Abstract
Objectives
The aim of this study was to investigate the effect of inhalation of Salvia sclarea (clary sage; clary) or Lavandula angustifolia (lavender) essential oil vapors on autonomic nervous system activity in female patients with urinary incontinence undergoing urodynamic assessment.
Study design, location, and subjects
This study was a double-blind, randomized, controlled trial carried out in 34 female patients with urinary incontinence.
Outcome measure
The subjects were randomized to inhale lavender, clary, or almond (control) oil at concentrations of 5% (vol/vol) each. Systolic blood pressure, diastolic blood pressure, pulse rate, respiratory rate, and salivary cortisol were measured before and after inhalation of these odors for 60 minutes.
Results
The clary oil group experienced a significant decrease in systolic blood pressure compared with the control (p=0.048) and lavender oil (p=0.026) groups, a significant decrease in diastolic blood pressure compared with the lavender oil group (p=0.034) and a significant decrease in respiratory rate compared with the control group (p<0.001). In contrast, the lavender oil group tended to increase systolic and diastolic blood pressure compared with the control group. Compared with the control group, inhalation of lavender oil (p=0.045) and clary oil (p<0.001) resulted in statistically significant reductions in respiratory rate.
Conclusions
These results suggest that lavender oil inhalation may be inappropriate in lowering stress during urodynamic examinations, despite its antistress effects, while clary oil inhalation may be useful in inducing relaxation in female urinary incontinence patients undergoing urodynamic assessments.
Introduction
Urinary incontinence is a voiding anomaly resulting in the involuntary leakage of urine, inhibiting social activity and causing health problems in some patients. Urinary incontinence occurs more frequently in women than in men, due in part to the shorter length of the female than the male urethra.1 Moreover, urinary incontinence in women may result from damage to the pelvic muscles that support the urethra and bladder occurring during pregnancy and birthing.1 Risk factors for female urinary incontinence have been reported to include obesity, aging, cystocele, uterine prolapse, weakened pelvic muscles, estrogen deficiency, pelvic infections, stroke, full paralysis, cognitive impairment, caffeine, tobacco, and alcohol abuse.2
Female urinary incontinence can be classified into several types, including stress urinary incontinence (SUI), caused by high abdominal stress such as coughing, sneezing, or laughing; urge urinary incontinence (UUI), occurring when abnormal contractions of the bladder cause urine leakage and accompanied by frequent and unbearable senses of urgency; and mixed urinary incontinence, a combination of SUI and UUI.1 Treatments for UUI include drugs that reduce bladder contractibility and voiding senses, especially antimuscarinic drugs, whereas treatments for SUI include α-adrenergic receptor agonists, which act on the contraction of the sphincter.3 Urodynamic assessments are useful to determine the causes of voiding anomalies and to select the most appropriate treatment methods. Tests used to determine whether voiding anomalies are due to abnormalities in reservoir or discharge function include urine speed tests, which measure discharge speed during voiding; assessments of bladder resilience; bladder-filling manometry, which determines the causes of urinary incontinence by assessing the presence of volumic and involuntary contractions that sense urine; and urinary flow-pressure tests, which determine whether discharge impairment is due to bladder muscle weakness or urinary tract obstruction.4
Patients undergoing urodynamic tests, however, are prone to experience shame arising from the need to urinate in front of a hospital tester.5 Patient stress during urination under these conditions is compounded by the insertion of a catheter into the rectum and urethra in the lithotomic position, with no lower-body clothing, in an unfamiliar environment in the hospital. Moreover, these patients experience anxiety and stress from embarrassment and discomfort.5 The increase in patient stress levels during urodynamic examination can stimulate the sympathetic nervous system and reduce bladder capacity to below maximum capacity, thus affecting the results of the examination.6 Although inhalation of the vapor of a 1:3 mixture of bergamot and Lavandula angustifolia (lavender) essential oils did not affect patient anxiety during urodynamic evaluation, it reduced the ratio of low-frequency to high-frequency, which reflect sympathovagal balance, although the latter change did not reach statistical significance.5 In other words, beat-to-beat heart rate variability displays two main components: a low-frequency one representing sympathetic and parasympathetic influence and a high-frequency component of parasympathetic origin. These results, however, may have been affected by a lack of separation of patients by gender, fertility history, and types of urinary incontinence, as well as the inclusion of patients with complications. Moreover, the effects of each component of the inhalant—bergamot oil and lavender oil—were not determined. Therefore, the present study sought to evaluate separately the effects of inhalation of lavender or Salvia sclarea (clary sage; clary) essential oil vapors, both of which are known to reduce stress,7,8 on the stress levels in female patients with urinary incontinence during urodynamic examination.
Lavender oil has been shown to relax mouse uteri and guinea pig smooth muscles and to inhibit muscle spasms.9 Although its mechanism of action has not been determined, it was reported to be similar to that of benzodiazepines, which increase the inhibitory neurotransmitter γ-aminobutyric acid in the amygdala.10 Moreover, linalool, one of the primary ingredients of lavender, has been shown to inhibit the secretion of acetylcholine into the neuromuscular junction areas of mice.11 Although lavender is less effective than benzodiazepines in a mouse anxiety model, it still has anti-anxiety effects in these animals.12
Clary oil was found to alleviate stress and have antidepressive effects in a mouse model, effects manifested by activation of paths with dopamine characteristics.8 In addition, inhalation of clary oil vapors for 20 minutes by patients with gingivitis was found to reduce the strain and stress of patients undergoing dental treatment.13
There have been few studies to date of interventions that can reduce stress levels in patients undergoing urodynamic examinations. In particular, there have been no comparisons of inhalation of clary and lavender oils. It was therefore examined whether inhalation of these aroma oils can induce relaxation in female urinary incontinence patients during urodynamic examination. Physiologic indicators of stress were compared, including salivary cortisol concentration, blood pressure, pulse rate, and respiration rate, during urodynamic examinations in female patients inhaling the vapors of clary oil, lavender oil, and, as a control, almond oil.
Materials and Methods
Study design
A double-blind, randomized, controlled trial was used. Each participant received 1 single-dose inhalation during urodynamic examination in random order: 5% (vol/vol, dissolved in almond oil) clary oil, 5% (vol/vol) lavender oil or almond oil (control). Randomization was performed and coded by an individual independent of the study, and the inhalations were blinded to the investigator and study participants before urodynamic examination. It was calculated that the minimum sample size needed to compare differences among the three groups was >14 subjects per group, based on an effect size of 0.50, a statistical power of 0.80, and a significance level of 0.05. Fifteen (15) subjects were originally assigned to the group; however, 5 patients in the control group, and 3 each in the clary and lavender groups dropped out. Patients who were examined for less or more than 60 minutes were excluded. Thus, collected were data from 10 patients in the control group and 12 each in the clary and lavender groups. Because the final number of subjects in trial was less than the designed sample size, the post hoc power was calculated. The observed power was 0.70 for the effect size of 0.5 and significance level of 0.05.
Participants
The study was approved by the Research Ethics Review Committee of Korea University Anam Hospital. Subjects who met the inclusion criteria and provided their written informed consent were enrolled in the study. The inclusion criteria for the enrollment of female patients were as follows: patients (1) diagnosed with urinary incontinence and undergoing urodynamic examination; (2) were aged ≥20 years; (3) had no trouble with sense of smell and were able to communicate; (4) had no allergic reaction to any of the aromas used in this study; (5) were not undergoing any drug, hormone, or aroma therapy due to psychiatric illnesses; (6) did not smoke or drink alcohol; (7) had no brain conditions such as stroke or Parkinson's disease; and (h) had no history of medical conditions, including diabetes, high blood pressure, and cardiovascular diseases. Patients who were examined for less or more than 60 minutes were excluded.
Gas chromatography–mass spectrometry analysis
Clary and lavender oil were supplied by Aromarant Co. Ltd. (Rottingen, Germany) and were derived from flowers of Salvia sclarea and Lavandula angustifolia, respectively. Compositions of clary and lavender oil were analyzed by gas chromatography–mass spectrometry (GC-MS, Hewlett-Packard 5890 series, Palo Alto, CA). Electron impact (70 eV) GC–MS measurements were carried out on a Hewlett-Packard HP 5890 gas chromatograph equipped with a 25 m polydimethylsiloxane (Chrompack CP Sil 5 CB) capillary column and coupled to a VG Analytical VG 70-250S mass spectrometer. Helium was used as carrier gas. The identification of the constituents was performed by Aromarnat Co., Ltd. (Rottingen, Germany). The results of compositions of clary and lavender oil are listed in Table 1. The primary volatile components of lavender oil are 38.5% linalyl acetate, 33.3% linalool, and 2.1% terpinen-4-ol. Clary oil is composed of 63.7% linalyl acetate, 17.7% linalool, and 2.5% α-terpineol.
Table 1.
Main Composition of the Essential Oils of Lavandula angustifolia (Lavender) and Salvia sclarea (Clary)
| Compounds of lavender oil | % | Compounds of clary oil | % |
|---|---|---|---|
| Linalyl acetate | 38.5 | Linalyl acetate | 63.7 |
| Linalool | 33.3 | Linalool | 17.7 |
| Caryophyllene | 3.9 | Sclareol | 4.5 |
| Myrcene | 3.9 | α-Terpineol | 2.5 |
| Trans-ocimene | 2.4 | Germacrene D | 2.0 |
| Lavandulyl acetate | 2.2 | Neryl acetate | 2.0 |
| Terpinen-4-ol | 2.1 | Geraniol | 1.2 |
| Lavandulol | 1.6 | α-Copaene | 0.8 |
| α-Terpineol | 1.3 | Myrcene | 0.8 |
| 1,8-Cineol | 0.9 | β-Caryophyllene | 0.8 |
| Geraniol | 0.5 | Nerol | 0.5 |
| Camphor | 0.5 | Limonene | 0.3 |
| Limonene | 0.4 | β-Pinene | 0.1 |
Aroma inhalation device
Almond oil (Aromarnat Co., Ltd.), 5% lavender oil, and 5% clary oil were prepared. A 2-mL aliquot of each was dropped onto aroma pads and positioned 30 cm from the nose tip.14 Patients breathed these vapors through natural nasal or oral breathing for 60 minutes during urodynamic examinations.
Stress scale
Stress was measured as described,15 using a questionnaire composed of 30 questions: 15 related to psychologic stress and 15 related to physical stress. Each question was graded according to a Likert-type 4-point scale, with 0 representing “no stress” and 3 representing “always felt stress.” Scores on each stress category ranged from 0 to 45 points. Scores of 0–5 points represented below-average-level stress levels with no specific problems; 6–12 points represented average stress levels for adult males and females; 13–19 points represented slightly above-average stress, requiring care; and >20 points represented dangerous levels of stress, requiring utmost care and consultation with a physician.
Blood pressure, pulse rate, and respiratory rate
Blood pressure, pulse rate, and respiratory rate were used as indicators of physiologic responses of the autonomic nervous system under stressful conditions.13 Before and after urodynamic examination, each participant was told to rest for 5 minutes in a sitting position, and blood pressure and pulse rate were measured in the right brachial area using an electronic sphygmomanometer (model BP 3BMI-3, Microlife, Switzerland). Respiratory rate for 1 minute was measured at the same time. For obtaining informed consent, all three measurements of blood pressure, pulse rate, and respiratory rate were averaged.
Salivary cortisol
To minimize the effects of circadian rhythms during the test day on salivary cortisol concentration, subjects were prohibited from eating any foods that stimulated salivary secretion 1 hour before saliva sample collection. All samples were collected from subjects between 8:00 am and 9:00 am. After completing the survey, before urodynamic examination, and after the completion of the urodynamic examination, each subject was made to sit for 5 minutes to relax and to hold their saliva without swallowing. A 1-mL sample of each subject's saliva was collected at each time using uncontaminated plastic sputum containers, and the samples were frozen immediately at −20°C. Saliva cortisol was measured using standard procedures (Spectra max plus384, MDS Analytical Technologies (US) Inc., USA) and cortisol EIA kit (Assay Designs, Inc, Ann Arbor, MI). A decrease in saliva cortisol concentration indicated a decrease in stress response.
Statistical analysis
SAS 9.2 was used for statistical analyses. Characteristics of each group were compared at baseline and final follow-up by Fisher's exact test and the χ2 test for categorical and continuous variables, respectively. Differences among the three groups in blood pressure and pulse rate were determined by one-way analysis of variance (ANOVA), differences in respiratory rate were compared using non-parametric Kruskal-Wallis tests, and differences in salivary cortisol were compared by analysis of covariance. Pooled data were presented as mean±standard deviation.
Results
General characteristics of subjects and the homogeneity test
All study subjects were females, with a mean age of 56.2 years (range 33–75 years old). Of the 34 subjects, 27 patients (79.4%) were married. Body mass index (BMI) was <18.5 kg/m2 in 2 subjects (5.9%), 18.5–23.0 kg/m2 in 11 subjects (32.4%), and >23.0 kg/m2 in 21 subjects (61.8%). Regarding symptoms, 13 patients (38.2%) had SUI, 6 patients (17.6%) had UUI, and 15 patients (44.1%) had mixed urinary incontinence; moreover, 9 patients (26.5%) had frequent urination and 3 patients (8.8%) had urgent urination. It was found that 23 patients (67.6%) were postmenopausal, 33 patients (97.1%) had given birth to at least 1 child, and 4 patients (11.8%) had undergone cesarean section. Following urodynamic examinations, 16 patients (47.1%) were diagnosed with SUI, 12 patients (35.3%) with mixed urinary incontinence, and 6 patients (17.6%) with UUI. Overall, the baseline demographic and disease-related characteristics of the lavender oil, clary oil, and control groups were similar (Table 2). Moreover, prior to urodynamic examination, there were no significant differences among the three groups in psychologic and physical stress scores, systolic blood pressure, diastolic blood pressure, pulse rate, or respiratory rate (Table 3).
Table 2.
Homogeneity Test for General Characteristics Among the Three Groups (n=34)
| Characteristics | Total n (%) | Control n (%) | Lavender n (%) | Clary n (%) | χ2orFisher's exact | p | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 31–40 | 2 (5.9) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 2.60 | 0.999 |
| 41–50 | 9 (26.5) | 3 (33.3) | 3 (33.3) | 3 (33.3) | |||
| 51–60 | 11 (32.4) | 3 (27.3) | 4 (36.4) | 4 (36.4) | |||
| 61–70 | 9 (26.5) | 2 (22.2) | 4 (44.4) | 3 (33.3) | |||
| ≥71 | 3 (8.8) | 1 (33.3) | 1 (33.3) | 1 (33.3) | |||
| BMI (kg/m2) | Under 18.5 | 2 (5.9) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2.06 | 0.883 |
| 18.5–23.0 | 11 (32.4) | 3 (27.3) | 3 (27.3) | 5 (45.5) | |||
| over 23.0 | 21 (61.8) | 6 (28.6) | 8 (38.1) | 7 (33.3) | |||
| Marital status | Single | 7 (20.6) | 3 (42.9) | 3 (42.9) | 1 (14.3) | 1.86 | 0.448 |
| Married | 27 (79.4) | 7 (25.9) | 9 (33.3) | 11 (40.7) | |||
| Symptomsa | Frequency | 9 (26.5) | 3 (30.0) | 4 (33.3) | 2 (16.7) | 0.67 | 0.716b |
| Urgency | 3 (8.8) | 0 (0.0) | 2 (16.7) | 1 (8.3) | 0.33 | 0.564b | |
| UI SUI | 13 (38.2) | 6 (60.0) | 4 (33.3) | 3 (25.0) | 1.07 | 0.584b | |
| UUI | 6 (17.6) | 1 (10.0) | 3 (25.0) | 2 (16.7) | 1.00 | 0.607b | |
| MUI | 15 (44.1) | 3 (30.0) | 5 (41.7) | 7 (58.3) | 1.60 | 0.449b | |
| Menstrual state | Menopause | 23 (67.6) | 5 (21.7) | 10 (43.5) | 8 (34.8) | 2.71 | 0.285 |
| Menstruation | 11 (32.4) | 5 (45.5) | 2 (18.2) | 4 (36.4) | |||
| Delivery number | None | 1 (2.9) | 1 (100) | 0 (0.0) | 0 (0.0) | 5.37 | 0.506 |
| 1–2 | 22 (64.7) | 6 (27.3) | 7 (31.8) | 9 (40.9) | |||
| 3–4 | 10 (29.4) | 2 (20.0) | 5 (50.0) | 3 (30.0) | |||
| ≥5 | 1 (2.9) | 1 (100.0) | 0 (0.0) | 0 (0.0) | |||
| Cesarean section | Yes | 4 (11.8) | 0 (0.0) | 3 (75.0) | 1 (25.0) | 2.84 | 0.297 |
| No | 30 (88.2) | 10 (33.3) | 9 (30.0) | 11 (36.7) | |||
| UI type | SUI | 16 (47.1) | 5 (31.3) | 5 (31.3) | 6 (37.5) | 1.09 | 0.957 |
| UUI | 6 (17.6) | 1 (16.7) | 3 (50.0) | 2 (33.3) | |||
| MUI | 12 (35.3) | 4 (33.3) | 4 (33.3) | 4 (33.3) |
Fisher's exact test.
Subjects could select more than one item.
χ2 test.
Clary, clary sage; BMI, body–mass index; UI, urinary incontinence; SUI, stress urinary incontinence; UUI, urge urinary incontinence; MUI, mixed urinary incontinence.
Table 3.
Homogeneity Test for Measurement Variables Among the Three Groups (n=34)
| Variables | Control | Lavender | Clary | p |
|---|---|---|---|---|
| Psy. stress | 11.4±4.58 | 11.8±5.87 | 12.8±4.33 | 0.809 |
| Phy. stress | 11.5±6.75 | 11.8±7.10 | 8.9±4.27 | 0.472 |
| sBP | 113.6±15.01 | 116.2±15.21 | 117.0±12.73 | 0.850 |
| dBP | 77.4±10.67 | 78.1±11.24 | 77.6±5.18 | 0.984 |
| PR | 78.3±6.40 | 80.8±9.53 | 77.0±8.11 | 0.532 |
| RR | 19.1±1.37 | 19.4±1.31 | 19.3±1.22 | 0.931a |
| Cortisol level | 4.15±0.35 | 3.97±0.48 | 3.74±0.40 | 0.032a |
One-way analysis of variance.
Kruskal-Wallis test.
Clary, clary sage; Psy. stress, psychological stress; Phy. stress, physical stress; sBP, systolic blood pressure; dBP, diastolic blood pressure; PR, pulse rate; RR, respiratory rate. Values are expressed as mean±standard deviation or median±interquartile range.
Effects of inhalation on blood pressure, pulse rate, and respiratory rate
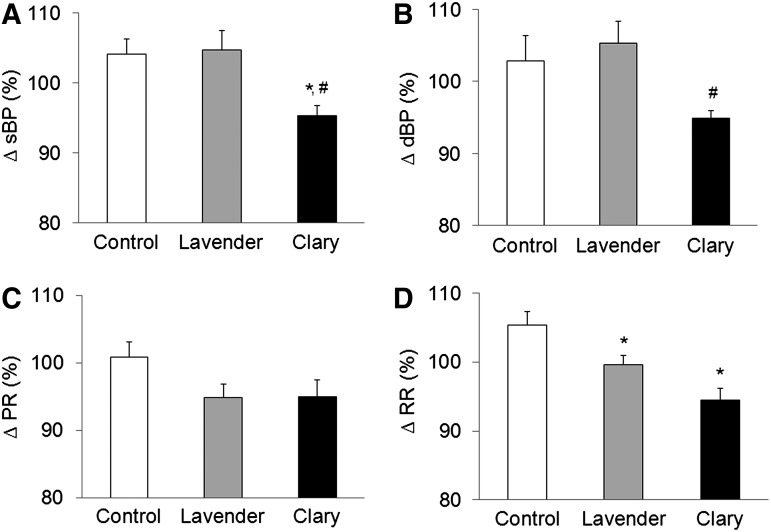
Compared with systolic blood pressure before inhalation, the control and lavender oil groups showed increases of 4.2% (113.6±15.01 mm Hg to 118.6±20.01 mm Hg) and 4.7% (116.2±15.21 mm Hg to 121.3±17.19 mm Hg), respectively, after inhalation, whereas the clary oil group showed a 4.7% (117.0±12.73 mm Hg to 111.2±11.30 mm Hg) decrease (F=4.54, p=0.019, Fig. 1A). The clary oil group experienced a significant decrease in systolic blood pressure compared with the control (p=0.048) and lavender oil (p=0.026) groups. It was found that diastolic blood pressure increased 2.9% (77.4±10.67 mm Hg to 79.5±13.64 mm Hg) and 5.3% (78.1±11.24 mm Hg to 81.8±11.14 mm Hg) in the control and lavender oil groups, respectively, after compared with before inhalation, whereas diastolic blood pressure decreased 5.1% (77.6±5.18 mm Hg to 73.6±5.74 mm Hg) in the clary oil group (F=4.73, p=0.016, Fig. 1B). The clary oil group experienced a significant reduction in diastolic blood pressure compared with the lavender oil group (p=0.034).
FIG. 1.
Effects of inhalation of lavender and clary oil vapors on (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse rate, and (D) respiratory rate. All values are expressed as mean±SE. Systolic blood pressure (sBP), diastolic blood pressure (dBP), and pulse rate (PR) were compared among the groups using one-way analysis of variance and post-hoc Tukey test, and respiratory rate (RR) was compared using the Kruskal-Wallis test. Clary, clary sage. Ten (10) patients were in the control group and 12 each were in the lavender and clary groups. *p<0.05 compared with the control group. #p<0.05 compared with the lavender group.
Compared with pulse rate before inhalation, it was found that pulse rate after inhalation increased 0.8% (78.3±6.40 beats/min to 79.0±9.65 beats/min) in the control group and decreased 5.2% (80.8±9.53 beats/min to 76.3±8.02 beats/min) in the lavender oil and 5.0% (77.0±8.11 beats/min to 73.3±10.38 beats/min) in the clary oil group, but the differences among the three groups were not statistically significant (F=2.38, p=0.109, Fig. 1C). When respiration rate after and before inhalation was compared, a 5.4% (19.1±1.37 breaths/min to 20.1±1.52 breaths/min) increase was observed in the control group, a 0.5% (19.4±1.31 breaths/min to 19.3±1.61 breaths/min) decrease in the lavender oil group, and a 5.5% (19.3±1.22 breaths/min to 18.2±1.11 breaths/min) decrease in the clary oil group, resulting in statistically significant differences among the 3 groups (F=14.29, p<0.001, Fig. 1D). Compared with the control group, inhalation of lavender oil (p=0.045) and clary oil (p<0.001) resulted in statistically significant reductions in respiratory rate.
Salivary cortisol
Salivary cortisol concentration increased from a mean 4.15±0.35 ng/mL before inhalation to 4.83±0.64 ng/mL after inhalation in the control group (mean change, 0.68±0.52 ng/mL) and from a mean 3.97±0.48 ng/mL to 4.14±0.51 ng/mL in the lavender oil group (mean change, 0.17±0.20 ng/mL), but decreased from 3.74±0.40 ng/mL to 3.65±0.39 ng/mL (mean change, 0.09±0.19 ng/mL) in the clary oil group, differences that were not statistically significant (F=1.35, p=0.280, Table 4).
Table 4.
Effects of Inhalation of Lavender and Clary Oil Vapors on Salivary Cortisol Concentrations
| Group | Control | Lavender | Clary | F | p |
|---|---|---|---|---|---|
| Pre | 4.15±0.35 | 3.97±0.48 | 3.74±0.40 | 1.35 | 0.280 |
| Post | 4.83±0.64 | 4.14±0.51 | 3.65±0.39 | ||
| Difference | −0.68±0.52 | −0.17±0.20 | 0.09±0.19 |
F-value of analysis of covariance with pretest value as covariate.
Values are median±interquartile range (ng/mL) (n=10, n=12, n=12, respectively).
Clary, clary sage.
Discussion
This study examined the effects of inhalation of clary oil or lavender oil vapors, both of which contain high concentrations of linalyl acetate, compounds that relieve stress and relax blood vessels, on systolic and diastolic blood pressure, pulse and respiration rate, and salivary cortisol concentrations. The study subjects consisted of middle-aged women with urinary incontinence, with a mean age of 56.2 years. Moreover, 67.6% were postmenopausal, 61.8% had BMI >23 kg/m2, and 61.8% were overweight, confirming the correlation of urinary incontinence with age and estrogen deficiency due to menopause and obesity.16 Of the randomly selected patients, 47% were diagnosed with SUI, 18% with UUI, and 35% with mixed urinary incontinence, similar to findings in gynecological patients, in whom 50% had SUI, 10%–20% had UUI, and 30%–40% had mixed urinary incontinence.17
It was found that blood pressure, pulse rate, and respiration rate increased in the control group after urodynamic examination, confirming that urodynamic examination itself induced stress. Inhalation of clary oil vapors resulted in significantly reduced systolic blood pressure compared with inhalation of lavender oil vapors, similar to findings in patients with gingivitis,13 and suggesting that linalyl acetate, the main component of clary oil, directly relaxed vascular smooth muscles and reduced blood pressure.18
In contrast, lavender oil, which contains esters that are antistress agents and relax blood vessels, did not significantly reduce systolic or diastolic blood pressure; rather, it tended to increase blood pressure compared with the control group. These differences between lavender and clary oil may be associated with the diuretic actions of lavender oil.19 The diuretic properties of lavender oil may have caused the bladder to become more sensitive, suggesting that this may have acted as a source of stress in patients with a sudden, unmanageable urge to urinate and urinary incontinence, resulting in an increase in blood pressure. These findings suggest that lavender oil may be inappropriate in lowering blood pressure during urodynamic examinations, despite its antistress effects.
In contrast to the small changes in pulse rate before and after inhalation of almond oil vapors in the control group, inhalation of clary oil and lavender oil vapors resulted in pulse rate reductions of 5.0% and 5.2% reduction, respectively, but these differences were not statistically significant. Clary oil and lavender oil contain 17.7% and 33.3% linalool, respectively, and inhalation of (R)-(-)-linalool and lavender oil vapors was found to significantly reduce heart rate in normal healthy subjects.20 Blood pressure and pulse are indicators of response of the autonomic nervous system to stress and are therefore closely correlated with physiologic changes.
The finding in this study that inhalation of clary oil vapors slightly reduced respiratory rate may be a manifestation of mental calm—through its association with smooth-muscle relaxation and the effects of clary oil on parts of the brain, including the limbic system, cerebral cortex, amygdala, and hippocampus—by transforming awareness or suppressing sympathetic nerves. Lavender has been reported to reduce anxiety by increasing the effects of inhibitory neurotransmitter γ-aminobutyric acid in the amygdala, whereas clary oil reduces depression by activating dopamine pathways, suggesting that the effects of these two oils are due to their activation of neurotransmitters.8,10,12
It was found that salivary cortisol concentration tended to decrease in the lavender and clary groups, but did not differ significantly when compared with the control group. Similarly, administration of 5% clary oil vapors to patients with gingivitis and to mice undergoing the forced swim test decreased corticosteroid concentrations, but the differences were not statistically significant.8,13 In contrast, inhalation of linalool by normal healthy persons resulted in a significant decrease in salivary cortisol levels.21 These differences in the effects of essential oil vapors inhalation on salivary cortisol concentration may be due to difference in times of salivary cortisol concentration measurements. Since salivary cortisol was consistently measured at its highest daily times (8:00 am–9:00 am), the antistress effects of clary oil may have been mitigated by the stress occurring during urodynamic examinations. Moreover, although there were no statistically significant differences between group differences, the decreasing trend of salivary cortisol concentrations in the clary oil compared with the control group suggests that clary oil has stress-mitigating effects.
Conclusions
This study's findings indicate that inhalation of 5% clary oil vapors effectively reduces the stress that occurs during urodynamic examination in female patients with urinary incontinence. Thus, inhalation of 5% clary oil may be a feasible intervention for inducing relaxation in patients undergoing urodynamic examinations.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (2011-0003685, 2012-0004065, 2012-007145).
Disclosure Statement
No competing financial interests exist.
References
- 1.Lee JG. Diagnosis and treatment of urinary incontinence in women. J Korean Med Assoc. 2005;4:354–374. [Google Scholar]
- 2.Herzog AR. Fultz NH. Normolle DP, et al. Methods used to manage urinary-incontinence by older adults in the community. J Am Geriatr Soc. 1989;4:339–347. doi: 10.1111/j.1532-5415.1989.tb05502.x. [DOI] [PubMed] [Google Scholar]
- 3.Wein AJ. Pharmacologic options for the overactive bladder. Urology. 1998;2A:43–47. doi: 10.1016/s0090-4295(98)90009-7. [DOI] [PubMed] [Google Scholar]
- 4.Korean Continence Society. Textbook of Voiding Dysfunction and Female Urology. Seoul: Ilchokak; 2009. [Google Scholar]
- 5.Kim MW. Yoo YS. Cho OH, et al. The effects of aroma inhalation therapy on anxiety and heart rate variability in the patients undergoing urodynamic study. J Korean Biol Nurs Sci. 2009;1:32–41. [Google Scholar]
- 6.Hong WS. Ham SY. Kim TW, et al. Usefulness of a sonographic bladder scan for uroflowmetry and the evaluation of the anxiety level associated with uroflowmetry. Korean J Urol. 2007;6:633–637. [Google Scholar]
- 7.Atsumi T. Tonosaki K. Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Res. 2007;1:89–96. doi: 10.1016/j.psychres.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Seol GH. Shim HS. Kim PJ, et al. Antidepressant-like effect of Salvia sclarea is explained by modulation of dopamine activities in rats. J Ethnopharmacol. 2010;1:187–190. doi: 10.1016/j.jep.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Lis-Balchin M. Hart S. Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P. Miller) Phytother Res. 1999;6:540–542. doi: 10.1002/(sici)1099-1573(199909)13:6<540::aid-ptr523>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Tisserand R. Lavender beats benzodiazepines. Int J Aromather. 1988;1:1–2. [Google Scholar]
- 11.Re L. Barocci S. Sonnino S, et al. Linalool modifies the nicotinic receptor-ion channel kinetics at the mouse neuromuscular junction. Pharmacol Res. 2000;2:177–181. doi: 10.1006/phrs.2000.0671. [DOI] [PubMed] [Google Scholar]
- 12.Shaw D. Norwood K. Leslie JC. Chlordiazepoxide and lavender oil alter unconditioned anxiety-induced c-fos expression in the rat brain. Behav Brain Res. 2011;1:1–7. doi: 10.1016/j.bbr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 13.You JH. Kim MY. Moon HK, et al. Effect of clary sage-inhalation on pain and stress during the treatment of periodontitis. J Korean Acad Oral Health. 2011;1:32–40. [Google Scholar]
- 14.Seol GH. Jung MH. Effect of bergamot essential oil-inhalation on chronic pain after surgery for lumbar spinal stenosis. J Korean Biol Nurs Sci. 2011;2:156–163. [Google Scholar]
- 15.Park SY. Stress and my health. Health Newslett Gwangju. 1999;84:7–9. [Google Scholar]
- 16.Choi JB. Urinary incontinence in women. Korean J Family Med. 2010;9:661–671. [Google Scholar]
- 17.Ortiz OC. Stress urinary incontinence in the gynecological practice. Int J Gynecol Obstet. 2004;86:S6–S16. doi: 10.1016/j.ijgo.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Koto R. Imamura M. Watanahe C, et al. Linalyl acetate as a major ingredient of lavender essential oil relaxes the rabbit vascular smooth muscle through dephosphorylation of myosin light chain. J Cardiovasc Pharmacol. 2006;1:850–856. doi: 10.1097/01.fjc.0000238589.00365.42. [DOI] [PubMed] [Google Scholar]
- 19.Elhajili M. Baddouri K. Elkabbaj S, et al. Diuretic activity of the flowers infusion of Lavandula officinalis. Reprod Nutr Dev. 2001;5:393–399. [PubMed] [Google Scholar]
- 20.Kuroda K. Inoue N. Ito Y, et al. Sedative effects of the jasmine tea odor and (R)-(-)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur J Appl Physiol. 2005;2–3:107–114. doi: 10.1007/s00421-005-1402-8. [DOI] [PubMed] [Google Scholar]
- 21.Hoferl M. Krist S. Buchbauer G. Chirality influences the effects of linalool on physiological parameters of stress. Planta Med. 2006;13:1188–1192. doi: 10.1055/s-2006-947202. [DOI] [PubMed] [Google Scholar]