INTRODUCTION
Since the middle of the 20th century, particle therapy has been in focus for patient treatments. In 1946, Robert Wilson proposed the use of charged particles for tumor therapy, and since then, the clinical use of protons and heavier ions, mainly carbon ions, has become more widespread [1]. The first clinical evidence was obtained in Berkeley, treating radiation-resistant targets with various ion species [2–14]. The main advantage of particle beams derive from their physical properties: through an inverted dose profile, regions within the entry channel of the beam can be spared of dose, while a steep dose deposition can be directed in an energy-dependent manner into the defined treatment volume (Bragg Peak). The following dose fall-off spares tissue behind the target volume, thus reducing integral dose significantly compared to when using photons. Heavier charged particles, such as carbon ions or oxygen, are additionally associated with an increased relative biological effectiveness (RBE), while the RBE of protons is commonly accepted to be about 1.1 [15, 16]. Recent observation, however, suggests that this may be an oversimplification.
Carbon ion radiotherapy is currently available in only a few centers worldwide: mainly in Japan, with two centers in Europe (CNAO, Pavia, Italy and HIT, Heidelberg, Germany). For proton therapy, treating centers are more widespread throughout the USA and Asia, with a few centers in Europe. Most clinical data has been reported as smaller series within Phase I/II trials, or retrospectively assembled data from patient subgroups, most indications being sarcomas, malignant salivary gland tumors or ocular melanoma [2, 17–22]. The only large randomized trial comparing protons to photons was performed in patients with prostate cancer, where proton therapy was applied as a boost in the experimental arm to a higher total dose than in the control arm [23, 24]; this trial did show a benefit in terms of biochemical progression-free survival, with overall comparable toxicity [25]. Over the years, photon treatments have improved and modern intensity-modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT) approaches enable the radiation oncologist to deliver doses of ∼80 Gy to the prostate safely. This is not the dose concept and technical background that was implemented in that trial. Therefore, controversy remains about the real value of this trial, i.e. comparing ‘protons vs photons’, or comparing ‘high dose vs low dose’.
Currently, the cost of particle therapy is a multiple of that of photon treatments, due to the size of structures, the complexity of technology required, the resulting investment and also the high running cost; additionally, capacity for treatment is still limited [18, 26, 27]. Therefore, current discussions focus on the indications for particle therapy.
Therefore, besides seeking technical improvement and developments to make particle therapy less costly and more widely available, four main clinical foci are of importance:
design of clinical trials comparing protons, carbon ions and advanced photon techniques for selected tumor indications;
identification of prognostic markers, i.e. molecular characteristics, imaging properties or normal tissue qualities stratifying patients;
better understanding of patient- and tumor-specific RBE for protons and heavier ions alike;
integration of particles into multimodal treatment with systemic approaches together with surgery.
Integrating such novel tools into modern radiation oncology requires large-scale preclinical and clinical research in the fields of biology, physics and medicine. To bring together the expertise of various researchers of all stages, and to educate newcomers into the field through dissemination of knowledge, joint European structures were generated as a platform to incorporate protons and heavier ions into modern radiation oncology concepts.
ENLIGHT
A consortium of clinical and preclinical researchers was founded forming the ‘European Network for LIGht ion Hadron Therapy’ (ENLIGHT); the major goal was to bring together the expertise of European researchers in the fields of biology, physics and clinical medicine, with a strong focus on particle therapy [28]. Formed initially in 2002, with 50 people from seven European countries, it now consists of over 400 members from over 20 countries [28–31]. The main strength of the consortium is in bringing together people of various nationalities with different experience and interests in particle therapy to work together and establish common projects. Thus, an innovative platform for European research with the possibility for European research funding could be established, bringing together all the necessary disciplines involved. Since then, regular meetings, common projects and exchange of research, results and data in an organized and beneficial fashion, have been taking place with the goal of developing radiation oncology, especially particle therapy. With general agreement, the ENLIGHT community focuses on complementary approaches, i.e. the combination of high-end research combined with networking essential to disseminate and educate so as to generate common standards and protocols for optimized patient treatment. Therefore, the primary mandate of ENLIGHT is generation of strategic planning to acquire the funding necessary for continuation of the initiative of its two main pillars—research and networking.
The ENLIGHT network has led to four main European Commission (EC)-funded projects: PARTNER (PARticle Training Network for European Radiotherapy), ULICE (Union of Light Ion Centres in Europe), ENVISION (European NoVel Imaging Systems for ION therapy) and ENTERVISION (European Training Network in Digital Medical Imaging for Radiotherapy). A common up-to-date homepage keeps members of ENLIGHT, as well as newcomers and non-members informed about acitivies, achievements and future plans (www.enlight.web.cern.ch).
PARTNER
The PARTNER project was designed as a unique effort for training scientific and clinical personnel in the field of particle therapy. The Seventh Framework Programme (FP7) provided the optimal basis for Marie Curie projects; the proposal was started in 2008, and was funded successfully, based on the previously established ENLIGHT platform and connections. The PARTNER project focused on education and dissemination of knowledge, as well as bringing young physicists, biologists and clinicians into hadron therapy research. Besides scientific training, development of the management, teaching and strategic skills necessary for successful careers in this field were part of the funding statutes. On the horizon was the goal of bringing together this interdisciplinary, multinational initiative of training to help to improve the overall efficacy of particle therapy in cancer treatment. More and more centers were being planned or under construction, but the personnel and expertise necessary for building, start-up and running of such centers were still scarce. Keeping this in mind, PARTNER also included research topics for subsequent clinical trials (bridging the gap to the successful follow-up project, ULICE).
The PARTNER project, which ended in September 2012, was one of the most successful EC-funded projects in the field. Research results and data generated from the various topics have been brought together as part of the present Supplement issue of the Journal of Radiation Research. To coordinate this huge effort via training courses and regular meetings good administration was necessary, which was taken over by CERN. With a funding volume of 5.6 million Euros, 25 early- and late-stage researchers were able to benefit from this unique European experience, and to demonstrate successul research, as reflected by the scientific publications and follow-up projects.
ULICE
Based on the research and education in the field of particle therapy, the next step was to form a project consortium more technically and facility-oriented. For much of the research beam time is required, and specific research concerning the optimization and development of innovative technologies to improve existing treatment centers is essential. Moreover, the ULICE consortium was formed to design and implement clinical trials. Therefore, the ULICE project was formed with special ‘transnational access’ to beam time for studies within the European research framework.
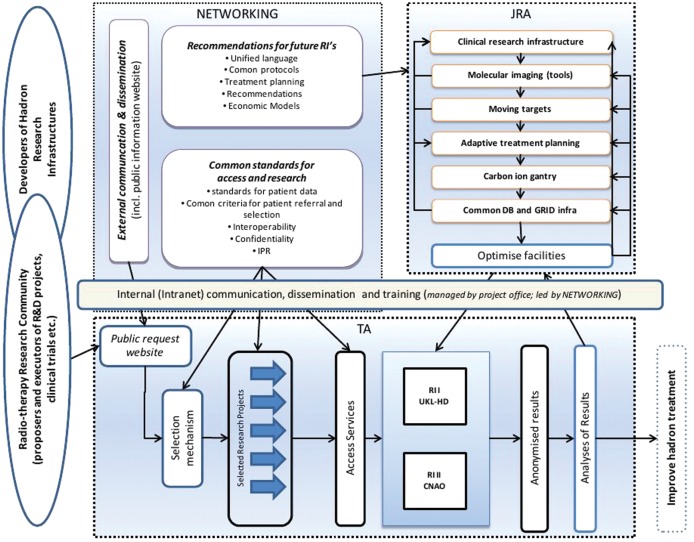
The project was set up with three main pillars: Joint Research Activities (JRA), Transnational Access (TNA) and Networking (Fig. 1).
Fig. 1.
Design of the ULICE-Project funded within FP7 of the European Union.
Research topics included issues in radiation biology, treatment planning, moving organs and 4D-imaging, and aspects of treatment, as well as establishment of standardized clinical approaches for patient treatment. The main facilities are the Heidelberg Ion Therapy Center (HIT) and the CNAO facility in Pavia, Italy, which provide particle treatment within clinical protocols, as well as beam time for preclinical research activities.
Clinical research is based on a common database structure [32]. In this database, data from all patients treated with particle therapy are collected and prospectively documented within the safe clinical database structures of the hospital information system interface (Fig. 2). Web-Access allows referring centers to upload and transfer their data, including imaging, treatment-planning data, as well as all other patient-related information, through safe and validated datalines. The database integrates all hospital information systems. For all patients enrolled into clinical trials, eCRFs are used which can be filled in directly at the center, or through the web-application server. This database system will allow for broad-scale evaluation of data including biology, imaging and physical details of particle treatment [33].
Fig. 2.
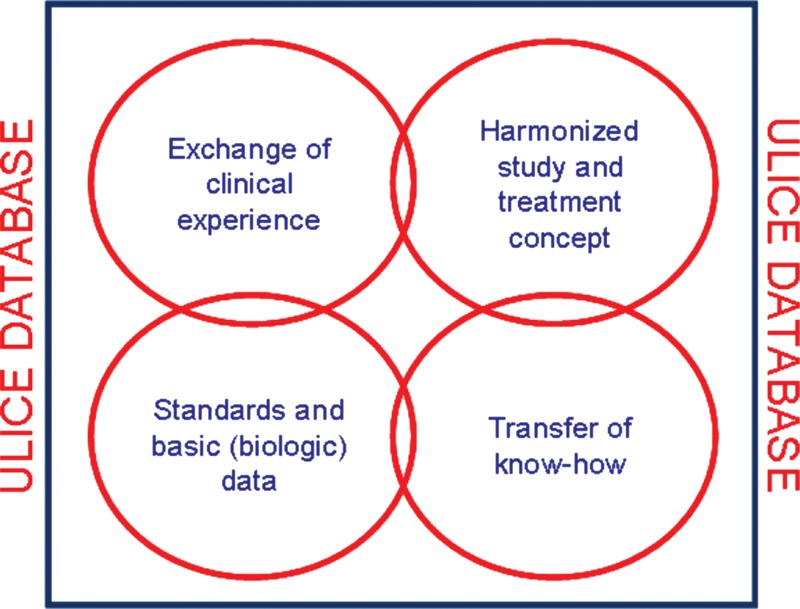
ULICE-Database includes clinical, physical and biological data for evaluation of patients treated with particle therapy and for subsequent correlation with e.g. molecular markers for subsequent personalized treatment approaches.
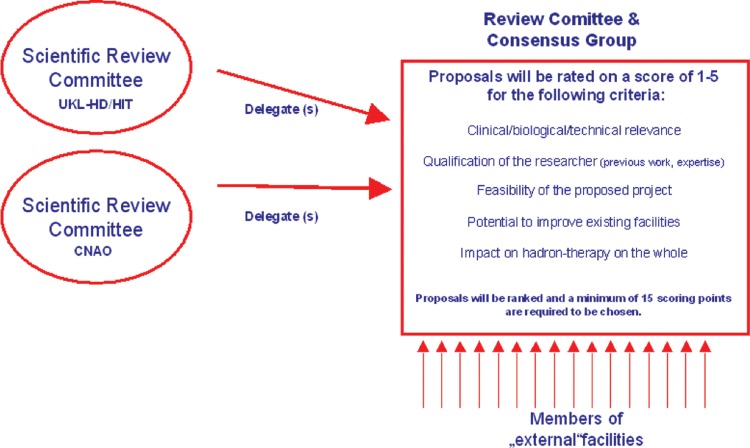
A review committee and consensus group gathers expertise from throughout Europe to define and establish clinical trials. The group reviews preclinical research proposals according to defined criteria. Positively evaluated projects are attributed beam time within the project through TNA. Thus, researchers are able to access the two running facilities (HIT and CNAO) to perform high-end preclinical and clinical research. The combination of clinical and preclinical aspects bridges the gap between the necessary data and development issues required for improvement, while enabling design of clinical trials based on recent clinical standards and indications. The next step will be the formation of the European Hadron Therapy Research Board, which is currently being set up and is intended to structure and coordinate future study concepts and developments.
ACHIEVEMENTS FOR CLINICAL INTEGRATION OF PARTICLE THERAPY
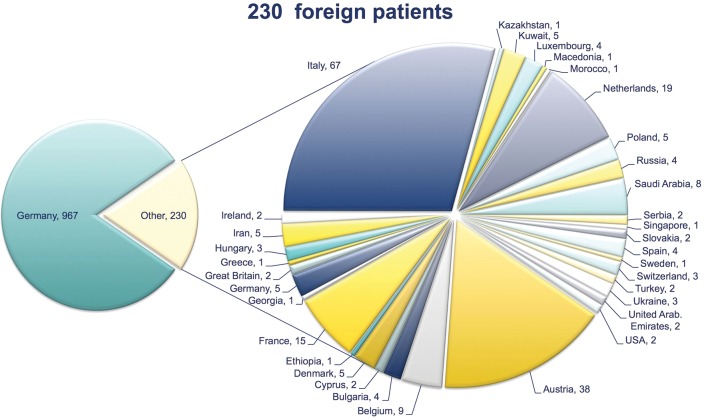
Through the EU-funded projects and beyond, particle therapy has been brought forward, pre-clinically, and especially clinically. Through increases in patient treatment at HIT in 2009, and at CNAO in 2011, more patients have been granted access to particle treatments (Fig. 3). By December 2012, over 1200 patients had been treated with protons and carbon ions at HIT. Access for European patients, both outside and within the ULICE project, has been successfully implemented, i.e. fully ‘living’ TNA (Fig. 4).
Fig. 3.
Foreign patients treated at HIT from 2009–2012, as proposed in Transnational Access (TNA).
Fig. 4.
Review Committee and Consensus group established to evaluate and decide on research proposals.
Currently, 12 clinical trials are recruiting at the HIT facility (Fig. 5) for different clinical indications [32, 34–44]. A purely ULICE-generated trial, jointly designed and set up according to the proposed ULICE-workflow, is the PANDORA-01 trial on recurrent rectal cancer, which started recruitment in December 2012 after full approval from all the relevant authorities in Germany and in Italy [35].
Fig. 5.
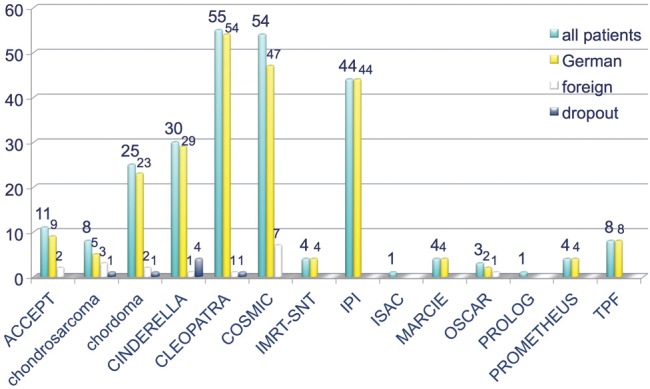
Clinical trials currently running at HIT.
FUTURE ASPECTS
The goal for the future is to bring particle therapy into modern radiation oncology, leading to a personalized treatment approach based on tumor and normal tissue biology. Currently, several randomized trials are running or are being designed; special effort in the field of particle therapy is being made within the RTOG-group, where trials with a sharp focus on protons, but also on carbon ion radiotherapy, are underway. Correlation of clinical outcome with molecular markers, molecular determinants of local control, metastases development, as well as toxicity is required. Prospective evaluation of treatment outcome, analysis of tumor tissue, and liquid biobanking will enable identification of patient- and tumor-specific signatures, determining treatment parameters, i.e. dose, fractionation, addition of chemotherapy or molecular-targeted substances, and even radiation quality.
This, in conjunction with modern IGRT techniques, offers the potential for Adaptive RadioTherapy (ART), with a special focus on Biology (BIO-ART) using particle beams. However, technical improvements in terms of smaller facilities, and faster and small-scale accelerator possibilities (e.g. laser-driven particle facilities), are needed to reduce the cost of facilities and subsequently of treatment cost. In the meantime, the issue of comparing particle therapy with high-end photon radiotherapy is of high priority.
FUNDING
We would like to thank all members of the PARTNER and ULICE consortium, as well as the European Commission (EC) for the funding opportunity.
ACKNOWLEDGEMENTS
The authors wish to thank Kerstin Kessel and Nina Bougatf, as well as Alexander Emig and Dieter Oetzel for their excellent support and setup of the database. Special thanks go to Kerstin Kessel for her meticulous work with patient documentation issues, as well as generation of eCRFs for the study protocols. Moreover, we thank Rebecca Klumpp, Anna-Lena Radecke and Eva Meyerhof for kind and dependable patient care and their input of data into the database system.
REFERENCES
- 1.Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487–91. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 2.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953–64. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 3.Castro JR, Linstadt DE, Bahary JP, et al. Experience in charged particle irradiation of tumors of the skull base: 1977–1992. Int J Radiat Oncol Biol Phys. 1994;29:647–55. doi: 10.1016/0360-3016(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 4.Castro JR, Saunders WM, Austin-Seymour MM, et al. A phase I–II trial of heavy charged particle irradiation of malignant glioma of the brain: a Northern California Oncology Group Study. Int J Radiat Oncol Biol Phys. 1985;11:1795–800. doi: 10.1016/0360-3016(85)90034-3. [DOI] [PubMed] [Google Scholar]
- 5.Castro JR, Chen GT, Blakely EA. Current considerations in heavy charged-particle radiotherapy: a clinical research trial of the University of California Lawrence Berkeley Laboratory, Northern California Oncology Group, and Radiation Therapy Oncology Group. Radiat Res Suppl. 1985;8 S263–71. [PubMed] [Google Scholar]
- 6.Castro JR. Heavy charged particle irradiation of human cancers. Radiat Med. 1983;1:70–5. [PubMed] [Google Scholar]
- 7.Castro JR, Saunders WM, Quivey JM, et al. Clinical problems in radiotherapy of carcinoma of the pancreas. Am J Clin Oncol. 1982;5:579–87. doi: 10.1097/00000421-198212000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Castro JR, Quivey JM, Lyman JT, et al. Current status of clinical particle radiotherapy at Lawrence Berkeley Laboratory. Cancer. 1980;46:633–41. doi: 10.1002/1097-0142(19800815)46:4<633::aid-cncr2820460402>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Castro JR, Quivey JM, Lyman JT, et al. Radiotherapy with heavy charged particles at Lawrence Berkeley Laboratory. J Can Assoc Radiol. 1980;31:30–4. [PubMed] [Google Scholar]
- 10.Kaplan ID, Castro JR, Phillips TL. Helium charged particle radiotherapy for meningioma: experience at UCLBL. University of California Lawrence Berkeley Laboratory. Int J Radiat Oncol Biol Phys. 1994;28:257–61. doi: 10.1016/0360-3016(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 11.Reimers M, Castro JR, Linstadt D, et al. Heavy charged particle therapy of bone and soft tissue sarcoma. A phase I–II trial of the University of California Lawrence Berkeley Laboratory and the Northern California Oncology Group. Am J Clin Oncol. 1986;9:488–93. doi: 10.1097/00000421-198612000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Saunders W, Castro JR, Chen GT, et al. Helium-ion radiation therapy at the Lawrence Berkeley Laboratory: recent results of a Northern California Oncology Group Clinical Trial. Radiat Res Suppl. 1985;8 S227–34. [PubMed] [Google Scholar]
- 13.Schoenthaler R, Castro JR, Halberg FE, et al. Definitive postoperative irradiation of bile duct carcinoma with charged particles and/or photons. Int J Radiat Oncol Biol Phys. 1993;27:75–82. doi: 10.1016/0360-3016(93)90423-s. [DOI] [PubMed] [Google Scholar]
- 14.Schoenthaler R, Castro JR, Petti PL, et al. Charged particle irradiation of sacral chordomas. Int J Radiat Oncol Biol Phys. 1993;26:291–8. doi: 10.1016/0360-3016(93)90209-e. [DOI] [PubMed] [Google Scholar]
- 15.Denekamp J, Waites T, Fowler JF. Predicting realistic RBE values for clinically relevant radiotherapy schedules. Int J Radiat Biol. 1997;71:681–94. doi: 10.1080/095530097143699. [DOI] [PubMed] [Google Scholar]
- 16.Ando K, Koike S, Uzawa A, et al. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res. 2005;46:51–7. doi: 10.1269/jrr.46.51. [DOI] [PubMed] [Google Scholar]
- 17.Delaney TF. Proton therapy in the clinic. Front Radiat Ther Oncol. 2011;43:465–85. doi: 10.1159/000322511. [DOI] [PubMed] [Google Scholar]
- 18.Combs SE, Laperriere N, Brada M. Clinical controversies: proton radiation therapy for brain and skull base tumors. Semin Radiat Oncol. 2013;23:120–6. doi: 10.1016/j.semradonc.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37–43. doi: 10.1038/nrclinonc.2009.183. [DOI] [PubMed] [Google Scholar]
- 20.Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol. 2012;42:670–85. doi: 10.1093/jjco/hys104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blomquist E, Bjelkengren G, Glimelius B. The potential of proton beam radiation therapy in intracranial and ocular tumours. Acta Oncol. 2005;44:862–70. doi: 10.1080/02841860500355934. [DOI] [PubMed] [Google Scholar]
- 22.Hocht S, Bechrakis NE, Nausner M, et al. Proton therapy of uveal melanomas in Berlin. 5 years of experience at the Hahn-Meitner Institute. Strahlenther Onkol. 2004;180:419–24. doi: 10.1007/s00066-004-1222-5. [DOI] [PubMed] [Google Scholar]
- 23.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 25.Gardner BG, Zietman AL, Shipley WU, et al. Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol. 2002;167:123–6. [PubMed] [Google Scholar]
- 26.Efstathiou JA, Trofimov AV, Zietman AL. Life, liberty, and the pursuit of protons: an evidence-based review of the role of particle therapy in the treatment of prostate cancer. Cancer J. 2009;15:312–8. doi: 10.1097/PPO.0b013e3181b14ec0. [DOI] [PubMed] [Google Scholar]
- 27.Zietman AL. The Titanic and the iceberg: prostate proton therapy and health care economics. J Clin Oncol. 2007;25:3565–6. doi: 10.1200/JCO.2007.11.9768. [DOI] [PubMed] [Google Scholar]
- 28.ENLIGHT. The European Network for LIGht ion Hadron Therapy. 2009 doi: 10.1097/HP.0b013e3182606520. https://enlight.web.cern.ch . [DOI] [PubMed] [Google Scholar]
- 29.Dosanjh MK., Hoffmann HF, Magrin G. Status of hadron therapy in Europe and the role of ENLIGHT. Nucl Instrum Methods Phys Res. 2007;A571:191–4. [Google Scholar]
- 30.Dosanjh M, Jones B, Mayer R. ENLIGHT and other EU-funded projects in hadron therapy. Br J Radiol. 2010;83:811–3. doi: 10.1259/bjr/49490647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dosanjh M, Cirilli M, Greco V, et al. ENLIGHT: the European Network for light ion hadron therapy. Health Phys. 2012;103–5:674–80. doi: 10.1097/HP.0b013e3182606520. [DOI] [PubMed] [Google Scholar]
- 32.Kessel KA, Bougatf N, Bohn C, et al. Connection of European particle therapy centers and generation of a common particle database system within the European ULICE-framework. Radiat Oncol. 2012;7:115. doi: 10.1186/1748-717X-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessel KA, Habermehl D, Bohn C, et al. Database supported electronic retrospective analyses in radiation oncology: establishing a workflow using the example of pancreatic cancer. Strahlenther Onkol. 2012;188:1119–24. doi: 10.1007/s00066-012-0214-0. [DOI] [PubMed] [Google Scholar]
- 34.Blattmann C, Oertel S, Schulz-Ertner D, et al. Non-randomized therapy trial to determine the safety and efficacy of heavy ion radiotherapy in patients with non-resectable osteosarcoma. BMC Cancer. 2010;10:96. doi: 10.1186/1471-2407-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Combs SE, Kieser M, Habermehl D, et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: the PANDORA-01 trial. BMC Cancer. 2012;12:137. doi: 10.1186/1471-2407-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combs SE, Kieser M, Rieken S, et al. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: the CLEOPATRA trial. BMC Cancer. 2010;10:478. doi: 10.1186/1471-2407-10-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Combs SE, Habermehl D, Ganten T, et al. Phase I study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: the PROMETHEUS-01 trial. BMC Cancer. 2011;11:67. doi: 10.1186/1471-2407-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Combs SE, Edler L, Burkholder I, et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. 2010;10:615. doi: 10.1186/1471-2407-10-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Combs SE, Burkholder I, Edler L, et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: the CINDERELLA trial. BMC Cancer. 2010;10:533. doi: 10.1186/1471-2407-10-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikoghosyan AV, Karapanagiotou-Schenkel I, Munter MW, et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer. 2010;10:607. doi: 10.1186/1471-2407-10-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikoghosyan AV, Rauch G, Munter MW, et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer. 2010;10:606. doi: 10.1186/1471-2407-10-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen AD, Nikoghosyan A, Hinke A, et al. Combined treatment of adenoid cystic carcinoma with cetuximab and IMRT plus C12 heavy ion boost: ACCEPT [ACC, Erbitux® and particle therapy] BMC Cancer. 2011;11:70. doi: 10.1186/1471-2407-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen AD, Nikoghosyan A, Windemuth-Kieselbach C, et al. Combined treatment of malignant salivary gland tumours with intensity-modulated radiation therapy (IMRT) and carbon ions: COSMIC. BMC Cancer. 2010;10:546. doi: 10.1186/1471-2407-10-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen AD, Krauss J, Potthoff K, et al. Phase II study of induction chemotherapy with TPF followed by radioimmunotherapy with Cetuximab and intensity-modulated radiotherapy (IMRT) in combination with a carbon ion boost for locally advanced tumours of the oro-, hypopharynx and larynx – TPF-C-HIT. BMC Cancer. 2011;11:182. doi: 10.1186/1471-2407-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]