Abstract
Background:
Long-term prognosis in patients with fulminant myocarditis can be favorable; however, for 32–36% of patients, this condition becomes fatal during the acute stages despite the use of mechanical circulatory support. Other therapeutic options may be needed for patients in whom these conditions are resistant to aggressive management.
Case Report:
We present a case of fulminant myocarditis that rapidly progressed to cardiogenic shock and in-hospital cardiac arrest in a 46-year-old male. The patient promptly received inotropic agents, intra-aortic balloon pump therapy, and extracorporeal membrane oxygenation. However, creatinine kinase (CK), C-reactive protein (CRP), and QRS width increased significantly between days 1 and 3 of treatment; the patient’s hemodynamic profile deteriorated despite this treatment regimen. Intravenous methylprednisolone was initiated on day 3 at a dose of 1,000 mg/day and maintained for an additional three days. Less than 24 h after methylprednisolone administration, the QRS width decreased significantly from 0.44 s to 0.18 s. In addition, CK and CRP levels declined sharply, which is associated with hemodynamic improvement.
Conclusions:
High doses of intravenous methylprednisolone may be considered a therapeutic option for patients with fulminant myocarditis that is refractory to usual management practices.
Keywords: immunosuppression, fulminant myocarditis, extracorporeal membrane oxygenation
Background
Fulminant myocarditis is characterized by acute deterioration of hemodynamics because of rapid progression of an inflammatory myocardial injury [1]. Other common findings include repetitive ventricular tachycardia and/or fibrillation [2,3]. For most cases, the combined approach of mechanical circulatory support and optimal medical treatments is sufficient for life-saving recovery of the injured myocardium. However, for 32–36% patients, the condition becomes fatal during the acute stages despite aggressive treatment [4,5]. Thus, other therapeutic options may be necessary for patients whose conditions are resistant to aggressive management practices.
Case Report
A 46-year-old male presented to our hospital with a three-day history of moderate fever and general malaise. His medical history included admission to another hospital at 38 years of age for complete atrioventricular block, where pacemaker implantation (VDD mode) was performed. There were no evidence of myocardial necrosis and inflammation that were suggestive of myocarditis. He was admitted to our hospital at 45 years of age for a non-ST-segment elevation myocardial infarction and treated by placement of a drug-eluting stent in the totally occluded middle portion of the high lateral branch. In response, the pre-discharge left ventricular ejection fraction (EF) visualized in the left ventriculogram decreased slightly to 54%.
Upon patient arrival, physical examination revealed low blood pressure (90/53 mmHg) and a third heart sound detected on the apex. The first chest radiograph revealed cardiomegaly with a cardiothoracic ratio of 54% in association with moderate pulmonary congestion. The patient was initially diagnosed with heart failure and admitted to a basic care unit. However, he became increasingly dyspneic, progressed to a state of shock within 1.5 h of admission, and was transferred to the cardiac intensive care unit (CICU). The electrocardiogram obtained in the CICU revealed an accelerated idioventricular rhythm with ST-segment elevation in II, III, and aVF leads and more prominent in V2–V6 leads (Figure 1A). Failure of both ventricular sensing and pacing was also noted. Elevated levels of cardiac troponin T (7.790 mg/mL) and creatinine kinase (979 IU/L) indicated myocardial necrosis. In addition, N-terminal B-type natriuretic peptide levels were elevated significantly (8,273 pg/mL). Rapid development of pulmonary edema was noted in the second chest radiograph. The patient underwent intratracheal intubation that was controlled by intermittent mandatory ventilation. His systolic blood pressure was approximately 80 mmHg despite continuous infusion of norepinephrine and dopamine. The patient experienced a transient cardiac standstill in the catheter laboratory; 15 min of cardiopulmonary resuscitation with chest compression and epinephrine injection was required for recovery of spontaneous circulation in the patient. We promptly introduced both extracorporeal membrane oxygenation with an initial flow rate of 2.5 L/min and intra-aortic balloon pump therapy to prevent hemodynamic disruption. Coronary artery trees were free from any lesions with ≥50% diameter stenosis. The left ventriculogram pictured in Figure 1B displays global systolic dysfunction with an EF of 22%. Because the patient experienced a tonic convulsion after resuscitation, mild therapeutic hypothermia of 34°C was initiated to protect his brain.
Figure 1.
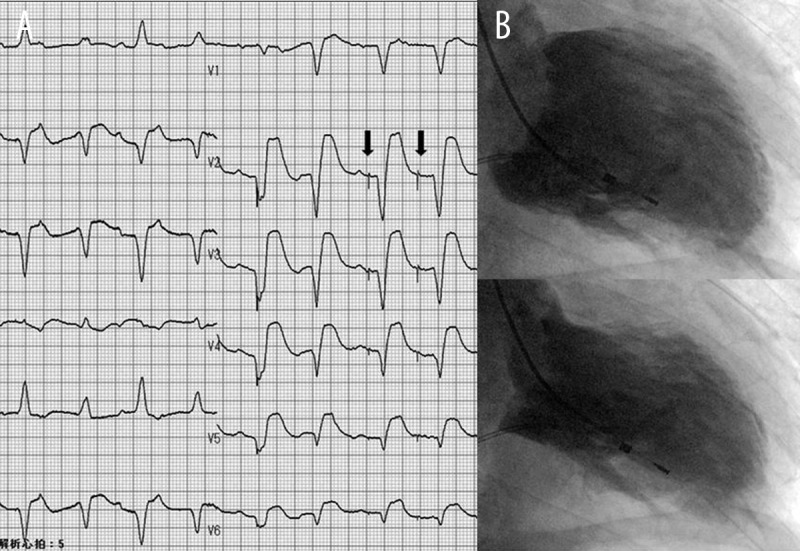
The 12-lead electrocardiogram and left ventriculogram obtained on admission during hemodynamic collapse. (A) The 12-lead electrocardiogram; arrow indicates the spike in ventricular pace. (B) The left ventriculogram viewed from the right, anterior, oblique perspective.
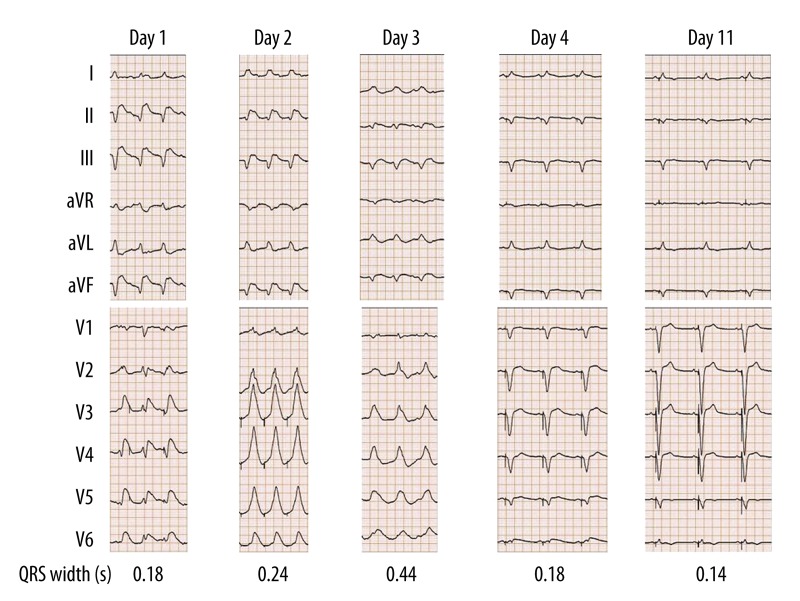
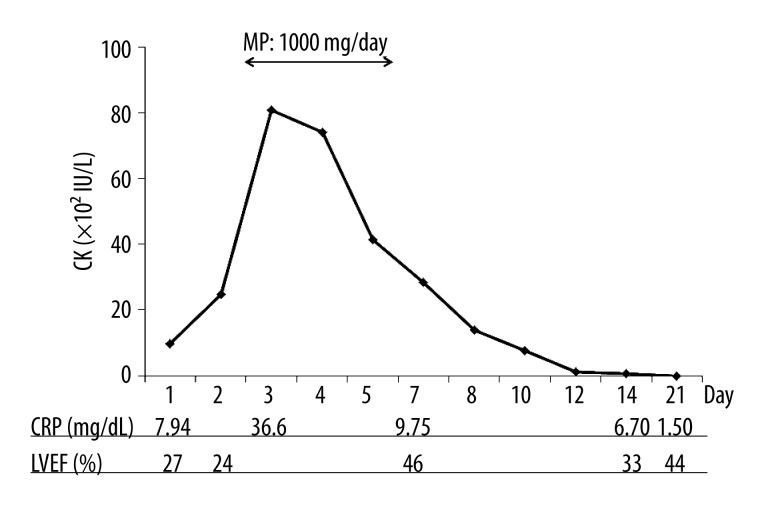
Shock persisted in the patient despite aggressive management. Between days 1 and 3 of treatment, the QRS width progressively increased from 0.18 s to 0.44 s (Figure 2). By day 3, CRP levels reached 36.6 mg/dL and CK levels were 8,078 IU/L (Figure 3). This unfavorable clinical course was suggestive of extended and activated myocardial injury and inflammation, and the patient appeared to be close to cardiac death. Thus, on day 3, we initiated intravenous methylprednisolone at a dose of 1,000 mg/day for the purposes of immunosuppression and anti-inflammation. The methylprednisolone treatment was maintained for three days and then immediately terminated.
Figure 2.
Serial changes in heart rhythm, QRS morphology, and QRS width.
Figure 3.
Time courses of CK, CRP, and LVEF. CK – creatinine kinase; CRP – C-reactive protein; LVEF – left ventricular ejection fraction; MP – methylprednisolone.
On day 4, the ventricular pacing rhythm was restored and the QRS width was decreased to 0.14 s, consistent with pre-admission QRS width until day 11 (Figure 2). As shown in Figure 3, the CK levels declined sharply and returned to its normal limit until day 12. The time courses of the CRP and CK levels were similar. In accordance with improved myocardial injury and inflammation, the left ventricular ejection fraction (LVEF) in the echocardiogram increased from 24% to 44%. Extracorporeal membrane oxygenation and intra-aortic balloon pump therapy were discontinued on days 14 and 16, respectively.
Although the patient exhibited moderately impaired LV function, he survived and was discharged without any neurological deficits.
Discussion
In the present case, although endomyocardial biopsy could not be taken, the recent viral illness, rapid development of cardiogenic shock, failure of ventricular pacing and sensing, diffuse impairment of LV contraction, and absence of flow-limiting coronary stenosis were highly suggestive of fulminant myocarditis. To prevent hemodynamic collapse and enable the recovery of injured myocardium, intra-aortic balloon pump therapy and/or extracorporeal membrane oxygenation are often used as adjuncts to optimized medical treatments and are considered first-line treatments for severe cases of fulminant myocarditis [4–6]. The long-term prognosis of fulminant myocarditis can be favorable [1]; however, the mortality rate is high during acute stages of the illness. Mirabel et al. [4] demonstrated that 13 of 41 patients (32%) who received mechanical circulatory assistance died, and four of the 41 (10%) required heart transplants. Hsu et al. [5] demonstrated that 27 of 75 patients (36%) treated with extracorporeal membrane oxygenation died during hospitalization. To decrease the rates of death and heart transplantation, development of alternative therapeutic interventions is necessary to treat patients who do not respond to usual aggressive treatment regimens.
In this case, the progressive expansion of the QRS width and progressive elevation of CK and CRP levels suggested a classification of the patient as a non-responder. A high dose of intravenous methylprednisolone seemed effective based on the results that followed. Failed ventricular pacing and sensing recovered within 24 h, and the QRS width had decreased to baseline levels for a brief period after methylprednisolone treatment. The time courses of the CK and CRP levels also exhibited sharp declines, which were associated with improved LVEF.
Immunosuppressive treatments, including prednisone and azathioprine, are reportedly effective for the recovery of LV systolic function in patients who exhibit virus-negative inflammatory cardiomyopathy [7]. In contrast, the use of immunosuppression to treat acute myocarditis has led to controversial results. Myocarditis is associated with a wide spectrum of clinical presentations, which may explain inconsistent results. No large clinical studies have examined the effects that immunosuppression has on the functional and clinical outcomes of fulminant myocarditis; however, several case reports and a small clinical study have highlighted the benefits of corticosteroid treatment [3,8–10]. In acute viral myocarditis, the major mechanisms that underlie tissue injury differ depending on the time elapsed from viral exposure [6,11]. Viral replication directly causes myocardial necrosis during the acute phase over a period of few days. After the acute phase, the activated immune system plays a major role in subsequent cardiac failure. Because of molecular mimicry, T-lymphocytes that had been sensitized by viral antigen may attack host myocardium. Activation of cytokines and creation of auto-antibodies against cardiac proteins also may also contribute to tissue injury. Immunosuppression may enhance viral replication and thus have deleterious effects during the acute phase. In contrast, immunosuppression may be beneficial during subsequent phases. Because methylprednisolone inhibited progressive aggravation of clinical findings and improved LV function and inflammation, the abrupt collapse of hemodynamics that occurred in our patient most likely developed in response to an immune-mediated myocardial injury.
Conclusions
We present intravenous methylprednisolone as an attractive therapeutic option for patient with fulminant myocarditis that is refractory to optimal medical treatment and mechanical circulatory support. Additional clinical studies are needed to determine the optimal timing, dosage, and duration of intravenous methylprednisolone to treat refractory fulminant myocarditis.
References:
- 1.McCarthy RE, III, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. New Eng J Med. 2000;342(10):690–95. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 2.Berte B, Eyskens B, Meyfroidt G, Willems R. Bidirectional ventricular tachycardia in fulminant myocarditis. Europace. 2008;10(6):767–68. doi: 10.1093/europace/eun105. [DOI] [PubMed] [Google Scholar]
- 3.Kohno K, Aoyama N, Shimohama T, et al. Resuscitation from fulminant myocarditis associated with refractory ventricular fibrillation. Jpn Circ J. 2000;64(2):139–43. doi: 10.1253/jcj.64.139. [DOI] [PubMed] [Google Scholar]
- 4.Mirabel M, Luyt CE, Leprince P, et al. Outcomes, long-term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit Care Med. 2011;39(5):1029–35. doi: 10.1097/CCM.0b013e31820ead45. [DOI] [PubMed] [Google Scholar]
- 5.Hsu KH, Chi NH, Yu HY, et al. Extracorporeal membranous oxygenation support for acute fulminant myocarditis: analysis of a single center’s experience. Eur J Cardiothorac Surg. 2011;40(3):682–88. doi: 10.1016/j.ejcts.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 6.Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol. 2012;59(9):779–92. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 7.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30(16):1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 8.Moreels M, Delforge ML, Renard M. Fulminant myocarditis with dramatic response to corticoids. Acta Cardiol. 2010;65(1):97–99. doi: 10.2143/AC.65.1.2045898. [DOI] [PubMed] [Google Scholar]
- 9.Dennert R, Velthuis S, Westermann D, et al. Parvovirus-B19-associated fulminant myocarditis successfully treated with immunosuppressive and antiviral therapy. Antivir Ther. 2010;15(4):681–85. doi: 10.3851/IMP1563. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima H, Katayama T, Ishizaki M, et al. Q wave and non-Q wave myocarditis with special reference to clinical significance. Jpn Heart J. 1998;39(6):763–74. doi: 10.1536/ihj.39.763. [DOI] [PubMed] [Google Scholar]
- 11.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99(8):1091–100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]