Abstract
Hemorrhagic pneumonia can be a major cause of mortality in farmed mink in the fall. In its classic form, hemorrhagic pneumonia is caused by the bacterium Pseudomonas aeruginosa. In recent years, however, outbreaks of this type of pneumonia that are associated with hemolytic Escherichia coli have also occurred in farmed mink. The purpose of this study was to compare histological lesions of acute hemorrhagic pneumonia associated with both P. aeruginosa and E. coli in mink, including a description of tissue distribution of pathogens, in an attempt to differentiate between the 2 disease entities based on histopathology. The study included material submitted for diagnostic investigation to the National Veterinary Institute in Denmark from 2006 to 2009. Altogether, 19 cases of hemorrhagic pneumonia with a pure lung culture of P. aeruginosa and 18 cases of hemorrhagic pneumonia with a pure lung culture of E. coli were examined. Formalin-fixed paraffin-embedded lung tissue obtained from the mink was examined by histology and fluorescence in-situ hybridization (FISH). It was possible to detect a slight histological difference between hemorrhagic pneumonia caused by P. aeruginosa and by E. coli, as P. aeruginosa was most often found surrounding blood vessels and lining the alveoli, while E. coli showed a more diffuse distribution in the lung tissue. Furthermore, P. aeruginosa often elicited a very hemorrhagic response in the lung, while infection with E. coli was associated with a higher frequency of alveolar edema and mild lymphoid cuffing in the lungs.
Résumé
À l’automne, la pneumonie hémorragique peut être une cause majeure de mortalité chez les visons d’élevage. Dans sa forme classique, la pneumonie hémorragique est causée par la bactérie Pseudomonas aeruginosa. Au cours des dernières années toutefois, des poussées de cas de ce type de pneumonie associées à des isolats hémolytiques d’Escherichia coli se sont produits chez des visons d’élevage. Le but de cette étude était de comparer les lésions histologiques de pneumonie hémorragique aiguë associée à P. aeruginosa et E. coli, incluant une description de la distribution tissulaire des agents pathogènes, dans une tentative de différencier les deux maladies en se basant sur l’histopathologie. L’étude incluait du matériel soumis pour diagnostic à l’Institut National Vétérinaire du Danemark entre 2006 et 2009. Au total, 19 cas de pneumonie hémorragique avec culture pure de P. aeruginosa et 18 cas de pneumonie hémorragique avec une culture pure d’E. coli ont été examinés. Du tissu pulmonaire provenant des visons fixé à la formaline et enrobé de paraffine a été soumis à un examen histologique et par hybridation avec fluorescence in-situ (FISH). Il a été possible de détecter une légère différence histologique entre la pneumonie hémorragique causée par P. aeruginosa et E. coli, P. aeruginosa étant retrouvé plus fréquemment entourant les vaisseaux sanguins et tapissant les alvéoles, alors qu’E. coli montrait une distribution plus diffuse dans le tissu pulmonaire. De plus, P. aeruginosa causait souvent une réponse très hémorragique dans le poumon, alors que l’infection par E. coli était associée avec une fréquence plus élevée d’oedème alvéolaire et de présence de manchons lymphoïdes dans les poumons.
(Traduit par Docteur Serge Messier)
Introduction
Outbreaks of fatal hemorrhagic pneumonia caused by P. aeruginosa have been described in farmed mink since the disease was first recognized in Denmark in 1953 (1). Hemorrhagic pneumonia due to P. aeruginosa spreads rapidly on the farm, with mortalities reaching 75% (2). The disease may also have a milder course, however, with lower mortality (1). In Denmark, hemorrhagic pneumonia occurs almost exclusively from September to December.
Over the last 5 to 10 y, pure cultures of hemolytic E. coli have been isolated from outbreaks of acute hemorrhagic pneumonia in Danish mink submitted for diagnostic investigations. Thus, E. coli has become an increasingly important differential diagnosis to hemorrhagic pneumonia caused by P. aeruginosa. As with P. aeruginosa pneumonia, pneumonia associated with E. coli occurs primarily in the autumn and the 2 disease entities appear to have very similar macroscopic and microscopic pathology.
In humans, both P. aeruginosa and E. coli are a rare cause of acute pneumonia in immune-competent individuals (3–5). Pseudomonas aeruginosa is mainly associated with acute lung infections in neutropenic humans or those who require mechanical ventilation (6,7), while community-acquired pneumonia due to E. coli is primarily seen in humans with underlying diseases (5). In animals, P. aeruginosa is a well-known spontaneous pathogen from chronic otitis externa in dogs (8), but is also found in acute deep pyoderma and ocular infections (9,10). Spontaneous lung infections are not described in healthy animals, however, with the exception of mink. The pathology and histopathology of hemorrhagic pneumonia in mink caused by P. aeruginosa have been described in detail (11–13).
Escherichia coli has been described as the cause of spontaneous fatal pneumonia in a few cases among dogs, cats, and a horse (14–16). In a study by Tibbetts et al (17), E. coli is associated with hemorrhagic pneumonia in mink, although the pathology or histopathology of the disease is not described. To our knowledge, this study is the first attempt to describe the pathology of E. coli associated with outbreaks of fatal hemorrhagic pneumonia in mink.
In this study, we compared the histopathology of hemorrhagic pneumonia in mink caused by P. aeruginosa and E. coli in an attempt to differentiate between the 2 diseases on the basis of histopathology and to possibly identify disparities in disease pathogenesis. We compared the distribution of E. coli and P. aeruginosa in the lung using fluorescence in-situ hybridization (FISH).
Materials and methods
Samples of lung tissue from 37 mink diagnosed with hemorrhagic pneumonia from 2006 to 2009 and with pure cultures of the bacteria of interest were included in this study. Hemorrhagic pneumonia was diagnosed at the National Veterinary Institute in Denmark based on macroscopic evaluation of the lungs, histology, and bacteriological investigations at the time of submission. Cultures from the lungs of mink included in this study yielded pure growth of either P. aeruginosa (n = 19) or β-hemolytic E. coli (n = 18). Samples for bacterial culture were obtained from the lungs at the time of submission by quickly burning the surface of the lung and making an incision in the burned area with a sterile scalpel before introducing a sterile swab, which was streaked onto blood agar and MacConkey agar.
One piece of lung tissue per mink from areas with macroscopic lesions was fixed in 10% buffered formalin for 1 to 3 d and embedded in paraffin as part of a routine diagnostic investigation. The formalin-fixed paraffin-embedded (FFPE) tissue was used in the study.
All mink included in this study were tested for antibodies against Aleutian disease virus using an additive counter-immunoelectrophoresis method (18) with negative results. According to records at the National Veterinary Institute in Denmark, the macroscopic findings in the lungs of the 37 mink included in this study all showed swelling, increased texture, and red discoloration, with tissue distribution reported as most often being diffuse and with some animals displaying diffuse lesions with focal, multifocal, or lobar necrosis.
From the paraffin blocks, 3-μm tissue sections were mounted on glass slides (SuperFrost Plus; Menzel-Gläser, Braunschweig, Germany). Each slide was stained with conventional hematoxylin and eosin (H&E) and examined for severity of lesions. The severity of lesions was graded as follows: 0 — no lesions; 1 — mild lesions (up to 33% of tissue involved) or a few cells, e.g., leukocytes or erythrocytes; 2 — moderate lesions (34% to 66% of tissue involved) or a moderate number of cells; and 3 — severe lesions (more than 66% of tissue involved) or a massive number of cells. The average scores for severity of lesions are given in Table I. The slides were examined twice in a blinded fashion by the same examiner. The entire piece of lung on the slide was included when deciding the histological grades assigned to each lung.
Table I.
Average scores for histopathological lesions. In these cases, vasculitis is regarded as transmural inflammation, while interstitial edema refers to perivascular and peribronchial edema
| E. coli | P. aeruginosa | |
|---|---|---|
| Extent of interstitial edema | 1.1 | 1.3 |
| Extent of alveolar edema | 1.9 | 0.9 |
| Amount of fibrin | 1.6 | 1.6 |
| Number of interstitial leukocytes | 1.3 | 1.2 |
| Number of alveolar leukocytes | 1.5 | 1.9 |
| Extent of necrosis | 1.5 | 1.4 |
| Number of erythrocytes in alveoli | 1.6 | 2.3 |
| Vasculitis | 0.3 | 0.6 |
| Thrombosis | 0.1 | 0.3 |
| Epithelial cell loss in conductive airways | 1.6 | 1.9 |
| Lymphoid cuffing | 0.6 | 0.2 |
The severity of lesions was graded as 0 — no lesions (0%), 1 — mild lesions (0% to 33% of tissue) or a few cells, 2 — moderate lesions (34% to 66% of tissue) or a moderate number of cells, and 3 — massive/severe lesions (> 66% of tissue) or a massive number of cells.
The 3-μm tissue sections from the paraffin blocks were mounted on SuperFrost Plus glass slides (Menzel-Gläser). A 6-FAM labeled P. aeruginosa probe with the sequence 5′-GGTAACCGTCCCCCTTGC-3′ (19) (DNA Technology, Aarhus, Denmark) and a Texas Red labeled E. coli probe with the sequence 5′-GCATAAGCGTCGCTGCCG-3′ (20) (Eurofins MWG Operon, Ebersberg, Germany) were used to detect P. aeruginosa and E. coli by fluorescence in-situ hybridization (FISH) targeting 16S rRNA in a hybridization concentration of 5 ng/μL of hybridization buffer (0.1M Tris pH 7.2, 0.9 M NaCl, 0.1% SDS) and at a hybridization temperature of 45°C. The oligonucleotide probe sequences were found on probeBase (21). It was noted in probeBase that the chosen E. coli probe would not bind to 16S rRNA in all E. coli strains. In the slides with tissue from lungs showing a pure culture of E. coli but without fluorescence when using the E. coli probe, the bacteria were visualized with a mixture of Texas Red labeled gamma-proteobacterium probes; GAM 42A, 5′-GCCTTCCCACATCGTTT-3′ and 5′-GCCTTCCCACTTCGTTT-3′ (22) (Eurofins MWG Operon).
The slides were washed 3 × 5 min (0.1M Tris pH 7.2, 0.9M NaCl) and mounted with Vectashield Mounting Medium for Fluorescence with DAPI (Vector Laboratories, Burlingame, California, USA). To verify the specificity of the probes, we initially used a double-staining protocol including a probe targeting the Bacterial Domain EUB338, 5′-GCTGCCTCCCGTAGGAGT-3′ (23) (Eurofins MWG Operon).
Results
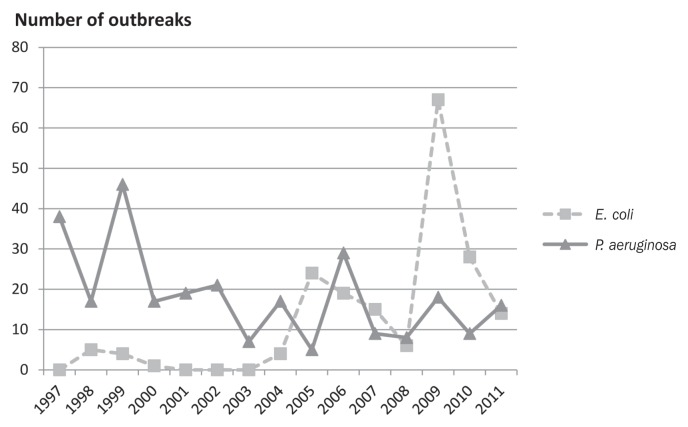
Records of diagnostic investigations at the National Veterinary Institute in Denmark clearly show that E. coli hemorrhagic pneumonia has been detected in mink with increasing frequency over the last 10 y (Figure 1).
Figure 1.
Prevalence of hemorrhagic pneumonia in Denmark caused by P. aeruginosa and E. coli.
Histology
The average scores for the categories of histopathological lesions are shown in Table I. The calculation of averages is not valid for statistical purposes since the data are categorical, but is used in this case only as a way of displaying the differences in scores. The calculations should therefore be interpreted with caution. The agreement between the 2 assessments of each slide was calculated based on each category and ranged from 62% to 100%.
The significance of the difference between the scores for E. coli and P. aeruginosa was assessed using Fisher’s exact test on a 2 × 4 contingency table (2 groups with 4 scores) and a significance level of 0.05. Only the first grading of the slides was included in the statistical evaluation to avoid working with 2 dependent scores, while both gradings were included in the calculation of the average shown in Table I in order to use as much information from the slides as possible. Three types of lesions differed with significance levels below 0.05, namely the extent of alveolar edema, the amount of erythrocytes in alveoli, and lymphoid cuffing.
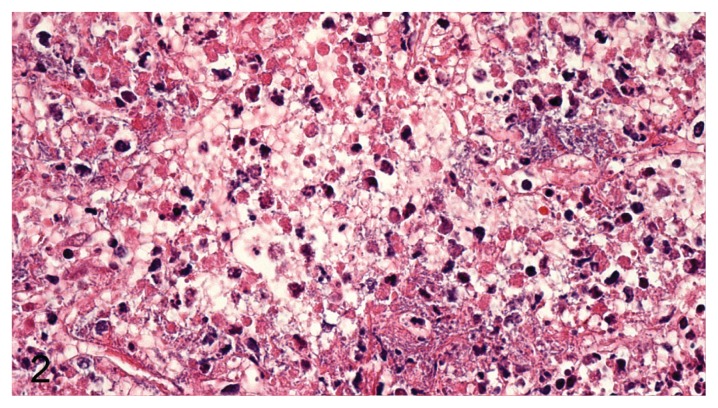
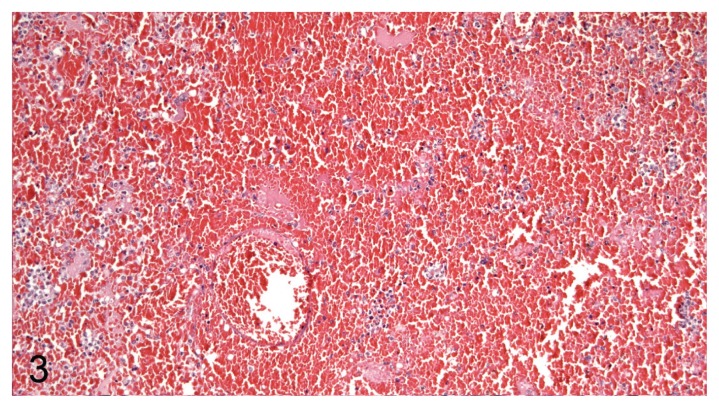
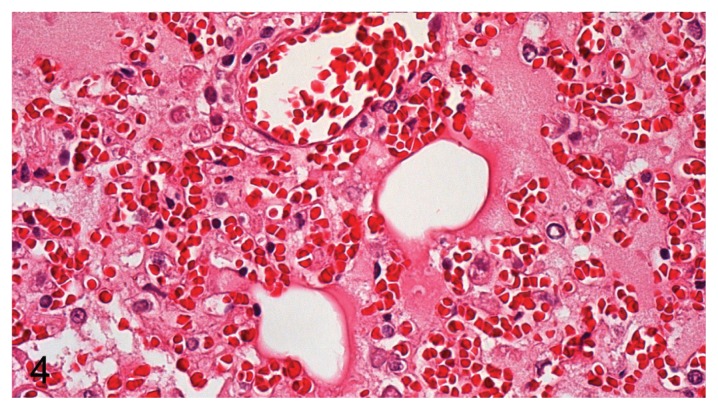
Based on morphology, the most predominant types of leukocytes were neutrophil granulocytes and macrophages, but lymphocytes were also observed. In P. aeruginosa pneumonia, the types of leukocytes consisted predominantly of neutrophils or a mix of neutrophils and macrophages. In E. coli pneumonia, the type of inflammation was dominated by a mixture of macrophages and neutrophils. Two main patterns and various mixtures of these patterns were found, with 1 pattern dominated by necrosis of alveolar septa and vascular walls (rarely penetrating the tunica elastic interna), neutrophils, and massive amounts of bacteria (Figure 2) and the other displaying massive intra-alveolar hemorrhage with few bacteria and inflammatory cells (Figure 3). The very hemorrhagic pattern was most often observed in lungs infected with P. aeruginosa, while severe alveolar edema (Figure 4) was most often encountered in lungs infected with E. coli.
Figure 2.
Massive necrosis of alveolar septa, degenerated neutrophils, and myriads of bacteria (P. aeruginosa) in mink lung. H&E, 400× magnification.
Figure 3.
Severe acute pulmonary hemorrhage (P. aeruginosa) in mink lung. H&E, 200× magnification.
Figure 4.
Massive alveolar edema and blood vessel without perivascularly located bacteria (E. coli) in mink lung. H&E, 400× magnification.
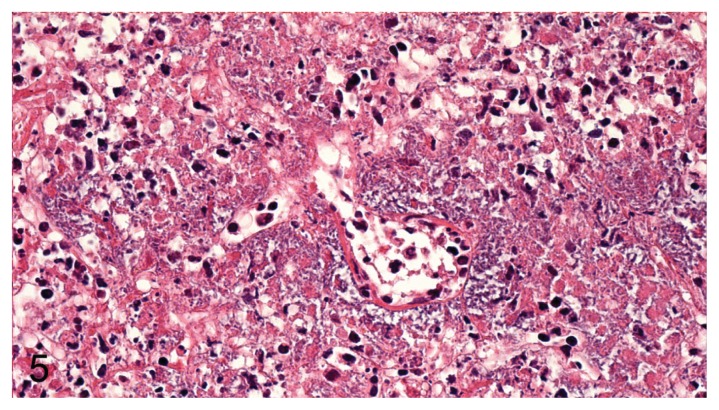
Epithelial cell loss was more prominent in the bronchioles than in the bronchi in all cases of pneumonia. Perivascular accumulation of bacteria around venules and arterioles was noted for pneumonia caused by P. aeruginosa (Figure 5), but was noted only in 1 case for pneumonia associated with E. coli.
Figure 5.
Blood vessel showing massive perivascular accumulation of bacteria, mild vasculitis, and margination of leukocytes along with severe necrosis of the surrounding tissue (P. aeruginosa) in mink lung. H&E, 400× magnification.
Fluorescence in-situ hybridization (FISH)
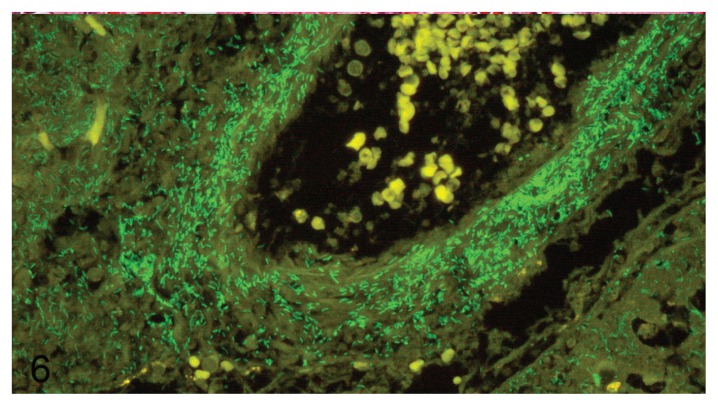
In most P. aeruginosa-positive mink lungs, the distribution of bacteria in the lung tissue could be regarded as perivascular with varying amounts of P. aeruginosa in alveoli, interstitium, and bronchial epithelium. The bacteria did not usually penetrate into the vascular lumen, although this was observed in a few cases in which massive amounts of bacteria were identified surrounding the vessel (Figure 6).
Figure 6.
Pseudomonas aeruginosa around and in blood vessel wall in mink lung. Fluorescence in-situ hybridization with 6-FAM labeled P. aeruginosa probe. 400× magnification.
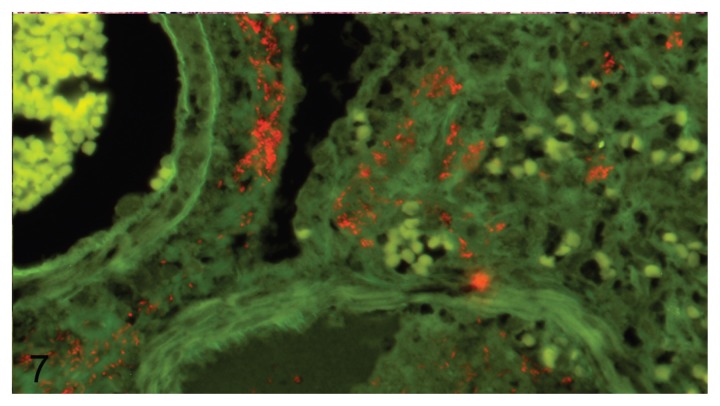
Fewer bacteria were generally found in pneumonia associated with E. coli than in pneumonia caused by P. aeruginosa. In addition, the distribution of E. coli in the tissue was more varied, with bacteria being found mostly in alveoli, bronchioles, and in interstitial spaces surrounding large vessels and bronchi (Figure 7).
Figure 7.
Escherichia coli between blood vessel (left upper corner) and bronchiole (right lower corner) in mink lung. Fluorescence in-situ hybridization with Texas Red labeled E. coli probe. 400× magnification.
Discussion
The average scores for most of the lesions observed in the lung tissue were quite similar for the 2 pathogens. A somewhat similar pattern was expected, since the inclusion criteria for this study were hemorrhagic pneumonia and infection with either P. aeruginosa or E. coli. Nonetheless, there were significant differences in alveolar edema, the amount of erythrocytes in alveoli, and lymphoid cuffing between the 2 infections. Severe alveolar edema and mild lymphoid cuffing were more often encountered in E. coli hemorrhagic pneumonia, while a severely hemorrhagic pattern was most often seen in P. aeruginosa hemorrhagic pneumonia. This indicates that P. aeruginosa and E. coli do not share the same pathological pathway. The animals may die at different time points in the infection, underlying viral infections may be present, or different virulence factors may be employed, all of which could lead to the differences in histological pattern observed between the 2 pathogens. The predominance of neutrophils in P. aeruginosa hemorrhagic pneumonia in mink does not agree with a previous study on rats (24), which showed that non-immunized rats developed a response dominated by macrophages and died, while immunized animals cleared an intratracheal challenge dose with P. aeruginosa by developing an inflammatory response dominated by neutrophils. The dependence on neutrophils for clearing P. aeruginosa infection has been underlined by other studies (25,26), which show that neutrophils are of paramount importance for clearing pulmonary infection with P. aeruginosa. In our study, mink displaying a predominantly neutrophil response also died of the infection, which raises the question of whether this dependency is also present in mink. Nevertheless, this cannot be taken as evidence of a lack of dependence on neutrophil leukocytes for clearing of the infection in mink, since no surviving animals were included in the study. This makes it impossible to reveal a difference in leukocyte response between survivors and non-survivors. Defect neutrophils are present in the Chediak-Higashi syndrome recognized in mink with the blue coat color type (27–29). If development of hemorrhagic pneumonia in mink was highly dependent on the activity of neutrophils, this type of mink should be more prone to develop hemorrhagic pneumonia. No coat color types were particularly prevalent in this study, although too few mink were represented to further explore this theory.
In 1968, Nordstoga described 2 histopathological patterns in mink lungs infected with P. aeruginosa(13). One pattern, which was found in mink from 1 farm, showed a hemorrhagic necrosis without inflammation and with only a few bacteria present. Mink from another farm showed a severe inflammation dominated by neutrophils. In these mink, high numbers of bacteria were often found in the perivascular space and inside the vessel wall but did not penetrate the tunica elastica interna and therefore were rarely seen in the vessel lumen. This study (13) is in good agreement with our finding of P. aeruginosa in the perivascular space and with our assessment of the slides as showing an either predominantly hemorrhagic picture or a pattern dominated by necrosis of the alveolar septa and vascular walls and massive numbers of neutrophils. The finding of a perivascular localization of P. aeruginosa is also in agreement with previously described septic lesions in rats (30). It has not been determined why P. aeruginosa shows a predominantly perivascular localization, but it could be speculated that the amount of certain nutrients is higher in this area or that P. aeruginosa targets specific receptors. Receptors for specific adherence to some carbohydrates have been identified in the capillaries and epithelium of mink lungs and pancreas (31). This might partially explain why mink are more easily infected with P. aeruginosa than other species.
The localization of E. coli primarily to the bronchioles, alveoli, and interstitial spaces around larger vessels and bronchi is consistent with the sparse literature on the subject (14,15). The perivascular distribution, as was observed in pneumonia caused by P. aeruginosa, was seen only once in our study, while a greater tendency for provoking alveolar edema was observed. It is not clear whether this reflects a difference in receptors or a tendency for evoking different responses by the immune system. Further investigations including experimental studies are necessary to investigate the pathogenesis of hemorrhagic pneumonia in mink.
Diagnostic material is not optimal for use in this type of study but, at present, is the only accessible source of tissue from this disease. The macroscopic lesions described in the materials and methods section are based on records used for diagnostic purposes. These records are subject to variations due to various personnel and handling procedures and should be assessed with caution.
Studies involving experimental infections have previously been conducted with P. aeruginosa in mink with the purpose of revealing pathogenesis (11,12,32–34) but this has never been attempted with E. coli in mink. It is well-known that both E. coli and P. aeruginosa possess a plethora of different virulence factors and a further study of the expression of these in bacterial isolates from mink could lead to a better understanding of the pathogenesis of this disease entity. Studies of recruitment of specific cell types and cytokines could also be relevant, since these factors apparently play an important role in the progression of P. aeruginosa pneumonia in humans (7,35).
This study revealed a difference in distribution of the pathogens that lead to hemorrhagic pneumonia in mink; P. aeruginosa showed a preference for perivascular localization compared to the more diffuse distribution of E. coli in the lungs. It was also recognized that P. aeruginosa more often evokes a predominantly hemorrhagic pattern in mink lungs, while E. coli was more often associated with higher amounts of alveolar edema and mild lymphoid cuffing. It remains to be determined whether these differences reflect aspects of pathogenesis of hemorrhagic pneumonia in mink caused by the 2 pathogens.
Acknowledgments
The authors thank Kopenhagen Fur and Nordvacc for their financial support of this work. The funding sources were not involved in study design, data collection, analysis, interpretation, writing, or the decision to publish the work. The authors thank Bodil Kruse, Christina Schmidt, Rikke Frandsen, Dorte Jensen, Annie Ravn Pedersen, and Joanna Zeitman Amenuvor for their excellent technical assistance and Mariann Chriél for discussions on the dataset.
References
- 1.Knox B. Pseudomonas aeruginosa som årsag til enzootiske infektioner hos mink. (Pseudomonas aeruginosa as the cause of enzootic infections in mink) Nord Vet Med. 1953;5:731. [Google Scholar]
- 2.Karlsson KA, Kull KE, Svanholm R. Infections in minks caused by Pseudomonas aeruginosa. Infection and vaccination trials. Nord Vet Med. 1971;23:345–351. [PubMed] [Google Scholar]
- 3.Hatchette TF, Gupta R, Marrie TJ. Pseudomonas aeruginosa community-acquired pneumonia in previously healthy adults: Case report and review of the literature. Clin Infect Dis. 2000;31:1349–1356. doi: 10.1086/317486. [DOI] [PubMed] [Google Scholar]
- 4.Okimoto N, Hayashi T, Ishiga M, et al. Clinical features of Escherichia coli pneumonia. J Infect Chemother. 2010;16:216–218. doi: 10.1007/s10156-010-0034-z. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz LA, Gomez A, Jaca C, Martinez L, Gomez B, Zalacain R. Bacteraemic community-acquired pneumonia due to gram-negative bacteria: Incidence, clinical presentation and factors associated with severity during hospital stay. Infection. 2010;38:453–458. doi: 10.1007/s15010-010-0058-4. [DOI] [PubMed] [Google Scholar]
- 6.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 7.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mekic S, Matanovic K, Seol B. Antimicrobial susceptibility of Pseudomonas aeruginosa isolates from dogs with otitis externa. Vet Rec. 2011;169:125. doi: 10.1136/vr.d2393. [DOI] [PubMed] [Google Scholar]
- 9.Hillier A, Alcorn JR, Cole LK, Kowalski JJ. Pyoderma caused by Pseudomonas aeruginosa infection in dogs: 20 cases. Vet Dermatol. 2006;17:432–439. doi: 10.1111/j.1365-3164.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- 10.Ledbetter EC, Mun JJ, Kowbel D, Fleiszig SM. Pathogenic phenotype and genotype of Pseudomonas aeruginosa isolates from spontaneous canine ocular infections. Invest Ophthalmol Vis Sci. 2009;50:729–736. doi: 10.1167/iovs.08-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long GG, Gallina AM, Gorham JR. Pseudomonas pneumonia of mink: Pathogenesis, vaccination, and serologic studies. Am J Vet Res. 1980;41:1720–1725. [PubMed] [Google Scholar]
- 12.Trautwein G, Helmboldt CF, Nielsen SW. Pathology of Pseudomonas pneumonia in mink. J Am Vet Med Assoc. 1962;140:701–704. [PubMed] [Google Scholar]
- 13.Nordstoga K. Pseudomonas infections in mink with special reference to Pseudomonas vasculitis in pulmonary lesions. Acta Vet Scand. 1968;9:33–40. doi: 10.1186/BF03547887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sura R, Van Kruiningen HJ, DebRoy C, et al. Extraintestinal pathogenic Escherichia coli-induced acute necrotizing pneumonia in cats. Zoonoses Public Health. 2007;54:307–313. doi: 10.1111/j.1863-2378.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 15.DebRoy C, Roberts E, Jayarao BM, Brooks JW. Broncho-pneumonia associated with extraintestinal pathogenic Escherichia coli in a horse. J Vet Diagn Invest. 2008;20:661–664. doi: 10.1177/104063870802000524. [DOI] [PubMed] [Google Scholar]
- 16.Handt LK, Stoffregen DA, Prescott JS, et al. Clinical and microbiologic characterization of hemorrhagic pneumonia due to extraintestinal pathogenic Escherichia coli in four young dogs. Comp Med. 2003;53:663–670. [PubMed] [Google Scholar]
- 17.Tibbetts RJ, White DG, Dyer NW, Giddings CW, Nolan LK. Characterization of Escherichia coli isolates incriminated in coli-septicaemia in mink. Vet Res Commun. 2003;27:341–357. doi: 10.1023/a:1024741719361. [DOI] [PubMed] [Google Scholar]
- 18.Uttenthal A. Screening for antibodies against Aleutian disease virus (ADV) in mink. Elucidation of dubious results by additive counterimmunoelectrophoresis. Appl Theor Electrophor. 1992;3:83–84. [PubMed] [Google Scholar]
- 19.Hogardt M, Trebesius K, Geiger AM, Hornef M, Rosenecker J, Heesemann J. Specific and rapid detection by fluorescent in situ hybridization of bacteria in clinical samples obtained from cystic fibrosis patients. J Clin Microbiol. 2000;38:818–825. doi: 10.1128/jcm.38.2.818-825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neef A, Amann R, Schleifer K. Detection of microbial cells in aerosols using nucleic acid probes. Syst Appl Microbiol. 1995;18:113–122. [Google Scholar]
- 21.Loy A, Maixner F, Wagner M, Horn M. probeBase — an online resource for rRNA-targeted oligonucleotide probes: New features 2007. Nucleic Acids Res. 2007;35:D800–804. doi: 10.1093/nar/gkl856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manz W, Amann R, Ludwig W, Wagner M, Schleifer K. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: Problems and solutions. Syst Appl Microbiol. 1992;15:593–600. [Google Scholar]
- 23.Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson NR, Dunkley ML, Buret A, Young B, Cripps AW. Histopathology of the lung following intratracheal challenge with live Pseudomonas aeruginosa in intestinally immunized rats. Immunol Cell Biol. 1995;73:440–445. doi: 10.1038/icb.1995.68. [DOI] [PubMed] [Google Scholar]
- 25.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun. 2009;77:5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennington JE, Ehrie MG. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J Infect Dis. 1978;137:764–774. doi: 10.1093/infdis/137.6.764. [DOI] [PubMed] [Google Scholar]
- 27.Hammer AS, Andersen TH, Eriksen T, Kortegaard HE, Dietz HH, Chriel M. Radiographic evaluation of destructive periodontal disease in blue mink in relation to age and blood morphology. Can J Vet Res. 2005;69:128–134. [PMC free article] [PubMed] [Google Scholar]
- 28.Clark RA, Kimball HR, Padgett GA. Granulocyte chemotaxis in the Chediak-Higashi syndrome of mink. Blood. 1972;39:644–649. [PubMed] [Google Scholar]
- 29.Gallin JI, Klimerman JA, Padgett GA, Wolff SM. Defective mononuclear leukocyte chemotaxis in the Chediak-Higashi syndrome of humans, mink, and cattle. Blood. 1975;45:863–870. [PubMed] [Google Scholar]
- 30.Teplitz C. Pathogenesis of Pseudomonas vasculitis and septic legions. Arch Pathol. 1965;80:297–307. [PubMed] [Google Scholar]
- 31.Kirkeby S, Wimmerova M, Moe D, Hansen AK. The mink as an animal model for Pseudomonas aeruginosa adhesion: Binding of the bacterial lectins (PA-IL and PA-IIL) to neoglycoproteins and to sections of pancreas and lung tissues from healthy mink. Microbes Infect. 2007;9:566–573. doi: 10.1016/j.micinf.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Homma JY, Abe C, Tanamoto K, et al. Effectiveness of immunization with single and multi-component vaccines prepared from a common antigen (OEP), protease and elastase toxoids of Pseudomonas aeruginosa on protection against hemorrhagic pneumonia in mink due to P. aeruginosa. Jpn J Exp Med. 1978;48:111–133. [PubMed] [Google Scholar]
- 33.Shimizu T, Homma JY, Abe C, et al. Effect of common protective antigen vaccination to protect mink from challenge exposure with Pseudomonas aeruginosa. Am J Vet Res. 1976;37:1441–1444. [PubMed] [Google Scholar]
- 34.Elsheikh LE, Kronevi T, Wretlind B, Abaas S, Iglewski BH. Assessment of elastase as a Pseudomonas aeruginosa virulence factor in experimental lung infection in mink. Vet Microbiol. 1987;13:281–289. doi: 10.1016/0378-1135(87)90090-3. [DOI] [PubMed] [Google Scholar]
- 35.Wolbeling F, Munder A, Kerber-Momot T, et al. Lung function and inflammation during murine Pseudomonas aeruginosa airway infection. Immunobiology. 2011;216:901–908. doi: 10.1016/j.imbio.2011.02.003. [DOI] [PubMed] [Google Scholar]