Abstract
Background: Pancreatic cancer is the fourth leading cause of cancer deaths, being responsible for 6% of all cancer-related deaths. Conventional radiotherapy with or without additional chemotherapy has been applied in the past in the context of neoadjuvant or adjuvant therapy concepts with only modest results, however new radiation modalities, such as particle therapy with promising physical and biological characteristics, present an alternative treatment option for patients with pancreatic cancer. Up until now the raster scanning technique employed at our institution for the application of carbon ions has been unique, and no radiobiological data using pancreatic cancer cells has been available yet. The aim of this study was to evaluate cytotoxic effects that can be achieved by treating pancreatic cancer cell lines with combinations of X-rays and gemcitabine, or alternatively with carbon ion irradiation and gemcitabine, respectively. Materials and Methods: Human pancreatic cancer cell lines AsPC-1, BxPC-3 and Panc-1 were irradiated with photons and carbon ions at various doses and treated with gemcitabine. Photon irradiation was applied with a biological cabin X-ray irradiator, and carbon ion irradiation was applied with an extended Bragg peak (linear energy transfer (LET) 103 keV/μm) using the raster scanning technique at the Heidelberg Ion Therapy Center (HIT). Responsiveness of pancreatic cancer cells to the treatment was measured by clonogenic survival. Clonogenic survival curves were then compared to predicted curves that were calculated employing the local effect model (LEM). Results: Cell survival curves were calculated from the surviving fractions of each combination experiment and compared to a drug control that was only irradiated with X-rays or carbon ions, without application of gemcitabine. In terms of cytotoxicity, additive effects were achieved for the cell lines Panc-1 and BxPC-3, and a slight radiosensitizing effect was observed for AsPC-1. Relative biological effectiveness (RBE) of carbon ion irradiation ranged from 1.5–4.5 depending on survival level and dose. Sensitizer enhancement ratio (SER) values calculated at 10% cell survival ranged from 1.24–1.66, depending on cell line, gemcitabine dose and irradiation modality. Experimentally ascertained survival curves matched those predicted by LEM-calculation. Conclusion: Our experiments have shown a combined treatment of irradiation and chemotherapy with gemcitabine to be a good means of achieving additive cytotoxic effects on pancreatic cancer cell lines. The data generated in this study will serve as radiobiological basis for further preclinical and clinical studies.
Keywords: carbon ion radiotherapy, pancreatic cancer, gemcitabine, RBE, local effect model (LEM)
INTRODUCTION
The pancreas is the tenth most common site of new cancers, and pancreatic cancer is the fourth leading cause for cancer deaths, being responsible for 6% of all cancer-related deaths [1]. The only potential cure has been surgery, and as the cancer is very hard to detect in an early stage, only 15–20% of the tumors are diagnosed in a resectable state. Even after undergoing an R0-resection, prognosis is still very poor, so that all patients are expected to eventually die from the disease [2].
Conventional radiotherapy, with or without additional chemotherapy, has been applied in the past in the context of neoadjuvant or adjuvant therapy concepts with no clear consensus in favor of one particular concept and with only modest results [3]. In those approaches photon radiotherapy and chemotherapy based on gemcitabine or 5-FU have been employed as leading therapeutic modalities. Recent works from our department have shown that neoadjuvant gemcitabine-based chemoradiation improves resectability and benefits overall survival in locally advanced pancreatic cancer [4]. However problems remain, including biological radioresistance in many cases, and limitations due to low radiotolerance of the surrounding organs.
New radiation modalities such as particle radiotherapy might offer the means of overcoming those obstacles. Due to the physical characteristics of high-LET particle radiation (such as protons or carbon ions) the maximum dose deposition occurs at the so-called Bragg peak, the depth of which can be altered within the target tissue. Based on this custom dose profile it should be possible to more accurately administer maximum doses to the tumor with minimum adverse effects to the surrounding tissue [5–7]. Additionally, particle radiation has been proven to have a higher relative biological effectiveness (RBE) achieving an increased cytotoxic effect when compared with conventional photon therapy, most likely due to the increased number of DNA double-strand breaks it generates [8–9].
At Heidelberg Ion-Beam Therapy Center (HIT) carbon ion irradiation has been performed successfully since 2009 on an increasing number of different tumor entities, including skull base chordomas and chondrosarcomas, salivary gland tumors and gliomas with good results and generally low toxicities [10–17]. Clinical trials are currently evaluating the potential for—among others—abdominal tumors such as hepatocellular carcinoma (HCC) and have shown first promising results [18].
It was the aim of this study thus to combine the promising radiobiological characteristics of carbon ion irradiation with the chemotherapeutic agent gemcitabine, which has already shown promising results in combination with photon irradiation, and to evaluate and compare the effect of combined chemo- and carbon ion radio-chemotherapy with conventional gemcitabine-based chemoradiation on pancreatic cancer cell lines in vitro. Furthermore, the experimentally ascertained clonogenic survival curves were to be compared to predicted curves that were calculated employing the local effect model (LEM), currently in use and under further development for biological plan optimization at our institution.
MATERIALS AND METHODS
Reagents and cell culture
The pancreatic cancer cell lines AsPC-1, BxPC-3 and Panc-1 were obtained via the American Type Culture Collection (ATCC, Manassas, VA, USA). The BxPC-3 and AsPC-1 cell lines were cultivated in RPMI media, whereas Panc-1 cells were cultivated in DMEM media. To all media 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin were added. Cells were kept at 37°C with 5% CO2 and 95% humidity. Mycoplasma screening was performed at regular intervals by a dedicated laboratory.
Photon radiotherapy
Photon irradiation was performed with a biological cabinet X-ray irradiator (XRAD 320 Precision X-ray Inc., N. Bradford, CT) at single doses of 2, 4, 6 and 8 Gy. Radiotherapy was performed at room temperature.
Carbon ion radiotherapy
Carbon ion radiotherapy was performed at the Heidelberg Ion-Beam Therapy Center with the horizontal beamline using the raster scanning technique developed by Haberer et al. [19]. Single doses of 0.125, 0.5, 1, 2 and 3 Gy were delivered with an extended Bragg peak (dose average LET, 103 keV/μm) that was adjusted using a 3.5-cm acrylic shield and positioning cell monolayers in the middle of the extended Bragg peak.
Treatment with gemcitabine
Chemotherapeutic treatment was performed by applying gemcitabine at different doses to the adherent cells and incubating them for 4 h, followed by a media change to stop substance exposition. Single doses of 10, 20, 50, 70, 100 and 200 nM were applied.
Clonogenic assay
Clonogenic survival essays were performed to evaluate the efficacy of the applied treatment and to measure cell death. Prior to treatment an increasing number of cells (from 500–6500 cells, varying in accordance with the dosage to be applied) were seeded into 25 cm2 flasks (Becton Dickinson, Heidelberg, Germany) as described previously [20]. After a period of 24 h for the cells to adhere to the flask, treatment was performed. Afterwards, the flasks containing the treated cells were incubated for a number of days ranging from 8–13 days, depending on the cell line, for clonogenic cell death to occur and the surviving cells to form colonies. For cell fixation 4 ml of 70% ethanol was then applied, followed by 5 ml of methylene blue for 10 min for staining. Counting the colonies formed by surviving cells was performed under the microscope with a threshold of 50 cells per colony for a colony to be considered surviving. From the so-determined surviving fractions plating efficiency and clonogenic survival were calculated, which were in turn used to generate clonogenic survival curves, to determine α- and β-parameters, and to calculate the RBE. Using triplicates of each dose, each experiment was repeated independently on at least two different days. Mean values were calculated only from independent experiments.
LEM calculation
The LEM-calculation was performed in three steps. First—as for the generation of clonogenic survival curves described above—the α- and β-parameters were determined by employing non-linear fits [21] to the survival data generated by photon irradiation. The second step comprised the generation of tables of energy-adjusted initial RBE-values for the different fragments generated by carbon ion irradiation by means of an LEM-IV-calculation similar to [22]. Finally, using the calculated initial RBE-values, the biologically applied dose and the resulting cell survival were ascertained by employing the treatment-planning system for ion therapy (TRiP) [23] and used to generate LEM-predicted cell-survival curves.
RESULTS
Clonogenic survival and RBE after carbon versus photon irradiation and gemcitabine treatment
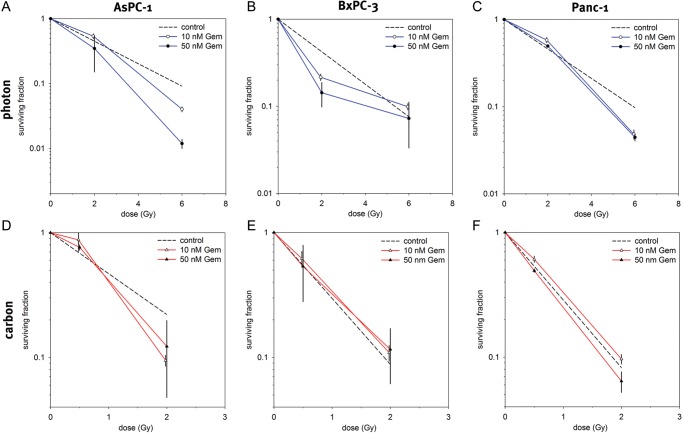
The surviving fractions determined by single-treatment experiments were used to perform linear quadratic fits (LQ-fits) and calculate clonogenic survival curves for each cell line in order to compare the effectiveness of photon and carbon ion radiotherapy. AsPC-1, BxPC-3 and Panc-1 cells showed a dose-dependent suppression of the survival fraction after both photon and carbon ion treatment. The survival fractions for the carbon ion radiotherapy treated cells were lower than those for the photon-irradiated groups. Carbon ion radiotherapy showed an enhanced efficacy in the three different pancreatic cancer cell lines as compared with standard photon radiotherapy (Fig. 1, Table 1).
Fig. 1.
Single-treatment survival curves for AsPC-1 (A), BxPC-3 (B) and Panc-1 (C). Carbon ion radiotherapy showed an enhanced cytotoxic effect as compared with standard photon radiotherapy.
Table 1.
α- and β-values from the LQ-fitting performed in order to generate clonogenic survival curves
| carbon |
photon |
|||||
|---|---|---|---|---|---|---|
| cell line | α | β | α/β-ratio | α | β | α/β-ratio |
| AsPC-1 | 0.871 5 | 0.184 1 | 4.73 | 0.190 1 | 0.010 3 | 18.46 |
| BxPC-3 | 0.900 7 | 0.198 7 | 4.53 | 0.278 1 | 0.019 4 | 14.34 |
| Panc-1 | 0.997 6 | 0.229 6 | 4.34 | 0.292 3 | 0.021 0 | 13.92 |
α/β-ratio was considerably lower with carbon ion irradiation.
A comparison of the experimentally determined survival fractions with those predicted by LEM-calculation yielded a satisfactory match of experimentally determined and predicted cell survival, as is shown in Fig. 2.
Fig. 2.

LEM calculation for cells AsPC-1 (A), BxPC-3 (B) and Panc-1 (C). The LEM-prediction for carbon ion survival showed a good match with our experimentally ascertained data.
From the linear quadratic fits (LQ-fits) that were performed, the RBE for carbon ion radiotherapy was determined. The calculated RBE ranged from 1.5–4.5, depending on cell line and survival level (Fig. 3). Treatment with gemcitabine also showed a dose-dependent suppression of survival level in which Panc-1 proved least responsive to gemcitabine treatment, especially on the low-dose end (Fig. 4).
Fig. 3.
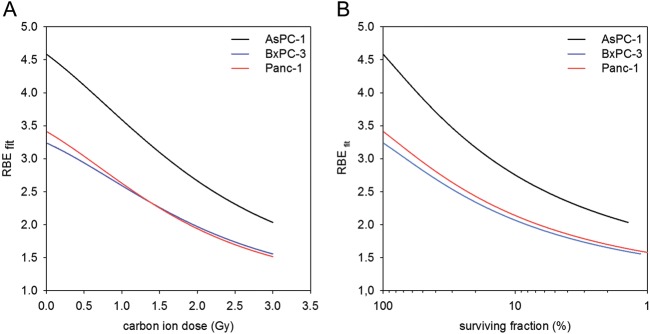
Relative biological effectiveness for carbon ion radiotherapy (calculated from LQ-fits) as a function of carbon ion dose (A) and survival level (B). The RBE ranges from 1.5–4.5 depending on survival level and dose.
Fig. 4.

Surviving fractions after treatment with gemcitabine. Panc-1 cells proved least responsive to the treatment compared to the other cell lines.
Evaluation of the cytotoxic effect of combined chemo- and radiotherapy
For combination experiments two doses each of photon irradiation, carbon irradiation and gemcitabine that achieved moderate and severe cell kill were chosen and combined with each other. Doses of 2 and 6 Gy of photon irradiation, 0.5 and 2 Gy of carbon ion irradiation and 10 and 50 nM of gemcitabine were used. Surviving fractions for the samples that underwent combination treatment were calculated and normalized to a drug control sample that was treated with only the respective dose of gemcitabine. The normalized fractions were then compared with another control sample that was only irradiated with X-rays or carbon ions, respectively, but without the application of gemcitabine (Fig. 5). In terms of cytotoxicity, additive effects were achieved for BxPC-3 cells by combining each photon and carbon irradiation with gemcitabine. In AsPC-1 cells, gemcitabine treatment showed a slight radiosensitizing effect for photon as well as for carbon ion irradiation. In Panc-1 cells the radiosensitizing effect could be observed only for photon irradiation. Sensitizer enhancement ratio (SER) values were calculated at 10% cell survival for AsPC-1 (photon and carbon irradiation) and Panc-1 (photon irradiation only). SER values ranged from 1.24–1.66 depending on cell line, gemcitabine dose and applied irradiation (Table 2).
Fig. 5.
Surviving fractions after combination of gemcitabine treatment with photon irradiation (A–C) and carbon ion irradiation (D–F). As the coloured curves have been normalized to a drug control sample, the decline in survival shown is to be attributed to irradiation only, letting a survival fraction identical to the control indicate additive (independent) toxicity, whereas a survival fraction lower than that of the control indicates a superadditive (radiosensitizing) effect for the chemotherapeutic agent. A slight radiosensitizing effect for gemcitabine could be observed in AsPC-1 for both carbon and photon irradiation and in Panc-1 for only photon irradiation.
Table 2.
Sensitizer enhancement ratio values determined from combination experiments
| Cell line | Gemcitabine dose | SER (10 % survival) |
|---|---|---|
| Photon irradiation | ||
| AsPC-1 | 10 nM | 1.27 |
| 50 nM | 1.66 | |
| Panc-1 | 10 nM | 1.56 |
| 50 nM | 1.35 | |
| Carbon irradiation | ||
| AsPC-1 | 10 nM | 1.24 |
| 50 nM | 1.27 | |
SER values ranged from 1.27–1.66 depending on cell line, gemcitabine dose and irradiation modality.
DISCUSSION
This study has evaluated in vitro the cytotoxic effect of combined carbon ion irradiation and gemcitabine-based chemotherapy on pancreatic cancer cell lines and compared it with the effect of a similar combined therapy employing conventional photon irradiation. It was performed in preparation for clinical trials soon to be conducted at our institution to investigate the potential of carbon ion irradiation for pancreatic cancer. In two out of three cell lines (BxPC-3 and Panc-1) additive effects for gemcitabine and carbon ion irradiation could be achieved as well as a slight radiosensitizing effect for the third cell line AsPC-1.
Clinical trials conducted in our department have already proven the great potential of carbon ion irradiation, especially in tumors where it is crucial to accurately apply high doses with a higher biological efficacy on a specific target volume without adversely affecting critical risk organs in the immediate surroundings, such as is the case for several tumors of the head and neck (salivary gland tumors, skull base chordoma and chondrosarcoma) [16, 24–25]. Current studies are evaluating the potential of carbon ion irradiation for other indications for which the above-mentioned precision and higher RBE are comparably crucial, such as various gliomas as well as HCC [11–13]. The adenocarcinoma of the pancreas is another entity with similar characteristics, being only unsatisfactorily treatable with photon irradiation and in the immediate neighborhood of risk organs with a low radiotolerance.
Consequently, clinical trials to evaluate carbon ion radiotherapy for pancreatic cancer are already being conducted at other institutions. Shinoto and colleagues have recently found out in a phase I study that preoperative, short-course carbon ion irradiation followed by surgery is feasible and tolerable, without unacceptable morbidity, and shows promising results for local tumor control [26]. Similar studies are on their way at our institution. However in order to estimate the impact of our different beam application technique with the raster scanning technique being employed at HIT, it is vital to conduct in vitro studies first, which has been one purpose of this evaluation. Additionally, having already shown that a combination of gemcitabine chemotherapy and photon irradiation benefits resectability [4], it was another aim to evaluate the effects of a combination of gemcitabine and carbon ions and in this study we have found out that additive effects, as well as a slight radiosensitizing effect in one cell line, can be achieved for this combination. Similar effects have already been confirmed at our department for glioblastoma cell lines using a combination of carbon ion irradiation and several chemotherapeutic agents including gemcitabine [27].
On the radiobiological end, our findings conform to the data recently published by Matsui and colleagues who showed that cell-killing effects of carbon ion beams on pancreatic cells are dependent on LET. Since our beam at 103 keV/μm has a slightly higher LET than that employed by Matsui and colleagues, we have been able to achieve a slightly steeper dose-dependent decrease of the surviving fractions for our carbon ion irradiated samples, as well as a comparable D10 RBE [28]. Recent data published by Oonishi and colleagues confirms the increased RBE of carbon ion irradiation for the pancreatic cancer cell line BxPC-3 and goes further, suggesting that carbon ions have a higher potential of killing cancer stem-cell like cells by prolonged induction of DNA double-strand breaks [29].
In addition to the higher RBE proven for carbon ion irradiation, recent studies at our institution have been able to detect further potentially promising effects of carbon ion irradiation on glioma cells. Rieken and colleagues have found that whereas photon irradiation enhanced ανβ3 and ανβ5 integrin expression and caused tumor cell hypermigration on both vitronectin and fibronectin, carbon ion irradiation suppressed integrin expression and inhibited cell migration [30]. The cell migration-inhibiting effect of carbon ion irradiation has in principle—although with another molecular focus—been confirmed by Fujita and colleagues for pancreatic cancer cell lines AsPC-1 and BxPC-3, and at the same time an increased invasiveness was shown for Panc-1 [31]. Additional potentially promising effects of carbon ion irradiation on pancreatic cancer cell lines on the cellular and molecular level shall be examined in further experimental studies.
The results and data generated by the current study will serve as basis for future experimental and clinical works, being the first to investigate and quantify the effect of the commonly employed chemotherapeutic agent gemcitabine when combined with carbon ion irradiation. With respect to the LEM that is being applied in Heidelberg for biological plan optimization in carbon ion radiotherapy and is under a process of constant further development [22], it is essential to generate radiobiological data that considers all agents eligible for use in future treatment prior to conducting clinical trials, as has herewith been done for gemcitabine and carbon ion irradiation in pancreatic cancer cells. Our results show that cell survival as predicted by LEM-calculation matches the results of radiobiological experiments, and so further confirms the accuracy of the model and its value for biological plan optimization in carbon ion radiotherapy.
CONCLUSION
In conclusion, a combination of gemcitabine and carbon ion radiation produces additive effects for pancreatic cancer cell lines BxPC-3 and Panc-1 and a slight radiosensitizing effect for AsPC-1. The data generated by this study will serve as a radiobiological basis for further preclinical and clinical studies.
FUNDING
This work was supported in part by the EU-Funded PARTNER project under FP7.
ACKNOWLEDGEMENTS
We thank Ass. Prof. Dr Amir Abdollahi and his group for providing us with the cell lines.
REFERENCES
- 1.Olson SH, Kurtz RC. Epidemiology of pancreatic cancer and the role of family history. J Surg Oncol. 2012;107:1–7. doi: 10.1002/jso.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 4.Habermehl D, Kessel K, Welzel T, et al. Neoadjuvant chemoradiation with Gemcitabine for locally advanced pancreatic cancer. Radiat Oncol. 2012;7:28. doi: 10.1186/1748-717X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedrich T, Scholz U, Elsasser T, et al. Calculation of the biological effects of ion beams based on the microscopic spatial damage distribution pattern. Int J Radiat Biol. 2012;88:103–7. doi: 10.3109/09553002.2011.611213. [DOI] [PubMed] [Google Scholar]
- 6.Scholz M, Kraft G. Track structure and the calculation of biological effects of heavy charged particles. Adv Space Res. 1996;18:5–14. doi: 10.1016/0273-1177(95)00784-c. [DOI] [PubMed] [Google Scholar]
- 7.Krämer M, Weyrather WK, Scholz M. The increased biological effectiveness of heavy charged particles: from radiobiology to treatment planning. Tech Canc Res Treat. 2003;2:427–36. doi: 10.1177/153303460300200507. [DOI] [PubMed] [Google Scholar]
- 8.Heilmann J, Taucher-Scholz G, Haberer T, et al. Measurement of intracellular DNA double-strand break induction and rejoining along the track of carbon and neon particle beams in water. Int J Radiat Oncol Biol Phys. 1996;34:599–608. doi: 10.1016/0360-3016(95)02112-4. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Eguchi-Kasai K, Sato K, et al. Differences in heavy-ion-induced DNA double-strand breaks in a mouse DNA repair-deficient mutant cell line (SL3-147) before and after chromatin proteolysis. J Radiat Res. 1995;36:258–64. doi: 10.1269/jrr.36.258. [DOI] [PubMed] [Google Scholar]
- 10.Rieken S, Habermehl D, Haberer T, et al. Proton and carbon ion radiotherapy for primary brain tumors delivered with active raster scanning at the Heidelberg Ion Therapy Center (HIT): early treatment results and study concepts. Radiat Oncol. 2012;7:41. doi: 10.1186/1748-717X-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combs SE, Habermehl D, Ganten T, et al. Phase I study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: the PROMETHEUS-01 trial. BMC Cancer. 2011;11:67. doi: 10.1186/1471-2407-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combs SE, Burkholder I, Edler L, et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: the CINDERELLA trial. BMC Cancer. 2010;10:533. doi: 10.1186/1471-2407-10-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Combs SE, Kieser M, Rieken S, et al. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: the CLEOPATRA trial. BMC Cancer. 2010;10:478. doi: 10.1186/1471-2407-10-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Combs SE, Edler L, Burkholder I, et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. 2010;10:615. doi: 10.1186/1471-2407-10-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen AD, Nikoghosyan A, Hinke A, et al. Combined treatment of adenoid cystic carcinoma with cetuximab and IMRT plus C12 heavy ion boost: ACCEPT [ACC, Erbitux® and particle therapy] BMC Cancer. 2011;11:70. doi: 10.1186/1471-2407-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen AD, Nikoghosyan AV, Ecker S, et al. Raster-scanned carbon ion therapy for malignant salivary gland tumors: acute toxicity and initial treatment response. Radiat Oncol. 2011;6:149. doi: 10.1186/1748-717X-6-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieken S, Habermehl D, Nikoghosyan A, et al. Assessment of early toxicity and response in patients treated with proton and carbon ion therapy at the Heidelberg ion therapy center using the raster scanning technique. Int J Radiat Oncol Biol Phys. 2011;81:e793–801. doi: 10.1016/j.ijrobp.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Combs SE, Kieser M, Habermehl D, et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: the PANDORA-01 trial. BMC Cancer. 2012;12:137. doi: 10.1186/1471-2407-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haberer T, Becher W, Schardt D, et al. Magnetic scanning system for heavy ion therapy. Nucl Instrum Meth A. 1993;330:296–305. [Google Scholar]
- 20.Abdollahi A, Lipson KE, Sckell A, et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res. 2003;63:8890–8. [PubMed] [Google Scholar]
- 21.CN/ASD Group. MINUIT – Users Guide, Program Library D506. CERN. 1993 [Google Scholar]
- 22.Elsasser T, Weyrather WK, Friedrich T, et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int J Radiat Oncol Biol Phys. 2010;78:1177–83. doi: 10.1016/j.ijrobp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Krämer M, Scholz M. Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol. 2000;45:3319–30. doi: 10.1088/0031-9155/45/11/314. [DOI] [PubMed] [Google Scholar]
- 24.Combs SE, Kalbe A, Nikoghosyan A, et al. Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother Oncol. 2011;98:63–7. doi: 10.1016/j.radonc.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Schulz-Ertner D, Haberer T, Jäkel O, et al. Radiotherapy for chordomas and low-grade chondrosarcomas of the skull base with carbon ions. Int J Radiat Oncol Biol Phys. 2002;53:36–42. doi: 10.1016/s0360-3016(01)02827-9. [DOI] [PubMed] [Google Scholar]
- 26.Shinoto M, Yamada S, Yasuda S, et al. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer. 2012;119:45–51. doi: 10.1002/cncr.27723. [DOI] [PubMed] [Google Scholar]
- 27.Combs SE, Zipp L, Rieken S, et al. In vitro evaluation of photon and carbon ion radiotherapy in combination with chemotherapy in glioblastoma cells. Radiat Oncol. 2012;7:9. doi: 10.1186/1748-717X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui Y, Asano T, Kenmochi T, et al. Effects of Carbon-ion beams on human pancreatic cancer cell lines that differ in genetic status. Am J Clin Oncol. 2004;27:24–8. doi: 10.1097/01.coc.0000046037.75545.ad. [DOI] [PubMed] [Google Scholar]
- 29.Oonishi K, Cui X, Hirakawa H, et al. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother Oncol. 2012;105:258–65. doi: 10.1016/j.radonc.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Rieken S, Habermehl D, Wuerth L, et al. Carbon ion irradiation inhibits glioma cell migration through downregulation of integrin expression. Int J Radiat Oncol Biol Phys. 2012;83:394–9. doi: 10.1016/j.ijrobp.2011.06.2004. [DOI] [PubMed] [Google Scholar]
- 31.Fujita M, Otsuka Y, Imadome K, et al. Carbon-ion radiation enhances migration ability and invasiveness of the pancreatic cancer cell, PANC-1, in vitro. Cancer Sci. 2012;103:677–83. doi: 10.1111/j.1349-7006.2011.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]