Abstract
Patient: Female, 94
Final Diagnosis: Malignant pleural effusion
Symptoms: —
Medication: —
Clinical Procedure: Cytology
Specialty: Oncology
Objective:
Unusual clinical course
Background:
The most common site of postoperative breast cancer recurrence is bone, followed by local relapse, lung, and liver. The majority of relapses occur within the first 3 years after surgery. Pleural recurrences more than 10 years after surgery are rare.
Case Report:
A 94-year-old woman who had undergone modified radical mastectomy for right breast cancer (invasive ductal carcinoma, pT2, pN1, ER+, PgR+) 12 years earlier presented to our hospital with carcinomatous pleuritis and a chief complaint of dyspnea. Endocrine therapy with oral letrozole was started and the pleural effusion had disappeared 3 months later.
Conclusions:
Oral endocrine therapy may be effective for the treatment of late recurrence of hormon receptor-positive breast cancer in elderly women.
Keywords: malignant pleural effusion, breast cancer, late recurrence, endocrine therapy
Background
Postoperative recurrence of breast cancer occurs in approximately 18% to 45% of patients [1–3]. The most common site of the relapse is bone, followed by local relapse, lung, and liver [1,4]. The majority of relapses occur within the first 3 years after surgery [1,3,5]. Pleural recurrences more than 10 years after surgery are rare [1,5–8]. We report herein the case of a patient who developed pleural recurrence from breast cancer 12 years after surgery and in whom letrozole was effective in treating the pleural effusion.
Case Report
A 94-year-old postmenopausal woman presented to our hospital with dyspnea. She had undergone modified radical mastectomy for right breast cancer at age 81. Pathological examination of the primary specimen revealed 25×25 mm in size, invasive ductal carcinoma with metastasis to the axillary lymph node, and estrogen and progesterone receptor positivity (Figure 1). According to the UICC-TNM classification, the tumor was pT2pN1M0 stage B. After the mastectomy, the patient had received adjuvant hormonal therapy with fadrozole hydrochloride hydrate followed by anastrozole and toremifene citrate for 5 years. She had remained well until 2 weeks before our hospital visit, when she reported experiencing difficulty breathing at rest.
Figure 1.
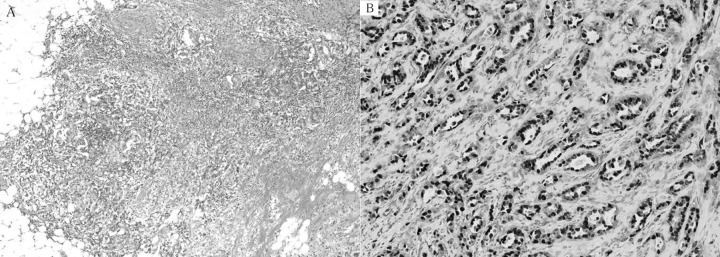
Pathological findings of the primary resected specimen showing invasive ductal carcinoma, papillotubular carcinoma with scirrhous carcinoma (A ×40). Tumor cells are positive for estrogen receptor (B ×100).
Chest X-ray and computed tomography showed bilateral pleural effusion, predominantly on the right side (Figure 2). Plural effusion in the right thoracic cavity was removed by thoracentesis, and cytological examination revealed adenocarcinoma cells. Immunohistochemical analysis revealed that the tumor cells were positive for estrogen receptor and progesterone receptor (Figure 3). Therefore, the patient was diagnosed with pleural recurrence of breast cancer. As intensive chemotherapy was judged to be too dangerous due to the patient’s advanced age, treatment with oral letrozole and diuretics (furosemide and spironolactone) was started. The pleural effusion had disappeared 3 months later (Figure 4). Chest X-ray demonstrated that the pleural effusion remained absent at 8 months after the start of treatment (Figure 5). No serious adverse effects were encountered during therapy. Although the patient was cured of her dyspnea, she died of natural causes about 9 months after the development of pleural effusion.
Figure 2.
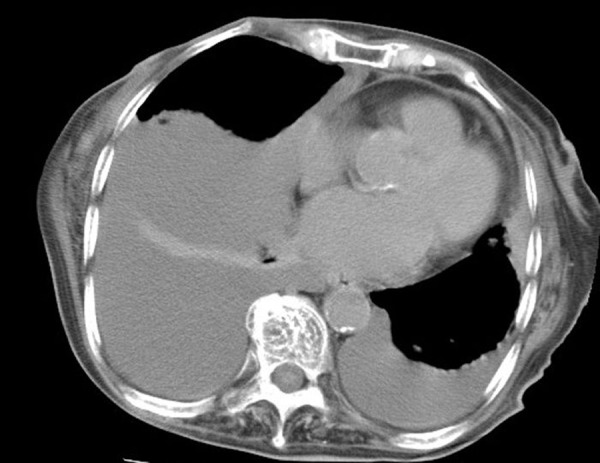
Chest CT scan showing bilateral and right massive pleural effusion.
Figure 3.
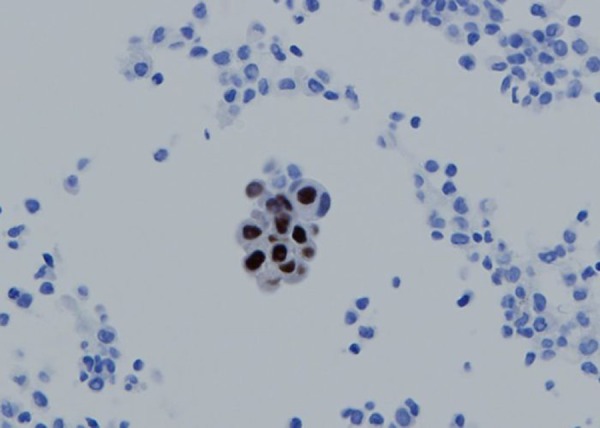
Immunocytological examinations of the thoracentesis fluid revealing estrogen receptor-positive adenocarcinoma cells.
Figure 4.
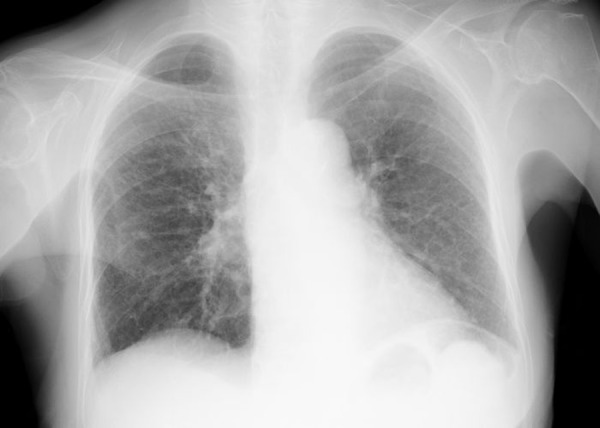
Chest X-ray film taken 3 months after starting oral hormonal therapy showing disappearance of bilateral pleural effusion.
Figure 5.

Re-accumulation of pleural fluid is not observed 8 months after the start of treatment.
Discussion
Malignant pleural effusions (MPEs) are defined as effusions that result from the direct infiltration of the pleura by cancer cells [9]. Breast cancer is the second most common cause after lung cancer of MPEs, accounting for approximately one third of all MPEs [10,11]. Moreover, breast cancer is often related to MPEs, with about 2% to 11% of patients with breast cancer developing MPEs during the disease course [12,13]. Approximately 80% of patients with pleural recurrences develop MPEs within the first 5 years after primary surgery, although pleural recurrences more than 10 years after surgery are rare [7,8].
MPEs are associated with a poor prognosis. In breast cancer patients, the median survival following pleural fluid accumulation is 5 to 13 months [7,11,14]. Many patients with malignant effusions experience dyspnea, and additional symptoms include weight loss, anorexia, malaise and fatigue, which disturb quality of life. Therefore, management of MPEs is important to improve the quality of life of patients, and inadequate management results in deterioration in respiratory function that can shorten expected survival time.
Therapeutic thoracentesis is the initial approach for patients with respiratory symptoms including dyspnea. However, pleural fluid usually recurs after simple aspiration [7]. Therefore, various approaches are used to prevent the re-accumulation of pleural effusions. Approaches to treatment of MPEs include chemical pleurodesis using various sclerosing agents [15], use of a chronic indwelling catheter [11,16], and pleuroperitoneal shunting [17,18]. Systemic therapy using with cytotoxic and/ or endocrine agents may also be effective in decreasing pleural fluid or relieving dyspnea in breast cancer patients [19]. Because the purpose of treatment for metastatic breast cancer is to maintain favorable quality of life and to improve survival, it is important to reduce the degree of treatment-related discomfort. In general, endocrine agents have fewer adverse effects than cytotoxic anticancer agents. In our case, the patient experienced no adverse effects with the hormonal therapy and was able to live comfortably for several months without dyspnea.
Conclusions
The hormonal therapy may be a useful treatment for elderly postmenopausal women with late recurrences of estrogen receptor-positive breast cancer.
References:
- 1.Elder EE, Kennedy CW, Gluch L, et al. Pattern of breast cancer relapse. Eur J Surg Oncol. 2006;32:922–27. doi: 10.1016/j.ejso.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;17:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–46. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 4.Patanaphan I, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109–12. [PubMed] [Google Scholar]
- 5.Takeuchi H, Tsuji K, Ueo H. Prediction of early and late recurrence in patients with breast carcinoma. Breast Cancer. 2005;12:161–65. doi: 10.2325/jbcs.12.161. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi H, Muto Y, Tashiro H. Clinicopathological characteristics of recurrence more than 10 years after surgery in patients with breast cancer. Anticnacer Res. 2009;29:3445–48. [PubMed] [Google Scholar]
- 7.Fentiman IS, Millis R, Sexton S, Hayward JL. Pleural effusion in breast cancer. Cancer. 1981;47:2087–92. doi: 10.1002/1097-0142(19810415)47:8<2087::aid-cncr2820470830>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Raju RN, Kardinal CG. Pleural effusion in breast carcinoma: analysis of 122 cases. Cancer. 1981;48:2524–27. doi: 10.1002/1097-0142(19811201)48:11<2524::aid-cncr2820481130>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Heffner JE. Diagnosis and management of malignant pleural effusions. Radiology. 2008;13:5–20. doi: 10.1111/j.1440-1843.2007.01154.x. [DOI] [PubMed] [Google Scholar]
- 10.Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion; factors affecting success. Ann Surg Oncol. 2009;16:745–50. doi: 10.1245/s10434-008-0263-x. [DOI] [PubMed] [Google Scholar]
- 11.Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362–68. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 12.Apffelstaedt JP, Van Zyl JA, Muller AG. Breast cancer complicated by pleural effusion: patient characteristics and results of surgical management. J Surg Oncol. 1995;58:173–75. doi: 10.1002/jso.2930580307. [DOI] [PubMed] [Google Scholar]
- 13.Kreisman H, Wolkove N, Finkelstein HS, et al. Breast cancer and thoracic metastases: review of 119 patients. Thorax. 1983;38:175–79. doi: 10.1136/thx.38.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest. 2000;117:79–86. doi: 10.1378/chest.117.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Lombardi G, Zustovich F, Nicoletto MO, et al. Diagnosis and treatment of malignant pleural effusion. A systematic literature review and new approaches. Am J Clin Oncol. 2010;33:420–23. doi: 10.1097/COC.0b013e3181aacbbf. [DOI] [PubMed] [Google Scholar]
- 16.van den Toorn LM, Schaap E, Surmont VFM, et al. Management of recurrent malignant pleural effusions with a chronic indwelling pleural catheter. Lung Cncer. 2005;50:123–27. doi: 10.1016/j.lungcan.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Schulze M, Boehle AS, Kurdow R, et al. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg. 2001;71:1809–12. doi: 10.1016/s0003-4975(01)02586-3. [DOI] [PubMed] [Google Scholar]
- 18.Genc O, Petrou M, Ladas G, Goldstraw P. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. Eur J Cardiothorac Surg. 2000;18:143–46. doi: 10.1016/s1010-7940(00)00422-x. [DOI] [PubMed] [Google Scholar]
- 19.Perrone F, Carlomagno C, De Placido S, et al. First-line systemic therapy for metastatic breast cancer and management of pleural effusion. Ann Oncol. 1995;6:1033–43. doi: 10.1093/oxfordjournals.annonc.a059068. [DOI] [PubMed] [Google Scholar]


