Abstract
Ion beam therapy, as an emerging radiation therapy modality, requires continuous efforts to develop and improve tools for patient treatment planning (TP) and research applications. Dose and fluence computation algorithms using the Monte Carlo (MC) technique have served for decades as reference tools for accurate dose computations for radiotherapy. In this work, a novel MC-based treatment-planning (MCTP) tool for ion beam therapy using the pencil beam scanning technique is presented. It allows single-field and simultaneous multiple-fields optimization for realistic patient treatment conditions and for dosimetric quality assurance for irradiation conditions at state-of-the-art ion beam therapy facilities. It employs iterative procedures that allow for the optimization of absorbed dose and relative biological effectiveness (RBE)-weighted dose using radiobiological input tables generated by external RBE models. Using a re-implementation of the local effect model (LEM), the MCTP tool is able to perform TP studies using ions with atomic numbers Z ≤ 8. Example treatment plans created with the MCTP tool are presented for carbon ions in comparison with a certified analytical treatment-planning system. Furthermore, the usage of the tool to compute and optimize mixed-ion treatment plans, i.e. plans including pencil beams of ions with different atomic numbers, is demonstrated. The tool is aimed for future use in research applications and to support treatment planning at ion beam facilities.
Keywords: ion beam therapy, treatment planning, Monte Carlo, FLUKA
INTRODUCTION
Dose calculations using the Monte Carlo (MC) method have the potential for the most accurate predictions of dose distributions for clinical radiotherapy [1]. Accurate predictions of dose distributions and associated ranges are of particular importance for radiation therapy with proton and ion beams due to the conformity of the treatment. This is specifically challenging for complex treatment situations, involving large heterogeneities or metal implants [2, 3]. A novel MC-based treatment-planning (MCTP) tool was presented and validated for protons using scanned pencil beams for realistic patient treatment conditions in a previous paper [4]. MCTP can support TP by helping to understand and improve inaccuracies due to approximations made by analytical dose and fluence algorithms, and allows provision of a ‘second opinion’ for selected patient cases. Furthermore, the developed MCTP tool seeks to enable TP and research studies for state-of-the-art ion beam therapy. An asset for such usage is the versatility of the MCTP tool, which is to some extent due to the usage of the MC method and the flexible interface to external relative biological effectiveness (RBE) models via ion charge and energy-specific tabulations of the linear-quadratic (LQ) model parameters, as predicted by the external model. In this work, an extension of this MCTP tool to perform TP with carbon ions is presented for realistic patient treatment conditions. Furthermore, its ability to perform TP mixing several ion species is demonstrated. This will enable future TP studies to be carried out using and comparing different ions and optimization techniques.
MATERIALS AND METHODS
The MCTP tool is composed of various program components that require a range of treatment- and facility-specific input information. A description of the workflow and details of the MCTP tool can be found in Mairani et al. [4], including in particular information about its general structure, the pre-optimization and pencil beam selection procedure, and the configuration of the MC code FLUKA [5, 6], that is used for dose and fluence computations in phantom and patient geometries. The tool allows the user to perform inverse optimization of single fields, and simultaneous multiple-fields optimization (often referred to as intensity-modulated particle therapy (IMPT) [7]) of absorbed and RBE-weighted doses using gradient-based methods. For the present work, the tool was extended to be able to perform TP for ion beams for treatment conditions at the HIT facility (Heidelberg Ion-Beam Therapy, Germany). This was achieved by merging the MCTP tool with parts of the FLUKA-based MC framework FICTION (FLUKA Integrated Framework for CT-based calculations in Ion Therapy), developed at HIT [8] within the framework of PARTNER (http://partner.web.cern.ch) and using the corresponding visualization tool [9]. The FICTION tool was validated and carefully fine-tuned to reproduce dosimetric measurements for HIT treatment conditions [8, 10]. FICTION previously allowed only a recalculation of a given proton or carbon ion treatment plan and not for the MC-based optimization of treatment plans using protons, carbon ions or other light ions. Differences in the biological effectiveness of ions were taken into account via tabulations of the linear and quadratic parameters of the LQ model, specific to the ion charge and its energy, which can be generated from external RBE models [11]. For this work, such input tables were obtained using a HIT re-implementation of the local effect model (LEM) in flavours of the LEM versions I and IV [4, 12, 13]. Further details about the calculation of absorbed and RBE-weighted dose with the MCTP tool can be found in [4, 8]. Calculation and optimization of absorbed and RBE-weighted doses performed by the certified analytical treatment planning system (TPS), presented as comparison for the test carbon ion patient, are largely based on the work of Krämer et al. [14].
RESULTS AND DISCUSSION
Examples of carbon ion treatment plans
Two test plans obtained from the MCTP tool are presented for carbon ions. The MCTP tool was used to compute and optimize a treatment field for a cube-shaped target volume in water. The pencil beams were optimized to yield a constant absorbed dose of 1 Gy in the target volume. Figure 1 shows the contour plots of the absorbed dose.
Fig. 1.
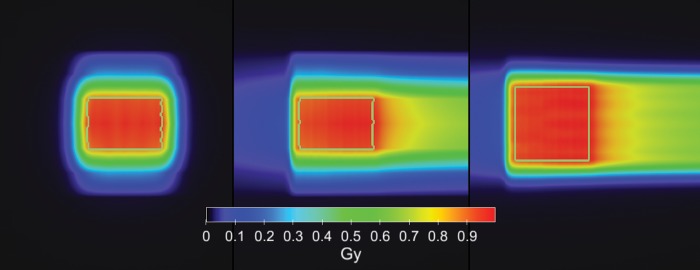
Carbon ion field computed and optimized by the MCTP tool for a cube-shaped target volume in water. The pencil beams were optimized to yield a constant absorbed dose of 1 Gy in the target volume.
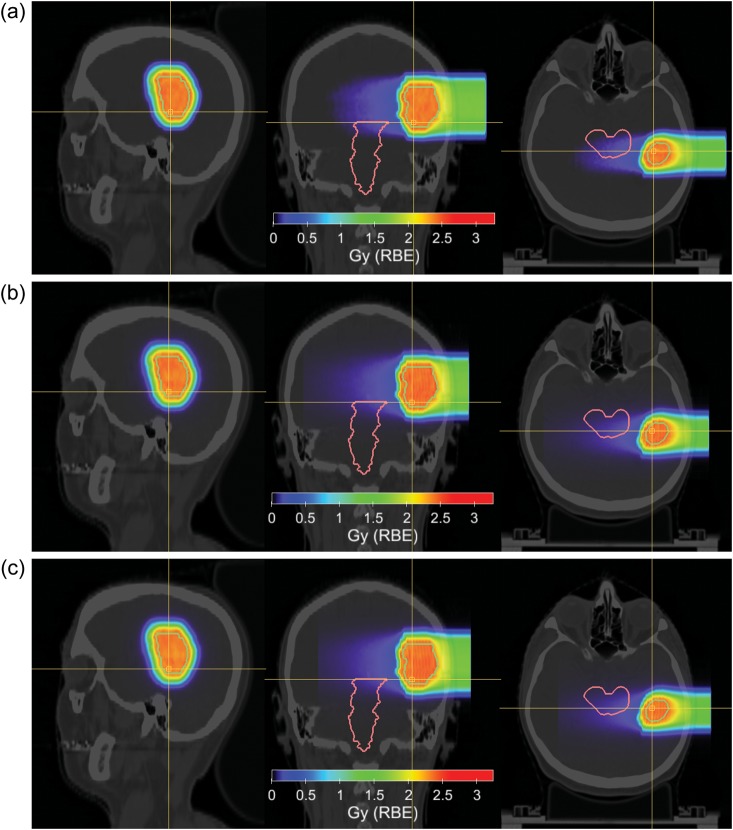
As a further example, a test patient plan for a carbon ion treatment of a brain tumour for HIT treatment conditions was created using the MCTP tool. A biological optimization was performed to obtain an RBE-weighted dose of 3 Gy (RBE) in the target volume using RBE values predicted by the LEM-I for chordoma [14]. Figures 2 and 3 show a comparison of the example treatment plan created by the certified analytical TPS used at HIT (SIEMENS Syngo RT Planning) and an MC recalculation of the same treatment plan using the FLUKA-based FICTION tool together with a treatment plan computed with the MCTP tool. For this test patient plan only minor differences between the treatment plan created using the analytical TPS, the MC recalculation of the same plan, and the treatment plan created with the MCTP tool are observable for the treated homogeneous tissue region. The MC recalculation of the plan created by the analytical TPS predicts a slight over-dosage in the clinical target volume of about 2%, which is, however, not clinically relevant. This over-dosage is corrected using the MCTP tool. Doses to the brain stem are very small. Observed differences in the DVH of the brain stem between the TPS and MC recalculation are therefore not addressed in the MCTP optimization.
Fig. 2.
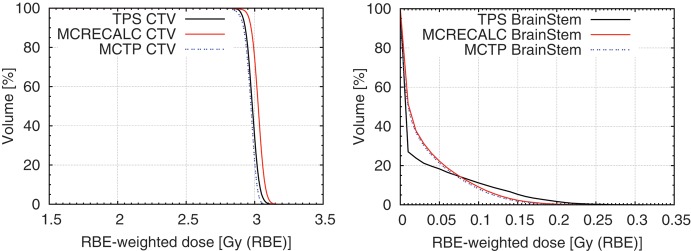
Test treatment plan of a brain tumour treated with carbon ions. DVHs of the clinical target volume (CTV) and the brain stem of a treatment plan created with an analytical treatment planning tool, the MC recalculation of the same plan, and a treatment plan created with the MCTP tool.
Fig. 3.
Test treatment plan of a brain tumour treated with carbon ions. Sagittal (left), coronal (middle) and axial (right) view are shown for (a) a treatment plan created with a TPS using an analytical dose engine, (b) the MC recalculation of the same plan, and (c) a treatment plan created with the MCTP tool. RBE-weighted doses are shown in colour-wash-scale. Hounsfield units of the planning CT are shown in grey-scale. Contours show the clinical target volume and the brain stem.
This test patient plan serves as a show-case and it is also a direct test of the MCTP implementation at HIT. Larger discrepancies between plans obtained using an MC recalculation of an analytical TPS and MCTP can be expected in the presence of tissue regions in the beam, with large heterogeneities and patient cases involving metallic implants [2, 3].
Treatment plans using new ions and combined ion beams of different charges
Coupled with an adequate RBE model, the MCTP tool is able to compute treatment plans not only for proton and carbon ion beams but also using other light ions, such as helium and oxygen, as well as treatment plans with several ion beams of different charges. The MC code FLUKA was shown to be able to simulate Bragg curves and fragmentation of ions beams such as oxygen beams with a satisfactory accuracy for investigative TP studies [15, 16]. TP involving ions other than protons and carbon ions, or even the usage of mixed-ion fields, could yield an improved therapeutic outcome for certain cases. For instance, ion beams with linear energy transfers (LET) higher than those of carbon ions, such as oxygen beams, are proposed for use to target radio-resistant tumours and to treat hypoxic tumour regions more efficiently [15, 17]. The simultaneous use of ions with different charges for patient treatment has been suggested: (i) to perform dose- and LET-painting; (ii) to decrease the fragmentation dose tail beyond the distal part of the spread-out Bragg peak (SOBP), while compromising to a lesser degree the high-LET component in the target, as well as to obtain benefit from the steeper lateral dose gradients achievable with ions heavier than protons; (iii) to avoid microscopic cold spots; and (iv) to reduce relative uncertainties of RBE for a given treatment protocol [18–20]. In the following, treatment plans using ion beams of two different charges for a cubic-shaped target in a water phantom are presented. They were created with the MCTP tool while using different optimization goals. Points (iii) and (iv) can be addressed by delivering a more homogeneous radiation quality in the target volume, which in turn can be achieved by plans using two ion species, even for mono-directional beams.
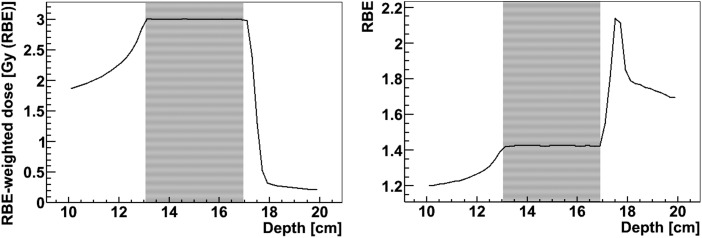
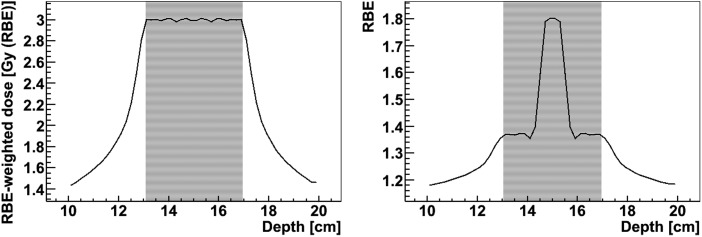
This is illustrated in Fig. 4, where a combination of mono-directional proton and carbon ion pencil beams were optimized to obtain a constant RBE, as a measure for homogeneity of radiation quality, throughout the SOBP. Similarly, dual ion fields of protons and carbon ions can be optimized to obtain a SOBP with a constant RBE-weighted dose in the target region, while delivering a ‘high-LET boost’ to a central hypoxic area with carbon ions, associated with a higher RBE, and delivering the dose around the boost region preferably by low-LET protons. This is shown in Fig. 5 using two opposed fields of proton and carbon ion pencil beams. The plans were optimized using the LEM-IV model for the proton and carbon ion beams with parameters for human salivary gland cells.
Fig. 4.
A 4-cm SOBP obtained using a combination of mono-directional proton and carbon ion pencil beams while optimizing for a constant RBE in the target volume. The left and right panels show RBE-weighted dose and RBE along the central axis of the field. The plan was optimized using the LEM-IV model for the proton and carbon ion beams with parameters for human salivary gland cells. The area with the fine stripes marks the target volume.
Fig. 5.
A 4-cm SOBP obtained using a combination of opposed fields of proton and carbon ion pencil beams. A constant RBE-weighted dose in the target volume is achieved while delivering a ‘high-LET boost’ to a central hypoxic area with carbon ions, associated with a higher RBE, and delivering the dose around the boost region preferably by low-LET protons. The left and right panels show the RBE-weighted dose and RBE along the central axis of the field. The plan was optimized using the LEM-IV model for the proton and carbon ion beams with parameters for human salivary gland cells. The area with the fine stripes marks the target volume including the boost volume.
CONCLUSION AND PERSPECTIVE
The prototype of a novel MCTP tool for ion beam therapy has been developed. It enables the user to perform TP with protons and carbon ions using realistic treatment conditions at HIT as well as with other light ions. Example treatment plans using carbon ions and mixed-ion treatments consisting of protons and carbon ions have been presented here. This versatile tool is useful for research applications and to support TP studies at state-of-the-art ion beam therapy facilities. In particular, studies comparing treatment plans created using different ion species and optimization techniques are envisaged.
FUNDING
This research project was supported by a Marie Curie Initial Training Network Fellowship of the European Community's Seventh Framework Programme under Contract No. PITNGA-2008-215840-PARTNER, by ENVISION, which is co-funded by the European Commission under FP7 Grant Agreement No. 241851 and by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie of Germany (DOTMOBI Project, Grant 01IB08002F).
REFERENCES
- 1.Chetty IJ, Curran B, Cygler JE, et al. Report of the AAPM Task Group No. 105: Issues associated with clinical implementation of Monte Carlo-based photon and electron external beam treatment planning. Med Phys. 2007;34:4818–53. doi: 10.1118/1.2795842. [DOI] [PubMed] [Google Scholar]
- 2.Paganetti H, Jiang H, Parodi K, et al. Clinical implementation of full Monte Carlo dose calculation in proton beam therapy. Phys Med Biol. 2008;53:4825–53. doi: 10.1088/0031-9155/53/17/023. [DOI] [PubMed] [Google Scholar]
- 3.Parodi K, Paganetti H, Shih HA, et al. Patient study of in vivo verification of beam delivery and range, using positron emission tomography and computed tomography imaging after proton therapy. Int J Radiat Oncol. 2007;68:920–34. doi: 10.1016/j.ijrobp.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mairani A, Böhlen TT, Schiavi A, et al. A Monte Carlo-based treatment planning tool for proton therapy. Phys Med Biol. 2013;58:2471–90. doi: 10.1088/0031-9155/58/8/2471. [DOI] [PubMed] [Google Scholar]
- 5.Battistoni G, Muraro S, Sala PR, et al. The FLUKA code: description and benchmarking. Proceedings of the Hadronic Shower Simulation Workshop, 2006. AIP Conf Proc. 2007;896:31–49. [Google Scholar]
- 6.Ferrari A, Sala PR, Fasso A, et al. FLUKA: A multi-particle transport code. CERN-2005-10, INFN/TC 05/11, SLAC-R-773, 2005. [Google Scholar]
- 7.Lomax A. Intensity modulation methods for proton radiotherapy. Phys Med Biol. 1999;44:185. doi: 10.1088/0031-9155/44/1/014. [DOI] [PubMed] [Google Scholar]
- 8.Sommerer F, Unholtz D, Brons S, et al. An easy-to-use Monte Carlo framework for ion therapy at the Heidelberg Ion-Beam Therapy Centre. 29th Annual Meeting of the European Society for Therapeutic Radiology and Oncology (ESTRO), Barcelona, Spain, September 12–16, 2010. Radiother Oncol. 2010;96 Suppl.1 S481. [Google Scholar]
- 9.Unholtz D, Sommerer F, Bauer J, et al. Post-therapeutical beta + -activity measurements in comparison to simulations towards in-vivo verification of ion beam therapy. In : Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2011 IEEE 2011. :2273–6. [Google Scholar]
- 10.Parodi K, Brons S, Cerutti F, et al. The FLUKA code for application of Monte Carlo methods to promote high precision ion beam therapy. Proceedings of the 12th International Conference on Nuclear Reaction Mechanisms, Varenna, Italy, 15–19th June, 2009. http://cds.cern.ch/record/1238366/files/p509.pdf. (April 2013, date last accessed). [Google Scholar]
- 11.Mairani A, Brons S, Cerutti F, et al. The FLUKA Monte Carlo code coupled with the local effect model for biological calculations in carbon ion therapy. Phys Med Biol. 2012;55:4273–89. doi: 10.1088/0031-9155/55/15/006. [DOI] [PubMed] [Google Scholar]
- 12.Scholz M, Kellerer AM, Kraft-Weyrather W, et al. Computation of cell survival in heavy ion beams for therapy. Radiat Environ Biophys. 1997;36:59–66. doi: 10.1007/s004110050055. [DOI] [PubMed] [Google Scholar]
- 13.Elsässer T, Weyrather WK, Friedrich T, et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int J Radiat Oncol. 2010;78:1177–83. doi: 10.1016/j.ijrobp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Krämer M, Scholz M. Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol. 2000;45:3319–30. doi: 10.1088/0031-9155/45/11/314. [DOI] [PubMed] [Google Scholar]
- 15.Kurz C, Mairani A, Parodi K. First experimental-based characterization of oxygen ion beam depth dose distributions at the Heidelberg Ion-Beam Therapy Center. Phys Med Biol. 2012;57:5017–34. doi: 10.1088/0031-9155/57/15/5017. [DOI] [PubMed] [Google Scholar]
- 16.Sommerer F, Parodi K, Ferrari A, et al. Investigating the accuracy of the FLUKA code for transport of therapeutic ion beams in matter. Phys Med Biol. 2006;51:4385–98. doi: 10.1088/0031-9155/51/17/017. [DOI] [PubMed] [Google Scholar]
- 17.Orecchia R, Krengli M, Jereczek-Fossa BA, et al. Clinical and research validity of hadrontherapy with ion beams. Crit Rev Oncol Hematol. 2004;51:81–90. doi: 10.1016/j.critrevonc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Bassler N, Jäkel O, Sondergaard CS, et al. Dose- and LET-painting with particle therapy. Acta Oncol. 2010;49:1170–6. doi: 10.3109/0284186X.2010.510640. [DOI] [PubMed] [Google Scholar]
- 19.Brahme A. Accurate description of the cell survival and biological effect at low and high doses and LET's. Radiat Res. 2011;52:389–407. doi: 10.1269/jrr.10129. [DOI] [PubMed] [Google Scholar]
- 20.Böhlen TT, Brons S, Dosanjh M, et al. Investigating the robustness of ion beam therapy treatment plans to uncertainties in biological treatment parameters. Phys Med Biol. 2012;57:7983–8004. doi: 10.1088/0031-9155/57/23/7983. [DOI] [PubMed] [Google Scholar]