Abstract
Traumatic brain injury (TBI) leads to acute functional deficit in the brain. Molecular events underlying TBI remain unclear. In mouse brains, we found controlled cortical impact (CCI) injury induced overexpression of the extracellular calcium-sensing receptor (CaSR), which is known to stimulate neuronal activity and accumulation of intracellular Ca2+ and concurrent down-regulation of type B or metabotropic GABA receptor 1 (GABA-B-R1), a prominent inhibitory pathway in the brain. These changes in protein expression preceded and were closely associated with the loss of brain tissue, as indicated by the increased size of cortical cavity at impact sites, and the development of motor deficit, as indicated by the increased frequency of right-biased swing and turn in the CCI mice. Mild hypothermia, an established practice of neuroprotection for brain ischemia, partially but significantly blunted all of the above effects of CCI. Administration of CaSR antagonist NPS89636 mimicked hypothermia to reduce loss of brain tissue and motor functions in the CCI mice. These data together support the concept that CaSR overexpression and overactivity play a causal role in potentiating TBI potentially by stimulating excitatory neuronal responses and by interfering with inhibitory GABA-B-R signaling and that the CaSR could be a novel target for neuroprotection against TBI.
Key words: calcilytics; calcium-sensing receptor; CaSR; controlled cortical impact; extracellular calcium-sensing receptor; hypothermia, neuroprotection; traumatic brain injury; type B GABA receptor
Introduction
Traumatic brain injury (TBI) is a leading cause of death and long-term morbidity throughout the world. Therapies for neuroprotection at the early phase of injury are limited. Induced mild hypothermia is a potential regimen to improve neurological outcome after TBI in preclinical animal models.1 Understanding the mechanisms that render the neuroprotective effect of hypothermia will help in identifying more specific neuroprotective targets in developing therapies for the disease.
Excitotoxicity and subsequent Ca2+ overload in the cytoplasm of the affected neurons are thought to be responsible for neuronal death after TBI,2–4 but the mechanism(s) underlying the initial cell membrane depolarization and aberrant Ca2+ mobilization remains unclear. It has been proposed that excess glutamate secretion, overactivity of ionotropic glutamate receptors/channels, and voltage-sensitive Ca2+ channel increased levels of reactive oxygen species,4 and the loss of signaling through the inhibitory transmitter γ-aminobutyric acid (GABA) contribute to the neuronal hyperactivity and eventually death. We recently showed that neuronal death is closely associated with concurrent reduction in the expression of type B or metabotropic GABA receptors (GABA-B-Rs) and increases in the expression of the extracellular Ca2+-sensing receptor (CaSR) in neurons in culture5 and in a transient global ischemia (TGI) mouse model.6
CaSR and GABA-B-Rs (R1 and R2) are close members of the family C of the G-protein coupled receptor (GPCR) superfamily,7 but they have distinct signaling properties. The CaSR was originally discovered in parathyroid cells (PTCs) where it senses minute changes in extracellular [Ca2+] ([Ca2+]e). In PTCs and other cell systems, activation of CaSR induces excitatory signaling responses, including activation of phospholipase C (PLC), Ca2+ releases from intracellular stores, and enhancement of membrane Ca2+ influx, leading to intracellular Ca2+ accumulation.7 These contrast to the inhibitory nature of GABA-B-R1/R2 signaling in the central nervous system. The CaSR is widely expressed and co-localized with GABA-B-R1 and R2 in brain regions involved in memory, cognition, motor reflexes, thirst, growth, and energy homeostasis.8
Most studies investigating the functional roles of neuronal CaSR were performed in vitro. In culture, activation of CaSR by high [Ca2+]e promotes axonal and dendritic growth of neurons from the supra cervical ganglion.9 Raising [Ca2+]e promotes chemotaxis and pulsatile release of GnRH in neuronal cell lines.10,11 Activation of CaSR by high [Ca2+]e enhances neuronal excitability in neurons in the subfornical organ.12 In cultured hippocampal neurons, the CaSR activates non-selective cation channels and Ca2+-dependent K channels and depolarizes their membrane potentials.13–15 These observations support a role for the CaSR in maintaining intracellular [Ca2+], ion permeability and secretory responses in neurons.
We have shown that GABA-B-R1 forms heteromeric complexes with the CaSR in neurons.5 Blocking GABA-B-R1 expression in hippocampal neurons in vivo or in culture increases CaSR expression and promotes cell death,5 while knocking out CaSR genes increases GABA-B-R1 expression in the same population of neurons,16 indicating mutual regulation of these two signaling molecules. Based on these observations, we hypothesize that increased CaSR expression promotes cell death by activating excitatory signaling responses and by down-regulating GABA-B-R1 expression and signaling and leads to damage in neurons subjected to ischemia and TBI. In support of this concept, we showed previously that the ischemia-induced CaSR overexpression and down-regulation of GABA-B-R1 in the injured hippocampal neurons were reduced by a hypothermia treatment.6
In the current study, we tested whether alterations in CaSR and GABA-B-R1 expression are associated with TBI-induced brain injury and motor deficits and whether these injury responses are reduced by therapeutic hypothermia or treatment with CaSR antagonist. Our data suggest that CaSR overexpression and perhaps its overactivity contribute to brain injury after TBI and that this receptor is a potential therapeutic target for the disease.
Methods
Three-months-old male C57/B6 mice (25–30g) (Simonson Laboratories) were housed and underwent surgery described in the protocol approved by the local Institutional Animal Care and Use Committee (IACUC) in accordance with NIH guidelines.
Controlled cortical impact (CCI)
CCI was performed according to a previously established protocol.17,18 Briefly, mice were anesthetized with isoflurane (5% for induction and 2% for maintenance via a nosecone) in a mixture of medical air:oxygen (3:1). Rectal temperatures were monitored throughout the procedure. For induction of CCI, anesthetized mice were fixed in a stereotaxic frame and a midline scalp incision was made, followed by a circular craniotomy (5 mm in diameter) in the left parietal plate immediately posterior to the bregma. The dura was not disrupted. CCI was performed with an automated impactor (Pinpoint Precision Cortical impactor, Hatteras Instruments, Cary, NC) with a tip size of 3 mm (in diameter) at 1.5 m/sec velocity to generate 2 mm penetration with a 100 msec dwell time.17 The excised cranial bone was replaced immediately, and the incision was then closed with suture.
Hypothermia regimen and treatment of CaSR antagonist
Injured mice were subjected to normothermia (CCI:37°C) or mild hypothermia (CCI:33°C) for 3 h, immediately after the CCI. Sham-operated mice were also subjected to normothermia (sham:37°C) or mild hypothermia (sham:33°C) as control.6,19 Mild hypothermia (33°C) was induced by applying 70% ethanol to the animal's torso, fanning, and placement on a cooling blanket. Core body temperature (monitored by a rectal probe) reached 33°C within 10 min and maintained for 3 h. We have previously established that a 33°C body temperature corresponds to a brain temperature of ≈30°C.20 The hypothermic mice were rewarmed on a heated blanket to 37°C within 30 minutes. Body temperatures in normothermic mice were maintained at 37°C for the same period, and their anesthesia was discontinued approximately 3 h later to account for the duration of cooling in the hypothermic groups.
Mice were recovered for 3 days before their brains were collected for immunohistochemical analyses or for a 14-day period during which neurobehavioral tests were performed at days 1, 3, and 7 post-injury. Cortical cavity volumes in the injured mice were measured at day 14 post-injury. Among 60 experimental mice, we observed 2 deaths (1 in the normothermic CCI group and 1 in the hypothermic CCI group).
The CaSR antagonist, NPS-89636 (NPS), was kindly provided by Dr. Edward Nemeth (MetisMedica, Toronto, Canada). This compound blocks CaSR-mediated signaling responses and cellular activities in human embryonic kidney (HEK)-293 cells transfected with the receptor cDNA and in osteoblasts that express the receptor endogenously.21–23 For drug treatment, NPS was applied topically at the site of injury immediately after CCI (1 μg in 10 μL phosphate-buffered saline, PBS), before closing the craniotomy, followed by daily doses of NPS via an intraperitoneal route (1 mg/kg body weight) for 3 days in mice for assessing CaSR and GABA-B-R1 expression in their brains and for 14 days in mice subjected to behavioral tests and measurement of brain lesion size.
Analyses of brain samples
For immunohistochemical detection of CaSR and GABA-B-R1, brain samples were prepared and analyzed 3 days after the surgery with or without hypothermia and/or NPS treatment (n=3 mice/group) as described previously.6 Briefly, mice were anesthetized and transcardially perfused with saline and then paraformaldehyde (PFA, 4%). Brains were dissected, post-fixed in 4% PFA, incubated in 20% sucrose at 4°C for 48 h, frozen, and cryosectioned (10 μm in thickness). Brain sections were incubated sequentially with 0.1% hydrogen peroxidase (3 min), a blocking buffer (0.5% Triton X-100, 0.1% BSA, 1.5% normal horse serum in PBS) for 30 min, and custom-made rabbit anti-CaSR (1:100) or guinea pig anti-GABA-B-R1 (1:1000) antibodies overnight at 4°C.5,6 Immunoreactivity was amplified and detected with biotinylated anti-rabbit immunoglobulin G (IgG) (1:200; Vector Laboratories, CA) or anti-guinea pig IgG (1: 500; Sigma, MO), peroxidase-conjugated avidin (ABC Elite, Vector Laboratories, CA) and diaminobenzidine substrate. Sections were counterstained with hematoxylin (Sigma, MO).
To measure the size of the lesion, brain samples were collected 14 days after surgery with or without hypothermia and/or NPS treatment (n=9 mice/group) and cut into 50-μm serial sections spanning the site of injury (∼2 mm, beginning near bregma). Sections were stained with hematoxylin and eosin (H&E). A total number (nt) of 30–35 sections were collected from each brain and divided into continuous groups (6 or 7 groups depending on the size of the lesion) with five serial sections per group. The first and second sections from each group of five (a total of 12–14 representative sections, ns), which were evenly distributed across the cavity, were chosen for analyses. Images of H&E-stained brain sections were taken by a Zeiss Axio Imager and analyzed using NIH Image J (v. 1.45) to estimate the cavity volume (in μm3). We measured the cavity area from digital images of the representative sections and multiplied it by the thickness to approximate the cavity volume in each section (As×50 μm=Vs). The averaged volumes (ΣVs/ns) from the representative sections were then multiplied by the total number of sections (nt) from each brain to estimate the total cavity volumes.
Behavior tests
For the swing test, mice were suspended vertically by the tail with their heads lifted 3 inches above the test bench. Swings were recorded when mice moved their heads >10 degrees away from the vertical axis.24 A total of 20 swings were counted in each trial, and frequency of right-biased swing was calculated for each animal.
The corner test was performed as previously described by our group.25 Two sheets of cardboard measuring 20×30 cm were placed in the animal's home cage and positioned to create a 30-degree corner with a 0.5–1 cm gap, which permitted light to shine through. Mice were placed between the boards and encouraged to enter the corner. Once the animals approached the corner, their vibrissae were stimulated by the cardboard, causing the animals to rear forward and turn away from the corner. The direction of turn was recorded. Turns made by mice that did not firmly press their faces into the corner opening were not counted. Each animal was tested 20 times, and the frequency of turn toward the ipsilateral injured hemisphere (right) was calculated.
Statistical analysis
Statistics were performed by Student t test for measurements of cortical lesion size and by one-way analysis of variance with Holm-Sidak post hoc test for behavioral tests (Systat Software, Inc., CA). A p value<0.05 was signed for >95% confidence interval between groups. All data were expressed as mean±standard error.
Results
Hypothermia reduced the size of cortical lesion following TBI
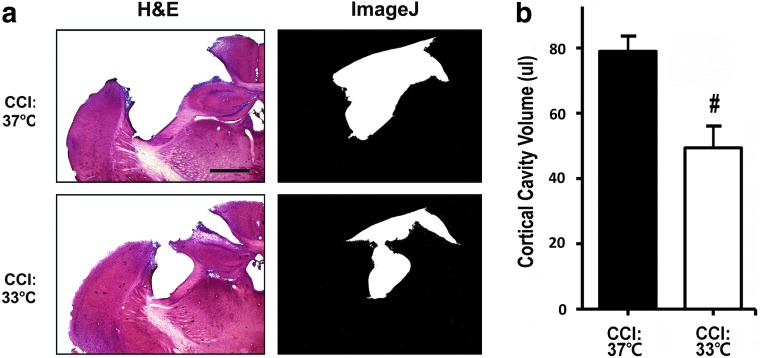
To study the neuroprotective effect of hypothermia against TBI, we compared the degree of injury in mouse brains subjected to CCI with or without a 3-h hypothermia treatment. After a 14-day recovery period, the size of cortical brain lesion in H&E-stained sections was quantified as detailed in Methods. As shown in Figure 1, at normal body temperature, CCI caused significant losses of neuron and tissue (both grey and white matter), as indicated by an increase in cortical cavity volume at the impact site (Fig. 1a,1b; CCI:37°C). Lowering the core body temperature from 37°C to 33°C significantly reduced the impact of CCI on the brain lesion size as indicated by a smaller cortical cavity volume (Fig. 1a,1b, CCI:37°C vs. CCI:33°C).
FIG. 1.
Effects of hypothermia on the development of cortical lesion in controlled cortical impact (CCI) mice. (a) Left panels show representative hematoxylin & eosin (H&E)-stained sections obtained from the center of the brain legion in C57B6 mice subjected to CCI at the normothermic (CCI:37°C, n=9 mice) or hypothermic (CCI:33°C, n=9 mice) condition for 3 h, followed by 14-day recovery. Right panels show delineation of cortical lesion area (white area) from the corresponding images on the left by using the NIH Image J software. Bar=100 μm (b). The volume of brain lesion in CCI mice was quantified using a series of H&E stained sections spanning the entire cortical cavity as described in Methods. Statistical analysis showed a significant reduction in the averaged lesion volume in hypothermic vs. normothermic CCI groups. #p<0.05.
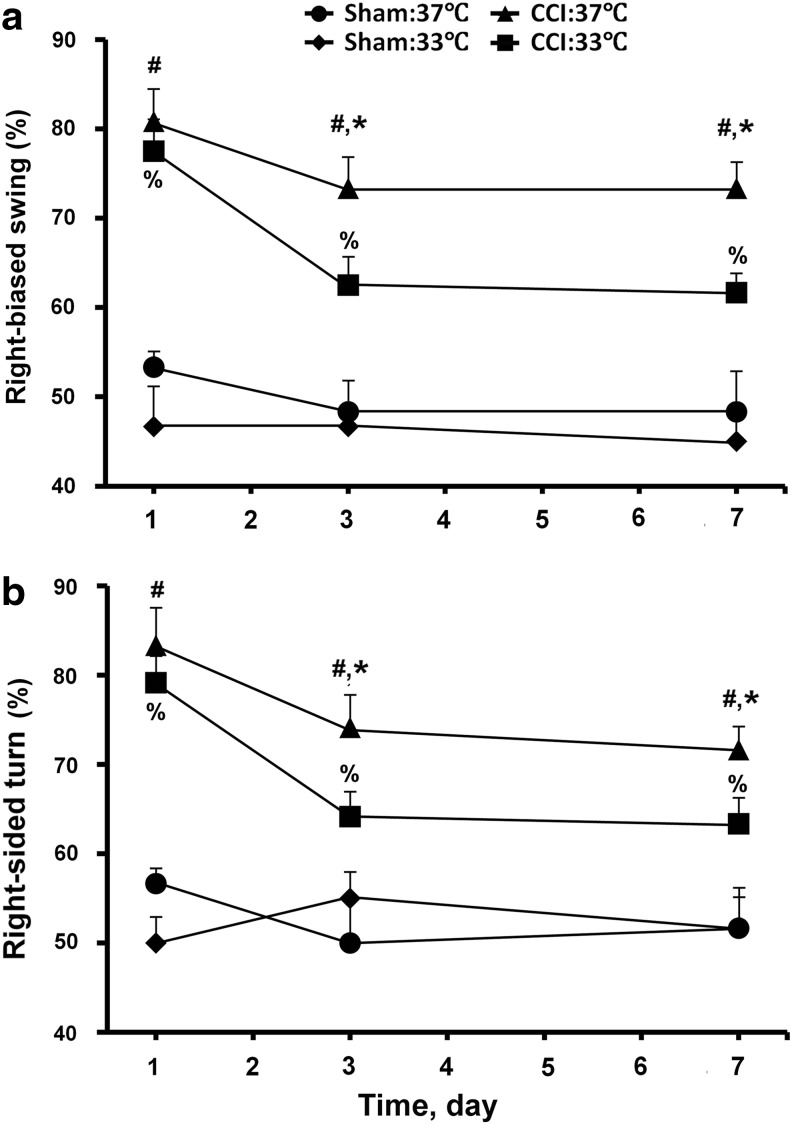
Hypothermia facilitates the recovery of motor functions in mice subjected to TBI
To determine whether the neuroprotective effects of hypothermia reduce the loss of motor function in the mice subjected to CCI, we performed tail-suspended swing and corner-turn tests on the mice with or without CCI and/or hypothermia treatment. In normothermic CCI-treated animals, we observe significant increases (p<0.05) in the frequency of right-bias swing (Fig. 2a, CCI:37°C vs. sham:37°C) and corner turn (Fig. 2b, CCI:37°C vs. sham:37°C) vs. control animals at days 1, 3, and 7 post-injury. While hypothermia did not alter these motor functions in sham-control mice (Fig. 2a,2b, sham:33°C vs. sham:37°C), it significantly reduced the ability of CCI to cause the right-bias swings (Fig. 2a, CCI:33°C vs. CCI:37°C) and corner turns (Fig. 2b, CCI:33°C vs. CCI:37°C) at days 3 and 7 post-injury. The effects of hypothermia were partial, however, becuse the frequencies of right-bias swing (Fig. 2a, CCI:33°C vs. sham:33°C) and corner turn (Fig. 2b, CCI:33°C vs. sham:33°C) remain significantly (p<0.05) higher in the hypothermic CCI vs. sham mice.
FIG. 2.
Effects of hypothermia on motor functions in controlled cortical impact (CCI) mice. (a) Swing and (b) corner tests were performed on mice subjected to CCI with 3 h of normothermia (CCI:37°C; n=6 mice) or hypothermia (CCI:33°C; n=6 mice) and on mice subjected to sham procedures with 3 h of normothermia (sham:37°C; n=3 mice) or hypothermia (sham:33°C; n=3 mice). The tests were performed at days 1, 3, and 7 post-injury. The frequency of right-biased swing and corner turn was calculated after 20 trials for each test on each animal. #p<0.01, CCI:37°C vs. sham:37°C; *p<0.01, CCI:37°C vs. CCI:33°C; %p<0.01, CCI:33°C vs. sham:33°C.
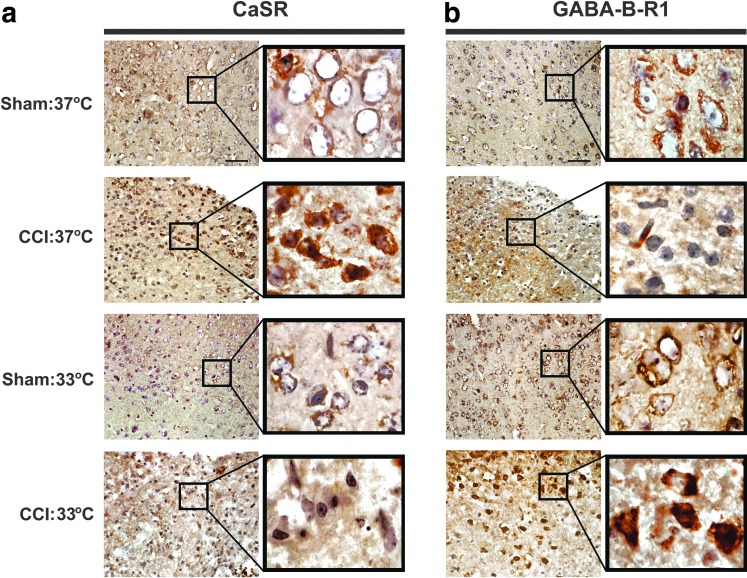
Hypothermia blunted the effects of TBI on CaSR overexpression and GABA-B-R1 down-regulation
Our previous observations, which closely associated neuronal death with increased CaSR and decreased GABA-B-R1 expression in mice subjected to TGI, prompted us to determine whether changes in CaSR and GABA-B-R1 expression precede and contribute to brain injury in the CCI mice. In sham controls, we detected CaSR expression at modest levels in the cell membrane of cortical neurons (Fig. 3a, Sham:37°C) and robust GABA-B-R1 expression in the same cell populations (Fig. 3b, Sham:37°C). In brain samples obtained from normothermic CCI mice at day 3 post-injury, we observed profound increases in CaSR expression in cortical neurons at the impact site with substantial amount of receptor protein localized intracellularly (Fig. 3a, Sham:37°C vs. CCI:37°C). In contrast, CCI at 37°C caused a decrease in GABA-B-R1 expression in the injured neurons (Fig. 3b, Sham:37°C vs CCI:37°C).
FIG. 3.
Effects of controlled cortical impact (CCI) and hypothermia on the expression of calcium-sensing receptor (CaSR) and GABA-B-R1 protein in neurons at impact sites. Immunohistological detection of (a) CaSR and (b) GABA-B-R1 in cortical neurons in mice subjected to CCI with hypothermia (CCI:33°C, n=3 mice) or normothermia (CCI:37°C, n=3 mice) vs. sham control mice with hypothermia (sham:33°C, n=3 mice) or normothermia (CCI:37°C, n=3 mice). Black boxes contain images of higher-power view at corresponding brain regions. Brown diamino benzidine stain depicts immunoreactivity in the brain sections counterstained with hematoxylin. Bar=50 μm.
While the hypothermic regimen had little impact on the CaSR and GABA-B-R1 expression in cortical neurons in sham-control mice (Fig. 3a,3b, Sham:37°C vs. Sham:33°C), it significantly blunted the ability of CCI to up-regulate CaSR and down-regulate GABA-B-R1 expression in the neurons at the impact site (Fig. 3a and 3b, CCI:37°C vs CCI:33°C). In fact, GABA-B-R1 expression was increased by CCI under the hypothermic condition. The data suggest that the CCI-induced CaSR overexpression and GABA-B-R1 down-regulation may play a role in causing brain injury and motor deficit.
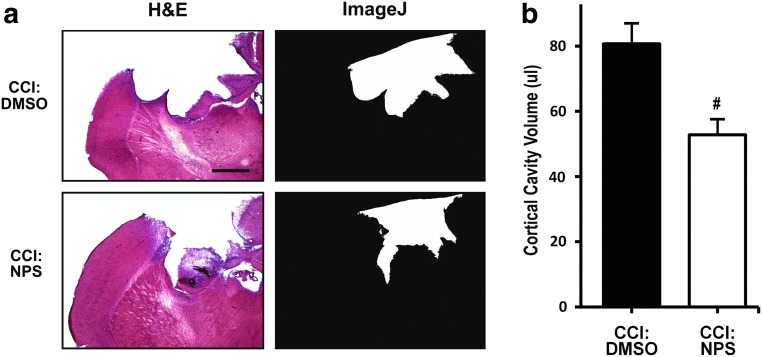
CaSR antagonists suppressed the effects of CCI on cortical lesion and motor deficit
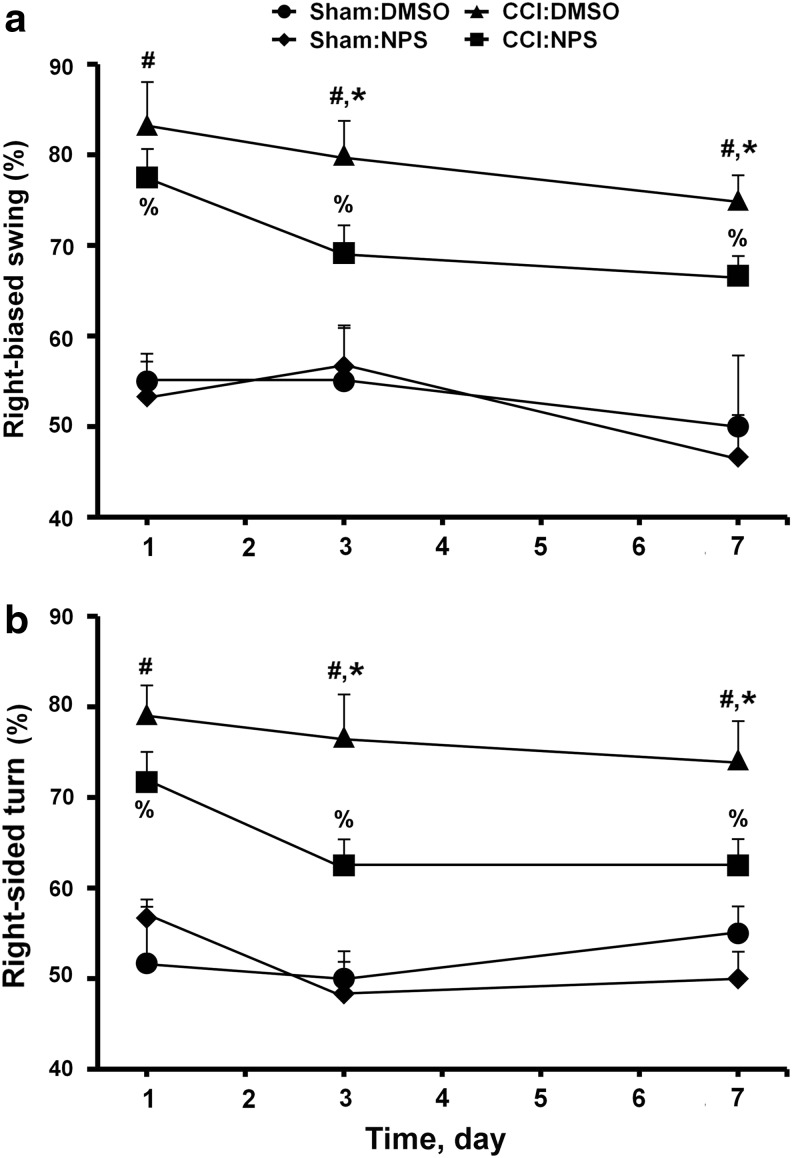
To determine whether CaSR overactivity from the overexpression of the receptor contributes to the CCI-induced neurotrauma, we tested the effects of CaSR antagonist NPS on the ability of CCI to induce tissue damage and motor deficit. NPS89636 is known to suppress the ability of CaSR to activate PLC and release Ca2+ from intracellular stores in native cells or HEK-293 cells transfected with the receptor cDNA.21–23 In normothermic CCI-treated mice, NPS treatment significantly reduced the size of the lesion, compared with that in the vehicle (dimethyl sulfoxide [DMSO])-treated mice at day 3 post-injury (Fig. 4a,4b, CCI:DMSO vs CCI:NPS). Consistent with this neuroprotective effect, NPS administration improved motor functions in the CCI-treated mice as indicated by a significant reduction in the frequency of right-biased swing and corner turn when compared with DMSO controls at day 3 and 7 post-injury (Fig. 5a,5b, CCI:DMSO vs. CCI:NPS). The compound has no significant effect on the motor functions in sham mice (Fig. 5a,5b, sham:DMSO vs. sham:NPS). The effects of NPS were also partial, because the frequencies of CCI-induced right-bias swing and corner turn remain significantly (p<0.05) higher in the NPS-treated mice (Fig. 4a,4b, CCI:NPS vs. sham:NPS). Overall, the degree of neuroprotection by NPS is comparable to that conferred by hypothermia.
FIG. 4.
Effects of calcium-sensing receptor (CaSR) antagonist (NPS89636) on cortical lesion in controlled cortical impact (CCI) mice. (a) Left panels show hematoxylin and eosin (H&E) staining of representative brain sections obtained from the center of the brain legion in mice subjected to CCI under normothermic condition with administration of NPS89636 (CCI:NPS; n=9 mice) or dimethyl sulfoxide (DMSO) (CCI:DMSO; n=9 mice) after 14 days of recovery. Right panels show delineation of cortical lesion area (white area) from the corresponding images on their left using the NIH Image J software. Bar=100 μm (b). The volume of the entire cortical cavity in CCI mice was quantified as described in Methods. Statistical analysis showed a significant reduction in the averaged lesion volume in NPS vs. DMSO groups. #p<0.05.
FIG. 5.
Effects of NPS compound on motor functions in controlled cortical impact CCI mice. (a) Swing and (b) corner tests were performed on mice subjected to CCI or with (CCI:NPS; n=6 mice) or without (CCI:dimethyl sulfoxide [DMSO]; n=6 mice) NPS89636 treatment and on mice subjected to sham procedures with (sham:NPS; n=3 mice) or without (sham:DMSO; n=3 mice) NPS89636 treatment. The tests were performed at days 1, 3, and 7 post-injury. The frequency of right-biased swing and corner turn was calculated after 20 trials for each test on each animal. #p<0.01, sham:DMSO vs. CCI:DMSO); *p<0.05, **p<0.01, CCI:NPS vs. CCI:DMSO; %p<0.01, CCI:NPS vs. sham:NPS.
Discussion
Studies on the early molecular events leading to brain injury are essential in developing effective therapies for neuroprotection against TBI. One approach for such investigations is to delineate mechanisms rendering the effects of the already known neuroprotective regimens, such as hypothermia.6,26,27 In this study, we confirmed the neuroprotective action of hypothermia in TBI by demonstrating its ability to reduce brain lesion size and to facilitate the recovery of motor function in an established CCI injury model. We further showed that CCI-induced CaSR overexpression and concurrent down-regulation of GABA-B-R1 occurred before apparent neurodegeneration and that these effects were partially averted by hypothermia, suggesting that alterations in CaSR and GABA-B-R1 expression may be a part of brain injury response after TBI.
The CCI-induced CaSR overexpression is anticipated to promote neuronal hyperactivity based on the ability of this receptor to stimulate excitatory signaling responses and to accumulate intracellular Ca2+. In addition, the reduced inhibitory GABA tone because of the decreased GABA-B-R1 expression could further exacerbate these neurodegenerative responses in the affected cells. In supporting a role for the CaSR overexpression/overactivity in promoting injury response to TBI, we showed that the CaSR-specific antagonist, NPS89636, was protective to an extent similar to that of therapeutic hypothermia. This protective effect is likely because of direct inhibition of the CaSR in the affected neurons, but not the ability of NPS89636 to transiently increase serum PTH level (from inhibition of CaSR in the parathyroid gland) and produce hypercalcemia, because the latter systemic effects will activate CaSR and are anticipated to counteract the inhibitory actions of the compound on the injured neurons. Whether these systemic effects (increasing serum PTH and Ca2+ levels) actually reduce the effectiveness of the compound in neuroprotection remains to be confirmed. Nevertheless, our data suggest that the CaSR could be a potential therapeutic target for neuroprotection, despite more selective CaSR antagonists that produce less calcemic effects may be needed to optimize the protection.
It remains unclear how TBI caused CaSR overexpression in the affected neurons in the first place. Our previous work showed that brain ischemia induced by transient occlusion of bilateral carotid arteries causes a robust increase in CaSR overexpression, suggesting that disrupted blood–brain barrier and blood flow and the eventually local ischemic stress may contribute to CaSR overexpression in TBI. Alternatively, physical insults to neurons and/or local inflammatory responses could also be a factor. Further investigations are needed to distinguish these possibilities.
Concurrent changes in CaSR and GABA-B-R1 expression suggest a close interplay between these two receptors in response to injury. Although detailed mechanisms underlying the mutual regulation of CaSR and GABA-B-R1 remain to be defined, our previous work suggests that physical interactions between these two receptor proteins can impact their trafficking and stability. CaSR, GABA-B-R1, and GABA-B-R2 are close members of the family C GPCR and have strong structural homologies.7 These three receptors can form heteromeric complexes in neurons expressing the receptors endogenously and in HEK-293 cells transfected with the receptor cDNAs.5 Co-expression of the GABA-B-R1 suppressed the CaSR expression and blunted its ability to activate PLC in the HEK-293 cells, suggesting that the GABA-B-R1 is a negative regulator of CaSR expression.5 This notion is further supported by the observation that knocking out the GABA-B-R1 gene in mouse brains in vivo and in cultured hippocampal neurons increased the CaSR expression.5 On the other hand, we found that conditional knockout of the CaSR genes in hippocampal neurons averted down-regulation of GABA-B-R1 in response to TGI (article in preparation), suggesting that CaSR is a negative regulator of GABA-B-R1 expression. These data suggest that interactions between CaSR and GABA-B-R1 destabilize these protein complexes.
On the other hand, in HEK-293 cells transfected with different combinations of receptor cDNA, we found that co-expression of GABA-B-R2 stabilizes the total and cell-surface expression of CaSR or GABA-B-R1 and that the CaSR tends to compete with GABA-B-R1 for binding to GABA-B-R2.5 It appears that changes in the stoichiometry of these three receptors may determine their overall and perhaps cell-surface expression and functionality through the formation of distinct protein complexes. We hypothesize that in neurons subjected to TBI or ischemia injury, the CaSR overexpression could have increased excitatory responses in the affected neurons not only by increasing CaSR/CaSR homomeric complexes but also by interfering with formation of GABA-B-R1/R2 heterodimers that are required for protein stability and GABA signaling.
The role of CaSR or GABA-B-Rs in brain injury that we observed in our animal model needs further validation in human subjects. Some clinical studies, however, suggest that those pathways may be relevant. Brain biopsies from patients with temporal lobe epilepsy, from trauma, anoxic injury, and other causes, show reduced GABA-B-R1 expression in the hippocampal formation.28 Previous studies on brain injury models have shown that increasing GABA-ergic tone through agonists acting on GABA-A receptors can limit the extent of brain injury.29 Clomethiazole, a compound thought to act on the GABA-A receptor, was studied at the preclinical level and shown to be neuroprotective in brain ischemia models.30–32 Clinical studies suggested a beneficial effect in a subgroup of stroke patients,33 but follow-up studies failed to demonstrate a therapeutic effect.34 Here, our studies point to additional therapeutic targets, namely CaSR, whose inhibition could potentially enhance neuroprotection against TBI and perhaps other forms of brain injury. Antagonists (calcilytics) of the CaSR have been developed to treat CaSR-mediated bone diseases,35 so these compounds are readily available for future trials in other clinically relevant TBI animal models and ultimately in patients with TBI.
Acknowledgments
We thank Dr. Edward Nemeth for providing the invaluable NPS89636 compound for this study. This work was supported by grants from: NIH NIA (AG21353 to WC), NINDS (NS40516, P50 NS014543 to MAY), the Department of Defense (WC, MAY), and Department of Veterans Affairs (WC, MAY).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dietrich W.D. Bramlett H.M. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young W. Role of calcium in central nervous system injuries. J. Neurotrauma. 1992;9(Suppl 1):S9–S25. [PubMed] [Google Scholar]
- 3.Farooqui A.A. Horrocks L.A. Involvement of glutamate receptors, lipases, and phospholipases in long-term potentiation and neurodegeneration. J. Neurosci. Res. 1994;38:6–11. doi: 10.1002/jnr.490380103. [DOI] [PubMed] [Google Scholar]
- 4.Delorenzo R.J. Sun D.A. Deshpande L.S. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol. Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang W. Tu C. Cheng Z. Rodriguez L. Chen T.H. Gassmann M. Bettler B. Margeta M. Jan L.Y. Shoback D. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J. Biol. Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.Y. Kim N. Yenari M.A. Chang W. Mild hypothermia suppresses calcium-sensing receptor (CaSR) induction following forebrain ischemia while increasing GABA-B receptor 1 (GABA-B-R1) expression. Transl. Stroke Res. 2012;2:195–201. doi: 10.1007/s12975-011-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang W. Shoback D. Extracellular Ca2+-sensing receptors—an overview. Cell Calcium. 2004;35:183–196. doi: 10.1016/j.ceca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Yano S. Brown E.M. Chattopadhyay N. Calcium-sensing receptor in the brain. Cell Calcium. 2004;35:257–264. doi: 10.1016/j.ceca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Vizard T.N. O'Keeffe G.W. Gutierrez H. Kos C.H. Riccardi D. Davies A.M. Regulation of axonal and dendritic growth by the extracellular calcium-sensing receptor. Nat. Neurosci. 2008;11:285–291. doi: 10.1038/nn2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay N. Jeong K.H. Yano S. Huang S. Pang J.L. Ren X. Terwilliger E. Kaiser U.B. Vassilev P.M. Pollak M.R. Brown E.M. Calcium receptor stimulates chemotaxis and secretion of MCP-1 in GnRH neurons in vitro: potential impact on reduced GnRH neuron population in CaR-null mice. Am. J. Physiol. Endocrinol. Metab. 2007;292:E523–E532. doi: 10.1152/ajpendo.00372.2005. [DOI] [PubMed] [Google Scholar]
- 11.Bandyopadhyay S. Jeong K.H. Hansen J.T. Vassilev P.M. Brown E.M. Chattopadhyay N. Calcium-sensing receptor stimulates secretion of an interferon-gamma-induced monokine (CXCL10) and monocyte chemoattractant protein-3 in immortalized GnRH neurons. J. Neurosci. Res. 2007;85:882–895. doi: 10.1002/jnr.21177. [DOI] [PubMed] [Google Scholar]
- 12.Washburn D.L. Smith P.M. Ferguson A.V. Control of neuronal excitability by an ion-sensing receptor (correction of anion-sensing) Eur. J. Neurosci. 1999;11:1947–1954. doi: 10.1046/j.1460-9568.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 13.Ye C.P. Yamaguchi T. Chattopadhyay N. Sanders J.L. Vassilev P.M. Brown E.M. Extracellular calcium-sensing-receptor (CaR)-mediated opening of an outward K(+) channel in murine MC3T3-E1 osteoblastic cells: evidence for expression of a functional CaR. Bone. 2000;27:21–27. doi: 10.1016/s8756-3282(00)00288-x. [DOI] [PubMed] [Google Scholar]
- 14.Chattopadhyay N. Ye C. Yamaguchi T. Nakai M. Kifor O. Vassilev P.M. Nishimura R.N. Brown E.M. The extracellular calcium-sensing receptor is expressed in rat microglia and modulates an outward K+ channel. J. Neurochem. 1999;72:1915–1922. doi: 10.1046/j.1471-4159.1999.0721915.x. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay N. Ye C.P. Yamaguchi T. Vassilev P.M. Brown E.M. Evidence for extracellular calcium-sensing receptor mediated opening of an outward K+ channel in a human astrocytoma cell line (U87) Glia. 1999;26:64–72. doi: 10.1002/(sici)1098-1136(199903)26:1<64::aid-glia7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.Y. Ho H. Nemeth E. Yenari M.A. Chang W. Pharmacological inhibition of the extracellular calcium-sensing receptor (CaSR) protects hippocampal neuronal death in a transient global ischemia mouse model. Presented at the 41th Society for Neuroscience Annual Meeting; Washington, D.C.. 2011. (Abstract). [Google Scholar]
- 17.Chang E.F. Wong R.J. Vreman H.J. Igarashi T. Galo E. Sharp F.R. Stevenson D.K. Noble-Haeusslein L.J. Heme oxygenase-2 protects against lipid peroxidation-mediated cell loss and impaired motor recovery after traumatic brain injury. J. Neurosci. 2003;23:3689–3696. doi: 10.1523/JNEUROSCI.23-09-03689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potts M.B. Adwanikar H. Noble-Haeusslein L.J. Models of traumatic cerebellar injury. Cerebellum. 2009;8:211–221. doi: 10.1007/s12311-009-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Povlishock J.T. Wei E.P. Posthypothermic rewarming considerations following traumatic brain injury. J. Neurotrauma. 2009;26:333–340. doi: 10.1089/neu.2008.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yenari M.A. Onley D. Hedehus M. deCrespigny A. Sun G.H. Moseley M.E. Steinberg G.K. Diffusion- and perfusion-weighted magnetic resonance imaging of focal cerebral ischemia and cortical spreading depression under conditions of mild hypothermia. Brain Res. 2000;885:208–219. doi: 10.1016/s0006-8993(00)02942-5. [DOI] [PubMed] [Google Scholar]
- 21.Dvorak M.M. Siddiqua A. Ward D.T. Carter D.H. Dallas S.L. Nemeth E.F. Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5140–5145. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies S.L. Gibbons C.E. Vizard T. Ward D.T. Ca2+-sensing receptor induces Rho kinase-mediated actin stress fiber assembly and altered cell morphology, but not in response to aromatic amino acids. Am. J. Physiol. Cell Physiol. 2006;290:C1543–C1551. doi: 10.1152/ajpcell.00482.2005. [DOI] [PubMed] [Google Scholar]
- 23.Davies S.L. Ozawa A. McCormick W.D. Dvorak M.M. Ward D.T. Protein kinase C-mediated phosphorylation of the calcium-sensing receptor is stimulated by receptor activation and attenuated by calyculin-sensitive phosphatase activity. J. Biol. Chem. 2007;282:15048–15056. doi: 10.1074/jbc.M607469200. [DOI] [PubMed] [Google Scholar]
- 24.Borlongan C.V. Sanberg P.R. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J. Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X.N. Wang Q. Koike M.A. Cheng D. Goris M.L. Blankenberg F.G. Yenari M.A. Monitoring the protective effects of minocycline treatment with radiolabeled annexin V in an experimental model of focal cerebral ischemia. J. Nucl. Med. 2007;48:1822–1828. doi: 10.2967/jnumed.107.041335. [DOI] [PubMed] [Google Scholar]
- 26.Dixon C.E. Markgraf C.G. Angileri F. Pike B.R. Wolfson B. Newcomb J.K. Bismar M.M. Blanco A.J. Clifton G.L. Hayes R.L. Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J. Neurotrauma. 1998;15:95–103. doi: 10.1089/neu.1998.15.95. [DOI] [PubMed] [Google Scholar]
- 27.Shah M.P. Zimmerman L. Bullard J. Yenari M.A. Therapeutic hypothermia after cardiac arrest: experience at an academically affiliated community-based veterans affairs medical center. Stroke Res. Treat. 20112011:791639. doi: 10.4061/2011/791639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz A. Arellano J.I. DeFelipe J. GABABR1 receptor protein expression in human mesial temporal cortex: changes in temporal lobe epilepsy. J. Comp. Neurol. 2002;449:166–179. doi: 10.1002/cne.10287. [DOI] [PubMed] [Google Scholar]
- 29.Sun J. Murphy E. Calcium-sensing receptor: a sensor and mediator of ischemic preconditioning in the heart. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1309–H1317. doi: 10.1152/ajpheart.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyden P. Jacoby M. Schim J. Albers G. Mazzeo P. Ashwood T. Nordlund A. Odergren T. The Clomethiazole Acute Stroke Study in tissue-type plasminogen activator-treated stroke (CLASS-T): final results. Neurology. 2001;57:1199–1205. doi: 10.1212/wnl.57.7.1199. [DOI] [PubMed] [Google Scholar]
- 31.Lyden P. Shuaib A. Ng K. Levin K. Atkinson R.P. Rajput A. Wechsler L. Ashwood T. Claesson L. Odergren T. Salazar-Grueso E. Clomethiazole Acute Stroke Study in ischemic stroke (CLASS-I): final results. Stroke. 2002;33:122–128. doi: 10.1161/hs0102.101478. [DOI] [PubMed] [Google Scholar]
- 32.Wahlgren N.G. Lyden P. Neuroprotectants in the treatment of stroke—an overview. J. Stroke Cerebrovasc. Dis. 2000;9:32–35. doi: 10.1053/jscd.2000.19320. [DOI] [PubMed] [Google Scholar]
- 33.Gilby K.L. Sydserff S.G. Robertson H.A. Differential neuroprotective effects for three GABA-potentiating compounds in a model of hypoxia-ischemia. Brain Res. 2005;1035:196–205. doi: 10.1016/j.brainres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Wahlgren N.G. Ranasinha K.W. Rosolacci T. Franke C.L. van Erven P.M. Ashwood T. Claesson L. Clomethiazole acute stroke study (CLASS): results of a randomized, controlled trial of clomethiazole versus placebo in 1360 acute stroke patients. Stroke. 1999;30:21–28. doi: 10.1161/01.str.30.1.21. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S. Matheny C.J. Hoffman S.J. Marquis R.W. Schultz M. Liang X. Vasko J.A. Stroup G.B. Vaden V.R. Haley H. Fox J. DelMar E.G. Nemeth E.F. Lago A.M. Callahan J.F. Bhatnagar P. Huffman W.F. Gowen M. Yi B. Danoff T.M. Fitzpatrick L.A. An orally active calcium-sensing receptor antagonist that transiently increases plasma concentrations of PTH and stimulates bone formation. Bone. 2009;46:534–542. doi: 10.1016/j.bone.2009.09.028. [DOI] [PubMed] [Google Scholar]