Abstract
Patient: Male, 76
Final Diagnosis: Aorto-enteric fistula
Symptoms: Abdominal pain • bloody vomiting • shock
Medication: —
Clinical Procedure: CT abdomen with contrast
Specialty: Gastroenterology • vascular surgery
Objective:
Rare disease
Background:
Primary Aorto-Enteric Fistula (PAEF) is a unique and rare life threatening communication between the Aorta and Gastrointestinal Tract with an unusually high mortality rate and delayed diagnosis. Aortic abdominal aneurysms are implicated in a vast majority of cases while cancer as an etiology of PAEF is extremely rare.
Case Report:
We present the first case report of a PAEF secondary to Large B-Cell Lymphoma, followed by a review of literature in regards to malignant aorto-enteric fistulae.
Conclusions:
We conclude that physicians should maintain a high index of suspicion for PAEF in any patient with unexplained anemia and history of aortic abdominal aneurysm complicated by cancer.
Keywords: gastrointestinal hemmorage, aortoenteric fistula, lymphoma
Background
Primary Aorto-Enteric Fistula (PAEF) is a life threatening communication between the Aorta and Gastrointestinal Tract that is ultimately fatal if left untreated. Aortic Abdominal Aneurysm is implicated in vast majority of cases and cancer constitutes only 1% of all causes of primary aorto-enteric fistulae. The most common cancers associated with PAEF are esophageal and pancreatic malignancies but case reports of metastatic testicular and recurrent cervical cancers have also been reported. We present the first case report of primary aorto-enteric fistula secondary to Large B-Cell Lymphoma, followed by a review of literature in regards to malignant aorto-enteric fistulae.
Case Report
A 76 year old Hispanic Male with a past medical history of thoraco-abdominal aneurysm and B-Cell Lymphoma presented with abdominal pain and hematemesis.
Two-and-a-half years ago he was diagnosed with a thoraco-abdominal aneurysm with maximum dilation of 4.7 cm and which ran from 4 cm past the left subclavian artery to just below the level of the SMA. The aneurysm was under active surveillance but did not meet criteria for surgical repair.
Three months prior to admission, the patient experienced 25-pound weight loss, associated with anorexia, weakness and pallor. A recent routine surveillance Abdominal CT followed by MRI (Figure 1) demonstrated a 9.2×8.0×7.9 cm retroperitoneal mass encasing the aorta and intimately associated with the 3rd and 4th portions of the duodenum as well as to parts of the jejunum and ileum.
Figure 1.
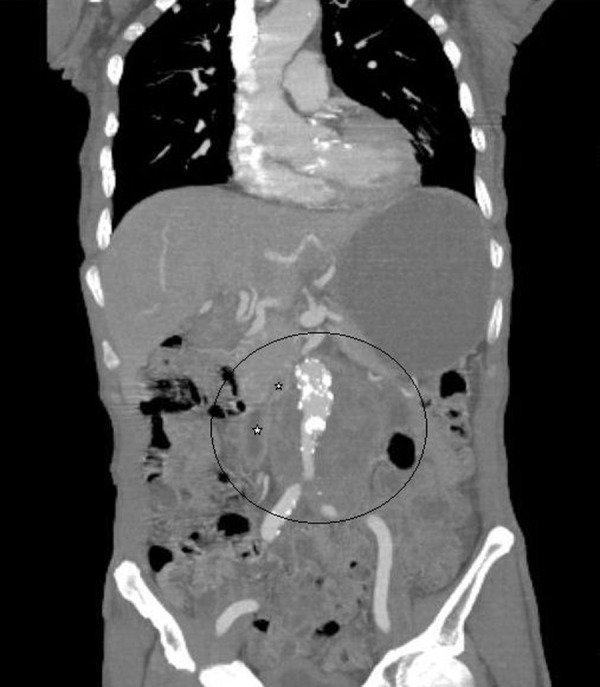
Abdominal CT Angiography showing abdominal aortic aneurysm and large retroperitoneal mass encasing abdominal aorta with close proximity to duodenum (star) and ileum (star).
CT-guided biopsy of the mass revealed an invasive, poorly differentiated, EBV positive, diffuse large B-cell lymphoma (Figure 2). Labs were notable for a hemoglobin level of 8.3 and hemoccult-positive stool. A gastroenterological evaluation found no massive gastrointestinal bleeding and occult bleeding was attributed to intraluminal lymphoma. The patient deferred surgery but agreed to chemotherapy to try and control the disease locally.
Figure 2.
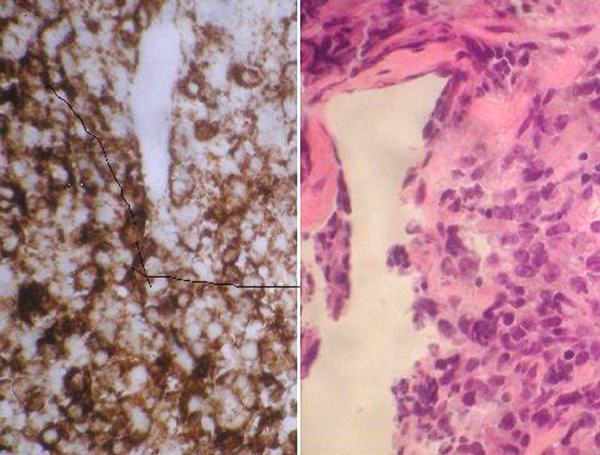
H&E and CD20 Antibody staining showing poorly differentiated lymphoid cells that are strongly CD20 positive.
Five days after receiving his first cycle of CHOP chemotherapy regimen the patient was admitted with a7/10 abdominal pain radiating to the back with multiple episodes of bloody emesis which lasted approximately 4 hours. Initial blood pressure was 81/45 with a pulse of 125. After two hours of resuscitation with IV saline, his blood pressure dropped to 64/29 while his abdominal pain increased to 10/10, and he developed shortness of breath, increased hematemesis, and profuse, dark red hematochezia. His hemoglobin was 7.5, platelet count 125,000 and INR 1.3.
A CT aortogram (Figure 3) showed an Aorto-Enteric Fistula at the duodenum, with extravasation of contrast mixed with bowel gas. A femoral line was placed for aggressive resuscitation with fluids and blood transfusions. The patient was intubated, and an NG tube consistently suctioned 500 cc per-hour of blood. Levophed and vasopressin drips were initiated, but unfortunately with limited effect. Vascular and General Surgery were consulted, as well as Gastroenterology. The patient was considered a poor surgical candidate in light of his moribund hemodynamic instability. After a discussion of his grave prognosis was held with the patient’s family, aggressive measures were withdrawn. The patient expired approximately 3 hours later.
Figure 3.
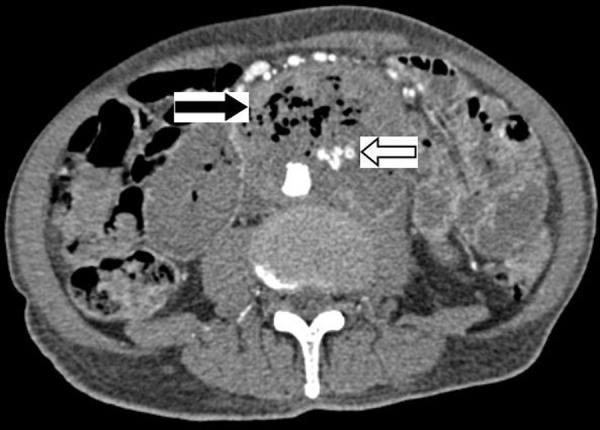
CT Angiography showing bowel gas and adjacent extravasations of contrast material with in the mass pathognomonic of a primary aortoenteric fistula.
Discussion
Primary Aorto-Enteric Fistula (PAEF) – a life-threatening, exsanguinating illness – was first reported by Sir Astley Cooper in 1829 [1,2]. He observed a lethal communication between the aorta and gastrointestinal tract causing massive upper gastrointestinal bleeding. The reported incidence is only 0.04–0.007%, however the true incidence of a PAEF is probably underreported [2,3]. More than 85% of PAEF are due to Aortic Abdominal Aneurysms [3] while cancer associated with primary aorto-enteric fistula is rare [1–3]. During the 1990’s, A. Gad described four cases of squamous esophageal cancer and two cases of pancreatic cancer associated with PAEF in the first autopsy case series [4]. Unusual malignant etiologies of AEF such as recurrent cervical cancer, colorectal cancer and testicular germ cell tumor were also reported on a level of case reports with close to 100% fatalities in almost all cases [5–9].
Mechanism of formation of PAEF due to malignancy is probably multifactorial and is related to susceptible anatomic location of the bowel and underlying pathological processes that directly weaken the aortic wall [7,9,10]. PAEF due to aortic abdominal aneurysms predominantly affect the 3rd and 4th parts of the duodenum. About 22% of the cases involve the esophagus and the rest of the cases involve other parts of gastrointestinal tract [10]. Cancer can directly erode the bowel and vasculature wall that is further compromised by an aneurysm or inflammation induced destruction of a vessel wall by metal-loproteinases [11]. Proximity of the esophagus to the aorta and anatomic fixation of the 3rd and 4th portion of the duodenum makes these GI tract sections more vulnerable to invasive processes-even in the absence of aortic aneurysms [12].
Primary AEF poses a tremendous diagnostic challenge and prompt recognition requires a high index of clinical suspicion [13]. Studies by Sweeny et al. and Saers et al. show that the classic clinical triad of abdominal pain, hemorrhage and pulsa-tile mass thought to be pathognomonic of aortoenteric fistula was insignificant with a reported incidence of only 0–11% in all cases [3,13]. Meanwhile gastrointestinal hemorrhage was the most commonly reported symptom in 64–94% of all patients followed by hematemesis from 64–74% [3,10,13]. Initial manifestation of PAEF hemorrhage can be a sentinel or herald bleed that may occur in a repetitive fashion culminating in full blown hemorrhagic shock [14]. In the case of malignancy, anorexia, weakness and pallor may be falsely attributed to cancer itself and sentinel bleeding episodes might be overlooked.
Imaging modalities are inaccurate when it comes to diagnosing AEF. In the early 1990s the diagnostic yield of CT and endoscopy were only 18% and 29%, respectfully. In the last two decades, the CT rate of detecting PAEF increased dramatically to 61% [3]. Although Esophagogastroduodenoscopy (EGD) is recommended to rule out other etiologies in a hemodynamically stable patient with high clinical suspicion for PAEF, EGD can be time consuming, may require a second look and there may be technical difficulties reaching the 4th portion of duodenum [1,3,4].
Since the most common etiologies of malignant PAEF are esophageal and pancreatic cancer, EGD followed by CT of the abdomen is a reasonable approach. The role of MRI and Endoscopic US in the diagnosis of PAEF due to cancer is yet to be determined. Exploratory laparotomy can both secure the diagnosis and provide treatment with local control of bleeding and graft repair. However early (<30 days) mortality rates are reported at 20–50% [16] and long-term survival is even lower. In poor surgical candidates, Endovascular Repair can be successful, short-term, but some patients will likely require a second, more definitive surgical procedure [17]. In malignant PAEF short-term survival is grim since most patients present with heavy tumor burden, disseminated disease and locally aggressive and inoperable malignancy.
Conclusions
To our knowledge, this is the first case report of Primary Aorto-Enteric Fistula due to Large B-cell Lymphoma. Anorexia and weakness with pallor may be attributed to cancer or to a herald bleed which delays diagnosis of PAEF in malignancy. Clinicians are advised to maintain a high index of suspicion for PAEF in all patients with occult gastrointestinal bleeding and especially those with an aortic abdominal aneurysm complicated by any malignancy. A timely diagnosis of PAEF with early surgical intervention may increase survival as opposed to a late presentation of hemorrhagic shock secondary to PAEF.
References:
- 1.Lemos DW, Raffetto JD, Moore TC, Menzoian JO. Primary aortoduodenal fistula: a case report and review of the literature. J Vasc Surg. 2003;37(3):686–89. doi: 10.1067/mva.2003.101. [DOI] [PubMed] [Google Scholar]
- 2.Voorhoeve R, Moll FL, et al. Primary aorto-enteric fistula: Report of eight new cases and review of the literature. Ann Vasc Surg. 1996;10:40–48. doi: 10.1007/BF02002340. [DOI] [PubMed] [Google Scholar]
- 3.Saers SJ. Primary aorto-enteric fistula. Br J Surg. 2005;92:143–52. doi: 10.1002/bjs.4928. [DOI] [PubMed] [Google Scholar]
- 4.Gad A. Aortoduodenal fistula revisited. Scand J Gastroenterol. 1989;24(Suppl.167):97–100. doi: 10.3109/00365528909091322. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor DK, et al. Primary Aorto/Iliac-Enteric Fistula: Report of 6 New Cases. Vasc Endovascular Surg. 2004;38(3):281–86. doi: 10.1177/153857440403800315. [DOI] [PubMed] [Google Scholar]
- 6.Huang JH, et al. Reccurrent cervical carcinoma presenting as a primary aortoduodenal fistula. Ann Vasc Surg. 2010;24(8):113.e5–7. doi: 10.1016/j.avsg.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Hansen KS, Sheley RC. Aorto-enteric fistula in advanced germ cell tumor: a rare lethal complication. J Urol. 2002;167(5):2131. [PubMed] [Google Scholar]
- 8.Armitage NC, et al. Primary aorto-enteric fistula due to recurrent colorectal cancer: Report of a case. Dis Colon Rectum. 1990;33:148–49. doi: 10.1007/BF02055546. [DOI] [PubMed] [Google Scholar]
- 9.Gaphrey AD, et al. Carcinoma of head of the pancreas with aortoduodenal fistula. Am J Surg. 1966;111:580–83. doi: 10.1016/0002-9610(66)90289-3. [DOI] [PubMed] [Google Scholar]
- 10.Saers SJ, Scheltinga MR. Primary aorto-enteric fistula. Br J Surg. 2005;92:143–52. doi: 10.1002/bjs.4928. [DOI] [PubMed] [Google Scholar]
- 11.Finch L, et al. Emergent Treatment of Primary Aorto-enteric Fistula with N-butyl 2-cyanoacrlyate and Endovasc ular Stent. J Vasc Radiology. 2002;13:841–43. doi: 10.1016/s1051-0443(07)61994-0. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita A, Noma T, Nakazawa A, et al. Enhanced expression of matrix metalloproteinase-9 in abdominal aortic aneurysm. World J Surg. 2001;25(3):259–65. doi: 10.1007/s002680020062. [DOI] [PubMed] [Google Scholar]
- 13.Sweeny S, Gadacz TR. Primary aortoduodenal fistula; Manifestation, diagnosis, and treatment. Surgery. 1984;96(3):492–97. [PubMed] [Google Scholar]
- 14.Perdue GD, Jr, Smith RB, III, et al. Impending aorto-enteric hemorrhage: the effect of early recognition on improved outcome. Ann Surg. 1980;192:237–43. doi: 10.1097/00000658-198008000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makhija M. Imaging of an actively bleeding aortoduodenal fistula. Clin Nucl Med. 1985;10:372–73. doi: 10.1097/00003072-198505000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Bustuttil SJ, Goldstone J. Diagnosis and management of aorto-enteric fistulas. Semin Vasc Surg. 2001;14:302–11. doi: 10.1053/svas.2001.27888. [DOI] [PubMed] [Google Scholar]
- 17.Lonn L, et al. Is EVAR the treatment of choice for aorto-enteric fistula. J Cardiovasc Surg (Torino) 2010;51(3):319–27. [PubMed] [Google Scholar]


