Abstract
There are no established biomarkers for mild traumatic brain injury (mTBI), in part because post-concussive symptoms (PCS) are subjective and conventional imaging is typically unremarkable. To test whether diffuse axonal abnormalities quantified with three-dimensional (3D) proton magnetic resonance spectroscopic imaging (1H-MRSI) correlated with patients' PCS, we retrospectively studied 26 mTBI patients (mean Glasgow Coma Scale [GCS] score of 14.7), 18- to 56-year-olds and 13 controls three to 55 days post-injury. All were scanned at 3 Tesla with T1- and T2-weighted MRI and 3D 1H-MRSI (480 voxels over 360 cm3, ∼30% of the brain). On scan day, patients completed a symptom questionnaire, and those who indicated at least one of the most common subacute mTBI symptoms (headache, dizziness, sleep disturbance, memory deficits, blurred vision) were grouped as PCS-positive. Global gray matter and white matter (GM/WM) absolute concentrations of N-acetylaspartate (NAA), choline (Cho), creatine (Cr) and myo-inositol (mI) in PCS-positive and PCS-negative patients were compared to age- and gender-matched controls using two-way analysis of variance. The results showed that the PCS-negative group (n=11) and controls (n=8) did not differ in any GM or WM metabolite level. The PCS-positive patients (n=15) had lower WM NAA than the controls (n=12; 7.0±0.6 versus 7.9±0.5mM; p=0.0007). Global WM NAA, therefore, showed sensitivity to the TBI sequelae associated with common PCS in patients with mostly normal neuroimaging, as well as GCS scores. This suggests a potential biomarker role in a patient population in which objective measures of injury and symptomatology are currently lacking.
Key words: diffuse axonal injury, magnetic resonance spectroscopy, mild traumatic brain injury, N-acetyl-aspartate, post-concussive symptoms
Introduction
The annual incidence of hospital-treated traumatic brain injury (TBI) in the US is 1.6 million,1 70–90% of which is diagnosed as mild TBI (mTBI) based on a Glasgow Coma Scale (GCS) score of 15-13, post-traumatic amnesia lasting fewer than 24 hours, and loss of consciousness (LOC) of less than 30 minutes duration.2 Although within this category a GCS score of 14-13 and findings on head computed tomography and T1/T2-weighted magnetic resonance imaging (MRI) predict poor outcome, there currently are no biomarkers for patients with a GCS score of 15, the mildest and most common mTBI,3 in which abnormalities on conventional imaging occur with frequency of only 5–8%.4,5 This results in an inability to predict or monitor a variety of subjective somatic and cognitive post-concussive symptoms (PCS), which in some cases become chronic.6 The lack of an objective metric for mTBI injury also prevents the ability to distinguish between neurological and psychiatric symptoms and between those of traumatic and non-traumatic origins.
These observations suggest that an mTBI biomarker must show (i) specificity to TBI injury, (ii) sensitivity to its mildest manifestation (i.e., to GCS of 15), and (iii) correlation with clinical symptoms. The mechanical strain associated with TBI results in multifocal dysregulation of the axonal ionic balance, which can progress to cytoskeletal damage and axotomy in more severe TBI, or may resolve without lasting damage in milder cases.7 Quantitative MR methods such as diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H-MRS) have shown sensitivity to injury missed by conventional imaging.3 DTI in particular has shown micro-structural alterations in axons even in mTBI.8 Subtle non-structural damage also occurs9 but can only be assessed via tissue biochemistry. 1H-MRS can investigate with high specificity cell status: neuronal integrity, cellular energy/density, membrane turnover and astroglial proliferation via their respective N-acetylaspartate (NAA), creatine (Cr), choline (Cho) and myo-inositol (mI) markers.10 Recently we developed a 1H-MRSI approach optimized for high sensitivity to diffuse changes in the brain's white matter (WM) and gray matter (GM)11 and applied it to 26 mTBI subjects with average GCS of 14.7, over 90% of which with unremarkable clinical MRI.12 We found decreased global WM levels of the neuronal marker NAA and no other metabolic differences in either WM or GM, suggesting a diffuse dysfunction confined only to axons, consistent with the type of subtle, yet characteristic TBI injury expected in this cohort, thus addressing (i) and (ii) above. The purpose of this study was to test whether the WM NAA decline correlated with patients' PCS.
Methods
Subjects
The previously described 26-patient cohort12 was recruited serially based on history of closed head trauma, GCS score of 13–15, LOC of 30 minutes' or less duration, and post-traumatic amnesia of less than 24 hours' duration. Twenty-five were enrolled following emergency department visits and one (# 13) was referred from a physician's office. Exclusion criteria were any MRI contraindications, other neurological disorders, human immunodeficiency virus infection, previous head trauma, lifetime alcohol or drug abuse, or vascular disease. Thirteen age- and gender-matched healthy controls were subject to the same exclusion criteria. All subjects gave Institutional Review Board-approved written consent.
Post-concussive symptoms
Based on a review of eleven original research articles, a 2004 report by the World Health Organization Collaborating Center Task Force on mTBI classified the following as the most common acute self-reported mTBI symptoms: headache, dizziness, sleep disturbance, memory problems, and blurred vision.13,14 On scan day, patients completed a “yes” or “no” questionnaire whether they were experiencing any of these five symptoms which they attributed to the trauma. Patients reporting at least one symptom were grouped as “PCS-positive,” whereas the rest were defined as “PCS-negative.” The questionnaire also asked about the presence of four symptoms other than the ones used in the PCS definition: neck stiffness, photophobia, nausea, and paresthesia.
Data acquisition and post-processing
All measurements and post-processing have been previously described.12 Experiments were done in a 3 T MR scanner. Sagittal 3D Magnetization Prepared RApid Gradient Echo (MP-RAGE) images were acquired for 1H-MRSI VOI guidance, for tissue segmentation and along with axial T2-weighted Fluid Attenuated Inversion Recovery images, for identification of any mTBI induced abnormalities. Following magnetic field shimming, the 10×8×4.5=360 cm3 1H-MRSI VOI was image-guided over the corpus callosum. At two averages, the 1H-MRSI took 34 minutes and the entire protocol less than an hour. After segmentation of the MP-RAGE images, the resultant cerebro-spinal fluid (CSF), GM, and WM masks were co-registered with the 1H-MRSI grid using in-house software, yielding their volume in every voxel in each subject. Absolute metabolite amounts were obtained using phantom replacement with correction for T1 and T2 relaxation time differences. Global GM and WM concentrations were calculated for each metabolite using linear regression.11
Statistical analysis
Two-way analysis of variance (ANOVA) was used to compare each patient group to the cohort of controls matched to them in terms of each metabolite within GM and WM. The indicator variable identifying subjects that were matched to each other was included as a blocking factor. The error variance was allowed to differ across comparison groups. A Shapiro-Wilk test applied to the residuals from the ANOVA models showed no significant departure from the normality assumption underlying the analysis (p>0.2).
The Cohen's d statistic was calculated as a measure of effect size for the difference between each patient group and the cohort of controls matched to them in terms of each metabolite within GM and WM.
Pearson correlations were used to assess the association of time from injury with the level of each metabolite within GM and WM for each patient group.
SAS 9.3 (SAS Institute, Cary, NC) was used for all computations.
Results
The demographic, imaging and symptomatology data for the PCS-positive (n=15) and PCS-negative (n=11) patients is shown in Table 1. There were no statistical differences in the GCS score or time from injury of the two groups. One patient in each group had positive MRI findings that may have been directly related to their injury.
Table 1.
Demographics, Symptoms and Imaging Findings of the PCS-Positive Patients (Top) and PCS-Negative Patients (Bottom), Sorted by Time From Mild Traumatic Brain Injury
| Patient | Age/gender | TBI cause | GCS | LOC duration1 | Time from injury2 | Symptoms | Magnetic resonance imaging findings |
|---|---|---|---|---|---|---|---|
| 1 | 40/M | Fall | 15 | 3 | 3 | V | Unremarkable |
| 2 | 41/M | Fall | 15 | <1 | 5 | H, D, S | ″ |
| 3 | 42/M | Fall | 14 | 5 | 5 | H, S, M | ″ |
| 4 | 22/M | Assault | 13 | 30 | 6 | H | ″ |
| 5 | 25/M | Assault | 15 | 25 | 10 | H | Right frontal convexity arachnoid cyst |
| 6 | 32/M | Assault | 15 | 2 | 17 | H, D, S, M | Unremarkable |
| 7 | 23/M | Assault | 15 | 30 | 18 | H, D, M | ″ |
| 8 | 18/M | Ped/Auto | 15 | 15 | 19 | H, D, M | ″ |
| 9 | 51/M | MVA | 14 | 30 | 19 | H | Few punctate foci of abnormal T2 hyperintensities in frontal and parietal lobe subcortical WM with nonspecific etiology |
| 10 | 37/M | Fall | 15 | 2 | 20 | H, D | Unremarkable |
| 11 | 51/F | Bike fall | 14 | 30 | 20 | H, D, S | Stable right cerebellopontine angle arachnoid cyst |
| 12 | 28/F | Bike/Auto | 15 | 20 | 29 | H, D, S | Unremarkable |
| 13 | 32/F | Fall | 15 | 1 | 43 | D, M | ″ |
| 14 | 44/F | Ped/Auto | 15 | <1 | 54 | D, M | ″ |
| 15 | 50/M | Fall | 15 | <1 | 55 | H, D, S, M | ″ |
| Avg. | 36±11 | 14.7±0.6 | 13±13 | 22±17 | |||
| 16 | 18/M | Assault | 15 | 20 | 10 | None | ″ |
| 17 | 27/M | Assault | 15 | <1 | 12 | ″ | ″ |
| 18 | 29/M | Bike fall | 15 | 15 | 13 | ″ | ″ |
| 19 | 25/F | Ped/Auto | 15 | 2 | 14 | ″ | ″ |
| 20 | 23/M | Assault | 14 | 30 | 18 | ″ | Two ovoid foci of abnormal T2 hyperintensities in left frontal lobe subcortical WM with nonspecific etiology |
| 21 | 24/M | Assault | n/a | 30 | 18 | ″ | Unremarkable |
| 22 | 19/M | Assault | 14 | 30 | 19 | ″ | ″ |
| 23 | 36/M | Fall | 15 | <1 | 23 | ″ | ″ |
| 24 | 35/M | Sport collision | 15 | <1 | 24 | ″ | ″ |
| 25 | 38/M | Fall | 15 | <1 | 31 | ″ | ″ |
| 26 | 56/M | Assault | 15 | <1 | 40 | ″ | ″ |
| Avg. | 30±11 | 14.8±0.4 | 12±13 | 20±9 |
Minutes; 2Days.
PCS, post-concussive symptoms; mTBI, mild traumatic brain injury; TBI, traumatic brain injury; GCS, Glasgow Coma Scale; LOC, loss of consciousness; V, blurred vision; H, headache; D, dizziness; S, sleep disturbance; M, memory deficits; Ped/Auto, pedestrian struck by car; MVA, motor vehicle accident; Bike/Auto, bicyclist struck by car.
Bold: Average±standard deviation.
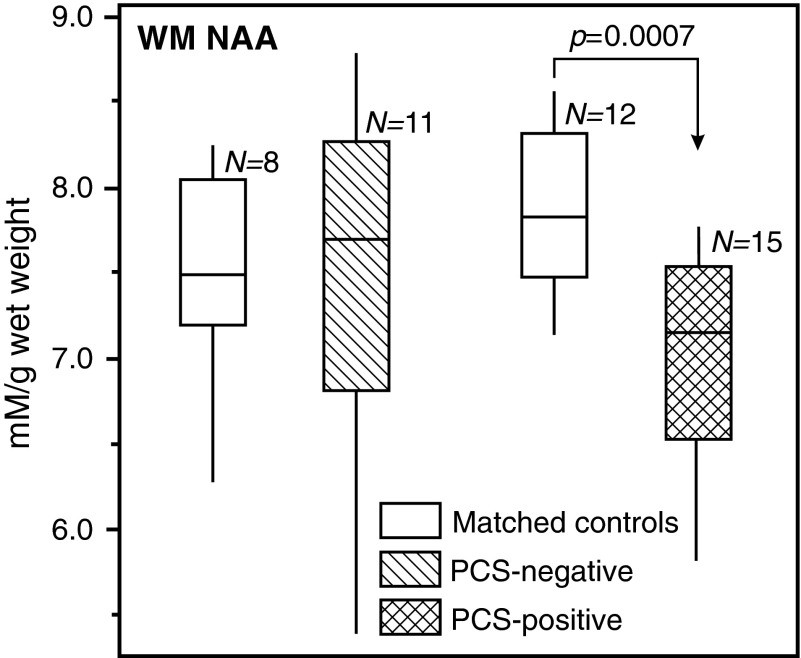
None of the concentrations of any metabolite either in GM or WM were different between the PCS-negative patients and their age- and gender-matched controls (n=8). PCS-positive patients had normal GM NAA, Cr, Cho and mI, as well as normal WM Cr, Cho and mI, but significantly lower WM NAA than their age- and gender-matched controls (n=12; 7.0±0.6 versus 7.9±0.5mM; p=0.0007, as shown in Fig. 1). Based on its Cohen's d value of 1.65, the effect size of this difference is defined as “large.”
FIG. 1.
Box plots displaying 25%, median and 75% (box) and 95% (whiskers) of the N-acetylaspartate (NAA) concentrations distributions in the white matter of post-concussive symptoms (PCS)-negative and PCS-positive mild traumatic brain injury patients, compared with their age- and gender-matched controls. Note that a highly significant (p<0.001) NAA deficit is observed only in the PCS-positive cohort (124×105 mm; 300×300 DPI).
Twelve of the PCS-positive and one of the PCS-negative patients reported at least one of the other symptoms included on the questionnaire. Exclusion of the PCS-negative patient from the analysis (#26, complaining of neck stiffness and photophobia) did not affect any of the results.
Finally, none of the metabolite's levels in the GM and WM of either the PCS-positive or PCS-negative patient group correlated with time from injury.
Discussion
In the neuropathological literature, axonal injury in TBI is referred to as “traumatic axonal injury,” a main outcome of which is diffuse axonal injury (DAI), classically defined as histopathologically observed axonal swellings. In the clinical setting, DAI is suspected when MRI is positive for vascular damage but unfortunately the sensitivity of the exam is low, and DAI is thought to occur across the entire clinical TBI spectrum, including patients with a GCS score of 15 and normal neuroimaging. To be able to detect it in this population, we previously used two 1H-MRSI post-processing approaches developed specifically for increased sensitivity to diffuse disease.11,15 We found decreased global WM NAA but no other metabolic abnormalities either in WM or GM, and these results were interpreted as DAI without glial involvement or cell body injury.12 In the current paper, we report that the WM NAA decrease can be entirely ascribed to those who reported at least one PCS at the time of scanning. The association was robust statistically (Cohen's d=1.65), even despite the relatively small sample sizes.
Usually, within hours of mTBI, abnormal neural function that can be measured by GCS returns to baseline and patients are often left with no objective clinical marker of having experienced mTBI or indicating injury recovery.16 Therefore, identifying biomarkers which correlate with (subjective) PCS has been a priority in TBI research.3,8 The majority of such mTBI studies have been done with DTI, which is sensitive to WM microstructural damage. In some instances, however, TBI results in secondary biochemical cascades that leave the axon intact but dysfunctional.9 Such non-structural, hence DTI-occult, changes are thought to contribute to clinical mTBI deficits.9 In contrast, 1H-MRS-detectable NAA levels reflect both axonal integrity and independent cellular processes, such as mitochondrial alterations,17 which may follow TBI.9 Our finding of lower WM NAA only in PCS-positive patients, therefore, may be due to the fact that its signal represents a wide range of clinically important TBI-related neuropathologies manifested by PCS.
Another explanation for the observed correlation is that unlike most previous 1H-MRS studies of mTBI,10 the measured WM NAA concentration is global, reflecting its status in approximately 40% of the brain's WM. This is important, because DAI is by definition “diffuse,” and measuring local metabolism in a subjectively-chosen region may, therefore, not be pertinent for all patients. A global measure is expected to correlate better with the varied types of PCS, which cannot all be traced back to a single brain region. Indeed, neither DTI nor 1H-MRS studies have shown a consistently injured locale in mTBI,10 probably due to the well known heterogeneity of human mTBI. Therefore, since all PCS presumably share common etiology, (e.g., axonal damage) they may be best assessed as a group by a global measure of that surrogate.
As such, lower global WM NAA was the only metric that distinguished PCS-positive patients from matched controls. The lack of any abnormalities in GM and WM levels of the glial markers Cr, Cho and mI corroborated the interpretation of our original study (on the entire patient population) of axonal dysfunction, rather than cell death (i.e., there were no differences in other markers that were “masked” due to grouping PCS-negative with PCS-positive patients). Importantly, mTBI patients who did not report PCS were indistinguishable from controls in all markers in both tissue types. Their mean WM NAA levels were identical to controls', suggesting no evidence of axonal injury in this clinically healthy cohort.
The World Health Organization's report13 was chosen as the source of the PCS symptom list because it pre-screened a large number of papers and its goal was to present the best scientific evidence on mTBI epidemiology. The symptoms on our questionnaire were defined as most common in acute/subacute mTBI and were prospectively chosen as appropriate for our cohort's time from mTBI. Diagnoses of post-concussional syndrome or disorder were not made, since they are intended for use only in establishing chronicity, more than one month post-TBI.6 There is, therefore, an important distinction between our PCS-positive cohort and chronic PCS patients. Indeed, the finding of approximately 50% PCS-positive patients in the acute/subacute stage is expected, but a much smaller proportion (7–30%) is expected to remain chronic. While this study suggests that WM NAA correlates with acute symptoms, only longitudinal observation can assess its utility in the chronic stage. Given the normal levels of all other 1H-MRS markers, and hence the hypothesis that lower WM NAA indicates axonal dysfunction and not death,12 normalization of its levels is plausible in a recovery scenario. Nevertheless, since physical symptoms tend to decrease at the expense of emotional and cognitive ones,18 PCS testing incorporating more components of the latter may be more appropriate for ascertaining WM NAA interactions in chronic PCS.
The main limitation of this study was its retrospective nature and that consequently it was not geared to comprehensively examine correlations between 1H-MRSI data and patient outcome. Although cognitive symptoms are common post mTBI, only one question addressed that domain. In addition, it was broadly defined as “memory deficits” and not objectively tested. This was the only symptom on our questionnaire, however, that could have been objectively confirmed. The rest of the symptoms were subjective in nature, reflecting the fact that this subset of PCS predominates in mTBI.
The relatively few symptoms studied also dictated a dichotomous classification of PCS status. This and the small sample size precluded using PCS criteria of having one or more symptoms in different categories.19 While a more stringent definition of PCS may increase the specificity, it reduces sensitivity: Patients complaining of only one or few symptoms would be excluded from such a definition, although their symptoms may be mTBI-related. We therefore opted for increased sensitivity instead. Indeed, because they are characteristic of subacute mTBI, there was only one patient who reported any symptoms other than those defining a PCS-positive status. The utility of the dichotomous approach is reflected in the results here, which reveal no trend for abnormalities in the PCS-negative patients. This is in contrast with studies in which abnormalities are found in patients deemed “PCS-free” by more rigorous PCS criteria,20 perhaps reflecting the presence of axonal injury giving rise to some, but not all characteristic mTBI symptoms.
In conclusion, in cohorts representative of patients for whom currently there are no established radiological or clinical measures of outcome, global WM NAA levels were lower in PCS-positive patients but were normal in the PCS-negative group. This indicates that global WM NAA is sensitive to the TBI sequelae underlying common subacute mTBI symptoms.
Acknowledgments
This work was supported by NIH Grants EB01015, NS39135, NS29029 and NS0050520. Dr. Tal acknowledges the support of the Human Frontiers Science Project.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M. Xu L. Wald M. Coronado V. Centers for Disease Control and Prevention; Atlanta, GA: 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths, 2002–2006. [Google Scholar]
- 2.Blyth B.J. Bazarian J.J. Traumatic alterations in consciousness: traumatic brain injury. Emerg. Med. Clin. North Am. 2010;28:571–594. doi: 10.1016/j.emc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belanger H.G. Vanderploeg R.D. Curtiss G. Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Borg J. Holm L. Cassidy J.D. Peloso P.M. Carroll L.J. von Holst H. Ericson K. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004:61–75. doi: 10.1080/16501960410023822. [DOI] [PubMed] [Google Scholar]
- 5.Culotta V.P. Sementilli M.E. Gerold K. Watts C.C. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38:245–250. doi: 10.1097/00006123-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ruff R.M. Mild traumatic brain injury and neural recovery: rethinking the debate. NeuroRehabilitation. 2011;28:167–180. doi: 10.3233/NRE-2011-0646. [DOI] [PubMed] [Google Scholar]
- 7.Biasca N. Maxwell W.L. Minor traumatic brain injury in sports: a review in order to prevent neurological sequelae. Prog. Brain Res. 2007;161:263–291. doi: 10.1016/S0079-6123(06)61019-4. [DOI] [PubMed] [Google Scholar]
- 8.Shenton M.E. Hamoda H.M. Schneiderman J.S. Bouix S. Pasternak O. Rathi Y. Vu M.A. Purohit M.P. Helmer K. Koerte I. Lin A.P. Westin C.F. Kikinis R. Kubicki M. Stern RA. Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson V.E. Stewart W. Smith D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2012 doi: 10.1016/j.expneurol.2012.01.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin A.P. Liao H.J. Merugumala S.K. Prabhu S.P. Meehan W.P., 3rd Ross B.D. Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2012;6:208–223. doi: 10.1007/s11682-012-9181-4. [DOI] [PubMed] [Google Scholar]
- 11.Tal A. Kirov I.I. Grossman R.I. Gonen O. The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. N.M.R. Biomed. 2012 doi: 10.1002/nbm.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirov I.I. Tal A. Babb J.S. Lui Y.W. Grossman R.I. Gonen O. Diffuse axonal injury in mild traumatic brain injury: a 3D multivoxel proton MR spectroscopy study. J. Neurol. 2012;260:242–252. doi: 10.1007/s00415-012-6626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll L.J. Cassidy J.D. Peloso P.M. Borg J. von Holst H. Holm L. Paniak C. Pepin M. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil. Med. 2004:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 14.McCrea M. Iverson G.L. McAllister T.W. Hammeke T.A. Powell M.R. Barr W.B. Kelly J.P. An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin. Neuropsychol. 2009;23:1368–1390. doi: 10.1080/13854040903074652. [DOI] [PubMed] [Google Scholar]
- 15.Kirov I.I. George I.C. Jayawickrama N. Babb J.S. Perry N.N. Gonen O. Longitudinal inter- and intra-individual human brain metabolic quantification over 3 years with proton MR spectroscopy at 3 T. Magn. Reson. Med. 2012;67:27–33. doi: 10.1002/mrm.23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigler ED. Mild traumatic brain injury: the elusive timing of “recovery.”. Neurosci. Lett. 2012;509:1–4. doi: 10.1016/j.neulet.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Paling D. Golay X. Wheeler-Kingshott C. Kapoor R. Miller D. Energy failure in multiple sclerosis and its investigation using MR techniques. J. Neurol. 2011;258:2113–2127. doi: 10.1007/s00415-011-6117-7. [DOI] [PubMed] [Google Scholar]
- 18.Dischinger P.C. Ryb G.E. Kufera J.A. Auman K.M. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J. Trauma. 2009;66:289–296. doi: 10.1097/TA.0b013e3181961da2. [DOI] [PubMed] [Google Scholar]
- 19.King N.S. Crawford S. Wenden F.J. Moss N.E. Wade D.T. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 20.Messe A. Caplain S. Pelegrini-Issac M. Blancho S. Montreuil M. Levy R. Lehericy S. Benali H. Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging Behav. 2012;6:283–292. doi: 10.1007/s11682-012-9159-2. [DOI] [PubMed] [Google Scholar]