkin A, et al. A prospective epidemiological study of new incident GISTs during two consecutive years in Rhone Alpes region: incidence and molecular distribution of GIST in a European region. Br J Cancer. 2010;103:165–70. doi: 10.1038/sj.bjc.6605743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247–58. doi: 10.1146/annurev-med-043010-091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 7.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045–52. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35:1646–56. doi: 10.1097/PAS.0b013e31822d63a7. [DOI] [PubMed] [Google Scholar]
- 9.Menard C, Blay JY, Borg C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–9. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- 10.Delahaye NF, Rusakiewicz S, Martins I, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–7. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 11.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48:202–8. doi: 10.1016/j.ejca.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–13. doi: 10.1158/1078-0432.CCR-10-2245. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–22. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 17.Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23:5795–804. doi: 10.1200/JCO.2005.11.601. [DOI] [PubMed] [Google Scholar]
- 18.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski P, Nowecki ZI, Debiec-Rychter M, et al. Predictive factors for long-term effects of imatinib therapy in patients with inoperable/metastatic CD117(+) gastrointestinal stromal tumors (GISTs) J Cancer Res Clin Oncol. 2007;133:589–97. doi: 10.1007/s00432-007-0202-4. [DOI] [PubMed] [Google Scholar]
- 20.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247–53. doi: 10.1200/JCO.2009.24.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10:3282–90. doi: 10.1158/1078-0432.CCR-03-0715. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Perez D, Hauswirth F, Jager D, et al. Protein expression of cancer testis antigens predicts tumor recurrence and treatment response to imatinib in gastrointestinal stromal tumors. Int J Cancer. 2011;128:2947–52. doi: 10.1002/ijc.25836. [DOI] [PubMed] [Google Scholar]
- 24.Perez D, Herrmann T, Jungbluth AA, et al. Cancer testis antigen expression in gastrointestinal stromal tumors: new markers for early recurrence. Int J Cancer. 2008;123:1551–5. doi: 10.1002/ijc.23698. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93:273–8. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atzpodien J, Royston P, Wandert T, Reitz M. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88:348–53. doi: 10.1038/sj.bjc.6600768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donskov F, Bennedsgaard KM, Hokland M, et al. Leukocyte orchestration in blood and tumour tissue following interleukin-2 based immunotherapy in metastatic renal cell carcinoma. Cancer Immunol Immunother. 2004;53:729–39. doi: 10.1007/s00262-004-0525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negrier S, Escudier B, Gomez F, et al. Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol. 2002;13:1460–8. doi: 10.1093/annonc/mdf257. [DOI] [PubMed] [Google Scholar]
- 29.Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45:1950–8. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 31.Souto JC, Vila L, Bru A. Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Med Res Rev. 2011;31:311–63. doi: 10.1002/med.20185. [DOI] [PubMed] [Google Scholar]
- 32.Richardson MA, Ramirez T, Russell NC, Moye LA. Coley toxins immunotherapy: a retrospective review. Altern Ther Health Med. 1999;5:42–7. [PubMed] [Google Scholar]
- 33.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


