Abstract
The efficacy of a piglet-specific inactivated Porcine circovirus type 2 (PCV2) vaccine was evaluated with clinical field trials, as recommended by the Republic of Korea’s Animal, Plant & Fisheries Quarantine & Inspection Agency. Three farms were selected on the basis of their history of postweaning multisystemic wasting syndrome. On each farm 60, 1-week-old pigs were randomly allocated to 1 of 2 treatment groups: vaccination at 1 and 3 wk of age or no vaccination. The 2-dose schedule of vaccination with inactivated PCV2 vaccine improved the average daily weight gain from birth to 16 wk of age, the PCV2 load in the blood, and the frequency and severity of lymph node lesions. Inactivated PCV2 vaccine seems to be very effective in controlling PCV2 infection under field conditions.
Résumé
L’efficacité d’un vaccin spécifique pour les porcelets à base de circovirus porcin de type 2 (PCV2) a été évalué dans des études cliniques, tel que recommandé par l’Agence d’inspection et de quarantaine des animaux, plantes et des pêcheries de la République de la Corée. Trois fermes ont été sélectionnées en fonction de leur historique relativement au syndrome de dépérissement multi-systémique en période post-sevrage. Sur chaque ferme, 60 porcelets de 1 semaine d’âge ont été répartis de manière aléatoire à un des 2 groupes de traitement : vaccination à 1 et 3 semaine d’âge, ou aucune vaccination. La cédule de vaccination à 2 doses avec le vaccin PCV2 inactivé a amélioré le gain quotidien moyen entre la naissance et l’âge de 16 semaines, la charge sanguine de PCV2, ainsi que la fréquence et la sévérité des lésions des noeuds lymphatiques. Le vaccin PCV2 inactivé semble être très efficace pour maîtriser les infections par PCV2 dans des conditions de terrain.
(Traduit par Docteur Serge Messier)
Porcine circovirus-associated diseases (PCVADs), caused by Porcine circovirus type 2 (PCV2), have been recognized as the most economically important diseases in the global swine industry (1,2). Since PCV2 vaccines were introduced into the world market in 2006, vaccination strategies have been used to control and prevent PCV2 infection. PCV2 vaccination was administered to approximately 97.5% of all piglets farrowed in Korea in 2010 (3).
Several commercial PCV2 vaccines are available in the global market. Field reports on some products indicate that vaccination has been highly efficacious in reducing the incidence of PCVADs in the production system (4–9). Recently, a piglet-specific commercial inactivated PCV2 vaccine has been developed by the Korean Pharmaceutical Company. The objective of this study was to evaluate the efficacy of this new vaccine after administration to piglets at 1 and 3 weeks of age with the use of clinical field trials, in accordance with the registration guidelines of the Republic of Korea’s Animal, Plant & Fisheries Quarantine & Inspection Agency (10).
The vaccine (CircoPrime; Komipharm International Company Ltd., Shiheung-shi, Kyongki-do, Republic of Korea) was prepared from an inactivated tissue homogenate and contained inactivated PCV2b (105 fluorescent antibody infectious dose50/mL) and aluminum hydroxide gel adjuvant (10% of volume). It was given as two 1.0-mL doses at 1 and 3 weeks of age.
The clinical field trial was conducted on 3 farms. Farms A, B, and C housed herds of 1100, 450, and 250 sows, respectively, that had consistently suffered losses due to PCV2 infection in several recent months. Farms A and B were 2-site production systems with separate nurseries and finishing units. Farm C was a 1-site production system. At all 3 farms the pigs were weaned into a nursery barn (which housed pigs from weaning until approximately 10 weeks of age) at an average age of 21 days, with approximately 2 farrowing house litters to a nursery pen. The pigs were moved to the finishing barns at approximately 10 weeks of age. All 3 farms tested seropositive for Porcine reproductive and respiratory syndrome virus (PRRSV), but the pigs were not vaccinated against PRRSV, and all 3 farms had been confirmed as positive for postweaning multisystemic wasting syndrome (PMWS) according to the diagnostic criteria of PMWS (1). No PCV2-associated reproductive problems had been reported, and the 3 farms had not previously used any commercial PCV2 vaccine.
On farm A the clinical signs of PCV2 infection first appeared at approximately 6 to 8 weeks of age, and the peak mortality rate (18%) occurred at approximately 9 to 11 weeks of age. On farm B, the clinical signs first appeared at approximately 7 to 8 weeks of age, and the peak mortality rate (20%) occurred at approximately 9 to 11 weeks of age. On farm C, the clinical signs first appeared at approximately 3 to 5 weeks of age, and the peak mortality rate (30%) occurred at approximately 7 to 8 weeks of age.
This study used a randomized, blinded, weight- and gender-matched, controlled clinical trial with a parallel-group design. To minimize sow variation, two 7-day-old piglets were randomly selected from each sow by means of a split-plot design and randomly assigned to vaccination and nonvaccination groups. The 30 pigs in the vaccination group were given the new vaccine at 7 and 21 days of age, and the 30 pigs in the non-vaccination group remained unvaccinated. The pigs in each group were randomly assigned by weight to pens (10 pigs per pen). All of the pigs were euthanized for necropsy at 16 weeks of age. All of the methods had been approved by the Seoul National University Institutional Animal Care and Use Committee.
Blood samples from each pig were collected by jugular venipuncture at 1, 3, 5, 7, 10, and 16 weeks of age. The serum was tested with the commercial PCV2 enzyme-linked immunosorbent assay Ingezim Circovirus IgG (Ingenasa, Madrid, Spain), and DNA extracted from the same samples was subjected to real-time polymerase chain reaction (11). The live weight of each pig was measured at 1, 5, 7, 10, 14, and 16 weeks of age. The average daily feed consumption (AFC) per pig was calculated from the feed consumption during the study period relative to the average number of pigs in the pen during this period. The pigs were monitored daily for clinical signs and scored weekly as 0 (normal), 1 (rough haircoat), 2 (rough haircoat and dyspnea), 4 (severe dyspnea and abdominal breathing), and 6 (death) (11). The histopathological lymphoid lesion score and immunohistochemical PCV2 antigen score were determined as previously described (11).
Summary statistics were calculated for all of the groups to assess the overall quality of the data, including normality. Statistical analysis was conducted for PCV2 DNA quantification, body weight, average daily weight gain (ADG), histopathological lymphoid lesion score, immunohistochemical PCV2 antigen score, serologic results, and clinical score, as previously described (11). A P-value < 0.05 was considered to be significant.
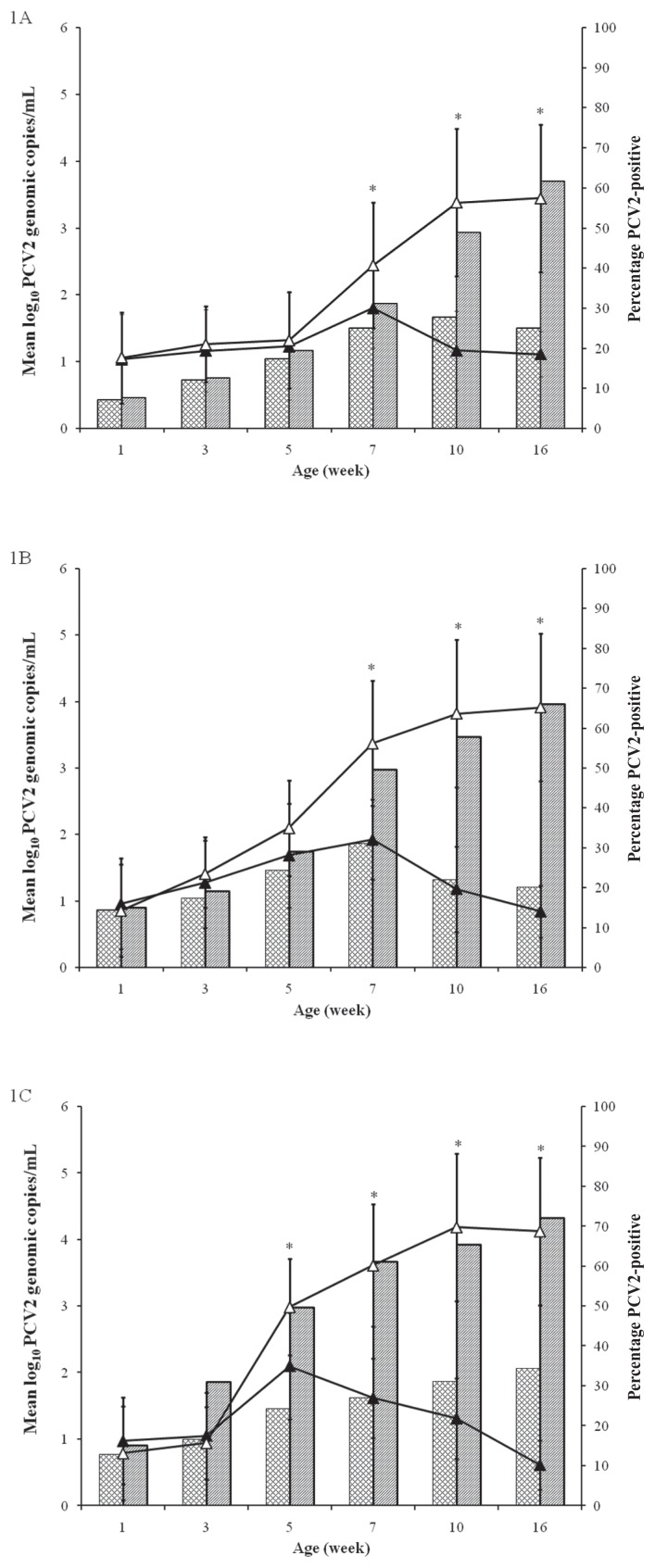
The anti-PCV2 IgG levels were significantly higher among the vaccinated animals than among the control animals on all 3 farms at 3, 5, and 7 weeks of age (P < 0.05). The vaccinated animals had a significantly lower number of genomic copies of PCV2 than the control animals on farm A at 7 (P = 0.026), 10 (P < 0.001), and 16 (P < 0.001) weeks of age (Figure 1), on farm B at 7 (P < 0.001), 10 (P < 0.001), and 16 (P < 0.001) weeks of age, and on farm C at 5 (P = 0.002), 7 (P < 0.001), 10 (P < 0.001), and 16 (P < 0.001) weeks of age.
Figure 1.
Mean group log10 load of DNA of Porcine circovirus type 2 (PCV2) in the blood of vaccinated (black triangles) and nonvaccinated (white triangles) piglets and percentage of PCV2-viremic pigs in the vaccinated (cross-hatched bars) and nonvaccinated (bars with diagonal lines) groups for farms A, B, and C. Asterisks indicate a significant (P < 0.05) difference between the 2 groups at the same time point.
The mean clinical scores were significantly lower in the vaccinated animals than in the control animals at 5, 6, 7, and 12 weeks of age (P < 0.05) on farm A, at 5 and 14 weeks of age (P < 0.05) on farm B, and at 4 weeks, at 5 weeks, and between 11 and 16 weeks of age (P < 0.05) on farm C. The ADG between 5 and 16 weeks of age was significantly greater in the vaccinated animals than in the control animals on farms A (P = 0.016), B (P = 0.001), and C (P < 0.001) (Table I). The mortality rate during the study period was significantly lower (P < 0.05) in the vaccinated animals than in the control animals only on Farm C (Table I). The typical granulomatous inflammatory reaction and lymphoid depletion associated with PCV2 infection in pigs, which is consistent with the histopathological lesions in PCVADs, was observed in the lymph nodes of the nonvaccinated pigs. The histopathological lymphoid lesion scores were significantly lower in the vaccinated animals than in the control animals on farms A (P = 0.007), B (P = 0.025), and C (P = 0.001), and these scores correlated with the number of genomic copies of PCV2 in the blood on farms A (rs = 0.687), B (rs = 0.758), and C (rs = 0.887) (Table I). The mean number of PCV2-positive cells per unit area of lymph node was significantly higher in the control animals than in the vaccinated animals on farms A (P < 0.001), B (P = 0.03), and C (P < 0.001), and this number correlated with the number of genomic copies of PCV2 in the blood on farms A (rs = 0.761), B (rs = 0.773), and C (rs = 0.888) and with the histopathological lymphoid lesion score on farms A (rs = 0.871), B (rs = 0.902), and C (rs = 0.877).
Table I.
Findings for piglets vaccinated or not vaccinated against Porcine circovirus type 2 (PCV2) on 3 farms affected by postweaning multisystemic wasting syndrome
| Mean ± standard deviation or proportion | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Vaccinated animals | Non-vaccinated animals | |||||
|
|
|
|||||
| Variable | Farm A | Farm B | Farm C | Farm A | Farm B | Farm C |
| Body weight (kg); age (week) | ||||||
| 1 | 2.9 ± 0.17 | 3.0 ± 0.18 | 2.9 ± 0.43 | 3.0 ± 0.42 | 2.9 ± 0.31 | 2.9 ± 0.59 |
| 5 | 8.3 ± 1.4 | 9.2 ± 1.41 | 8.0 ± 1.62 | 8.4 ± 1.30 | 8.2 ± 2.1 | 7.1 ± 1.89 |
| 7 | 12.9 ± 1.91 | 14.6 ± 2.42 | 12.0 ± 2.64 | 11.7 ± 1.72 | 12.8 ± 2.40 | 11.1 ± 2.35 |
| 10 | 19.7 ± 2.47 | 26.0 ± 3.50 | 21.6 ± 3.95 | 18.2 ± 2.71 | 24.0 ± 3.45 | 18.3 ± 3.90 |
| 14 | 35.8 ± 5.85 | 46.6 ± 6.61 | 37.8 ± 6.80 | 32.7 ± 4.45 | 43.0 ± 4.92 | 33.6 ± 7.30 |
| 16 | 47.9 ± 7.88 | 59.2 ± 7.13 | 48.1 ± 5.75 | 43.5 ± 4.64 | 53.3 ± 4.22 | 39.3 ± 6.12 |
| ADGa (kg/day); 5 to 16 week | 0.52 ± 0.10c | 0.65 ± 0.10c | 0.52 ± 0.07c | 0.45 ± 0.06 | 0.58 ± 0.06 | 0.42 ± 0.08 |
| ADFb (kg/day); 5 to 16 week | 1.04 | 1.21 | 0.95 | 1.04 | 1.22 | 1.01 |
| Mortality rate | 2/30 | 1/30 | 3/30d | 6/30 | 6/30 | 12/30 |
| Lymphoid lesion score | 0.7 ± 0.48d | 0.5 ± 0.53d | 0.8 ± 0.4d | 1.8 ± 0.92 | 1.4 ± 0.97 | 2.1 ± 0.74 |
| PCV2-antigen score | 12.2 ± 2.49d | 21.9 ± 5.79d | 31.1 ± 8.30d | 61.3 ± 13.58 | 78.8 ± 16.58 | 74.4 ± 17.89 |
Average daily weight gain.
Average daily feed consumption.
Significantly higher in the vaccinated animals than in the non-vaccinated animals (P < 0.05).
Significantly lower in the vaccinated animals than in the non-vaccinated animals (P < 0.05).
In agreement with a previous study using a different type of PCV2 vaccine (4–9), the inactivated PCV2 vaccine used in this study significantly reduced the frequency and severity of PCV2-associated clinical signs, the frequency and severity of histopathological lymphoid lesions, the immunohistochemical PCV2-antigen scores, and the incidence of PCV2 viremia among the vaccinated animals on the 3 farms. The anti-PCV2 IgG and genomic PCV2 levels in the blood samples suggest that the litters on the 3 farms experienced different PCV2 infections at different ages. For example, on farm C the anti-PCV2 IgG and genomic PCV2 levels were increased significantly in the nonvaccinated animals at 3 weeks of age, and the clinical signs peaked in the nonvaccinated animals at 7 weeks of age, indicating that the nonvaccinated animals were naturally infected with PCV2 at approximately 3 weeks of age. Hence, the earlier the pigs were vaccinated, the greater their chance of avoiding PCV2 infection before vaccination.
The daily weight gain was 70 to 100 grams higher in the vaccinated animals than in the nonvaccinated animals between 5 and 16 weeks of age in the herds with severe PMWS. Our results are similar to those of previous studies in which the ADG was improved by 71.8 grams/day in vaccinated animals between 3 and 26 weeks of age (8) and by 70 grams/day in vaccinated animals between 12 and 26 weeks of age (12). However, the difference in ADG between the vaccinated and nonvaccinated animals was much lower (between 16 and 30 grams/day) in other field studies (4,6,9). The farms in our study and the former 2 studies (8,12) had higher mortality rates and more severe clinical signs of PMWS than the farms in the latter studies (4,6,9). Therefore, the discrepancy could be due to differences in the severity of PMWS in the herds used in the field trials. Moreover, our study finished at 16 weeks of age owing to the experimental design recommended by the Republic of Korea’s Animal, Plant & Fisheries Quarantine & Inspection Agency. This design renders comparison with other field trials difficult and limited because most of the other studies finished at the end of the fattening period.
Under field conditions, the prevalence of PCV2 seropositivity in sows has reached almost 100%; consequently, most piglets vaccinated against PCV2 at 1 and 3 weeks of age have variable levels of PCV2 maternal antibodies (13). Therefore, swine practitioners have raised concerns that detectable levels of passively acquired PCV2 antibodies in 1- and 3-week-old pigs in the present study may interfere with the induction of a protective immune response with the PCV2 vaccine. The present data indicate that the inactivated PCV2 vaccine used in this study is effective in reducing the amount of PCV2 in the blood and PCV2-associated lesions, even when used in pigs with passively acquired antibodies at the time of vaccination, as in previous experimental and field studies (9,12,14).
The results of this study provide pig producers with important new information regarding how to improve growth performance using PCV2 vaccine under field conditions. A 2-dose schedule for inactivated PCV2 vaccine improves the average daily weight gain from birth to 16 weeks of age and reduces mortality rate, serum PCV2 load, and the frequency and severity of lymph node lesions in clinical field trials.
Acknowledgments
This research was supported by the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea, by contract research funds from the Research Institute for Veterinary Science of the College of Veterinary Medicine, and by the Brain Korea 21 Program for Veterinary Science in the Republic of Korea.
References
- 1.Chae C. Postweaning multisystemic wasting syndrome: A review of aetiology, diagnosis and pathology. Vet J. 2004;168:41–49. doi: 10.1016/j.tvjl.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169:326–336. doi: 10.1016/j.tvjl.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Chae C. Commercial porcine circovirus type 2 vaccine: Efficacy and clinical application. Vet J. 2012;194:151–157. doi: 10.1016/j.tvjl.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Fachinger V, Bischoff R, Jedidia SB, Saalmüller A, Elbers K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine. 2008;26:1488–1499. doi: 10.1016/j.vaccine.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Horlen KP, Dritz SS, Nietfeld JC, et al. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. J Am Vet Med Assoc. 2008;232:906–912. doi: 10.2460/javma.232.6.906. [DOI] [PubMed] [Google Scholar]
- 6.Segalés J, Urniza A, Alegre A, et al. A genetically engineered chimeric vaccine against porcine circovirus type 2 (PCV2) improves clinical, pathological and virological outcomes in postweaning multisystemic wasting syndrome affected farms. Vaccine. 2009;27:7313–7321. doi: 10.1016/j.vaccine.2009.09.084. [DOI] [PubMed] [Google Scholar]
- 7.Desrosiers R, Clark E, Tremblay D, Tremblay R, Polson D. Use of a one-dose subunit vaccine to prevent losses associated with porcine circovirus type 2. J Swine Health Prod. 2009;17:148–154. [Google Scholar]
- 8.Pejsak Z, Podgórska K, Truszczyński M, Karbowiak P, Stadejek T. Efficacy of different protocols of vaccination against porcine circovirus type 2 (PCV2) in a farm affected by postweaning multisystemic wasting syndrome (PMWS) Comp Immunol Microbiol Infect Dis. 2010;33:e1–e5. doi: 10.1016/j.cimid.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Fraile L, Grau-Roma L, Sarasola P, et al. Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: Improvement of production parameters and interaction with maternally derived immunity. Vaccine. 2012;30:1986–1992. doi: 10.1016/j.vaccine.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Guidelines of the Republic of Korea’s Animal, Plant & Fisheries Quarantine & Inspection Agency. [Last accessed January 25, 2013]. http://www.qia.go.kr/bbs/lawAnn/viewLawWebAction.do?id=323&type=0.
- 11.Kim D, Kim CH, Han K, et al. Comparative efficacy of commercial Mycoplasma hyopneumoniae and porcine circovirus 2 (PCV2) vaccines in pigs experimentally infected with M. hyopneumoniae and PCV2. Vaccine. 2011;29:3206–3212. doi: 10.1016/j.vaccine.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Martelli P, Ferrari L, Morganti M, et al. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet Microbiol. 2011;149:339–351. doi: 10.1016/j.vetmic.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Opriessnig T, Yu S, Thacker EL, Halbur PG. Derivation of porcine circovirus type 2-negative pigs from positive sow herds. J Swine Health Prod. 2004;12:186–191. [Google Scholar]
- 14.Opriessnig T, Patterson AR, Elsener J, Meng XJ, Halbur PG. Influence of maternal antibodies on efficacy of porcine circovirus type 2 (PCV2) vaccination to protect pigs from experimental infection with PCV2. Clin Vaccine Immunol. 2008;15:397–401. doi: 10.1128/CVI.00416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]