Abstract
Background:
We experienced a case in which Cronkhite-Canada Syndrome presented with complications of multiple gastric cancers and multiple colon adenomas.
Case Report:
Our case is a 64-year-old male who visited a nearby hospital with diarrhea and weight loss. The patient was anemic and hypoproteinemic, with multiple polyps in the stomach, duodenum, and large intestine. He also presented with alopecia, onychatrophia, cutaneous pigmentation, and dysgeusia, and was diagnosed with Cronkhite-Canada Syndrome. Follow-up examinations found multiple gastric cancers and colon adenomas. We performed a total gastrectomy and a polypectomy of the large intestine lesions, revealing 4 well-differentiated adenocarcinomas in the resected stomach, and tubular adenomas in the large intestine lesions. Intraoperative findings included scattered melanoid pigmentation on the mesentery and the small intestinal wall. Tumor cells were positive for p53 and Ki67 and partially positive for MUC5AC and MUC2. Cronkhite-Canada Syndrome polyps are generally classified as juvenile type polyps, and these polyps rarely become cancerous. However, of the 383 cases of Cronkhite-Canada Syndrome reported in Japan, complications of gastric cancer were found in 39 cases (10.2%), and only 8 cases with multiple gastric cancer were reported in Japan. including the cases we have personally experienced. There were only two English literatures on Cronkhite-Canada Syndrome complicated with gastric cancer. So it is necessary to notify this information of Cronkhite-Canada Syndrome to the world.
Conclusions:
Close gastrointestinal examination and strict follow-up are believed to be essential for Cronkhite-Canada Syndrome patients.
Keywords: Cronkhite-Canada syndrome, gastric cancer, colon adenoma
Background
Cronkhite-Canada Syndrome (CCS), which was first reported by Cronkhite and Canada in 1955, is an idiopathic, non-hereditary syndrome with gastrointestinal polyposis and ectodermal changes including alopecia, onychatrophia, and pigmentation [1]. Unlike neoplastic polyposis, such as familial adenomatous polyposis, CCS polyps are classified as juvenile type polyps, and malignancy is thought to be rare [2]. We have had a case in which CCS presented complications of multiple gastric cancers and multiple colon adenomas. This study reports such a case while including some discussion of relevant literature.
Case Report
The patient described here is a 64-year-old Japanese male with a past history of low anterior resection for rectal cancer at age 55, and a polypectomy for a colonic polyp at age 58. Family history was unremarkable. The patient has smoked 10 cigarettes per day and consumed 4 go (0.72 L) of shochu (a Japanese distilled spirit, 25% alcohol by volume) per day for 40 years. The patient has presented symptoms of muddy diarrhea since approximately January 2011, and visited a local doctor upon losing 4 kg of weight. A blood test revealed anemia and hypoproteinemia (hemoglobin: 6.7 g/dL, albumin (Alb): 3.2 g/dL), while a gastrointestinal (GI) endoscopy found multiple polyps in the stomach, duodenum, and large intestine. The patient therefore began attending the Digestive Disease Center at our hospital from March 2011.
Multiple polyps found by lower GI endoscopy, combined with the presence of associated symptoms (alopecia, onychatrophia, pigmentation, and dysgeusia), resulted in the diagnosis of CCS (Figure 1). A histopathological examination revealed the gastric lesions to be hyperplastic polyps, and the small and large intestine lesions to be inflammatory polyp. The base of the polyp and adjacent mucosa showed extensive edema and increased eosinophils in the lamina propria mucosa and dilated glands. These are typical histological features of CCS polyps. Hypoproteinemia caused due to liver cirrhosis and nephrotic syndrome was excluded because liver dysfunction, ascites, and proteinuria were not observed. Steroid treatment was not conducted due to the following factors: the patient was a heavy drinker, there was a possibility that the hypoproteinemia had not been corrected, and the patient had previously tested positive for tuberculosis with the QuantiFERON® test, which put him at risk for recurrence. During observation, the patient was therefore administered H2 blocker orally. Regular follow-up examinations revealed multiple gastric cancers and multiple colon adenomas, at which point the patient was admitted to our hospital for additional treatment in January 2012. A blood test revealed hypoproteinemia and undernutrition (total protein (T.P.): 5.08 g/dL, Alb: 3.08 g/dL, cholinesterase (CHE): 102 U/L), as well as abnormalities in trace elements (Fe: 18 g/dL, Zn: 60 g/dL, Ca: 8.12 mg/dL, P: 2.32 mg/dL). Immunoglobulin values, IgG (887 mg/dL) and IgE (1150 IU/mL) were high, while IgA (189 mg/dL) and IgM (82 mg/dL) were within normal limits. Carcinoembryonic antigen (CEA), a tumor marker, increased slightly from 5.0 to 7.6 ng/mL. An upper GI series found a dense growth in the stomach of small protrusions 10 mm or less, including 10–25 mm type 0-I lesions primarily in the gastric antrum, and type 0-IIc+III lesions in the gastric angle in the lesser curvature (Figure 2). Upper GI endoscopy found no abnormalities in the esophageal mucosa; however, large and small diffuse inflammatory polyps were found from the antrum cardiacum to the descending limb of the duodenum. Several 12–20 mm large protruding lesions indicative of type 0-I lesions were also found in the gastric antrum in the greater curvature, the posterior wall, and the pylorus in the posterior wall, which led us to suspect well-differentiated adenocarcinomas (Figure 3). Endoscopy of the small intestine revealed large and small inflammatory, partially villous polyps accompanied by oblong gland ducts with low duct density for 100 cm, from Bauhin’s valve to the oral end. No polyps were found in the jejunum. Endoscopy of the large intestine revealed approximately 50 large and small inflammatory polyps with rugged surfaces predominantly in the right side of the colon (Figure 4).
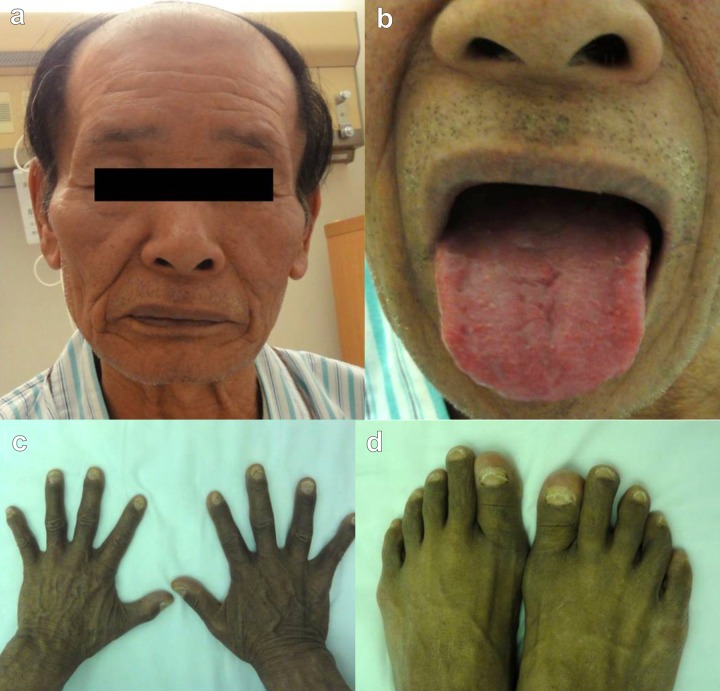
Figure 1.
Physical characteristics of the case. (A) Alopecia; (B) Tongue atrophy; (C, D) Onychatrophia.
Figure 2.
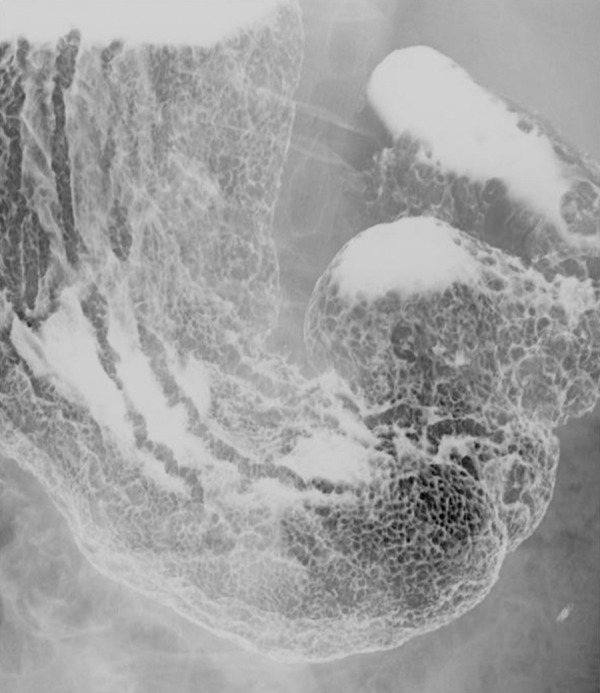
Upper gastrointestinal (GI) series. A dense growth of small protrusions 10 mm or less are found in the stomach, including 10–25 mm type 0–I lesions, which are primarily found in the gastric antrum. In addition, type 0–IIc+III lesions are found in the gastric angle in the lesser curvature.
Figure 3.
Upper GI endoscopy. Several 12–20 mm large protruding lesions indicative of type 0-I lesions are found in the gastric antrum in the greater curvature, the posterior wall of the stomach, and the pylorus in the posterior wall.
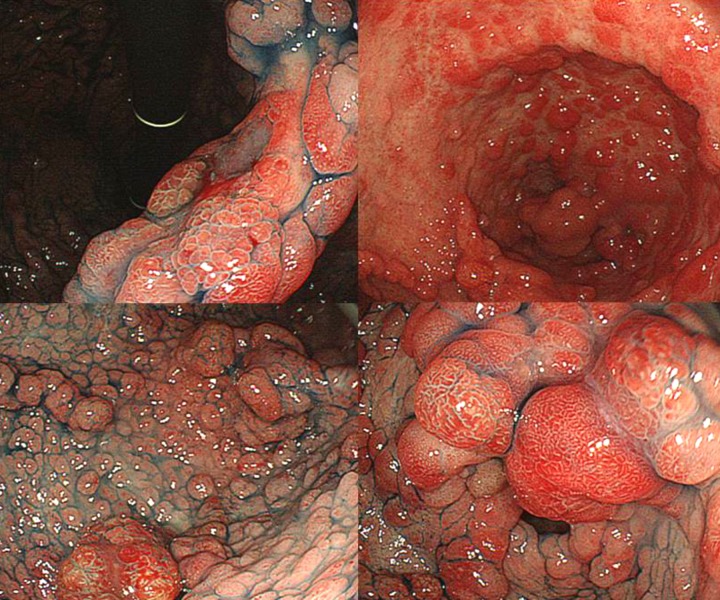
Figure 4.
Large intestine endoscopy. Large intestine endoscopy reveals several large and small inflammatory polyps with rugged surfaces predominantly in the right side of the colon.
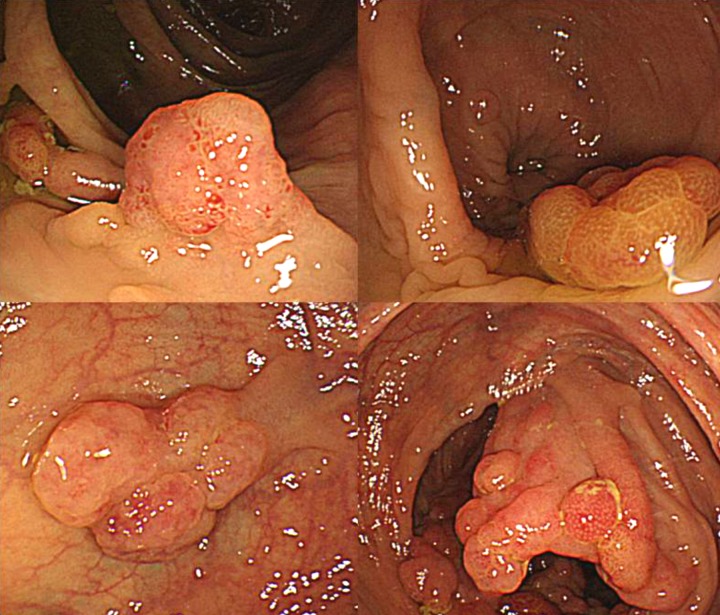
Polypectomy was performed on 4 lesions that were diagnosed as adenomas in a biopsy taken at the time of the large intestine endoscopy. Of these 4 lesions, 2 were at the location of gland duct growth indicating neoplastic change on part of the mucosal epithelium, which in turn indicates hyperplastic change; these were diagnosed as low-grade tubular adenomas (Figure 5). A total gastrectomy was performed on the gastric lesions, because there was a possibility of cancer lurking in other polyps, and if the polyps were not removed, they may have become cancerous. Intraoperative findings included scattered melanoid pigmentation on the mesentery and the small intestinal wall. Large and small polyps were present in the surgical margin of the duodenum. Blinding the surgical margin would have resulted in the possibility of an anastomotic leak, thus making regular observation of duodenal polyps difficult. Therefore, we used the double tract method for reconstruction (Figure 6). The postoperative pathological report found atypical gland duct growth, inflammatory cell infiltration primarily into lymphocytes, and 4 gastric cancer lesions. All of these lesions were well-differentiated adenocarcinomas. The main lesion was well-differentiated tubular adenocarcinoma, intermediate type, 10×20 mm in size, invading submucosal layer (sm2), positive lymphatic invasion (ly1) and negative vascular invasion (v0). The tumor showed expanding growth and a distinct border with the surrounding tissue (Infiltration Alpha). No metastasis to the lymph nodes was observed (n0/52). Tumor cells were positive through full thickness for p53 and Ki67 and partially positive for MUC5AC and MUC2 (Figures 7 and 8). In addition, pathological examination detected Helicobacter pylori infection. The patient was discharged from the hospital 18 days after surgery with no postoperative complications.
Figure 5.
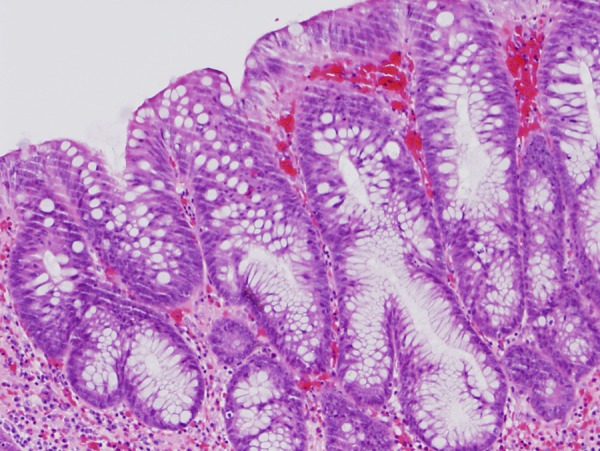
Image of large intestinal lesion tissue. Gland duct growth indicating neoplastic change on part of the mucosal epithelium, which in turn indicates hyperplastic change (H&E, 40× magnification).
Figure 6.
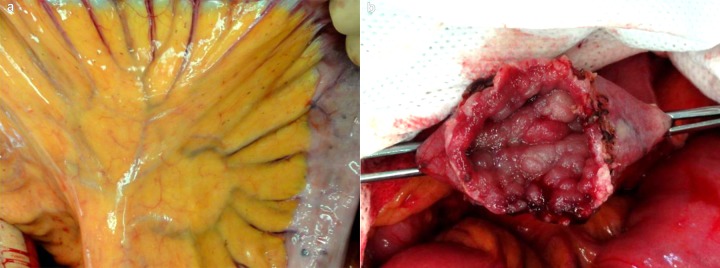
Intraoperative findings. (A) Pigmentation is observed on the mesentery and the small intestinal wall. (B) Several polyps are observed in the surgical margin of the duodenum.
Figure 7.
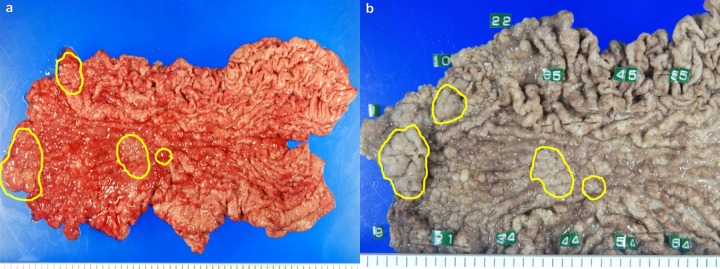
Findings of the macroscopic examination of stomach resection using total gastrectomy. (A) Isolated sample; (B) Formalin-fixated isolated sample. Cancer cells are highlighted in yellow.
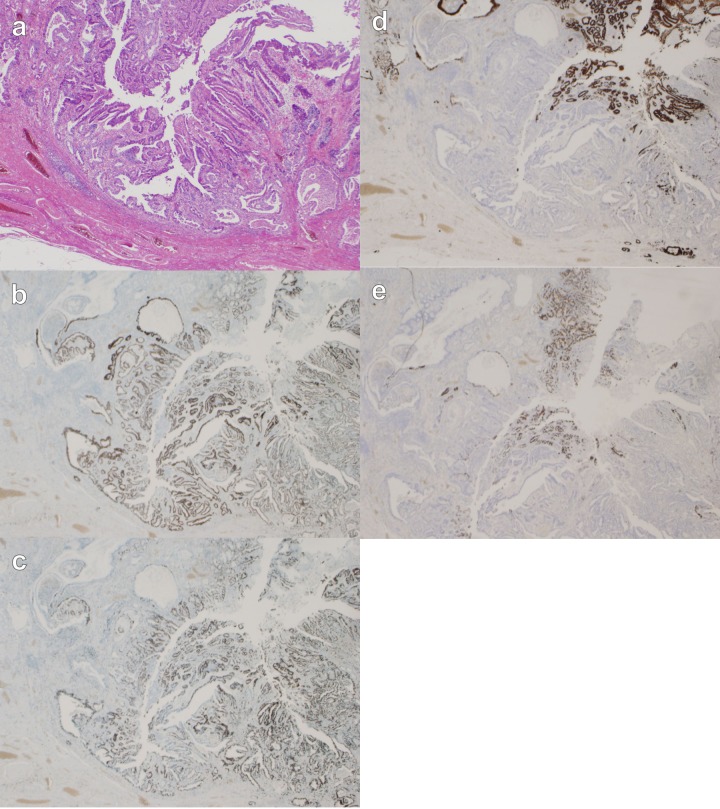
Figure 8.
Image of resected gastric tissue. A well-differentiated adenocarcinoma; submucosal (sm)2, intermediate type, lymphatic invasion (ly)1, venous invasion (v)0. The tumor shows expanding growth and a distinct border with the surrounding tissue (Infiltration Alpha). Tumor cells are positive for p53 and Ki67 through full thickness, and partially positive for MUC5AC and MUC2. (12.5× magnification). (A) H&E; (B) p53; (C) Ki67; (D) MUC5AC; (E) MUC2.
Discussion
Since Cronkhite and Canada reported the first two cases of CCS in 1955, most cases of CCS have been reported in middle-aged and older males. Although several potential causes of CCS have been hypothesized, including infection, vitamin deficiency, immunosuppression, and stress, the exact cause remains unclear [3]. Characteristics include ectodermal changes such as alopecia, onychatrophia, and pigmentation. Digestive symptoms include diarrhea, abdominal pain, decreased appetite, dysgeusia, impaired digestion and absorption in the digestive tract, resulting in hypoproteinemia and weight loss. Depleted electrolytes such as calcium and potassium, and abnormal levels of trace elements such as copper, zinc, and magnesium as well as abnormalities in vitamins and immunoglobulins have occasionally been found in CCS patients [4–6].
Although esophageal polyps are rare in CCS, polyps can occur elsewhere throughout the digestive tract, particularly in the stomach and large intestine. Polyps in the stomach and large intestine are often diffuse and clustered in dense growths; many of these polyps are sessile, inflammatory, and edematous. Histological characteristics include: cystoid gland duct enlargement; hyperplasia of the glandular epithelium; and hyperplasia, edema, and inflammatory cell invasion of the interstitial cells. Similar findings have been observed in nonpolypoid mucosae, which appear outwardly normal [7–10].
In their first report, Cronkhite and Canada described benign polyps as “adenomatous polyps”. However, the current view is that they are non-neoplastic polyps accompanied by inflammation, which resemble hyperplastic, hamartomatous, or juvenile polyps. Therefore, coincidences of cancer in CCS have been comparatively rare thus far [11]. However, since DaCruz’s [7] report of CCS cases involving coincident multiple cancers in the descending colon and the ileocecum, there have been reports of digestive system cancer complications in 38 of 204 cases (18.6%) [6], and gastric cancer coincidence in 19 of 374 cases (5.1%) [12]. Thus, it may be inaccurate to state that cancer coincidences are rare. Our investigations found 383 reported cases of CCS in Japan from 1980 through 2011. Of these, coincident gastric cancer occurred in 39 cases (10.2%), while coincident multiple gastric cancers occurred in 8 cases. The rate of coincident gastric cancer among CCS patients is significantly higher than the prevalence of gastric cancer in the general population [4,5,13–15]. Table 1 summarizes the clinical pathology of these gastric cancer complications. There have been 39 cases (54 lesions) in Japan with coincident gastric cancer. The average age of these patients was 61.4 years; 35 of them were male and 4 were female. An examination of the depth of invasion (n=54 lesions) showed that 72.2% of cases (39 lesions) were early cancers, and 28.8% (15 lesions) were advanced cancers. A high percentage of these were protruded type, well-differentiated adenocarcinomas (78.7%).
Table 1.
Reported cases of Cronkhite-Canada syndrome with gastric cancer.
| No. | Year | Author | Age (years) | Sex | Macroscopic type | Depth of invasion | Histological type |
|---|---|---|---|---|---|---|---|
| 1 | 1979 | Nakamura | 46 | F | Type 1 | m | tub1 |
| 2 | 1983 | Yokoyama | 70 | M | Type 1 | ss | tub1+sq |
| 3 | 1983 | Sagara | 71 | M | Type 1 | sm | tub1 |
| 4 | 1984 | Nishiki | 58 | M | |||
| 5 | 1984 | Sugimura | 51 | M | ss | ||
| 6 | 1985 | Isobe | 72 | F | m | tub2+por | |
| 7 | 1985 | Tsushita | 61 | M | sm | ||
| 8 | 1986 | Uchida | 59 | F | Type 1 | m | tub1 |
| 9 | 1986 | Koido | 78 | M | |||
| 10 | 1988 | Yoshida | 59 | M | Type 1 | tub1 | |
| 11 | 1990 | Nouchi | 68 | M | m | tub1 | |
| 12 | 1990 | Hasegawa | 72 | M | m | tub1 | |
| 13 | 1990 | Ogawa | 78 | F | |||
| 14 | 1991 | Kumano | 70 | M | ss | por+sig | |
| 15 | 1991 | Kaneko | 69 | M | Type 0 IIc | sm | tub1 |
| 16 | 1994 | Fushida | 54 | M | Type 2 | ss | tub2 |
| Type 1 | m | tub1 | |||||
| 17 | 1994 | Yano | 50 | M | ss | ||
| 18 | 1997 | Yabushita | 72 | M | Type 2 | ss | por+sig |
| 19 | 1997 | Nasu | 54 | M | |||
| 20 | 1998 | Daidou | 62 | M | m | tub1 | |
| 21 | 1998 | Konishi | 50 | M | Type 3 | mp | tub1+tub2 |
| 22 | 1998 | Tokubayashi | 61 | M | m or sm | tub1 | |
| m or sm | pap | ||||||
| m or sm | muc | ||||||
| 23 | 1999 | Shiraishi | 52 | M | mp | tub2+por | |
| 24 | 1999 | Watanabe | 70 | M | Type 1 | sm | tub1 |
| Type 1 | m | tub1 | |||||
| Type 1 | m | tub1 | |||||
| 25 | 2000 | Ogami | 73 | M | Type 2 | ss | tub2 |
| 26 | 2000 | Egawa | 52 | M | Type 1 | mp | por |
| 27 | 2000 | Kurisu | 46 | M | Type 1 | sm | tub2 |
| 28 | 2002 | Kazuki | 62 | M | sm | muc | |
| m | tub1+tub2 | ||||||
| m | pap | ||||||
| 29 | 2002 | Kazuki | 59 | M | Type 1 | mp | por |
| 30 | 2003 | Ikeda | 73 | M | ss | tub2 | |
| 31 | 2003 | Yokoyama | 68 | M | Type 1 | ss | por, AFP(+) |
| 32 | 2004 | Hayashi | 71 | M | Type 1 | m | tub1 |
| 33 | 2005 | Abe | 71 | M | Type 2 | sm | por |
| Type 0 IIb | tub1 | ||||||
| Type 0 IIb | tub1 | ||||||
| Type 1 | m | tub1 | |||||
| 34 | 2009 | Karasawa | 59 | M | Type 1 | m | tub1 |
| 35 | 2010 | Kanei | 56 | M | mp | tub1 | |
| sm1 | por1 | ||||||
| mp | tub1 | ||||||
| 37 | 2011 | Nakayama | 60 | M | Type 1 | sm2 | tub1 |
| Type 1 | m | tub1 | |||||
| 38 | 2011 | Watari | 74 | M | Tyoe 0 IIa | m | tub1 |
| 39 | 2012 | Present case | 64 | M | Type 1 | sm2 | tub1 |
| Type 1 | m | tub1 | |||||
| Type 0 IIa | m | tub1 | |||||
| Type 0 IIc | m | tub1 |
m – mucosa; sm – submucosa; mp – muscularis propria; ss – subserosa.
In our patient, the histological structure of well differentiated adenocarcinoma in the tumor resembled that of hyperplasia in other CCS polyps, which led us to suppose that the carcinoma had arisen from the hyperplastic CCS polyps. Recently, the concept of “gastric-type” well differentiated adenocarcinoma, a disease entity which is different from “intestinal-type” and which arises in nonintestinalized gastric mucosa, has been established following the discovery of MUC genes coding core proteins of mucin, although differentiated adenocarcinomas of the stomach have been commonly thought to arise from intestinal metaplasia [16]. MUC2 is the main mucin of the intestinal mucosa and respiratory system, and does not show any staining in normal gastric epithelium; however, de novo expressions appear in the areas of intestinal metaplasia and in malignancies. MUC5AC, a gastric-type mucin of the cardia and corpus of stomach, is widely expressed in normal gastric epithelium [17]. In our patient, Both MUC5AC and MUC2 were partially positive. These results suggested that this cancer could be classified as mixed-type, well differentiated adenocarcinoma. We also validated by Ki67 and p53 staining, in which only cancer cells were stained with Ki67 and p53, indicating that only the cancer cells had proliferative activity and that there was mutated p53 protein without intermediate changes between the surrounding mucosa and cancer nests.
In some of the reports, adenomatous changes were found around gastric cancer nests in patients with CCS suggesting that some gastric cancers may be related to the adenoma-carcinoma sequence, while other gastric cancers may have occurred coincidentally. The etiology of gastric cancer in CCS is debatable, regarding whether it is related to the adenoma-carcinoma sequence shown in colon cancer or whether it is coincidental (de novo). Recently, H. pylori infection has been indicated to be one of the causes of gastric cancer, and it has also been shown to cause several auto-immune diseases, such as idiopathic thrombocytopenic purpura and autoimmune pancreatitis. In the present case also, H. pylori infection was detected. It is therefore possible that H. pylori infection is associated with the development of gastric cancer in CCS patients. However, it is difficult to confirm the association of H. pylori with gastric cancer in CCS patients, as there are few reports that discuss the association of gastric cancer and this infection in CCS patients [5]. Future research is required to elucidate this association.
Conclusions
Here, we report a case of CCS presenting with complications of multiple gastric cancers and multiple adenomatous polyps. Based upon this case as well as previously reported cases of CCS that presented with gastric cancer complications discussed here, we recommend close examination and strict follow-up observation at the time of onset to improve the chances of a favorable prognosis for CCS patients.
Footnotes
Consent
Written informed consent has been obtained from the patient for publication of this case report and any accompanying images.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References:
- 1.Cronkhite LW, Jr, Canada WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med. 1955;252:1011–15. doi: 10.1056/NEJM195506162522401. [DOI] [PubMed] [Google Scholar]
- 2.Goto A, Shimokawa K. Cronkhite-Canada syndrome associated with lesions predisposing to development of carcinoma. J Jpn Soc Cancer Ther. 1994;29:1767–77. [Google Scholar]
- 3.Lin HJ, Tsai YT, Lee SD, et al. The Cronchite-Canada syndrome with focus on immunity and infection report of a case. J Clin Gastroenterol. 1987;9:568–70. doi: 10.1097/00004836-198710000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Sagara K, Fujiyama S, Kamuro Y, et al. Cronkhite-Canada syndrome associated with gastric cancer: report of a case. Gastroenterol Jpn. 1983;18:260–66. doi: 10.1007/BF02774970. [DOI] [PubMed] [Google Scholar]
- 5.Karasawa H, Miura K, Ishida K, et al. Cronkhite-Canada syndrome complicated with huge intramucosal gastric cancer: report of a case. Gastric Cancer. 2009;12:113–17. doi: 10.1007/s10120-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 6.Goto A. Cronkhite-Canada syndrome: an analysis of clinical features and follow-up studies of 204 cases reported in Japan. Hashima City Hosp. 1994;3:1–25. [PubMed] [Google Scholar]
- 7.Gomes da Cruz GM. Generalized gastrointestinal polyposis. An unusual syndrome of adenomatous polyposis, alopecia, onychorotrophia. Am J Gastroenterol. 1967;47:504–10. [PubMed] [Google Scholar]
- 8.Sassatelli R, Bertoni G, Serra L, et al. Generalized juvenile polyposis with mixed pattern and gastric cancer. Gastroenterology. 1993;104:910–15. doi: 10.1016/0016-5085(93)91031-c. [DOI] [PubMed] [Google Scholar]
- 9.Longo WE, Touloukian RJ, West AB, Ballantyne GH. Malignant potential of juvenile polyposis coli. Report of a case and review of the literature. Dis Colon Rectum. 1990;33:980–84. doi: 10.1007/BF02139111. [DOI] [PubMed] [Google Scholar]
- 10.Daniel ES, Ludwig SL, Lewin KJ, et al. The Cronkhite-Canada syndrome: an analysis of clinical and pathologic features and therapy in 55 patients. Medicine. 1982;61:293–309. [PubMed] [Google Scholar]
- 11.Rubin M, Tuthill RJ, Rosato EF, Cohen IS. Cronkhite-Canada syndrome: report of an unusual case. Gastroenterology. 1980;79:737–41. [PubMed] [Google Scholar]
- 12.Nakamura Y, Takagi S, Omoto R, et al. A case of Cronkhite-Canada syndrome associated with cancer development of a gastric polyp. I To Cho (Stomach and Intestine) 1979;14:1217–22. (in Japanese) [Google Scholar]
- 13.Egawa T, Kubota T, Otani Y, et al. Surgically treated Cronkhite-Canada syndrome associated with gastric cancer. Gastric Cancer. 2000;3:156–60. doi: 10.1007/pl00011711. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Kudo M, Shirane H, et al. Cronkhite-Canada syndrome associated with triple gastric cancers: a case report. Gastrointest Endosc. 1999;50:688–91. doi: 10.1016/s0016-5107(99)80022-4. [DOI] [PubMed] [Google Scholar]
- 15.Watari J, Morita T, Sakurai J, et al. Endoscopically treated Cronkhite-Canada syndrome associated with minute intramucosal gastric cancer: an analysis of molecular pathology. Dig Endosc. 2011;23:319–23. doi: 10.1111/j.1443-1661.2011.01150.x. [DOI] [PubMed] [Google Scholar]
- 16.Kushima R, Vieth M, Borchard F, et al. Gastric-type well-differentiated adenocarcinoma and pyloric gland adenoma of the stomach. Gastric Cancer. 2006;9:177–84. doi: 10.1007/s10120-006-0381-8. [DOI] [PubMed] [Google Scholar]
- 17.İlhan Ö, Han Ü, Önal B, Çelık SY. Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk J Gastroenterol. 2010;21:345–52. doi: 10.4318/tjg.2010.0119. [DOI] [PubMed] [Google Scholar]