Abstract
Background:
Moyamoya disease (MMD) is a progressive cerebrovascular occlusive disease of the bilateral internal carotid arteries that leads to a compensatory abnormal vascular network at the base of the brain. Its average annual incidence 0.54 per 100,000 population but it is the most common pediatric cerebrovascular disease in East Asia. The reported incidence in USA is approximately 0.086 per 100,000 patients.
Case Report:
We present a case of Moyamoya disease that was to detected in a 7-year-old female who presented with transient altered mental status.
Conclusions:
Moyamoya disease can be diagnosed if history, physical exam and brain imaging is highly suspicious. Conventional angiography remains the gold standard for diagnosis and aids in surgical planning for patients with suspected Moyamoya disease.
Keywords: Moyamoya disease, altered mental status, silent infarction, familial MMD
Background
Takeuchi and Shimizu [1] (1957) defined Moyamoya disease (MMD) as hypoplasia of bilateral internal carotid arteries. Twelve years later Suzuki and Takaku [2] named it Moyamoya, which means puff of smoke that represents angiographic representation of collaterals. Current definition is progressive internal carotid arteries occlusion with compensatory basal cerebral collaterals development [3]. Its incidence ranges between 0.086 in USA to 0.54 per 100,000 patients globally [4,5]. It accounts for one fifth of the identifiable cerebral arteriopathies in childhood stroke up to most common cerebrovascular disease in children in East Asia [6,7]. In children, unilateral involvement occurs about 18% [8] and progress to bilateral involvement within 2 years [9].
Case Report
A 7-year-old African American girl presented to the pediatric emergency department with acute mental status changes. Her parents reported that she had woken up after a nap agitated and confused. She was observed to stand up from the car seat, was restless and wanted to exit the car. She was able to speak a few words although not in a proper context, and was not able to follow any directions. However, She was able to walk supported. No convulsive movements, head or eye deviation was seen. There was no incontinence or tongue biting.
Review of symptoms was negative except what mentioned previously. There was no observed ingestion of drugs or toxins, although there was potential access to drugs in the house including hydrocodone/acetaminophen, alprazolam, and amitriptyline. There was no history of any past head injury, seizures or central nervous system infections. She was born full-term vaginally with Apgar scores of 8 and 9. There was no history of previous neurological deficit, developmental delay or other significant medical illness. The paramedic staff found persistent confusion and agitation with sluggishly reactive dilated pupils.
Family history was negative for seizure disorder or chronic medical condition including sickle cell disease or trait and neurofibromatosis. There was a paternal history of psychiatric illness, unspecified, treated with multiple medications.
In the emergency department, she was found to be afebrile with following vitals: BP 110/68, HR 123 bpm and RR 24. Her weight was 32.7 kg (75th centile) and her height was 152.4 cm (>97th centile). Child was asleep but arousable, with brief eye contact. Pupils were equal and reactive at 8 mm. She had a normal cranial nerve examination, including sharp disc margins on funduscopy. She moved all 4 extremities well with symmetrical 3+ deep tendon reflexes in the lower limb and 2 + in the upper extremities. Plantar responses were flexor bilaterally, without ankle clonus. Chest, heart and abdomen examination was within normal limits with Tanner stage I pubertal stage. Skin and mucous membrane did not reveal any unusual pigmentation, rash or pallor.
Complete blood picture were WNL as well as comprehensive metabolic panel that showed bicarbonate 18 mmol and anion gap 18 mmol that respond to intravenous saline bolus. Urine drug screen was negative. Acetaminophen and ethanol blood levels were below detectable levels. Hemoglobin electrophoresis showed hemoglobin A1 96.1%, A2 3.2% and F 0.7%. Both electrocardiography and bedside electroencephalography were normal for age. Renal ultrasonography with Doppler flow study showed no evidence of renal artery stenosis.
Non-contrast computerized tomography of brain showed an area of decreased density consistent with encephalomalacia in left parietal cortex (Figure 1). Magnetic resonance imaging (MRI) brain with contrast showed an old ischemic infarct involving left parietal cortex with attenuation and narrowing of cavernous and supra-clinoid internal carotid arteries bilaterally as well as horizontal portion of middle cerebral arteries bilaterally with increased prominence and enhancement of lenticulo-striate and thalamo-perforating arteries bilaterally favoring a diagnosis of MMD (Figure 2).
Figure 1.
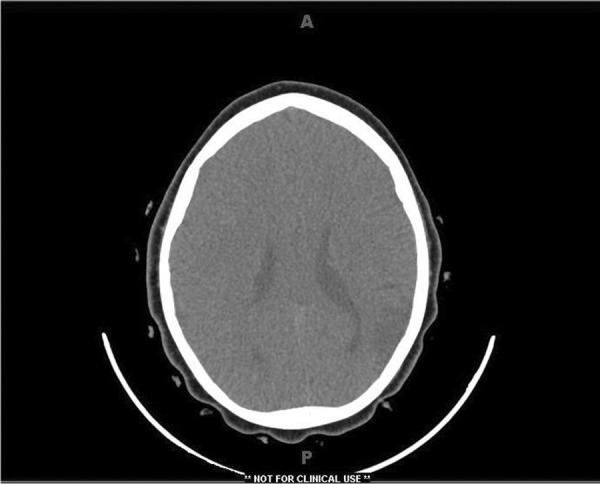
CT brain showing left posterior parietal encephalomalacia.
Figure 2.
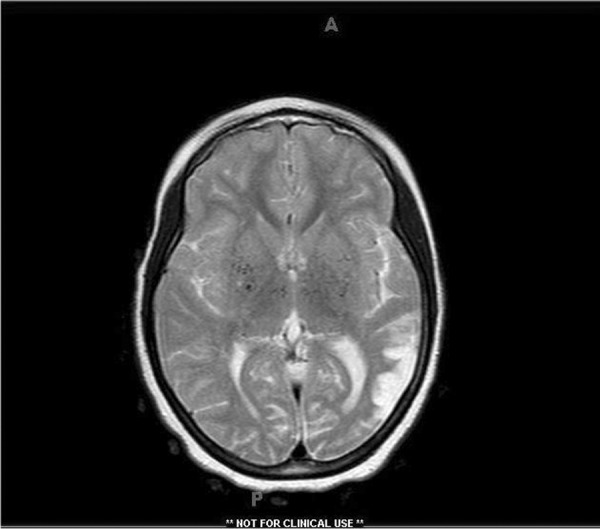
MRI brain with gadolinium showing old left parietal cortical infarct with prominence of blood vessels in basal ganglia and thalami bilaterally.
A trans-femoral four vessel cerebral angiogram showed extra-cranial intracranial collateral anastomoses derived from branches of the external carotid artery to bypass internal carotid obstruction in both sides especially left side. (Figures 3A,B and 4A,B). After this patient had been stabilized and diagnosed, she was discharged on aspirin 1 mg daily and to follow up with pediatric neurosurgeon and neurologist.
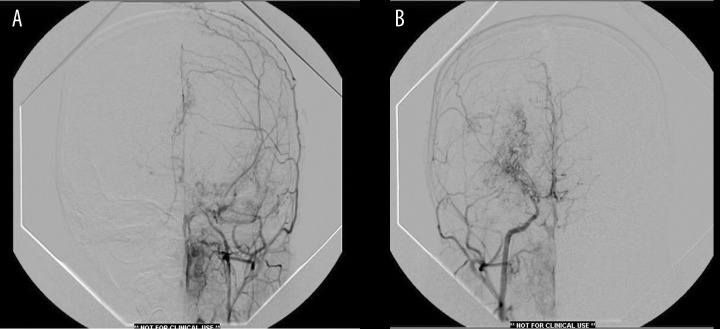
Figures 3.
(A) Left common carotid angiography showing extensive external carotid lepto-meningeal collateralization (occipital artery-ethmoidal branch of maxillary division-middle meningeal-anterior falcine). There is complete occlusion of internal carotid proximal to ophthalmic segment. (B) Right common carotid angiography showing attenuation of internal carotid with developed deep lenticulo-striate vessels as well as lepto-meningeal collaterals from external carotid. There is also crossover flow from right to left into the contralateral anterior cerebral artery.
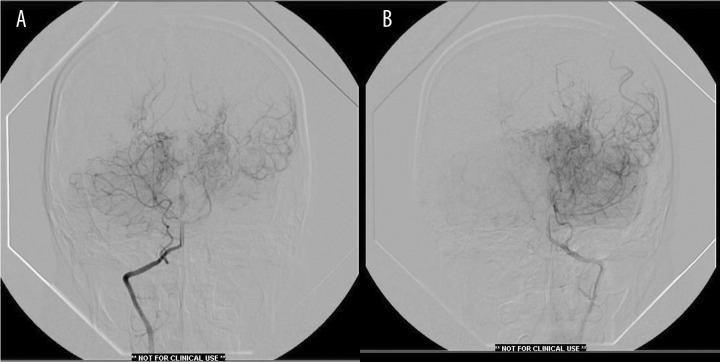
Figure 4.
(A) Right vertebral angiography showing extracranial origin of PICA. There is patent left posterior communicating artery with flow into middle cerebral artery distribution and evidence of choroidal vessel hypertrophy. (B) Left vertebral angiography showing typical findings with vertebral and posterior cerebral arteries poorly visualized.
Discussion
The differential diagnosis for altered mental status in children is vast. It can be attributed to structural or medical causes including toxic, infectious and metabolic. Structural lesions cause altered mental state by diffusely affecting both cerebral hemispheres and more locally the ascending reticular activating system. Medical ones cause an encephalopathic state by causing diffuse dysfunction of both cerebral hemispheres. Structural causes can produce unequal or unreactive pupils as well as focal neurological findings but sometimes occur without focality such as with bilateral cerebrovascular disease or early hydrocephalus [10]. Most general medical conditions were excluded by normal blood, urine studies and negative history of fever or trauma. A normal physical exam ruled out increased intracranial pressure as well as meningitis. Usage of contrast during brain imaging is important to differentiate inflammatory versus ischemic lesions. The presence of prominent lenticulo-striate and thalamo-perforating arteries on magnetic resonance imaging strongly suggested the presence of MMD, which was confirmed on trans-femoral four-vessel cerebral angiography. The child’s mother disclosed that she had the same diagnosis made at a younger age once she was made aware of the child’s diagnosis, and that she was asymptomatic at the time.
Moyamoya disease
Reported incidence rate ratios are 4.6 for Asian Americans, 2.2 for blacks and 0.5 for Hispanics as compared with whites [5]. The incidence peaks in two age groups: children who are approximately 5 years of age and adults in their mid 40s [11]. Females are affected nearly twice as often as males [12]. Familial occurrence accounts for about 15% of patients [13]. Pediatric MMD usually presents with cerebral ischemia (80%) rather than hemorrhage (20%) [14]. Immature verbal skills in younger children make it difficult for early diagnosis (transient ischemic attacks versus infarction) [15]. Ischemic events can occur secondary to crying, blowing or hyperventilation. This behavior induces hypocapnic vasoconstriction or vasospasm in an already compromised cerebral circulation [16]. Patients can also present with headaches, seizures, involuntary movements and progressive decline in intellectual ability [17,18].
The typical histopathology of Moyamoya cerebral vasculopathy includes thickened intima with smooth muscle proliferation with tortuous duplicated internal elastic lamina [19]. Abnormalities of vascular growth factor and inflammatory mediators have been reported [20–22].
Ischemic symptoms occur most often during the first 4 years of diagnosis then tend to decline in contrast to intellectual deterioration that increase with time [18,23]. Good prognostic indicators are age greater than 3 years old, TIA on presentation [24], increased cerebral blood volume on perfusion MRI [25], decreased vascular reserve only with normal basal perfusion on SPECT [26] and rebuild phenomenon on EEG [27] in which there is a slowed recovery to baseline after hyperventilation-induced physiologic slowing.
A head CT is typically the first study obtained which shows areas of hypodensity consistent with infarction, and less commonly hemorrhage or atrophy [17]. MRA is very useful for diagnosing MMD with sensitivity of 73% and specificity of 100% [28]. Sensitivity increases to 92% when MRA is combined with MR imaging [29,30]. However, the smaller Moyamoya collaterals are visualized more clearly with conventional cerebral angiography [31] that is still the gold standard for diagnosing and surgical decision making for MMD [5].
Cerebral blood flow techniques are very useful in diagnosis and management of MMD, which include Xe-enhanced CT, PET and SPECT that assess cerebrovascular reserve as well as disease progression [32–34].
Currently there is no definitive medical treatment to reverse or stabilize the course of MMD (except when associated with sickle cell disease with transfusion protocol). Aspirin and calcium channel blockers [35–37] play a supportive role in the medical management of MMD.
At the present time, there is no standardized surgical approach for the treatment of MMD in children and numerous revascularization procedures have been used [38]. They aim to prevent further ischemic injury by increasing collateral flow using external carotid circulation as a donor supply [39–43].
Revascularization procedures can be divided into 3 main groups: indirect (non-anastomotic) bypass techniques, direct (anastomotic) bypass techniques or combined. Indirect procedure group was compared with pooled data from the direct and combined procedure groups, good collateralization was seen more significantly more often in the direct or combined group [38]. Also data proved that addition of bifrontal encephalogaleo-periosteal synangiosis (EGPS) to indirect procedures could improve outcome in pediatric Moyamoya [44].
Conclusions
A high index of suspicion, consideration of wide range of differential diagnosis, complete physical exam and subsequent work up is mandatory to manage pediatric patients with altered mental status. Early diagnosis and intervention will alter the prognosis and life expectancy in pediatric patients with MMD. Conventional cerebral angiography is still the gold standard for diagnosing and surgical decision making for MMD.
There is no definitive medical treatment to reverse or stabilize the course of MMD. At the present time, there is no standardized surgical approach for the treatment of MMD in children and numerous revascularization procedures have been utilized with fairly good results in slowing disease progression.
References:
- 1.Takeuchi K, Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve. 1957;9:37–43. [Google Scholar]
- 2.Suzuki J, Takaku A. Cerebrovascular MoyaMoya disease: disease showing abnormal net-like vessels in the base of brain. Arch Neurol. 1969;20:288–99. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki J, Kodama N. Moyamoya disease – a review. Stroke. 1983;14:104–9. doi: 10.1161/01.str.14.1.104. [DOI] [PubMed] [Google Scholar]
- 4.Kim SK, Wang KC, Kim DG, et al. Clinical feature and outcome of pediatric cerebrovascular disease: a neurosurgical series. Childs Nerv Syst. 2000;16:421–28. doi: 10.1007/pl00007286. [DOI] [PubMed] [Google Scholar]
- 5.Uchino K, Johnston SC, Becker KJ, Tirschwell DL. Moyamoya disease in Washington state and California. Neurology. 2005;65:956–58. doi: 10.1212/01.wnl.0000176066.33797.82. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyama S, Kusaka Y, Fujimura M, et al. Prevalence and clinic-epidemiological features of Moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008;39:42–47. doi: 10.1161/STROKEAHA.107.490714. [DOI] [PubMed] [Google Scholar]
- 7.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathies in children with arterial ischemic stroke: Results of the international pediatric stroke study. Circulation. 2009;119:1417–23. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly ME, Bell-Stephens TE, Marks MP, et al. Progression of unilateral Moyamoya disease: a clinical series. Cerebrovasc Dis. 2006;22:109–15. doi: 10.1159/000093238. [DOI] [PubMed] [Google Scholar]
- 9.Kawano T, Fukui M, Hashimoto N, Yonekawa Y. Follow-up study of patients with unilateral Moyamoya disease. Neurol Med Chir (Tokyo) 1994;34:744–47. doi: 10.2176/nmc.34.744. [DOI] [PubMed] [Google Scholar]
- 10.Lehman R, Mink J. Altered mental status. Clin Ped Emerg Med. 2008;9:68–75. [Google Scholar]
- 11.Baba T, Houkin K, Kuroda S. Novel epidemiological features of Moyamoya disease. J Neurol Neurosurgery Psychiatry. 2008;79:900–4. doi: 10.1136/jnnp.2007.130666. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraja D, Verma A, Taly AB, et al. Cerebrovascular disease in children. Acta Neurol Scand. 1994;90:251–55. doi: 10.1111/j.1600-0404.1994.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi T, Houkin k, Tada M, Abe H. Familial occurrence of Moyamoya disease. Clin Neurol Neurosurg. 1997;99(2 Suppl):S162–67. doi: 10.1016/s0303-8467(97)00054-1. [DOI] [PubMed] [Google Scholar]
- 14.Nishimoto A, Ueta K, Onbe H. Cooperative study on Moyamoya disease in Japan, in Abstracts of the 10th Meeting on Surgery for stroke; Tokyo: Nyuuron-sha; 1981. [Google Scholar]
- 15.Jea A, Smith ER, Robertson R, Scott RM. Moyamoya syndrome associated with Down syndrome outcome after surgical revascularization. Pediatrics. 2005;116(5):e694–701. doi: 10.1542/peds.2005-0568. [DOI] [PubMed] [Google Scholar]
- 16.Ueki K, Meyer FB, Mellinger JF. Moyamoya disease: The disorder and surgical treatment. Mayo Clin Proc. 1994;69:749–57. doi: 10.1016/s0025-6196(12)61094-5. [DOI] [PubMed] [Google Scholar]
- 17.Smith JL. Understanding and treating Moyamoya disease in children. Neurosurg Focus. 2009;26(4):1–11. doi: 10.3171/2000.01.FOCUS08306. [DOI] [PubMed] [Google Scholar]
- 18.Williams T, WestMacott R, Dlamini N, et al. Intellectual ability and executive function in pediatric Moyamoya vasculopathy. Dev Med Child Neurol. 2012;54:30–37. doi: 10.1111/j.1469-8749.2011.04144.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology. 2000;20(Suppl):S61–64. doi: 10.1046/j.1440-1789.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 20.Soriano SG, Cowan DB, Proctor MR, Scott RM. Levels of soluble adhesion molecules are elevated in the cerebrospinal fluid of children with Moyamoya syndrome. Neurosurgery. 2002;50:544–49. doi: 10.1097/00006123-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Malek AM, Connors S, Robertson RL. Elevation of cerebrospinal fluid levels of basic fibroblast growth factor in Moyamoya and central nervous system disorders. Pediatr Neurosurg. 1997;27:182–89. doi: 10.1159/000121249. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Takaku A. Cerebrovascular Moyamoya disease. Arch Neurol. 1969;20:288–99. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa T, Tomita S, Ueda K, et al. Prognosis of occlusive disease of the circle of Willis in children. Pediatr Neurol. 1985;1:274–77. doi: 10.1016/0887-8994(85)90027-x. [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Cho BK, Phi JH, et al. Pediatric Moyamoya disease: An analysis of 410 consecutive cases. Ann Neurol. 2010;68:92–101. doi: 10.1002/ana.21981. [DOI] [PubMed] [Google Scholar]
- 25.Kim SK, Wang KC, Oh CW, et al. Evaluation of cerebral hemodynamics with perfusion MRI in childhood Moyamoya disease. Pediatr Neurosurg. 2003;38:68–75. doi: 10.1159/000068050. [DOI] [PubMed] [Google Scholar]
- 26.So Y, Lee HY, Kim SK, et al. Prediction of clinical outcome of pediatric Moyamoya disease with postoperative basal/acetazolamide stress brain perfusion SPECT after revascularization surgery. Stroke. 2005;36:1485–89. doi: 10.1161/01.STR.0000170709.95185.b1. [DOI] [PubMed] [Google Scholar]
- 27.Touho H, Karasawa J, Ohnishi H. Preoperative and postoperative evaluation of cerebral perfusion and vasodilatory capacity with 99m Tc-HMPAO SPECT and acetazolamide in childhood Moyamoya disease. Stroke. 1996;27:282–89. doi: 10.1161/01.str.27.2.282. [DOI] [PubMed] [Google Scholar]
- 28.Katz DA, Marks MP, Napel SA, et al. Circle of Willis: evaluation with spiral CT, angiography, MRA and conventional angiography. Radiology. 1995;195:445–49. doi: 10.1148/radiology.195.2.7724764. [DOI] [PubMed] [Google Scholar]
- 29.Yamada I, Suzuki S, Matsushima Y. Moyamoya disease: comparison of assessment with MR angiography and MR imaging versus conventional angiography. Radiology. 1995;196:211–18. doi: 10.1148/radiology.196.1.7784569. [DOI] [PubMed] [Google Scholar]
- 30.Yamata I, Himeno Y, Nagaoka T, et al. Moyamoya disease: evaluation with diffusion weighted and perfusion echo-planer MR imaging. Radiology. 1999;212:340–47. doi: 10.1148/radiology.212.2.r99au08340. [DOI] [PubMed] [Google Scholar]
- 31.Chang KH, Yi JG, Han MH, Kim IO. MR imaging findings of Moyamoya disease. Korean Med Sci. 1990;5:85–90. doi: 10.3346/jkms.1990.5.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Peng S, Li Y. The preoperative and postoperative cerebral blood flow and vasoreactivity in childhood Moyamoya disease. Keio J Med. 2000;49(1 Suppl):A86–A89. [PubMed] [Google Scholar]
- 33.Nambu K, Suzuki R, Hirakawa K. Cerebral blood flow measurement with Xenon-enhanced dynamic helical CT. Radiology. 1995;195:53–57. doi: 10.1148/radiology.195.1.7892495. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi S, Tanaka R, Ishii R, et al. Cerebral hemodynamics in patients with Moyamoya disease: a study of regional cerebral blood flow by Xe-133 inhalation method. Surg Neurol. 1985;23:468–74. doi: 10.1016/0090-3019(85)90241-1. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahimi D, Tamargo R, Ahn E. Moyamoya disease in children. Childs Nerv Syst. 2010;26:1297–308. doi: 10.1007/s00381-010-1209-8. [DOI] [PubMed] [Google Scholar]
- 36.Scott RM. Moyamoya syndrome: a surgically treatable cause of stroke in the pediatric patient. Clin Neurosurg. 2000;47:378–84. [PubMed] [Google Scholar]
- 37.Scott RM. Surgery of Moyamoya syndrome? Yes. Arch Neurol. 2001;58:128–29. doi: 10.1001/archneur.58.1.128. [DOI] [PubMed] [Google Scholar]
- 38.Fung LW, Thompson D, Ganesan V. Revascularization surgery for pediatric Moyamoya: a review of the literature. Childs Nerv Syst. 2005;21:358–64. doi: 10.1007/s00381-004-1118-9. [DOI] [PubMed] [Google Scholar]
- 39.Veeravagu A, Guzman R, Path CG, et al. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurg Focus. 2008;24(2):1–9. doi: 10.3171/FOC/2008/24/2/E16. [DOI] [PubMed] [Google Scholar]
- 40.Golby AJ, Marks MP, Thompson RC, Steinberg GK. Direct and combined revascularization in pediatric Moyamoya disease. Neurosurgery. 1999;45:50–58. doi: 10.1097/00006123-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa T, Houkin K, Kamiyama H, Abe H. Effects of surgical revascularization on outcome of patients with pediatric Moyamoya disease. Stroke. 1997;28:1170–73. doi: 10.1161/01.str.28.6.1170. [DOI] [PubMed] [Google Scholar]
- 42.Karasawa J, Touho H, Ohnishi H, Miyamoto S. Long-term follow up study after extracranial –intracranial bypass surgery for anterior circulation ischemia in childhood Moyamoya disease. J Neurosurg. 1992;77:84–89. doi: 10.3171/jns.1992.77.1.0084. [DOI] [PubMed] [Google Scholar]
- 43.Scott RM, Smith JL, Robertson RL, et al. Long-term outcome in children with Moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100(2 Suppl):142–49. doi: 10.3171/ped.2004.100.2.0142. [DOI] [PubMed] [Google Scholar]
- 44.Kim SK, Wang KC, Kim IO, et al. Combined encephaloduroateriosynagiosis and bifrontal encephalogaleo (periosteal) synangiosis in pediatric Moyamoya disease. Neurosurgery. 2002;50:88–96. doi: 10.1097/00006123-200201000-00016. [DOI] [PubMed] [Google Scholar]