Abstract
Patient:
Female, 21
Final Diagnosis:
Tuberculous lyphadenitis
Symptoms:
Cough dry • fever • subcutaneous mass • weight loss
Medication:
—
Clinical Procedure:
—
Specialty:
Pulmonology
Objective:
Unusual clinical course
Background:
Enlargement of lymph nodes during treatment of Tuberculous lymphadenitis is well recognized phenomenon in HIV infected patient with ample literature to help guide management. On the contrary, it poses a clinical challenge to distinguish between paradoxical reaction and treatment failure in HIV-seronegative patients and require high index of suspicion.
Case Report:
We report a case of 21 year old female of Bangladeshi origin with tuberculous lymphadenitis diagnosed on the basis of strong clinical history and radiologic findings. Patient’s clinical symptoms and lymphadenopathy initially improved but worsened after three months of RIPE therapy. This prompted re imaging and excision biopsy of enlarging lymph node to exclude other masqueraders. Patient was continued on same antituberculous treatment. Oral prednisone was added with subsequent clinical improvement and decrease in the size of lymphadenopathy.
Conclusions:
Paradoxical reaction during anti tuberculous treatment must be considered after careful exclusion of medication non adherence, development of resistance and other similar conditions.
Keywords: paradoxical reaction (PR), highly active anti-retroviral therapy (HAART), rifampin, isoniazid, pyrazinamide and ethambutol (RIPE), tuberculosis (TB), Kikuchi’s disease (KD), tuberculosis lymphadenitis (TL)
Background
Transient worsening of clinical symptoms and lesions of tuberculosis following antituberculous therapy termed as paradoxical reaction has previously been described as uncommon occurrence [1]. It is defined as clinical or radiological worsening of existing TB lesions or development of new lesions which are not secondary to TB infection or other similar diseases in patients who initially responded to antimycobacterial chemotherapy [1]. The reaction is attributable to hypersensitivity response to dying Mycobacterium tuberculosis organism; this has been described in studies of HIV-seropositive patients with TB after introduction of HAART [2].
We report a case of 21 year old HIV negative otherwise healthy female who suffered from paradoxical worsening of TB lymphadenitis during anti TB treatment.
Case Report
KM is a 21 year old female from Bangladesh who immigrated to the United States seven months earlier with no previous significant past medical history presented to the Emergency department with complains remitting and relapsing cervical and axillary masses for past 2 years associated with low grade fever, weight loss and dry cough. The masses first appeared two year ago which were resolved after spontaneous drainage of serosanguinous and purulent material. Subsequently she noticed new masses a year ago which has since progressed in size and now became painful for past 2 weeks prompting her to visit emergency room. She reported 15 pounds weight loss in past 7 months. There was no sputum production, night sweats, anorexia, joint pains, muscle aches or history of cat bites. Review of systems was unremarkable except enlarging cervical and axillary masses. Social and family history was unremarkable except treated pulmonary tuberculosis in sister two years ago.
Physical examination revealed blood pressure 119/70 mm Hg, heart rate 79 beats/min, respiratory rate 16/min, and temperature 97.8F and oxyhemoglobin saturation 99% on Room Air. Pertinent positive findings on exam are as follows; well-developed well-nourished female of average height and built, appears comfortable with palpable 2×3 cm submandibular and posterior cervical lymph nodes, 4×3 cm mobile, tender, soft lymph node at right supra clavicular region and 5x4 cm mobile, nontender, soft lymph node at right axilla.
The basic investigation including complete blood count, basic metabolic profile, liver function studies, coagulation profile, thyroid stimulating hormone, urinalysis were all within normal limits. Patient was admitted to medical floor in negative pressure room with respiratory isolation in view of high suspicion of contagious tuberculosis. HIV test was negative. Three sputum samples for AFB smears were negative. PPD was 15 mm induration. CT scan of neck and chest revealed multiple enlarged calcified Lymph nodes predominantly along the lateral margin of right sternocleidomastoid muscle measuring 3.3×2 cm in dimension with central low attenuation, a 4×2 cm right axillary lymph node and a sub centimeter right sub pectoral lymph node. Multiple small calcified lymph nodes are also seen at mediastinal, bilateral hilar, subcarinal, gastrohepatic and porta hepatic region. In addition combination of calcified and non-calcified sub centimeter scattered pulmonary nodules were visualized as well.
Subsequently patient underwent FNA cytology of right neck mass which revealed inflammatory exudates and necrotic debris with no viable cells present. Smear and cultures were negative for bacteria, acid fast bacillus and fungi. On the basis of strong clinical and radiologic suspicion of tuberculous lymphadenitis with above cytologic examination, patient was started RIPE treatment and followed up in pulmonary clinic. Her clinical symptoms and lymphadenopathy improved and patient gained ten pounds.
Three months into RIPE treatment she developed enlarging painful right cervical lymphadenopathy despite initial improvement. Those enlarged cervical lymph nodes were noticed to be recurrently draining purulent material with spontaneous healing. After ensuring medication adherence, repeat CT scans neck and chest revealed significant decrease in size of previously enlarged cervical and right axillary lymph node but preexisting medial right axillary and right sub pectoral lymph nodes were markedly increased in size. Excision biopsy of right neck lymph node revealed soft tissue with necrotizing and non-necrotizing granulomatous inflammation. Notably AFB and fungal stains and cultures were negative. Patient was then initiated on 60 mg of daily prednisone with subsequent improvement in pain and size of right axillary and sub pectoral lymphadenopathy (Figures 1 and 2).
Figure 1.

CT scan of chest without IV contrast (4×2.5 cm Right axillary LN).
Figure 2.
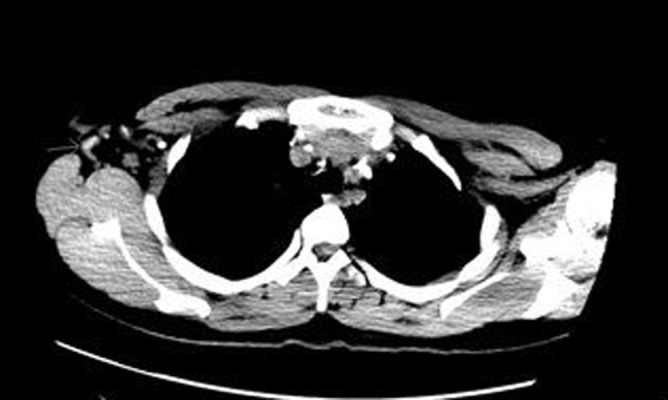
CT scan of chest without IV contrast (1.5×2 cm Right axillary LN).
Discussion
Worsening of existing TB lesions or development of new lesions termed as paradoxical reaction has been observed during or after the treatment of TB lymphadenitis. The mechanism of PR is not well understood but it has been attributed to host immunologic reactions, with possible mechanisms including delayed hypersensitivity response, decrease in immune suppression and a response to mycobacterial antigens [3]. In one study 74% of patients who were initially anergic had reversion of their previously negative tuberculin skin reaction within two weeks of starting anti tuberculous treatment suggest change in immunologic response to tuberculous protein [4]. The reaction is frequent in patients with extra pulmonary and disseminated tuberculosis [1]. It occurs in 20–30% of patients with TB lymphadenitis [5]. Young age, male gender and local tenderness at the time of diagnosis were independently associated with PR [5]. In one retrospective study, higher levels of circulating monocytes at baseline were associated with the risk of subsequent PR. These monocytes were thought to be stimulated by poorly cleared insoluble lipid cell wall antigens of Mycobacterium tuberculosis[6].
Clinical manifestation may include lymph node enlargement (12 percent), fluctuance (11 percent), overlying erythema, discharge (7 percent) and pleural effusion (16 percent) [7]. Patient may experience fever, night sweat, unexplained weight loss. To distinguish Paradoxical reaction from treatment failure may represent a diagnostic challenge. In our patient fine needle aspiration for acid fast bacillus microscopy and cultures was pursued from accessible lymph nodes which came back inconclusive. Yield of positive cultures from Fine needle aspirates is highest in HIV positive patients and has been reported as high as 77% [7]; however it is largely unknown in immunocompetent patients. The initial favorable clinical response to anti tuberculosis treatment experienced by our patient allowed us to wait for further invasive procedure before deciding whether to change treatment. Excision biopsy of the right neck lymph nodes was performed after enlarging regional lymph nodes were noticed which revealed necrotizing granulomatous inflammation. This again corroborated with our initial presumptive diagnosis of the patient with positive PPD and compatible clinical syndrome [8]. There was no evidence of other diseases with similar presentation such as actinomycosis, toxoplasmosis, cat scratch disease, lymphoma, Sarcoidosis, Brucellosis or Kikuchi disease. Among these diseases Kikuchi’s disease is thought to closely mimic tuberculosis lymphadenitis which sometimes is difficult to differentiate on the basis of histology alone. One study showed the presence of indistinct margins of necrotic foci in lymph nodes on computed tomography CT scan as an independent predictor in differentiating KD from TL with an accuracy of 80% [9]. Similarly our patient’s age, ethnic background, immune status and presenting clinical features made other alternative diagnoses very less likely.
There are no consensus guidelines for management once diagnosis is established. Options include observation, aspiration or surgical excision of Lymph nodes, a trial of anti-inflammatory agents such as NSAIDs or steroids. Monoclonal antibodies have also been used [10].
Immunotherapy with steroid and anti TNF alpha may help to resolve PR by inhibiting granuloma formation which interferes with penetration of ant tuberculosis medications [10]. In HIV negative patients, 56% showed spontaneous resolution according to one study [10]. The use of steroid in patients with PR is not uncommon. However there is an insufficient date regarding the use of immunotherapy in patients with lymph node tuberculosis [6]. The advantage of corticosteroid therapy in reducing edema around enlarging intracranial tuberculomas is apparent, but the advantage associated with the use of such therapy for lymph node TB is less clear. Rademacher at al review of literature recommended oral prednisone 1 mg/kg body weight for the duration of 4 to 8 weeks in patients with TB meningitis, TB pericarditis, Pulmonary TB, TB pleurisy but found insufficient data to recommend the same in peripheral lymph node TB [11]. In the same literature review two prospective studies using triamcinolone and prednisone tapering doses over 10 weeks and 6 weeks respectively showed faster resolution of mediastinal lymph nodes [12]. The decision to start steroid in our patient was mainly based upon painful lymphadenopathy with recurrent spontaneous drainage and uncomfortable constitutional symptoms. The patient was given a trial of 1 mg/kg body weight prednisone with subsequent clinical improvement and decrease in the size of her lymph nodes.
In HIV positive patients, time interval between the initiation of HAART to onset of PR associated with MTB has been varied from 10 to 180 days [13] but it tends to occurs within first two months [14]. This data is lacking in HIV negative patients. PR in our patient began three months after RIPE treatment and interestingly persisted more than six months while on corticosteroids. For this reason she was continued on prolonged course of oral steroids along with RIPE. She was started on 1 mg/kg body weight daily which was slowly tapered to 0.5 mg/kg body weight daily over 12 weeks followed by dose reduction to 0.25 mg/kg body weight daily in next 12 weeks before complete taper as guided by clinical improvement. The decision to continue prednisone for symptom control was made after excluding other inflammatory, malignant and infectious diseases.
Conclusions
Since there is no diagnostic test for PR, confirmation of the disease relies heavily upon case definitions incorporating clinical and laboratory data. This has hindered further clinical research in immunocompetent patient presenting with PR despite high incidence reports [6]. There are however, consensus case definitions related PR in patients on HAART which were developed during international meeting in Kampala, Uganda, in November 2006 conducted by International Network for the Study of HIV-associated PR (INSHI) [15]. Disseminated TB or other associated conditions such as HIV infection, immunosuppressive therapy etc. could represent risk factors for development of PR, because such conditions allow high load of bacillus in granulomatous lesions. Paradoxical reaction during anti tuberculous treatment must be considered after careful exclusion of medication non adherence, development of resistance and other similar conditions. The addition of short course of steroids may be necessary to control severe forms of reaction with or without percutaneous drainage or surgical excision of enlarged lymph nodes to avoid fistula formation. We believe the role of corticosteroids can only be defined by a randomized placebo controlled trial. Further studies are also needed to better understand the pathogenesis and risk factors in immunocompetent patients, which would help identify patients at risk of developing PR during TB treatment and better control of its clinical manifestation.
Statement
This work is not funded by any Government or Nongovernment organization. There is no conflict of interest for any author of this manuscript.
References:
- 1.Carvalho AC, De iaco G, Saleri N, et al. Paradoxical reaction during tuberculosis treatment in HIV-seronegative patients. Clin Infect Dis. 2006;42(6):893–95. doi: 10.1086/500459. [DOI] [PubMed] [Google Scholar]
- 2.Breen RA, Smith CJ, Bettinson H, et al. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59(8):704–7. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell IA, Dyson AJ. Lymph node tuberculosis: a comparison of various methods of treatment. Tubercle. 1977;58(4):171–79. doi: 10.1016/0041-3879(77)90041-1. [DOI] [PubMed] [Google Scholar]
- 4.Rooney JJ, Crocco JA, Kramer S, Lyons HA. Further observations on tuberculin reactions in active tuberculosis. Am J Med. 1976;60(4):517–22. doi: 10.1016/0002-9343(76)90718-x. [DOI] [PubMed] [Google Scholar]
- 5.Cho OH, Park KH, Kim T, et al. Paradoxical responses in non-HIV-infected patients with peripheral lymph node tuberculosis. J Infect. 2009;59(1):56–61. doi: 10.1016/j.jinf.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Hawkey CR, Yap T, Pereira J, et al. Characterization and management of paradoxical upgrading reactions in HIV-uninfected patients with lymph node tuberculosis. Clin Infect Dis. 2005;40(9):1368–71. doi: 10.1086/429317. [DOI] [PubMed] [Google Scholar]
- 7.Polesky A, Grove W, Bhatia G. Peripheral tuberculous lymphadenitis: epidemiology, diagnosis, treatment, and outcome. Medicine (Baltimore) 2005;84(6):350–62. doi: 10.1097/01.md.0000189090.52626.7a. [DOI] [PubMed] [Google Scholar]
- 8.Lee KC, Tami TA, Lalwani AK, Schecter G. Contemporary management of cervical tuberculosis. Laryngoscope. 1992;102(1):60–64. doi: 10.1288/00005537-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Yoo JH, Lee SW. Kikuchi disease: differentiation from tuberculous lymphadenitis based on patterns of nodal necrosis on CT. AJNR Am J Neuroradiol. 2012;33(1):135–40. doi: 10.3174/ajnr.A2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47(10):e83–85. doi: 10.1086/592695. [DOI] [PubMed] [Google Scholar]
- 11.Dooley DP, Carpenter JL, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin Infect Dis. 1997;25(4):872–87. doi: 10.1086/515543. [DOI] [PubMed] [Google Scholar]
- 12.Nemir RL, Cardona J, Lacoius A, David M. Prednisone therapy as an adjunct in the treatment of lymph node-bronchial tuberculosis in childhood. a double-blind study. Am Rev Respir Dis. 1963;88:189–98. doi: 10.1164/arrd.1963.88.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Shelburne SA, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81(3):213–27. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57(2):167–70. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- 15.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


