Abstract
Patients with immunodeficiency disorders have an increased incidence of lymphoproliferative disorders; however, only 4 such patients with DiGeorge/chromosome 22q11.2 deletion syndrome have been reported. We report a case of a pulmonary Epstein-Barr virus–negative extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in a child with this syndrome.
The patient is a 15-year-old female with chromosome 22q11.2 deletion/DiGeorge syndrome diagnosed at age 9 years, documented by cytogenetic fluorescence in situ hybridization studies. She has cardiovascular defects with congenital ventricular septal defect, patent ductus arteriosus, and right-sided aortic arch, hypoparathyroidism, subtle dysmorphic facial features, immunodeficiency (including low peripheral CD4+ T cell count, hypogammaglobulinemia, frequent recurrent pneumonias and other infections), and Evan syndrome diagnosed at the age of 3 years. She also has chronic eczema and lichen planus. Currently, she is maintained on amoxicillin and weekly to monthly gamma globulin.
Approximately 2 years ago, the patient presented with fever, chronic cough, and progressive respiratory symptoms. Chest radiograph revealed lung consolidation thought to represent pneumonia. Antibiotic therapy produced some improvement; however, follow-up chest radiograph remained abnormal, and a chest computed tomography (CT) scan showed consolidation involving the right lower lobe and multiple scattered bilateral pulmonary nodules. Pulmonary function testing revealed restrictive changes. Analysis of bronchoalveolar lavage fluid was nondiagnostic.
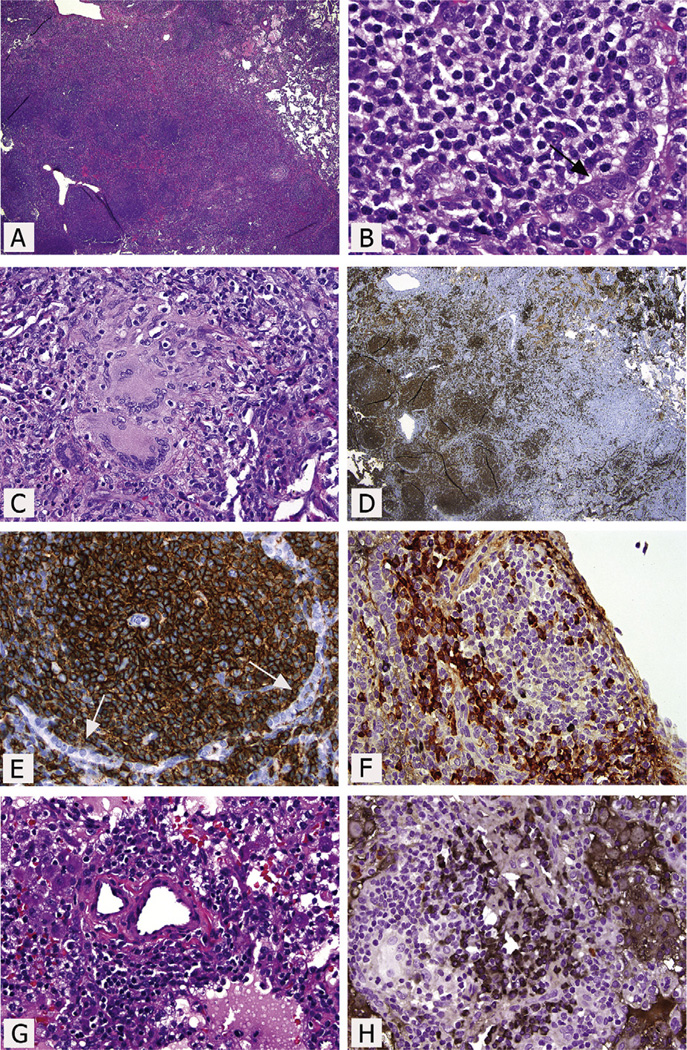
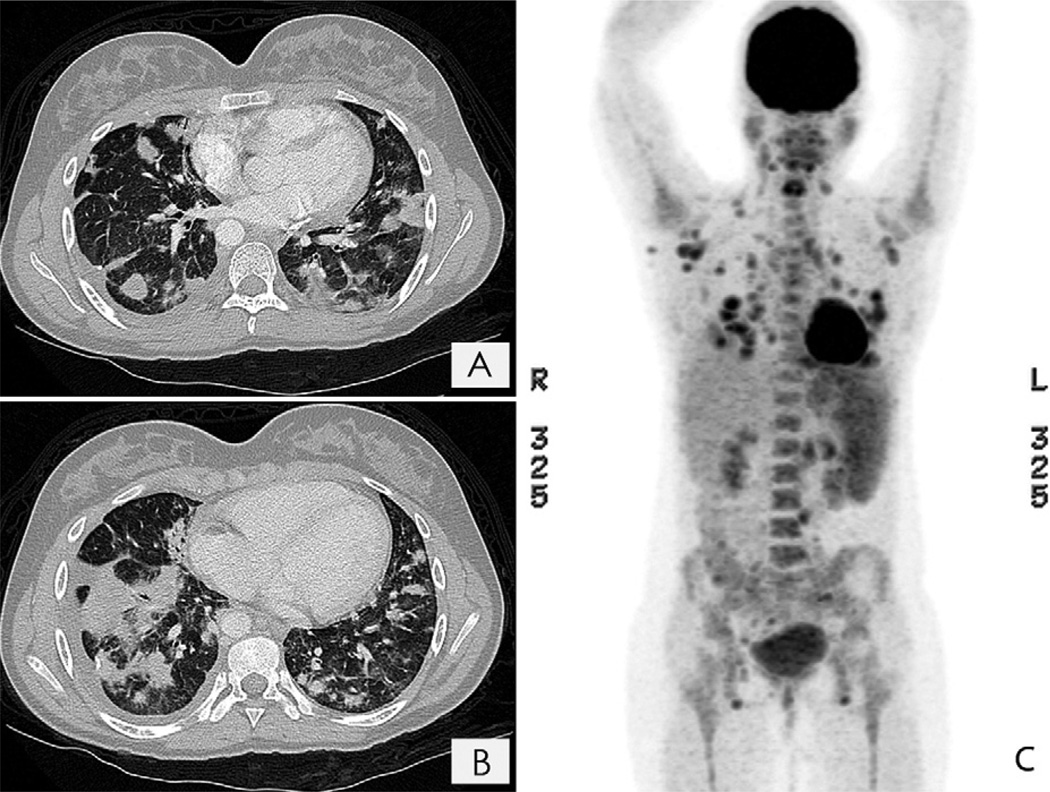
Three months later, progressive enlargement of the pulmonary nodules was noted. The patient underwent thoracoscopic wedge biopsies, which were initially diagnosed as inflammation and granulomatous disease. Two months later, a repeat chest CT showed that the pulmonary nodules had increased in size. Pathological re-review of the more abnormal right lower lobe biopsy specimen revealed an extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma with plasmacytic differentiation (Figure 1, A–C). Elsewhere a patchier infiltrate, granulomas with negative acid-fast and Grocott staining, prominent intraalveolar macrophages, and some interstitial and pleural fibrosis were seen. Immunohistochemistry analysis identified many CD20+ B cells in nodules; variable numbers of internodular CD5−, CD10−, BCL6−, CD43−, cyclin D1−, and BCL2+ B cells; IgM+ λ light chain-restricted plasma cells in the main mass, and scattered CD3+ T cells that were focally more numerous (CD4>CD8) (Figure 1, D–F). Elsewhere there were polytypic plasma cells and 2 small foci that appeared to be κ light chain-restricted (Figure 1, G and H). Epstein-Barr virus (EBV)-encoded RNA in situ hybridization for EBV and human herpesvirus 8 immunohistochemistry were negative. Polymerase chain reaction–based immunoglobulin heavy chain and T-cell receptor gene rearrangement analyses using BIOMED-2 protocols supported the presence of a monoclonal B-cell population but polyclonal T cells.1 Staging of bone marrow, including flow cytometry studies, showed nonnecrotizing granuloma, but no evidence of lymphoma. Staging positron emission tomography (PET)/CT imaging showed extensive hypermetabolic lymphadenopathy and numerous hypermetabolic pulmonary nodules (Figure 2; available at www.jpeds.com).
Figure 1.
Pulmonary wedge excision of the right lower lobe. A, Mass-like dense infiltrate with scattered follicular-type structures. B, Predominantly small lymphoid cells, including some with monocytoid and plasmacytoid features, infiltrating the epithelium. Note the residual epithelial remnants (arrow). Other focal areas show numerous plasma cells. C, Epithelioid granulomas with Langhans giant cells. D, Numerous CD20+ cells in the follicles and a moderate number in the interfollicular regions. E, At higher magnification, numerous CD20+ small lymphocytes in and around the follicle, also surrounding the residual CD20− epithelial cells (arrows). F, Double immunostaining for κ and λ immunoglobulin light chains showing numerous λ+ plasma cells (red/brown), but only rare κ+ plasma cells (black). The λ restriction was confirmed by κ and λ single immunostains and in situ hybridization stains. The plasma cells were IgG−, IgA−, IgM+, and IgD−. G, Small aggregates of small lymphocytes and plasma cells away from the main mass. H, In contrast to the main mass, the plasma cells here show κ light chain restriction in this double-immunohistochemical stain. (A, B, C, and G, hematoxylin and eosin staining; D and E, immunoperoxidase staining for CD20; F and H, double-immunoperoxidase staining for κ and λ immunoglobulin light chains).
Figure 2.
A and B, Chest CT scans showing multiple nodules throughout the lung parenchyma. C, Fluorodeoxyglucose PET showing extensive hypermetabolic cervical, axillary, mediastinal, abdominal, and pelvic lymphadenopathy, along with diffusely increased bone marrow, splenic, and gastric activity.
The patient was treated with 6 cycles of rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone (R-CHOP), and achieved complete remission. Restaging bilateral bone marrow examinations were negative. Posttherapy skin biopsy analysis revealed only granulomas. Analysis of gastric biopsy specimens obtained at the time of the restaging because of high fluorodeoxyglucose activity detected on the initial PET/CT was unremarkable, with negative immunostaining for Helicobacter pylori.
The patient subsequently developed new lymphadenopathy; however, biopsy demonstrated only granulomas and florid follicular hyperplasia. At the time of this report, she has received 4 weekly doses of rituximab, with a plan to begin azathioprine once her platelet count is stable. The cause of the granulomas remains uncertain. Although sarcoidosis remains a possibility, the lack of progression with such extensive granulomatous disease does not favor that diagnosis, even recognizing that the patient received steroids and chemotherapy. Although an angiotensin-converting enzyme test was requested, it was not performed. The patient is currently receiving calcium supplementation because of her hypoparathyroidism and hypocalcemia.
Discussion
Many patients with a primary immunodeficiency disorder are at increased risk for lymphoproliferative disorders (LPDs), often EBV-associated aggressive B-cell neoplasms, although both T-cell and indolent B-cell lymphomas have been reported as well.2 However, despite a suggested increased incidence of malignancies in patients with DiGeorge syndrome,3 few cases of LPD have been reported to date. In addition to 1 patient with B-lineage lymphoblastic lymphoma, 3 4 patients with DiGeorge syndrome who developed an LPD have been reported (Table).4–7 The patient reported herein had a documented autoimmune disorder, a feature also reported in DiGeorge syndrome.8 We did not identify prior reports of MALT or any other type of low-grade lymphomas occurring in patients with DiGeorge syndrome. It is possible that other patients with indolent MALT lymphomas have gone unrecognized, given that many of the changes and the association with more extensive granulomatous and other inflammatory diseases (as seen in the present case) may be interpreted as reactive changes. Only after her progressively growing pulmonary nodules were identified was our patient diagnosed with an LPD.
Table.
Reported patients with DiGeorge syndrome and an LPD
| Ramos et al5 | Sato et al6 | Hong et al7 | Itoh et al4 | Present case | |
|---|---|---|---|---|---|
| Age at LPD diagnosis/sex | 23 months/female | 7 months/male | 14 years/female | 25 years/male | 15 years/female |
| Cytogenetics | Not done | Not done | Equivocal chromosome 22q11.2 deletion (method not specified) | Hemizygous chromosome 22q11.2 deletion (FISH) | Hemizygous chromosome 22q11.2 deletion (FISH) |
| DiGeorge syndrome features | Thymic and parathyroid aplasia; no cardiac anormaly | Dysmorphic face, hypothyroidism, TOF, thymic and parathyroid aplasia | TOF, undetectable parathyroid hormone, thymic hypoplasia | Dysmorphic face, TOF, major aortopulmonary collateral artery, VSD, markedly hypoplastic thymus and parathyroid glands | Dysmorphic face, hypoparathyroidism, VSD, PDA, right-sided aortic arch |
| Evidence of immunodeficiency | Low peripheral T-cell counts, several episodes of thrush and otitis media | Low peripheral T-cell counts, hypogammaglobulinemia | Low peripheral T-cell counts, recurrent otitis media and sinopulmonary infection (led to bronchiectasis) | Recurrent fever and diarrhea for several months | Low peripheral CD4+ T-cell counts, recurrent sinusitis and pneumonia, hypogammaglobulinemia |
| Autoimmune phenomenon | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Evans syndrome |
| Major complications | Not mentioned | Not mentioned | None | Hemophagocytic syndrome, DIC, multiorgan failure | None |
| Nature of LPD | EBV+ DLBCL involving mediastinal lymph nodes, brain, liver, and kidneys | EBV+ DLBCL involving mediastinal and mesenteric lymph nodes, lung, trachea, larynx, small intestine, and liver | EBV+ DLBCL manifested with generalized lymphadenopathy | EBV+ peripheral T-cell lymphoma (CD3+, CD5+, CD8+, CD56−, TIA1+), involving mediastinal, para-aortic, and inguinal lymph nodes | MALT lymphoma (EBV−) involving lungs and presumptive cervical, axillary, mediastinal, abdominal, and pelvic lymph nodes (no pretreatment lymph node biopsy results to document lymphoma) |
| Therapy for LPD | Refused therapy for malignancy | Not mentioned | Chemotherapy (modified French LMB 89), thymus transplantation at 8 months after complete course of chemotherapy, autologous anti-EBV cytotoxic T-cell infusion (for relapsed disease) | None | Immunochemotherapy (6 cycles of R-CHOP) |
| Outcome* | Died 1 month after diagnosis of LPD | Died 1 month after diagnosis of LPD | Relapsed 16 months after thymus transplantation but later achieved remission for at least 29 months | Died at 12 hours after admission | In complete remission at least 22 months after diagnosis of LPD, lymph node biopsy at 17 months showed only granulomas. |
DIC, disseminated intravascular coagulation; DLBCL, diffuse large B-cell lymphoma; FISH, fluorescence in situ hybridization; PDA, patent ductus arteriosus; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
All patients who died underwent autopsy.
The present case is also unusual in the sense that MALT lymphomas usually occur in immunocompetent adults, with children affected only rarely.9, 10 Moreover, they are reported only infrequently in association with immunodeficiency, with most cases EBV − , although EBV+ MALT lymphomas have been described in the posttransplantation setting.2,11–13 Pulmonary MALT lymphomas, like other MALT lymphomas, have an indolent clinical course.9 MALT lymphomas arise in the setting of acquired MALT, related to infection, autoimmune disorders, or sometimes unknown factors.14 Pulmonary MALT lymphomas have been associated with autoimmune disorders, as well as with chronic inflammatory diseases.9,15 Perhaps this patient’s immunodeficiency with resulting chronic infections led to the acquired pulmonary MALT that ultimately developed into a MALT lymphoma. The development of lymphoma also might have been fostered by a lack of immune regulation related to the patient’s chromosome 22q11.2 deletion/DiGeorge syndrome. There are also rare reports of MALT lymphomas arising in patients with sarcoidosis,16–18 although the incidence must be extremely low, given that a large population-based study identified no such cases.19 In addition, the most recent large population-based studies have not confirmed an increased incidence of lymphoma in patients with sarcoidosis.20 Finally, clinically it is not likely that this patient has sarcoidosis, given that there have been only several reports of sarcoidosis or possible sarcoidosis in patients with DiGeorge syndrome (1 with mediastinal adenopathy, 21 1 with “sarcoid dermatitis” after isoniazid therapy with a negative “sarcoidosis workup,”22 and 1 “suggestive of ocular sarcoidosis”23).
In summary, this case expands the type of LPDs that may be seen in the setting of the chromosome 22q11.2 deletion/DiGeorge syndrome, and illustrates the difficulty in diagnosing MALT lymphomas in the setting of a chronic inflammatory disorder. Although this patient was apparently treated effectively with R-CHOP, the optimal therapy for MALT lymphomas in this setting remains to be established.
Glossary
- CT
Computed tomography
- EBV
Epstein-Barr virus
- LPD
Lymphoproliferative disorder
- MALT
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue
- PET
Positron emission tomography
- R-CHOP
Rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone
Footnotes
The authors declare no conflicts of interest.
References
- 1.Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481–499. doi: 10.1097/PAS.0b013e31824433d8. [DOI] [PubMed] [Google Scholar]
- 2.van Krieken JH, Onciu M, Elenitoba-Johnson KSJ, Jaffe ES. Lymphoproliferative disorders associated with primary immune disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008. pp. 336–339. [Google Scholar]
- 3.McDonald-McGinn DM, Reilly A, Wallgren-Pettersson C, Hoyme HE, Yang SP, Adam MP, et al. Malignancy in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Am J Med Genet A. 2006;140:906–909. doi: 10.1002/ajmg.a.31199. [DOI] [PubMed] [Google Scholar]
- 4.Itoh S, Ohno T, Kakizaki S, Ichinohasama R. Epstein-Barr virus–positive T-cell lymphoma cells having chromosome 22q11.2 deletion: an autopsy report of DiGeorge syndrome. Hum Pathol. 2011:2037–2041. doi: 10.1016/j.humpath.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Ramos JT, Lopez-Laso E, Ruiz-Contreras J, Giancaspro E, Madero S. B cell non-Hodgkin’s lymphoma in a girl with the DiGeorge anomaly. Arch Dis Child. 1999;81:444–445. doi: 10.1136/adc.81.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato T, Tatsuzawa O, Koike Y, Wada Y, Nagata M, Kobayashi S, et al. B-cell lymphoma associated with DiGeorge syndrome. Eur J Pediatr. 1999;158:609. doi: 10.1007/s004310051160. [DOI] [PubMed] [Google Scholar]
- 7.Hong R, Shen V, Rooney C, Hughes DP, Smith C, Comoli P, et al. Correction of DiGeorge anomaly with EBV-induced lymphoma by transplantation of organ-cultured thymus and Epstein-Barr–specific cytotoxic T lymphocytes. Clin Immunol. 2001;98:54–61. doi: 10.1006/clim.2000.4948. [DOI] [PubMed] [Google Scholar]
- 8.Jawad AF, McDonald-Mcginn DM, Zackai E, Sullivan KE. Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) J Pediatr. 2001;139:715–723. doi: 10.1067/mpd.2001.118534. [DOI] [PubMed] [Google Scholar]
- 9.Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002;20:750–762. doi: 10.1183/09031936.02.00404102. [DOI] [PubMed] [Google Scholar]
- 10.Taddesse-Heath L, Pittaluga S, Sorbara L, Bussey M, Raffeld M, Jaffe ES. Marginal zone B-cell lymphoma in children and young adults. Am J Surg Pathol. 2003;27:522–531. doi: 10.1097/00000478-200304000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raphael M, Said J, Borisch B, Cesarman E, Harris NL. Lymphomas associated with HIV infection. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008. pp. 340–342. [Google Scholar]
- 12.Hsi ED, Singleton TP, Swinnen L, Dunphy CH, Alkan S. Mucosa-associated lymphoid tissue-type lymphomas occurring in posttransplantation patients. Am J Surg Pathol. 2000;24:100–106. doi: 10.1097/00000478-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Gibson SE, Swerdlow SH, Craig FE, Surti U, Cook JR, Nalesnik MA, et al. EBV-positive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the posttransplant setting: a distinct type of posttransplant lymphoproliferative disorder? Am J Surg Pathol. 2011;35:807–815. doi: 10.1097/PAS.0b013e3182190999. [DOI] [PubMed] [Google Scholar]
- 14.Isaacson PG, Chott A, Nakamura S, Muller-Hermelink HK, Harris NL, Swerdlow SH. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008. pp. 214–217. [Google Scholar]
- 15.Imai H, Sunaga N, Kaira K, Kawashima O, Yanagitani N, Sato K, et al. Clinicopathological features of patients with bronchial-associated lymphoid tissue lymphoma. Intern Med. 2009;48:301–306. doi: 10.2169/internalmedicine.48.1438. [DOI] [PubMed] [Google Scholar]
- 16.Cauvain A, Delmer A, Godeau MJ, Molina T, Durdux C, Marinho E, et al. MALT (mucosal-associated lymphoid tissue) lymphoma of the palpebral conjunctiva. Ann Dermatol Venereol. 2006;133:168–170. doi: 10.1016/s0151-9638(06)70871-7. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda T, Sato K, Tachikawa S, Ohnuki K, Ohtani H, Suzuki T. Mucosa-associated lymphoid tissue lymphoma coexisting with epithelioid granulomas in the stomach of a patient with systemic sarcoidosis. Pathol Int. 1997;47:870–875. doi: 10.1111/j.1440-1827.1997.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 18.Qiu L, Unger PD, Dillon RW, Strauchen JA. Low-grade mucosa-associated lymphoid tissue lymphoma involving the kidney: report of 3 cases and review of the literature. Arch Pathol Lab Med. 2006;130:86–89. doi: 10.5858/2006-130-86-LMLTLI. [DOI] [PubMed] [Google Scholar]
- 19.Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 20.Romer FK, Hommelgaard P, Schou G. Sarcoidosis and cancer revisited: a long-term follow-up study of 555 Danish sarcoidosis patients. Eur Respir J. 1998;12:906–912. doi: 10.1183/09031936.98.12040906. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb C, Li Z, Uzel G, Nussenblatt RB, Sen HN. Uveitis in DiGeorge syndrome: a case of autoimmune ocular inflammation in a patient with deletion 22q11.2. Ophthalm Genet. 2010;31:24–29. doi: 10.3109/13816810903426249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jyonouchi H, Lien KW, Aguila H, Spinnato GG, Sabharwal S, Pletcher BA. SAPHO osteomyelitis and sarcoid dermatitis in a patient with DiGeorge syndrome. Eur J Pediatr. 2006;165:370–373. doi: 10.1007/s00431-006-0082-7. [DOI] [PubMed] [Google Scholar]
- 23.Saeed A, Khan M, Irwin S, Fraser A. Sarcoidosis presenting with severe hypocalcaemia. Ir J Med Sci. 2011;180:575–577. doi: 10.1007/s11845-009-0277-9. [DOI] [PubMed] [Google Scholar]