Abstract
The classical model of metabolic regulation of blood flow in muscle tissue implies the maintenance of basal tone in arterioles of resting muscle and their dilation in response to exercise and/or tissue hypoxia via the evoked production of vasodilator metabolites by myocytes. A century-long effort to identify specific metabolites responsible for explaining active and reactive hyperemia has not been successful. Furthermore, the metabolic theory is not compatible with new knowledge on the role of physiological radicals (e.g., nitric oxide, NO, and superoxide anion, O2−) in the regulation of microvascular tone. We propose a model of regulation in which muscle contraction and active hyperemia are considered the physiologically normal state. We employ the “bang-bang” or “on/off” regulatory model which makes use of a threshold and hysteresis; a float valve to control the water level in a tank is a common example of this type of regulation. Active bang-bang regulation comes into effect when the supply of oxygen and glucose exceeds the demand, leading to activation of membrane NADPH oxidase, release of O2− into the interstitial space and subsequent neutralization of the interstitial NO. Switching arterioles on/off when local blood flow crosses the threshold is realized by a local cell circuit with the properties of a bang-bang controller, determined by its threshold, hysteresis and dead-band. This model provides a clear and unambiguous interpretation of the mechanism to balance tissue demand with a sufficient supply of nutrients and oxygen.
Keywords: metabolic regulation, nitric oxide, on/off regulation, oxygen, superoxide anion
INTRODUCTION
Despite more than a century of research efforts, the mechanism of functional adaptation of local blood flow in cardiac and skeletal muscle to a multi-fold change of the metabolic rate remains an unresolved problem of physiology (234). The intercellular signal that increases local blood flow in response to the onset of contraction in muscle fibers and reduces blood flow to baseline level during the transition back to rest is still unknown. Currently, there is consensus on the fundamental role of oxygen in the change of vascular resistance in the microvascular network of a muscle (48). However, there is no agreement on the signaling mechanism that implements matching supply and demand over the wide dynamic range of tissue respiration in skeletal muscle. A clear understanding of the principle of local blood flow regulation in vital organs is a key to the prevention and correction of cardiovascular pathologies. Modern cardiovascular texts present a model of intercellular communication, which represents the historical consensus and provides a mnemonic scheme to help organize the disparate knowledge in this area.
The metabolic theory of local blood flow regulation, sometimes called the metabolic hypothesis, is a century-old descriptive model. According to this theory, dioxygen is a limited resource in the tissue and the rate of oxygen supply to cells depends on the intensity of tissue respiration. Therefore, the regulation of local blood flow in muscles is a way of maintaining an adequate oxygen flux to the mitochondria in muscle fibers. Thus, the structure and dynamic behavior of the microvascular system in an organ is determined by satisfying the task of adequate oxygen delivery. Usually, the hypothesis is explained by the example of the regulation of local blood flow in skeletal muscle, having a regular parallel arrangement of myocytes and capillaries, but the generality of this mechanism is implied for most organs and tissues. In order to avoid using the term "autoregulation," traditionally reserved for the mechanism of stabilization of organ blood flow in response to variation in the input blood pressure (132), we will use the terms “local regulation” and “supply and demand balance” or “matching supply and demand in tissue” for the mechanism responsible for adaptation of microcirculatory perfusion in conformity with the intensity of muscle contractions and changes in metabolic rate.
According to textbooks, oxygen is delivered to capillaries by red blood cells and then transferred by diffusion into a "tissue cylinder" surrounding each capillary. In resting muscle oxygen consumption is low and most of the capillaries are not perfused, so that the average radius of the tissue cylinder is relatively large. With the onset of contractile activity by muscle, oxygen consumption by myocytes increases dramatically and the amount of oxygen delivered to the periphery of the tissue cylinder is no longer sufficient for active cell metabolism. A hypothetical biochemical process that is sensitive to hypoxia begins to produce one or more low molecular weight chemical messengers (metabolites), which are exported from muscle fibers and reach smooth muscle cells in “pre-capillary sphincters” and terminal arterioles, causing their relaxation. This opens previously closed capillaries to blood flow and reduces the average radius of the tissue cylinders, thereby reducing the diffusion distances for oxygen transport from capillaries to mitochondria in cells. The blood flow increase in the capillary network is ensured by increasing the total number of the capillaries, rather than by increasing the linear velocity of red blood cells moving through them. In other words, the residence time of red blood cells in capillaries (duration of their deoxygenation) in this model does not decrease with an increase in perfusion. Dilation of arterioles reduces the resistance of the microvascular network to blood flow, which increases the total blood flow and oxygen delivery to tissues. Any external disturbance of organ blood flow triggers the same feedback mechanism, restoring the match between consumption of oxygen and its delivery. The term "metabolic" has two meanings: 1) the regulation of blood flow in response to changes in the rate of tissue metabolism and 2) the regulation mediated through the generation of metabolites related to the level of local hypoxia. To date, a single, exclusive metabolite has not been found, so there is general agreement on the combined action of several substances comprising the list of metabolic vasodilators.
Automatic augmentation of blood flow in response to increased activity of a group of myocytes is known as active, functional or exercise hyperemia, as opposed to reactive hyperemia, which is caused by a temporary shutdown and subsequent restoration of local perfusion. Reactive hyperemia is important for understanding pathological processes and is widely used in experimental physiology to study the regulation of local blood flow (18, 166, 235, 236). In both cases (active and reactive hyperemia) the sharp increase in organ blood flow is explained in the metabolic theory by the accumulation of tissue metabolites in the interstitial space, which implies the identity of the underlying physiological mechanisms.
Origins of the metabolic hypothesis
The metabolic hypothesis contains elements dating back to the physiological tradition of the late 19th and early 20th centuries and bears the imprint of the prevailing thought of the era. By all indications, the metabolic theory is stagnating, so it is useful to go back to its historical roots to investigate the origin of the principles of its foundations.
In the middle of the 19th century physiologists imagined the distribution of microvascular activity in the same way as modern scientists. Only microvessels containing smooth muscle in their walls were capable of constriction and dilation. Capillaries consisting predominantly of endothelial cells were considered unable to actively change their luminal caliber (235). This view was revised after the discovery of the independent contraction of capillaries in the nictitating membrane of frogs, made by Stricker (263). According to the authoritative sources of the late 19th and early 20th centuries (153, 235, 246) the active constriction of capillary walls in amphibian skin had been confirmed by other authors (58, 59, 99, 271). The work of Roy and Brown (1880) won a special place in the history of the microcirculation, combining the original bio-microscopic techniques with theoretical generalizations of the contemporary literature (235). The basic ideas and misconceptions presented in this article determined the direction of the study of local blood flow regulation for the next century. Roy and Brown formulated their findings as follows, showing that: "a) … the capillaries are contractile; b) … the individual capillaries contract or expand in accordance with the requirements of the tissue through which they pass; c) … temporary anemia is followed by dilation of the arteries, capillaries and veins; d) a mechanism whereby the degree of dilation of the vessels is regulated independently of the cerebro-spinal vasomotor centres; e) … the presence in increased amount of certain of the product of tissue change … as the cause which leads to the dilation of the vessels which results from temporary anemia; f) This action on the vessels … is more probably produced by direct action on the walls of the vessels."
Capillariomotor regulation theory of Krogh
Forty years later August Krogh added the concept of diffusive oxygen transport from the capillary into the surrounding cells to Roy and Brown’s metabolic theory. This gave a new meaning to the recruitment of reserve capillaries as a way of regulating the radius of the tissue cylinder in response to a change of its oxygen demand. In the Kroghian model an increase in local blood flow due to the increasing density of functioning capillaries can occur without a significant rise of linear velocity of red blood cells in the capillary, thus, the transit time of blood cells through the capillaries during hyperemia is not reduced significantly. Krogh gave experimental confirmation for the changing capillary density during hyperemia by in vivo observations and by the distribution of India ink injected into vascular beds, thus demonstrating the efficient performance of the proposed mechanism of flow regulation (152). In 1920 the Nobel Prize in Physiology or Medicine was awarded to August Krogh "for his discovery of the capillary motor regulating mechanism."
The fate of this theory was not very successful because of the emergence of new experimental data that did not fit into the logic of the Kroghian scheme. Shortly after the publication of Krogh’s book, The Anatomy and Physiology of Capillaries (151), doubts in the ability of the capillary wall to actively constrict and thus change the density of perfused capillaries had appeared. Experiments with an implantable chamber documented the absence of contractile activity in capillaries using intravital-microscopic observations at high optical resolution (58, 59, 246, 317). By 1940 data on the inability of the capillary wall to actively contract were accumulated and a quest for alternative ideas to salvage Krogh’s theory was started. The solution to the problem of flow control in individual capillaries was found by Lutz and Fulton (90) who discovered and described a smooth muscle structure in the capillary wall located near its origin from the arterioles. This sphincter-like muscular ring encircling the arterial end of the capillary was attributed to have the function of neuro-motor regulation of capillary blood flow. Later Zweifach (318) identified the initial muscular sections (20–30 µm) of capillaries as a separate class of microvessels, "pre-capillaries," acting like sphincters to control capillary blood flow. Interest in these "pre-capillary sphincters" was revived in 1967 after the electron-microscopic study of their ultra-structure by Rhodin (226) and after the publication of the mechano-chemical hypothesis of local blood flow regulation proposed by Honig (121). The role of pre-capillary sphincters in the management of the density of functioning capillaries was actively discussed in the 1970s (10, 113, 185, 299, 316).
Technological breakthroughs in the methods for in vivo study of the microcirculation, which occurred in the 1960s–1970s, brought up a large volume of experimental data that led to a more realistic view of the regulation of local blood flow (133). The existence of pre-capillary sphincters in skeletal muscle and their functional significance was questioned (96, 100, 101, 169) and now they receive almost no mention in the literature. However, in textbooks of physiology the pre-capillary sphincter remains a key element of the description of local regulation of blood flow via the recruitment of “reserve” capillaries which are not perfused with red blood cells under baseline conditions (34, 109).
Experimental intravital microscopic studies of active hyperemia at the level of single muscle fibers and associated capillaries, as well as studies of reactive hyperemia caused by occlusion of a single capillary and arteriole in muscles, revealed a dramatic contrast between the microcirculatory response and the behavior predicted by the theory of “capillariomotor regulation” (152). It was shown that hyperemia is mainly related to increased velocity of blood in previously perfused capillaries and an additional amount of opened capillaries (~30%) did not exceed the size of the statistical variability in capillary density (41, 122). Complete cessation of capillary blood flow in the muscle caused by its exposure to atmospheric oxygen showed the lack of pre-capillary sphincters for control of the opening of capillaries (170). Occlusion of an individual capillary, as a rule, did not evoke local hyperemia, while the occlusion of arterioles led to a marked hyperemic response in one-third of the cases (96). In another study, the density of erythrocyte-perfused capillaries could be doubled compared with resting muscle by the combined effects of oxygen-free superfusion, electrical stimulation at a rate of 8 Hz and the topical administration of 0.1 mM adenosine (150). In resting muscle about half of the capillaries contained only plasmatic flow, so the increase in erythrocyte-perfused capillaries was not the real opening of previously unperfused capillaries, but rather the appearance of red blood cells in the lumen of those capillaries (101). In recent years, debate about the possibility of recruitment of reserve capillaries reached a final round (218) with the conclusion that there was a lack of significant functional capillary reserve (219). In order to control tissue perfusion within the wide dynamic range of metabolic demand in muscle, the Kroghian mechanism requires a capillary reserve of at least 50% and such a reserve in muscles does not exist.
According to current concepts, local blood flow is controlled by an arteriole-motor mechanism, which is artificially merged with the Kroghian capillariomotor model (96, 101, 150, 198, 247). However, in Krogh’s own opinion (152) these systems are different in principle, so that such a synthesis is not justified: "Evidence is brought forward pointing to the arteriomotor and capillariomotor systems being able to act in opposite directions."
In search of metabolic vasodilators
The hypothesis of metabolic regulation gives a leading role to skeletal muscle cells that are able to produce a chemical signal that is related to their contractile activity, the degree of hypoxia, or oxygen demand in the cell. In response to the received signal, smooth muscle cell relaxation opens the arteriolar lumen proportionally to the concentration of a chemical transmitter in the interstitium. Metabolic signals of skeletal muscle activity have not been identified yet; however, several chemical mediators have traditionally been suggested, such as CO2, lactate, H+, K+ and adenosine, because each is known as a vascular smooth muscle relaxant (30, 136, 137, 247). Additional substances, including inorganic phosphate, prostaglandins and cytokines, have been proposed for this list (118, 234, 281), “a veritable laundry list of substances” (60). Since the relative importance of different mechanisms of metabolic dilation has not been established convincingly, "it is best to simply lump all of the usual suspects together" (257). According to modern ideas, all the mediators work synergistically and "there is clearly no single signaling molecule that drives the response." (247). The question “How is it possible to perform proportional regulation of local vascular tone via integration of signals from multiple heterogeneous vasodilators?” has not been discussed in the literature yet.
In parallel with the quest for mediators of vasodilation, the verification of their requirement for active and reactive hyperemia in skeletal muscle and myocardium has been carried out intensively. A substantial data set has been accumulated in this area, demonstrating preservation of the normal hyperemic response with multiple or selective blockade of the candidates for metabolic signals that are presented in the "laundry list" (137, 148, 281, 282). It is likely that the list of mediators will continue to be expanded in order to find an explanation for this paradox.
From a biological point of view the hypothesis of multiple mediators is in contradiction with the principle of parsimony. The presence of multi-molecular signals requires multiple sources for their generation in the cells, different mechanisms of trans-membrane export into the interstitial environment, several types of specific receptors in the smooth muscle cells and a computational system for the quantitative integration of these signals in the arteriolar smooth muscle. These complex tasks have to be performed under conditions of developing intracellular hypoxia at the moment of exercise onset, when increased functional activity of the muscle fibers is not yet supported by hyperemia. The question of which mechanisms provide proportionality between tissue demand and concentration of each signaling agent and which provide adequate vasodilation due to the action of multiple dilators remains unclear (137). Is such a cumbersome system capable of robust maintenance of an adequate oxygen supply to tissue (sometimes referred to as “precise matching of muscle blood flow to metabolic demand”), considering the wide dynamic range of muscle blood flow (up to a 100-fold increase over values observed at rest) (219)?
In contrast to the capillariomotor mechanism, the arteriole-motor regulation mechanism is characterized by a longer diffusion distance between the contracting myocytes, generating the metabolic chemical signal, and the vascular smooth muscles responding to this signal by relaxation (29, 41, 96, 100, 199). Due to the difference in diffusion coefficients between the various signaling molecules, the smallest signaling molecules would reach the arteriolar smooth muscle first at the onset of muscle contraction. The role of other metabolites in this case would be redundant or functionally distinct, for example, associated with long-term maintenance of dilation, i.e., "transient vasodilation" versus "maintained vasodilation" (101). Thus, the possibility exists that the regulatory pathways associated with different chemical mediators operate on different characteristic time scales (e.g., s, min, h). This suggests that the analysis of local regulation can be simplified by separating regulatory pathways on the basis of their characteristic time scales and then dealing individually with those of a particular time scale (9, 114).
Hypothetical oxygen sensors
An alternative hypothetical mechanism of regulation is based on the existence of a special oxygen sensor, which responds rapidly to changes in intracellular PO2 and translates the change in PO2 into a signal that controls arteriolar smooth muscle. The simplest hypothesis assumes a direct relationship between the smooth muscle tone of resistance vessels and oxygen tension in their wall, which causes relaxation of the arterioles following a deficiency of oxygen and ATP in the walls of arterioles (18, 63, 75, 80, 105, 217, 233). This hypothesis has not withstood direct experimental testing (100), but other candidates for the role of the oxygen sensor have been later proposed, such as O2-sensitive ion channels, NADPH-oxidase and whole mitochondria (22, 52, 292).
The sensitivity of the mitochondria and cells to oxygen supply is typically presented as a graph describing the oxygen dependence of cellular metabolism, well described by Hill’s equation (52, 97, 259, 302). These nonlinear relationships typically contain a region where oxygen consumption is relatively independent of PO2 (at high PO2’s) and a dependent region at low values of oxygen tension. The metabolic rate of isolated mitochondria and cells remains relatively independent of oxygen over a wide range of PO2, while due to the higher diffusion resistance in cells, the oxygen-dependent region of the curve is located at significantly higher PO2 than in the mitochondria (51, 117, 144, 176, 302). A suspension of isolated mitochondria is practically insensitive to PO2 above 1 mmHg (51, 97, 248, 303) and the Km (or P50) for cytochrome c oxidase is in the range 0.03–0.3 mmHg (57, 286). Isolated muscle cells show values of apparent P50 higher than those in mitochondria, yet lower than the oxygen tension in venous blood, which is close to interstitial PO2 (97, 135, 145, 227, 240). Thus, mitochondria themselves can hardly serve as oxygen sensors to monitor tissue oxygenation because of their low critical PO2 (301). The oxygen dependence of metabolism for skeletal muscle fibers in situ is characterized by a wider interval of sensitivity to oxygen than isolated cells (i.e., <1 mmHg); however, their P50 in situ (~10 mmHg) is still too low for participation in O2 sensing via diffusional restriction of oxygen transport to the mitochondria given the normal observed range of interstitial PO2 (~40–80 mmHg) in resting skeletal muscle (98, 228).
Despite problems with identification of the oxygen sensor, the sensitivity of arterioles to oxygen tension in the environment is a firmly established fact. Direct experimental evidence for the regulation of tissue oxygenation by constriction of small and terminal arterioles at the upper limits of PO2 in a superfusate were presented in 1972 for the hamster cheek pouch (74, 217). These results indicated an upper limit on the regulation of tissue oxygen tension (~80 mmHg) by means of a direct PO2 signal or a signal which is closely related to PO2. In later experiments, Duling and coworkers investigated the functional dilation of arterioles, but the results of their experiments with tissue hyperoxia demonstrated a maximum arteriolar constriction at the normal atmospheric level of PO2 and above, using a superfusion solution equilibrated with 20 to 50% oxygen (150). Hyperoxic vasoconstriction in all orders of arterioles and the associated cessation of blood flow has been reported in muscles of mammals at superfusion solution PO2’s up to 150 mmHg (170, 220, 222, 265, 266, 280). It has also been suggested that vasoconstriction could be caused by oxygen indirectly, by reducing the production of vasodilator metabolites (76, 217). However, later it was shown that the oxygen sensitivity of the arterioles was retained following removal of the parenchyma (128). It was found in microcirculatory studies that the response time of vasodilation to local electrical stimulation was 5–8 s (101) and the response time of hyperoxic vasoconstriction was 33 s (265). This fast action confirms the mechanism of signal transmission through the intercellular spaces by rapidly-diffusing low molecular weight agents.
There is an obvious difficulty with the integration of different hypotheses for oxygen sensing mechanisms using the metabolic approach in matching supply to demand in tissues. The signal for the hypothetical oxygen sensor should be dependent on the concentration of oxygen, so it has to be converted into a signal which codes the severity of hypoxia by normalization and inversion (i.e., “inversion” because the degree of hypoxia is inversely related to the degree of oxygenation), in order to represent the mismatch between required and available tissue PO2’s in this metabolic feedback system. It appears that this problem and the hypothetical mechanism for signal inversion are not presented in the available literature which discusses the metabolic hypothesis.
In summary, a technological breakthrough in intravital microscopic methods and quantification techniques led to significant advances in the study of the microcirculation. Paradoxically, none of the elements of a logically coherent theory of capillariomotor regulation of supply and demand balance was supported by unambiguous experimental evidence, while the phenomenon of local regulation of the microcirculation has been demonstrated by many studies at the level of individual myocytes and microvessels. Numerous attempts to integrate new knowledge with the traditional scheme has resulted in cumbersome "redundant" multi-signaling models, which cannot be easily combined with knowledge of the rapid and robust switching on of active and reactive hyperemia in muscular organs.
Implied proportional control for local blood flow
The reason for the discrepancies of the experiments and the Kroghian theory may lie in a longtime misunderstanding regarding the feedback control model. “On the failure of any paradigm, a return to historical and natural first principles is required” (164). It is therefore useful to turn to the origins of the metabolic hypothesis completely formulated in the last quarter of the 19th century (235, 236).
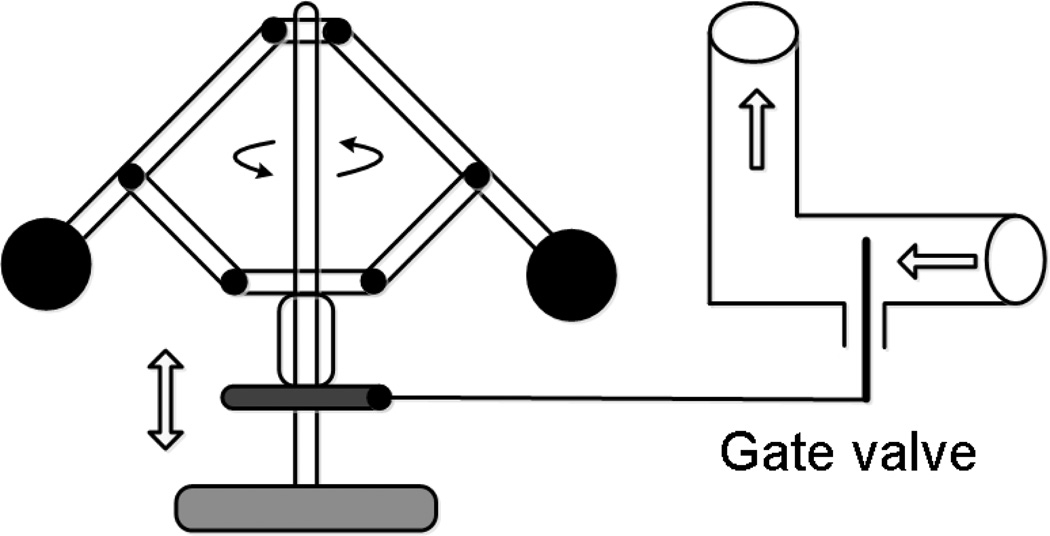
The 19th century in Europe and America was the age of the Industrial Revolution, driven by Boulton-Watt’s steam engine which converted thermal energy into mechanical energy transmitted by means of a rotating shaft. Work to improve steam engine performance led to the development of basic research in the fields of statistical physics, thermodynamics, theoretical mechanics and physical chemistry. The wide distribution of steam engines was initially prevented by the need to manually control the rotational speed of the shaft with a steam valve. The problem was solved by adopting a centrifugal governor (Fig. 1), invented by T. Mead, for automatically controlling the rotational speed in a steam engine (33). A reduction in the rotational speed was converted into an increase of the steam valve aperture. Thus, automatic stabilization of the rotational speed of the shaft was achieved. The centrifugal governor was mounted openly on the top of the steam engine, so its performance was open to public observation. Perhaps this fact caused a deep rooting of the idea of regulatory feedback in the minds of contemporaries: "On other hand, the operation of the flyball governor was clearly visible even to untrained eye, and its principle had an exotic flavor which seemed to many to embody the nature of the new industrial age. Therefore, the governor reached the consciousness of the engineering world and became a sensation throughout Europe. This was the first use of the feedback control of which there was popular awareness. "(164).
Figure 1.
Schematic diagram represents the principle of negative feedback regulation in a steam engine using the centrifugal governor. Reducing the steam flow through the valve reduces the speed of the flyballs and causes a proportional downward shift of the gate in the valve, thereby increasing the flow of steam and speed of rotation. The principle of proportional regulation via negative feedback has been transferred to physiology as a model of local blood flow regulation via metabolic vasodilators. In this metaphor, a parenchymal cell is the sensor of the oxygen delivery rate, acting as the flyballs unit, which produces the metabolic vasodilators in response to hypoxia, and the smooth muscle cells in the arteriolar wall act as the gate valve. In this model active regulation is initiated by the lack of oxygen delivery and realized by reducing the arteriolar tone. The disadvantage of this model of regulation is the permanent state of tissue hypoxia required to produce metabolites and the failure to find an elusive set of metabolic vasodilators to account for the observed vasodilation.
The principle of automatic control via negative feedback soon found application in different scientific fields, including chemistry and biology. In the historical “Ternate essay” sent to Charles Darwin in 1858, Alfred Wallace formulated the principle of biological evolution: "The action of this principle is exactly like that of the centrifugal governor of the steam engine, which checks and corrects any irregularities almost before they become evident;…” (256).
The centrifugal governor was mounted also on the top of the gearbox in the Ludwig-Baltzar kymograph, which became a world standard instrument for physiologists and psychologists since the 1870’s (276). This visual aid has contributed to the promotion of the concept of "governor" in the description of physiological regulation. A.V. Hill , Nobel laureate in physiology (1922), formulated a hypothesis of blood flow regulation: "This mechanism would tend, to some degree, to act as a “governor”, maintaining a reasonably high degree of saturation of the blood;…”(116).The development of central physiological concepts such as "the constancy of the internal environment" (Claude Bernard (207)), "the maintenance of the normal" (106) and "homeostasis" (44) might well have been directly stimulated by this material embodiment of the negative feedback regulation principle.
Biological science in the 19th century led to the confrontation between two philosophical approaches, vitalistic and mechanistic (107). A particularly strong battle occurred in physiology over the existence of a special life force or "entelechy" to control physiological functions. At that time the demonstration of a simple mechanism able to regulate the dynamic performance of a complex machine was a strong argument against vitalism.
Coming back to the hypothesis of metabolic regulation of local blood flow proposed in1880 by Roy and Brown (235) and later (1919) adapted by Krogh (152), it should be noted that it literally reproduced the principle of the flyball governor (Fig. 1). Indeed, oxygen is delivered by blood to muscle fibers via the capillaries serving as a "steam valve" which opens in response to a metabolic signal from muscle fibers to increase the supply of nutrients and oxygen for muscle contraction. In the modern version of this model the arteriole takes the role of a gate valve, opening the aperture proportionally to the magnitude of the metabolic feedback signal, thereby adjusting blood flow through capillaries and delivering an adequate supply of oxygen to muscle cells. Thus, by cross-pollination, the principle of regulation by negative feedback was transferred in the 19th century from technology to physiology. Broad recognition of the governor operation greatly facilitated the formulation of the homeostasis model and its perception by prepared scientific communities. A clear mental scheme of metabolic feedback control provided an opportunity to synthesize and organize the accumulated empirical knowledge in vascular physiology and then plant this knowledge deeply into the educational environment, where it successfully reigns as a cornerstone of the theory of microcirculatory regulation.
However, what has been omitted from the discussion is that the widespread use of Watt’s governor revealed a number of fundamental weaknesses in proportional feedback control. The main problems are: slow response to rapid load changes, hunting or limit cycling behavior, failure to remove the offset and low sensitivity at the set point. By Maxwell’s definition (184), this regulatory device is a “moderator” but not a “regulator.” If the regulation of local blood flow is based on a similar principle of proportional feedback control it would have all the flaws of the proportional controller. Even in a stationary situation, in resting muscle, the low sensitivity of a proportional controller at its set point would cause spontaneous variations in tissue blood flow. Paradoxically, in the case of active hyperemia in steady state contracting muscle, the achievement of an adequate blood flow should stop the production of the vasodilator by contracting muscle fibers, since the error signal is zero. Thus, according to the metabolic hypothesis, the recovery of oxygenation in a previously hypoxic tissue is due to active hyperemia, and this would lead to restoration of vasoconstriction and reduction of the blood supply. So, the final equilibrium reached is always an inevitably permanent state of hypoxia.
Let us assume, that in a thought experiment the muscle cells were replaced with glass fibers; then the arterioles (which are the gate valves) in the absence of metabolic vasodilators produced by muscle fibers would be maximally constricted. In a technical sense, the arterioles in a metabolic theory are normally closed valves. Returning muscle fibers to their rightful place would reduce the vascular tone and restore typical muscle blood flow to a level adequate to the resting state of the muscle. This indicates that proportional type regulation keeps the muscle cells in a hypoxic state, even at rest. Based on this paradoxical property of the proportional model of regulation, some authors (243) have proposed that the theory of feedback in local blood flow regulation be abandoned and replaced with a feed-forward system. The frustration of the authors and their desire to find a way out of the crisis of the metabolic theory is understandable, but such an approach may be considered too radical. The existence of local automatic regulation of blood flow is a firmly established fact, and the failure of a proportional control approach does not mean the failure of feedback regulation in general.
Bang-bang feedback regulators
Watt's steam engine contained two more automatic control devices, hidden from view inside the machine. These were a float regulator of the water level and a safety valve to control the maximum steam pressure in the boiler, ancient and reliable devices, which were simple in design and provided fast and accurate maintenance of a predetermined threshold set point. These regulators belong to the class of “bang-bang” control systems. The history of their use began in ancient Greece (Ktesibios’ float regulator; (164)) and they are still widely used in electronic circuits, space technology and the household. Well known examples of “bang-bang” regulators are: the float regulator controlling the fluid level in carburetors and water tanks, a pressure cooker safety valve, thermostatic regulators and switcher power-supplies. Their common feature is the existence of discrete states which switch at the crossing of a preset level. Despite their apparent simplicity, they have a complicated dynamic behavior, which is described by the mathematical theory of bang-bang control systems (279).
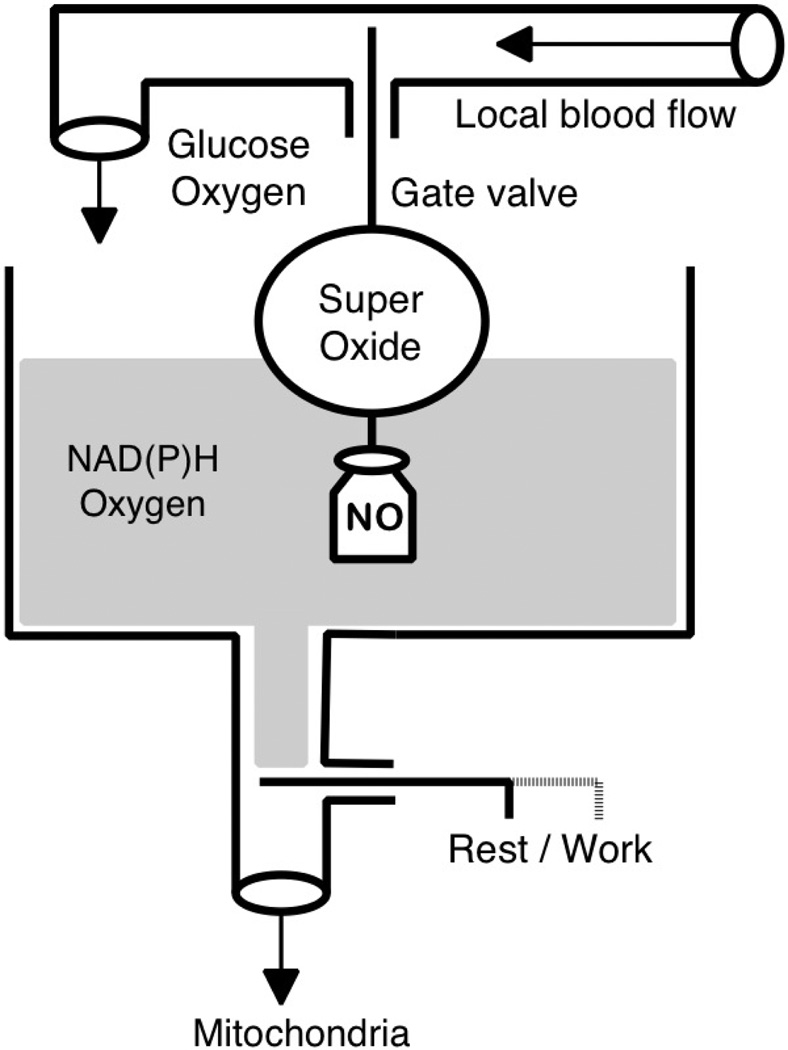
Consider the action of the float valve regulator of the fluid level in a tank (Fig. 2). When the tank is empty, the float is in the down position and the inlet gate valve is fully open (normally open valve), fluid enters the tank at a maximum rate determined by the pressure in the pipeline. Upon reaching a set point level the float approaches its upper position and shuts off the valve. If the fluid level decreases due to use or a leak, the float moves down and the valve opens, restoring the fluid level. Note that this system is characterized by a stable set point, fast response, simple design and minimal energy loss for regulation. To prevent frequent switching, the controller usually exhibits hysteresis determined by a deadband, the range of insensitivity to the error signal.
Figure 2.
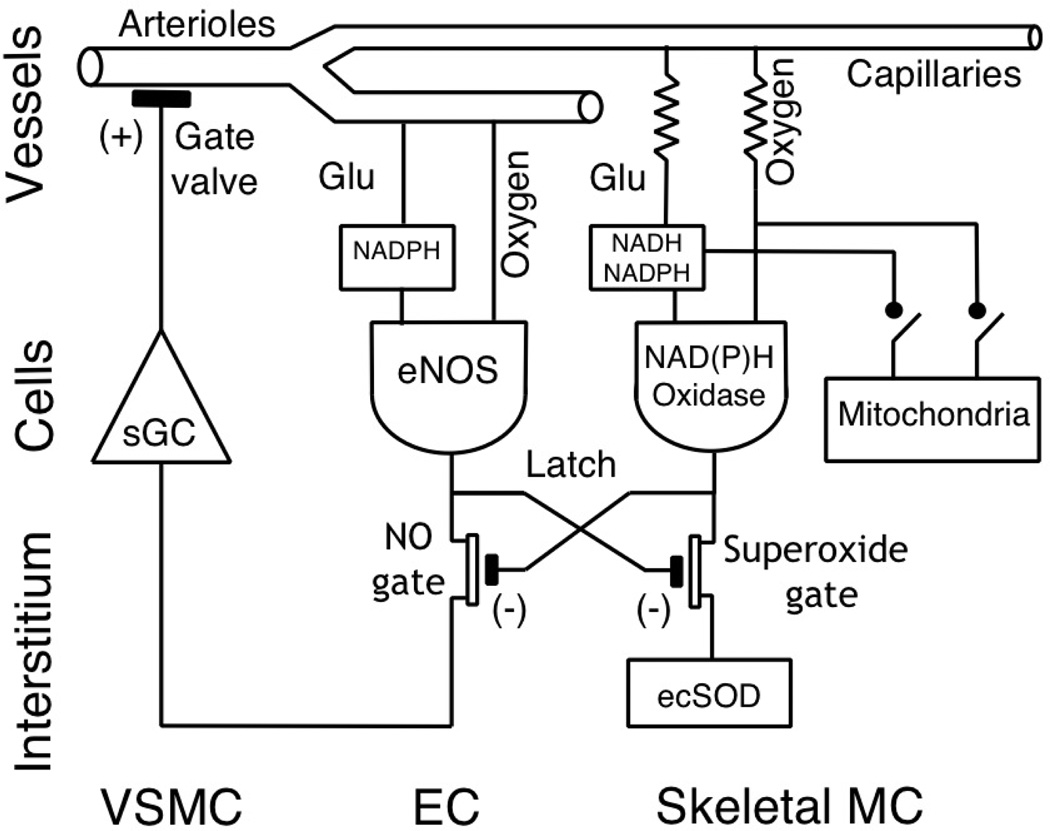
Bang-bang regulator of the fluid level is a system with negative feedback that closes the inflow when the set-point level is reached. When the level is low, the gate is passively opened by the weight (labeled NO), which provides a permanent opening force. When the liquid level rises to the set point, the float closes the gate valve, stopping the flow. We propose the concept of local control of blood flow, which is built on the interaction of three types of cells on a principle similar to the bang-bang liquid level controller. The role of the permanent weight is performed by microvascular endothelial cells continuously producing NO. The flux of nitric oxide to the smooth muscle cells relaxes them and keeps the arterioles (gate valve) in a normally open state. Under conditions of adequate oxygen and glucose supply the cytosolic level of NAD(P)H and oxygen (fluid level) in the parenchymal cells is sufficient for the production of superoxide by membrane NAD(P)H oxidases (the float). The superoxide is injected into the interstitial space to neutralize NO and evoke arteriolar vasoconstriction (closed gate valve). At the activation of mitochondrial respiration (sink valve “rest / work” is open) the cytosolic reducing agents are sequestered by mitochondria, and at the same time the PO2 on the cell surface is reduced. Because of these changes superoxide production goes down, the NO flux reaches VSMCs and dilates the arterioles (opens the gate valve). Thus, in this scenario dilation is the normal state for the arterioles, and the upper limit of the oxygen and glucose supply is actively regulated. Therefore, the cells are sufficiently supplied at rest and the upper limit of delivery for oxygen and glucose is controlled in order to avoid excessive tissue perfusion.
Are there any bang-bang controls in biological systems? To answer this question, let us express the design of the float control in the language of biological regulation (Fig. 2). Normally an open gate valve corresponds to the constant diffusive flow of activator molecules from their source to an effector, which produces the response necessary for the regulation of a biological variable. It is also necessary to produce an inhibitor (inactivator), whose concentration strongly depends on the level of the controlled variable. The inhibitor deactivates the activator on its way to the effector in a rapid and specific mode. If the level of the controlled variable is low, the activator is freely transported to the effector, which supports the regulated process by increasing the level of the controlled variable. Accordingly, this is accompanied by an increase in the level of inhibitor (inactivator) to a threshold concentration at which a constant flux of activator produced by the source is fully neutralized by the inhibitor before it reaches the effector. Thus, the set point is determined by the rate of activator production by its source. Because the activator is produced at a constant rate, the effector is activated immediately after the withdrawal of the inhibitor. This is the advantage of bang-bang control compared to the proportional system of metabolic feedback, which needs time to produce, deliver and accumulate the signaling metabolite. At the same time, to ensure optimal control, the activator and/or inhibitor should have a limited lifetime due to spontaneous decay or an effective mechanism of removal by rapid chemical reaction. The circuits of automatic regulation of the cell, known in systems biology as “network motifs” and formed by diffusive flows of interacting signaling molecules, function on a certain time scale (9, 114). Isolation of elementary regulatory circuits in biological signaling networks based on time scale allows one to simplify their analysis in terms of systems biology, separating, for example, the matching of supply and demand at the onset of muscle contractions from slower adaptive processes in the tissues and vascular networks.
Example of bang-bang regulation: HIF-1 signaling
Bang-bang control apparently occurs in the regulation of the hypoxic response in cells via the hypoxia-inducible factor HIF-1, which activates expression of many angiogenic genes, thus eliminating tissue hypoxia by increasing the number of microvessels (177, 250, 251). HIF-1 is a protein composed of two different sub-units HIF-1α and HIF-1β. The sub-unit HIF-1α has a short lifetime (about five minutes). It is produced at a constant rate and can be deactivated by hydroxylation of the oxygen-dependent domain. After that, hydroxylated HIF-1α labeled with von Hippel-Lindau protein is directed to further destruction in proteosomes. At a sufficient cellular oxygen supply the HIF-1α degradation pathway inhibits the signal carried by HIF-1α. Under hypoxic conditions oxygen-dependent hydroxylation is blocked and the HIF-1α level builds up. Then, after binding with the HIF-1β subunit, the complex HIF-1 activates the transcription of specific genes responsible for adaptation to hypoxia. The network motif of this regulation is the combination of the constant production rate of the activator HIF-1α and its oxygen-dependent degradation, thus forming bang-bang type regulation with inversion of the oxygen signal to produce a signal for hypoxia. Since the launch of angiogenesis requires the simultaneous turns (on or off) of a large group of genes, bang-bang control is optimal for this purpose.
NO/O2− CONTROL OF LOCAL CIRCULATION (PARTS AND COMPONENTS)
Is it possible to build an effective system of local blood flow control based on available cell types in a skeletal muscle and available signaling molecules released into the intercellular space, without resorting to hypothetical and unidentified metabolic vasodilators? Yes, if that system is built on the bang-bang control principle and composed of interacting cells in the “cell circuits” of a specific topology (114). The reliability and simplicity of a bang-bang controller makes it a potential mechanism for regulation of supply and demand balance in skeletal muscle and other organs, but to recognize this possibility, we must examine this alternative approach to traditional metabolic theory.
Because the transmission of paracrine signals between muscle fibers and adjacent microvessels occurs by the diffusion and accumulation of signaling molecules in the interstitial space, the properties of an activator molecule should include: 1) small size for ease of synthesis in the source cell and its rapid diffusion to the effector cell; 2) high solubility in lipid and aqueous environments to facilitate migration through the cell membrane and propagation through the interstitium; 3) the ability for spontaneous rapid degradation and/or inactivation via a specific inhibition mechanism; 4) good selectivity and rapid reaction with an inhibitor molecule; and 5) presence in cells of a special constitutive enzyme to provide a basal rate of activator release. The inhibitor (inactivator) substance must: 1) be produced by a special enzyme at a rate related to the sufficient supply of metabolic resources; 2) have a high affinity for the activator; 3) have a high rate of diffusion in the interstitial environment; 4) have a short lifetime and highly selective deactivation system localized in the interstitial space; and 5) be compartmentalized in the interstitium to avoid cross-talk with intracellular regulatory processes. Advances in biochemistry and molecular biology leave little hope for the discovery of currently unknown small molecules acting as paracrine messengers or antagonists, so the hypothetical cell circuit providing bang-bang control of supply and demand matching should be based on the interaction of familiar cells, substances and pathways.
Nitric oxide, an ultimate vasodilator
The weakness of the traditional hypothesis of metabolic regulation was manifested by its inability to predict and incorporate the powerful natural vasodilator NO, long used in medical practice for dilation of the coronary arteries (15). In 1980 it was found that the relaxation of contracted rabbit aortic strips in response to acetylcholine is mediated by endothelial cells that produce an endothelium derived relaxing factor, EDRF, (93, 94). Later EDRF was identified as nitric oxide, NO, a signaling molecule which carries out communication between endothelial cells and smooth muscle cells in blood vessels (82, 91, 92, 123, 124, 196, 212). Paradoxically, the source of the vasodilator was found in the endothelial cells located on the interface with the arterial blood and, therefore, was generally well supplied with oxygen and nutrients. This finding was inconsistent with the metabolic theory and has been allocated to a specific mechanism of regulation, in addition to the classic metabolic vasodilation (6). Because nitric oxide is involved in a large number of intracellular processes and the total number of publications and reviews on the biological effects of NO amounts to tens of thousands, we will mention only the main paths and targets, which are the crucial issues for local regulation of blood flow.
Nitric oxide is a stable diatomic radical with a high rate of diffusion in aqueous solutions: D = 3.0 – 3.8·10−5 cm2/s at 37 °C (159, 172, 273, 309). The solubility of nitric oxide in saline is lower than in water (1.75 vs. 1.94 µM/(cm3 atm), and its lipid solubility is about five times higher than this (189, 309). Thus, the signal transmitted by NO molecules in the tissue is propagated rapidly and easily passes through cell membranes. The lifetime of NO in biological media is greatly limited by the very high rate of its reaction with superoxide anion, O2−, (1.9 × 1010 M−1 s−1; (147)) and oxyhemoglobin (3.4 × 107 M−1 s−1 (77)) and a relatively low rate of reaction with oxygen (2.4 × 106 M−2 s−1 (165)).
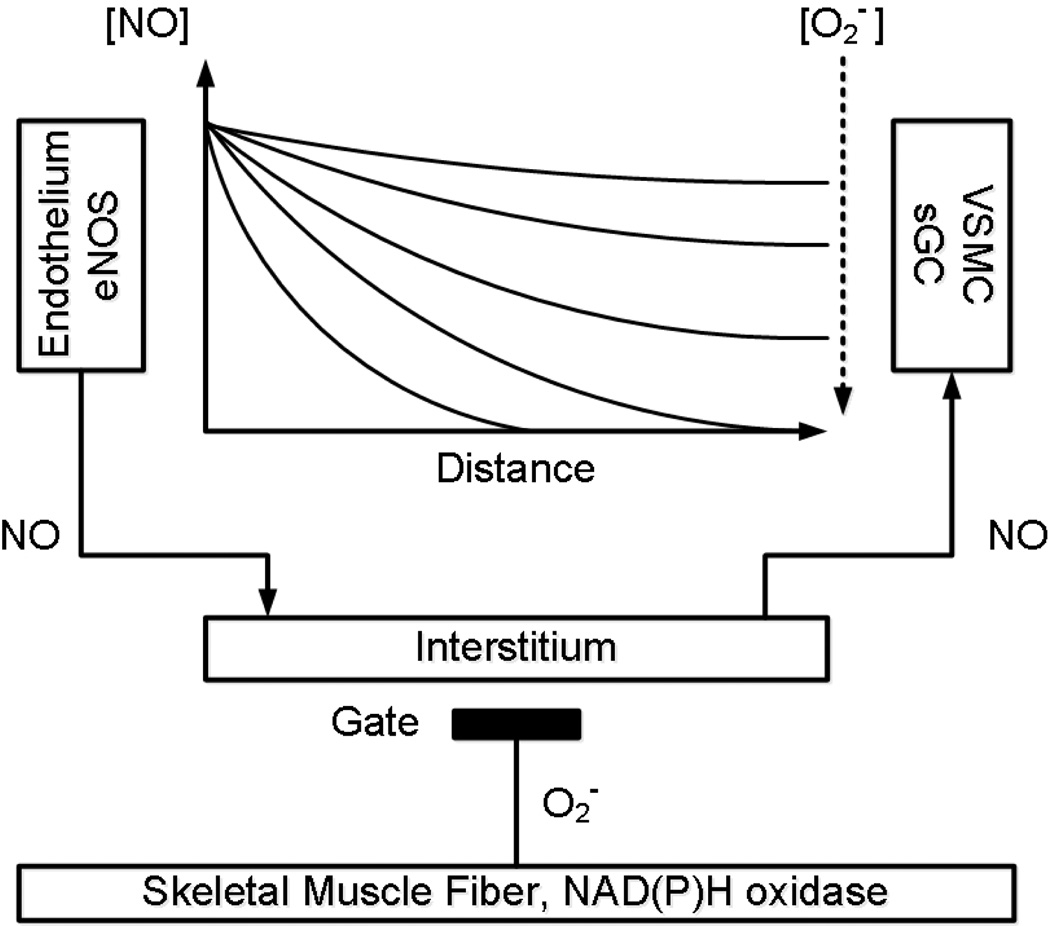
Thus, the half-life of NO in a solution of free oxyhemoglobin is about 2 µs, and in the blood with intact erythrocytes, about 2 ms (159, 172, 273). The half-life of NO in the extravascular space of tissues ranges from 0.01 to > 2 s, corresponding to an estimated diffusion distance of NO of 100–200 µm from a vessel. Reducing the PO2 in the interstitium can lead to an increase of the distance of penetration of NO into the tissue to > 300–400 µm. The concentration of NO in the tissues is a dynamic quantity that depends on many factors and is reported to be within 0.1–5 nM (108). Methods for determining the concentration of NO at a microscopic scale are under development, so details of the distribution of NO in the microcirculation are known mainly from mathematical models (38–40, 54, 71, 142, 143, 159, 273, 278), which are in good agreement with respect to the lifetime and penetration distance limit for the diffusion of NO. Note that the distance of 300–400 µm is in good agreement with the size of the tissue region supplied by a terminal arteriole, and the 2 sec time coincides with the time scale of the hyperemic response to muscle contraction (96, 101).
The ability of NO to activate soluble guanylate cyclase (sGC) and thus increase the production rate of guanosine 3', 5'-cyclic monophosphate (cGMP) in cells has been demonstrated as a pathway for NO action in smooth muscle cells (15). In the cytoplasm of smooth muscle cells the molecule NO reversibly binds to a heme in sGC, thus allowing sGC to increase the rate of cGMP generation from GTP (15, 154). In turn, cGMP interacts with specific effectors in vascular smooth muscle cells, of which the primary one is a family of cGMP-dependent protein kinases, PKGs, mediating control of smooth muscle tone. Activated cGMP-dependent protein kinase Iα influences the contractile activity of smooth muscle cells by myosin light chain phosphatase, thereby regulating smooth muscle tone (291). Another target for cGMP is the cyclic nucleotide-gated channels that regulate the influx of Na+ and Ca2+ into cells (154). In addition to these actions, cGMP is involved in the modulation of activity of cGMP regulated phosphodiesterase, which is responsible for the hydrolysis of cyclic nucleotides (154). The main result of increasing the intracellular level of cGMP is the activation of signaling pathways that lead to the reduction of [Ca2+], caused by the release of K+ from the cell through the large conductance channels (BKCa) and subsequent hyperpolarization of the smooth muscle cell membrane, closing voltage-gated calcium channels (155, 270). The physiological response to the signal carried by NO is relaxation of smooth muscle cells, leading to passive widening of the arteriolar lumen and increasing of local blood flow.
Nitric oxide production
Nitric oxide is synthesized in cells from L-arginine by the enzyme nitric oxide synthase (NOS) with the participation of cofactors NADPH, FAD and FMN (flavin di- and mono-nucleotides, respectively), BH4 (tetrahydrobiopterin), heme and oxygen (190, 211). In mammals, the production of nitric oxide is catalyzed by three isoforms of NOS, which differ in their cell-specific distribution and whose genes are localized in different chromosomes (183, 190). Two isoforms are constitutively expressed: endothelial (eNOS) and neuronal (nNOS) NOS. The endothelial isoform is the main source of NO acting as an endothelium derived relaxing factor for vascular smooth muscle cells (82, 183, 191, 308). The sustainable production of nitric oxide by the endothelium is provided by the preferred supply of the endothelium with oxygen, glucose and L-arginine, through contact with flowing arterial blood, and the independence of eNOS activity on oxygen in the physiological range of arteriolar blood PO2 (Km = 4 µM for eNOS reaction with oxygen (264)). The basal production and export of NO by vascular endothelium provides a constant and significant vasodilator tone in the vasculature, the lack of which inevitably leads to vasoconstriction of blood vessels in all organs (191). Removal of the endothelium or pharmacological inhibition of eNOS necessarily leads to vasoconstriction and increased vascular resistance (94, 223). A key activator of eNOS in the vessels is wall shear stress (11, 40, 237), which is supposed to be [Ca2+]-independent, in contrast to the activation by agonists such as bradykinin and acetylcholine, which depends on [Ca2+] (191). The expression of constitutive eNOS in endothelial cells has been found in all microvessels in skeletal muscle (249). In other studies a strong expression of eNOS in the endothelium of arterioles was reported, while the capillary endothelium was stained less eNOS-positive (12, 49). But in this case, the lower activity of eNOS in capillaries can be compensated by the greater fraction of capillary endothelium in the overall balance of endothelial cells in the vascular network.
In the past decade experimental and theoretical studies have confirmed the significant contribution of nNOS, located in nerve fibers and skeletal muscle cells, in maintaining the dilator tone in microvessels at a level of 17–30% of resting blood flow (61, 142, 186). It is known that the NO production rate by nNOS strongly depends on oxygen concentration with a Km = 350 µM (264). The oxygen dependence of NO production by nNOS implied by this Km, which far exceeds the physiological concentration of oxygen, yields a surprisingly high contribution of NO production. Interest in the issue of nNOS participation in the maintenance of vasodilation is awakened in connection with the finding of a lack of eNOS expression in endothelial cells of toads and frogs. In these poikilothermic vertebrates, the control of microvascular tone by NO is mediated by perivascular nitrergic nerves (35, 277). In contrast, the vascular tone of homeothermic vertebrates, birds and mammals is regulated by endothelial-derived NO.
Mathematical modeling of NO generation in the endothelium and its transport to arteriolar smooth muscle, combined with NO scavenging in the tissue environment, has shown that this rapidly diffusing radical is able to transmit a signal to a distance of 100–400 µm for a few seconds of its existence (159, 172, 278, 288). Thus, NO is the best messenger for local regulation of blood flow in the microcirculation, able to integrate signals from multiple sources in the tissue volume perfused by single terminal arterioles. The short lifetime of NO in tissue is in agreement with the time scale of the vascular response needed to switch on active hyperemia. In a complex multi-tiered system of biological regulation, the allocation of a specific time-scale for the elementary process, such as a switching transient in a cell circuit, allows one to understand the dynamics of the interaction of the components of regulation of supply and demand balance, using the stationary approximation for slower processes (9, 114). In the case of active hyperemia, transients associated with the onset of muscle contraction are considered as a more rapid process compared with the time scale of the activation of eNOS by agonists, shear stress and other modulating factors (191). In a resting muscle the basal production of nitric oxide by the constitutive enzyme eNOS under conditions of an abundant supply of L-arginine and oxygen from blood is constant on the time scale of the onset of active hyperemia. Investigation of the maintenance of vasodilation during long-term steady state exercise requires the transition to a larger time scale in order to include factors which modulate eNOS activity.
Role of NO signaling in matching supply and demand in tissues
The concentration of NO in the arteriolar wall is a function of the rate of its synthesis by endothelial cells, which are contained in the reference volume of tissue and the speed of NO deactivation due to its instability, as well as its scavenging and specific inactivation by the cells surrounding the microvessels. In order to virtually eliminate the effects of the parenchyma, let us again pretend that muscle cells are replaced with glass fibers, but this time not in the framework of the metabolic hypothesis, but taking into account the basal production of NO by the endothelium. It is obvious that, due to the high interstitial NO concentration, the arterioles should be dilated, thus the resistive arterioles work as normally open gate valves without any dilating metabolites from muscle cells. While there is no impact of the imaginary "glass" muscle fibers, arterioles are dilated and their blood flow is maximal, determined by the anatomic limits of diameters in the microvessels. Replacing the “glass” muscle fibers by “living” muscle fibers, for resting muscle the local blood flow would be reduced to a level corresponding to the resting state, which is far below the maximum flow. Since endothelial cells are located at the interface with normally oxygenated blood, the rate of production of nitric oxide is not expected to decline in resting muscle. A “network motif” for this situation looks similar to that for the mechanism of HIF-1α regulation, but is rather based on the oxygen-dependent degradation of NO on its way to a smooth muscle cell, with the direct participation of resting muscle fibers, well supplied with blood.
Assuming that the normal physiological state of skeletal muscles is steady exercise in “cruise” mode, when the metabolic needs are matched with increased blood flow, the dilation of arterioles should be regarded as their normal state with a minimum consumption of oxygen in the walls of arterioles. From this point of view, vasodilation and hyperemia are the normal state of affairs, while restriction of blood flow during the transition to the resting state is the act of regulation. The goal of local control of blood flow would be a state of normal, or slightly excessive, supply compared with demand. At the achievement of this goal, local blood flow is restricted via energy-dependent constriction of the arterioles. In the bang-bang model of regulation, the upper limit of tissue supply with oxygen and nutrients is monitored (to ensure the cell’s well being), rather than reacting to hypoxia and maintaining the deficit state as predicted by the metabolic theory of local regulation. In the transition from activity to rest, muscle blood flow decreases, just as the inflow of fluid is slowed down upon reaching a specified set point in the float regulator of fluid level. In a healthy organism, active hyperemia is a normal physiological state of working muscles, so the purpose of regulation is to prevent excessive levels of oxygen in the tissue at reduced load and at rest, protecting the tissue against hyperoxia and avoiding excessive perfusion. This upper level control provides “well supplied” conditions to the tissue, because the feedback signal carries information of excess blood flow, in contrast to a lack of supply, as in the metabolic regulation theory.
In the bang-bang model, the initiating vasoconstriction requires only a decline of the NO concentration in the smooth muscle cells of the arteriolar wall. That effect can be achieved by inactivation of NO through the production of a specific inactivator, depending on the status of energy supply in all cells represented in the reference volume of tissue. This design provides to all cells an opportunity to participate in the formation of the flow of an inactivator of nitric oxide into the interstitium in the vicinity of arterioles. This simple mechanism could provide for the averaging of chemical signals from individual cells in the interstitial space, which appears as an opportunity for “cell voting” on the level of nutrient supply to these parenchymal cells.
Superoxide anion (O2−) as a physiological signaling radical
Surprisingly, the discovery of an NO inactivator happened shortly before the identification of EDRF as NO. In a study of free radicals generated by endothelial cells in culture, it was shown that cells produce superoxide anion, but not hydroxyl radical (232). In experiments on acute hypertension, superoxide generation and its release into the extracellular space were detected in the brain. The appearance of superoxide completely suppressed acetylcholine-induced dilation of arterioles due to its interaction with or destruction of EDRF (296). It was observed in coronary artery rings and thoracic aortic spiral strip bioassays that superoxide dismutase (SOD) considerably prolongs the biological half-life of EDRF, particularly at low PO2 (104, 238, 239). Thus, a strong PO2 dependence of the production of the NO inactivator was established in arterial preparations, and this inactivator was identified as the unstable radical superoxide anion, O2− (141). The pivotal role of this inactivator of EDRF, produced by vascular and parenchymal cells at increased perfusion or oxygenation, was realized immediately: “Therefore, it appears that O2− is a potent inactivator of EDRF and that it is either present or produced in sufficient quantities in our system to inactivate EDRF rapidly. The idea that the short half-life of EDRF may be a function (at least in part) of the concomitant release of EDRF and O2− by vascular endothelial cells merits further study. It may explain the reported differences in the half-life of EDRF, as these may in turn be affected by the ratio of EDRF to O2− in a particular experimental system“ (104).
That report marked the discovery of the nitric oxide/superoxide signaling system, which is based on the interaction of two unstable, rapidly diffusing radicals possessing an extremely high mutual affinity and selectivity. One of these radicals, NO, is a mediator that transmits a relaxation command to its specific target, vascular smooth muscle cells, while the other radical, O2−, has the first radical as its target. This discovery could have immediately revolutionized our understanding of the local regulation of blood flow, but this did not happen for several reasons. First, neither of the complementary pair of radicals fit into the dominant metabolic theory, and their sources were also discordant to the metabolic regulation concept, which required the generation of one or more vasodilator metabolites by the parenchymal cells under hypoxic conditions.
Second, the concept of “oxidative stress” interpreted the biological roles of these radicals as damaging and toxic agents (1, 2, 6, 86, 110, 194, 293), which did not encourage the study of their normal biological roles as signaling molecules (197). Superoxide anion was considered to be a dangerously reactive byproduct of normal aerobic metabolism that caused oxidative damage in cell structure and signaling (6, 86, 87, 110, 111).
The superoxide anion is the main oxygen radical produced by various cells. This radical, O2−, wrongly called super-oxide, is not a super oxidant, because in most reactions it acts as a nucleophilic agent and a reducing agent (5, 6). In recent decades, the emphasis in studies of the biological role of superoxide anion has moved in the direction of its signaling functions, implemented in conjunction with NO as its partner in signaling processes (6, 73). According to recent trends, the superoxide anion and its counterpart NO are relatively harmless radicals, which can be considered as "physiological" free radicals because they are formed and used in normal physiological processes (3–5).
Superoxide anion, in contrast to its counterpart NO, is a negatively charged molecule and cannot permeate cell membranes at normal pH, so intracellular and extracellular superoxide can generally be considered to be compartmentalized (284). There is the hypothetical possibility for a small amount of superoxide to cross the cell membrane through anion channels, such as the ones reported in mitochondria (178). However, for the interstitial balance of these two radicals, a loss of extracellular NO via crossing the cell membranes could be more substantial. Intercellular chemical signals that determine control of arteriolar tone are integrated and averaged in the interstitial space, so the release of superoxide into the extracellular compartment is of particular interest. Generation of superoxide into the extracellular space was demonstrated in isolated muscle strips and bundles of muscle fibers (224, 313) and in primary cultures of myotubes (313). In these studies, muscle cells were exposed to high oxygen tension in the medium equilibrated with air and produced superoxide in a passive state and during stimulated contractions. The physical and chemical properties of superoxide and its availability in the extracellular space indicate its role as a possible inactivator of NO in the bang-bang regulation model of supply and demand balance.
Hyperoxic vasoconstriction and superoxide
Because our hypothesis suggests bang-bang regulation of the upper limit of oxygen supply to tissues, it is natural to consider data on the role of superoxide anion to the increase of microcirculatory PO2 to a hyperoxic level and the associated response of local blood flow and vascular tone. The mechanism of hyperoxic vasoconstriction based on selective blockade of EDRF was found in vitro in isolated vessels (104, 141, 181, 192, 239, 307). In these studies, using a selective superoxide dismutase (SOD) test, the superoxide radical was identified as an inactivator of NO. Subsequent studies, conducted on rats under hyperbaric conditions, also proved that the constriction of arterioles in the brain and reduced cerebral blood flow are mediated via NO inactivation by superoxide radical (68–70, 310). These authors hypothesized that at hyperbaric PO2 the increased production of superoxide is capable of neutralizing the basal production of NO, thereby producing vasoconstriction. These workers successfully used an exogenous erythrocyte SOD (i.v.) to eliminate vasoconstriction and to increase cerebral blood flow (with a maximum effect after 20 min of administration) (310). Recently, they have demonstrated the role of the extracellular SOD3 isoform in reducing the effect of superoxide on the bioavailability of NO, and in maintaining a balance between NO and O2− in the regulation of cerebral blood flow (70).
The hypothesis of NO/O2− regulation of matching the supply and demand in tissue was experimentally verified in skeletal muscle; however, there was a negative conclusion on the role of superoxide in controlling arteriolar tone via the destruction of NO as it diffused from the endothelium to smooth muscle cells (221, 222). Administration of SOD in the superfusate and blood did not relieve the hyperoxic constriction of arterioles, as expected. Among the assumed reasons that explained this lack of effect of SOD was the presence of a substantial amount of endogenous ecSOD (extracellular isoform), but this explanation was initially rejected on the basis of theoretical arguments (222). However, direct measurement of extracellular SOD in the arterial wall revealed a large concentration of the endogenous enzyme (262). Even under conditions of “oxidative stress,” there was no observed shortage of activity of this enzyme (138). Thus, the abundance of endogenous ecSOD in the vascular wall and interstitium could explain the insensitivity of arterioles to exogenous SOD in muscles, while the sensitivity of cerebral arterioles to exogenous SOD remains a brain-specific finding.
The concept of vascular tone regulation via the interplay between nitric oxide and superoxide radicals was successfully developed during the last few decades and detailed information has been accumulated on the sources and sinks of extracellular superoxide.
Generation of superoxide by NAD(P)H oxidase
In order to ensure a fast vascular response to rapid transitions between states of rest and exercise in muscle, the sources of superoxide anion should be directed into the interstitial space, which is a medium for intercellular communications. Compartmentalization of superoxide anion in the interstitial space (284), in combination with the relatively small volumes of that compartment in skeletal muscle (approximately 14% (56, 253)), are the necessary conditions for rapid changes in peri-vascular superoxide concentrations and effective modulation of local NO availability for arterioles.
The main source of superoxide anion production into the intercellular space is NAD(P)H oxidase (Nox), expressed in mammals by several isoforms. However, only those isoforms which are localized in the plasma membrane and generate the radical in the direction of the intercellular space are of special interest. The constitutive enzyme form is a heterodimer, consisting of two plasmalemmal-bound subunits gp91phox and p22phox (as in the first discovered phagocytic isoform), which is activated by translocation of the cytosolic subunits p47phox, p67phox, and p40phox to the gp91phox/p22phox complex (8, 26, 149, 157, 160). There are variations in the amount of subunits in the complex, depending on the type of Nox isoform, which can replace the trans-membrane subunit gp91phox (also known as Nox2) (8, 26, 284). In cells of blood vessels the basic functional unit, Nox2, can be replaced by the isoforms Nox1 or 4 (8, 149). However, the main generator of superoxide anion in the arterioles is the Nox2 containing isoform of NAD(P)H oxidase (26, 102). According to another literature source, the phagocytic Nox2 isoform is stimulated and produces oxidative bursts, while other forms are constitutive and produce O2− continuously (6).
The physiological function of each isoform of NAD(P)H oxidase is determined by the direction of O2− generation, intra- or extracellular, which is still a controversial matter. In the literature there is general agreement that gp91phox (Nox2)-containing NAD(P)H oxidase generates O2− into the extracellular space (149, 157, 160), although there are exceptions to this opinion (8, 284). Other authors believe that the isoforms Nox1 and Nox4 produce O2− into the intracellular compartment (149, 284).
It is assumed that the active membrane subunit of the Nox1–5 family consists of six alpha-helices crossing the membrane, and helices 3 and 5 coordinate iron atoms at the centers of two heme molecules, which are the prosthetic groups of the oxidases (8, 157, 160). These two hemes are located at the level of the lipid bilayer plasma membrane and together they form a channel for electrons to cross the membrane. These electrons come from the cytoplasmic domain containing binding sites for cofactors FAD and NAD(P)H. After crossing the membrane they are involved in the reduction of dioxygen to superoxide anion on the outer side of the membrane. Simultaneously, an appropriate number of protons are transferred in order to maintain the electrical neutrality of this process (160). It seems that this mechanism of enzyme function does not leave open the possibility for the generation of superoxide by the membrane NAD(P)H oxidase into the cytoplasm, and the production of O2− into the extracellular space is the rule for all trans-membrane forms of Nox (26, 157, 179). This conclusion is corroborated by reports that the same oxidases located in the membrane of intracellular vesicles release superoxide rather inside endosomes, but not into the cytosol (160, 179).
In membranes of cells involved in the regulation of supply and demand balance (i.e., skeletal muscle fibers, cardiomyocytes, vascular smooth muscle cells and endothelial cells) NAD(P)H oxidases containing Nox2, Nox4 and Nox5 are known to be present. Nox2 appears to be the predominant form generating superoxide in the arteriolar wall (8, 26, 102, 160) and muscle fibers (298). Inter-species variability in the expression of different isoforms of Nox (157) and their specific distributions among cell types has also been reported (6, 26, 179).
In the different cells forming an organ, membrane NAD(P)H oxidases are a source of interstitial superoxide. The producers of superoxide are the cells of the vasculature, including the endothelium and smooth muscle cells (23, 26, 102, 157, 160, 162, 167, 305, 306), as well as cells of the musculature (26, 126, 127, 129, 298). The active generation of superoxide has been shown in isolated bundles and strips of muscle fibers (224, 313), single isolated myofibers (298, 315) and in primary cultures of myotubes (213).
The presence of NAD(P)H oxidase in the plasma membrane of various cell types suggests the possibility of their joint participation in the formation of interstitial O2−, which determines the bio-availability of NO to arteriolar smooth muscle cells and thus, vascular tone (102, 195). The quantitative contribution of each cell pool in the integrated O2− signal controlling local blood flow has not been studied yet, but the localization of NAD(P)H oxidase in the plasma membrane suggests that there is a connection between the cell surface area and the contribution of cells in the total pool of interstitial O2−. Morphological data on the ratio of the perimeters of capillaries/myofibers in skeletal muscle (182, 267) show that the surface area of muscle cells is several times larger than that of microvascular endothelium.
Regulation of membrane NAD(P)H oxidase
A correct interpretation of the information carried by superoxide requires consideration of the basic relations that determine the rate of O2− production and disposal. The strong oxygen dependence of superoxide production by phagocytic cells, which are equipped with the Nox2 isoform of NAD(P)H oxidase, was described in a classic paper in 1978 (16). However, the detailed characteristics of this dependence remain uncertain because of the multiplicity of isoforms of the enzyme and the influence of many activating and inhibiting factors, except oxygen.
It was found in cultures of vascular smooth muscle cells and cardiomyocytes that the rate of superoxide production by NAD(P)H oxidase is highly dependent on the concentration of environmental oxygen (55). In homogenates of kidney and left ventricle the PO2 dependence of O2− production was shown to be well described by the Hill equation, with a Km of about 12–15 mmHg, and maximal production rate was achieved at a PO2 of about 80 mmHg. Since the homogenates of kidney and left ventricle contained several isoforms of NAD(P)H oxidase (26), the obtained dependences reflect rather the total effect of oxygen on the rate of O2− production in these tissues. A direct relationship between the O2− production rate and concentration of oxygen was revealed in thick slices of hippocampus superfused with oxygenated solutions at high (20–95%) O2 content (65). The oxygen dependence was also demonstrated in experiments where superoxide production decreased with a decrease in PO2. A gradual decrease in production of superoxide radicals induced by hypoxia was found in isolated lungs (13) and in swine granulosa cells (21). Due to the sensitivity of NAD(P)H oxidase to oxygen concentration, this enzyme was considered as a candidate for the role of regulator of vascular function in response to PO2 variations, possibly via the vasoactive effects of H2O2, produced by dismutation of O2− (306). In addition to data on the direct link between the production of O2− and PO2, evidence for the paradoxical increase in superoxide production at very low oxygen levels was reported. This small peak of O2− generation was assumed to be associated with a significant accumulation of reducing agents NADH and FADH in the cells (22, 57, 314).
Study of the stimulating effect of angiotensin II on superoxide production in cultured vascular smooth muscle cells showed that the rate of O2− production increases by activation of NADH and NADPH oxidases via an AT1 receptor-dependent mechanism (31, 103, 202, 230, 297). Regulation of superoxide generation by angiotensin II is biphasic; the first phase reaches a peak at 30 seconds, whereas the second phase reaches a peak half an hour later (252). In isolated aortic smooth muscle cells it was also reported that a rapid and transient peak of fluorescence was associated with the generation of superoxide (230). Long-term stimulation going on for hours and days is associated with the modulation of gene expression (103). Short-term stimulation of NAD(P)H oxidase activity involves assembly of its subunits and takes minutes (112, 252). And a very important finding is that superoxide-generating membrane oxidases use both NADH and NADPH cofactors to transfer electrons to oxygen (285). Studies of the role of NAD(P)H oxidases have been focused on vascular oxygen sensing, and thus the generated superoxide signal was preferably associated with the level of oxygen in the environment of the cells forming the vascular wall, thereby excluding the contribution of the parenchymal cells to the interstitial pool of superoxide (304–306).
Since cytosolic NADH and NADPH are the substrates for membrane NAD(P)H oxidase, which generates superoxide anion into the interstitial space, the availability of reducing agents in the cytosol is a modulator of superoxide production by membrane NAD(P)H oxidases (72, 304). The main source of cytosolic NADH is glycolysis, which produces one molecule of NADH from one molecule of glucose. To continue glycolysis it is necessary to recycle NAD+ from NADH and that requires the participation of mitochondria. If the activity of mitochondria lags behind the rate of glycolysis, then the NADH/NAD+ balance is shifted to NADH, and the reduction of pyruvate to lactate is necessary for re-oxidation of NADH. Thus, the lactate/pyruvate ratio reflects the cytosolic ratio NADH/NAD+ (19, 20, 36, 206, 241). Increased lactate concentration in the extracellular medium at a sufficiently high PO2 significantly increases the production of superoxide in isolated myocytes (188). Lactate increases the production of hydrogen peroxide in vascular smooth muscle cells and causes their H2O2-mediated relaxation (304). Skeletal muscles, especially the glycolytic type, contain significant amounts of the enzyme lactate dehydrogenase that converts pyruvate into lactate and back.
Cytosolic NADH molecules cannot directly penetrate into the matrix of the mitochondrion and their recycling is produced by electron transfer from cytosolic NADH to mitochondrial NAD+ by the reversible malate-aspartate shuttle (19, 79). This transfer can only be done if the ratio NADH/NAD+ in the cytoplasm is substantially higher than that in the mitochondria, which is typical for a glycolytic muscle at rest. In contrast, the glycerol-3-phosphate dehydrogenase shuttle is not reversible; it transfers electrons directly to the mitochondrial electron transport chain. This type of shuttle is present mostly in white muscle and brain, but is very low in myocardial cells (200). In skeletal muscles, glycolytic fibers possess both types of shuttles to import cytosolic reducing equivalents into the mitochondrial matrix, while the mitochondria from slow oxidative fibers have only the malate-aspartate shuttle (216).
Another substrate for NAD(P)H oxidase, NADPH, is produced by catabolic processes, mainly in the pentose phosphate cycle. NADPH production is associated with a high level of glucose and oxygen supply for cells, and the presence of an energetic excess, sufficient for energy accumulation in the synthesis of fatty acids. It is known that the content of enzymes for the pentose phosphate cycle in skeletal muscle is very low (78, 200); however, in smooth muscle cells the role of this pathway is significant (304). An alternative pathway for NADPH production is through the export of citrate from mitochondria to the cytosol, converting it into oxaloacetate with citrate lyase, then conversion to malate with malate dehydrogenase and, finally, to pyruvate and CO2 with the malic enzyme. The latter reaction also produces one molecule of NADPH and a proton. Citrate can be exported from the mitochondria to the cytosol only at a high concentration in the matrix, which happens only with a plentiful supply of carbohydrates. Otherwise citrate is not exported to the cytosol (78).
Thus, the high levels of NADH and NADPH in the cytosol are indicators of active glycolysis and a sufficient supply of glucose to muscle cells, equal to or exceeding the needs of the mitochondria at a given level of activity. The excessive rate of glycolysis in relation to the consumption of reducing equivalents by mitochondria and/or plenty of lactate in the cytoplasm and high concentrations of citrate in the mitochondria result in high substrate availability for the NAD(P)H oxidase located in the sarcolemma. At high oxygen tension on the surface of cells, superoxide anion generated into the interstitium by NAD(P)H oxidase is a signal of sufficient supply of glucose and oxygen to the striated muscle cells.
Contraction of muscle is followed by release of phosphate and ADP, which activates the mitochondrial respiration rate and import of reducing equivalents from the cytosol to the mitochondria. Depletion of cytosolic NADH and NADPH then causes a decrease of extracellular superoxide production by sarcolemmal NAD(P)H oxidase. The high oxygen consumption rate decreases the PO2 at the surface of myocytes, thus the oxygen dependence of NAD(P)H oxidase can also contribute to a decrease of superoxide production into the interstitial space. The traditional view of NAD(P)H oxidase as an oxygen sensor only (304, 306) seems incomplete; rather, it is a sensor of the functional state of cells related to the sufficiency of their supply with substrates and oxygen. Thus, the controlled parameters in the local regulation of blood flow are not the concentrations of oxygen or glucose alone, but rather the metabolic status of the cells, expressed as an equilibrium between the rates of glycolysis and respiration. In this way biochemical processes of intracellular metabolism in the musculature can be coordinated with physiological adaptations of blood flow in the microvasculature (301).
Since the state of a sufficient supply with oxygen and nutrients (“satiety of a cell”) can occur at different PO2 in different cells, no standard value of “tissue PO2” exists which can be used as a set point for regulation. There is no contradiction here with the fact that the state of the cell depends on an abundance of oxygen in the tissue.
Sinks for interstitial superoxide
The fate of superoxide anion in the interstitium is determined by its very high affinity for another physiological signaling radical, NO (258). The reason why superoxide can rapidly inactivate NO is clear, because both of these radicals contain unpaired electrons and react at a diffusion-limited rate to form peroxynitrite (ONOO−) (258, 311, 312). The rate of reaction obtained from different sources ranges from 6.7 × 109 M−1 s−1 (25) to 1.9 ×1010 M−1 s−1 (147). Peroxynitrite is not a radical, being stable in an alkaline medium, but at neutral pH, it is a strong toxic oxidant, participating in a variety of biochemical, physiological and pathological processes (6, 24, 25, 244, 269). The half-life of peroxynitrite at physiological pH in a cellular environment is short (~ 9 ms) which limits its diffusion distance to ~ 9 µm (24, 269). During the last decade the attention of researchers has been attracted to the study of the involvement of peroxynitrite in mechanisms of intracellular regulation which are not related to its toxic properties. Due to its short lifetime and short diffusion distance, the effect of peroxynitrite is limited by intracellular processes (6, 168), although a growing number of publications report the effect of peroxynitrite on vascular tone in various organs (81, 203, 204, 231, 283). Current data on the vasoactive properties of peroxynitrite and its role in modifying responses to other vasoactive stimuli are quite controversial (81, 203, 231, 283).
A second active sink for superoxide is the enzyme superoxide dismutase (SOD), which is traditionally considered to be the main antioxidant defense against superoxide radical (84, 87–89). Only the extracellular form, ecSOD, is present in the interstitial space; it is tetrameric and glycosylated unlike its dimeric cytosolic counterpart (88). It was found that the interstitium of the human vascular wall contains "very large amounts" of ecSOD (in some vessels more than 70% of total SOD activity) evenly distributed through the wall (210, 262). Extracellular SOD in rats is significantly different from that in humans. It is a dimer with low molecular weight (i.e., 85 kD) and with the absence of a heparin-binding domain, which determines its rapid turnover and very low concentration in the interstitium (46, 139). What is more, ecSOD activity in rat blood plasma is a hundred times higher than that in human plasma (139, 140). This carries with it the ability to delivery ecSOD to tissue with increased blood flow and high permeability of capillaries. The difference in mechanisms by which ecSOD controls superoxide concentration in humans and rats opens an opportunity for experimental studies manipulating superoxide with ecSOD and its mimetic. However, these special properties of ecSOD in rats impose certain restrictions on the transfer of experimental results obtained in rats to humans.
The rate of superoxide degradation by ecSOD is estimated at 2 to 4×109 M−1 s−1 (25, 88, 180), which is 4–5 orders of magnitude faster than that of artificial antioxidants. However, the rate of superoxide elimination by ecSOD is lower than that of the reaction of superoxide with nitric oxide.
The large amount of ecSOD in the vessel wall and its high specific activity may explain the lack of response of blood flow in skeletal muscle to administration of additional SOD (221, 222). A distinctive feature of the brain parenchyma is a low level of ecSOD compared with other organs (50). That explains the pronounced effect of additional administration of exogenous SOD on the tone of cerebral vessels (68–70, 310).
Hydrogen peroxide produced by dismutation of superoxide
Paradoxically, superoxide, which in most reactions acts as a weak reducing agent, is converted with the "antioxidant" enzyme SOD into a strong oxidant, hydrogen peroxide. This implies an alternative point of view: that by eliminating superoxide, SOD acts as an antireductant enzyme, having a direct protective antireductant role (214). Hydrogen peroxide is a stable substance and can accumulate in the tissue to a cytotoxic concentration, so a mechanism is needed to eliminate it. The enzyme catalase is necessary for the decomposition of H2O2; however, catalase activity in skeletal muscle is very low compared with the liver: 39 times lower in rabbit muscles (14) and 62-fold lower in rat muscles (131). Fast oxidative glycolytic muscles have half the amount of catalase that is present in slow oxidative muscles (130, 161). Given the absence of catalase activity in smooth muscle and endothelial cells of blood vessels (255), as well as the fact that the rate of decomposition of H2O 2 (3× 107 M−1 s−1, (67)) is lower than the rate of its production by ecSOD by two orders of magnitude, it can be assumed that the accumulation of H2O2 in muscle is quite possible and may be dependent on the rate of its removal by local blood flow. The experimentally established concentration of H2O2 in the dialysate obtained from a resting muscle is ~ 1.5 µM (287), and according to other workers the extracellular concentration in muscle can reach 5–8 µM (126, 261).
It is generally accepted that H2O2 is a signaling molecule that regulates diverse biological processes (85, 260, 289). Changes in vascular tone in response to hydrogen peroxide reported by different authors are quite heterogeneous. Arteriolar constriction was observed in isolated kidney in vivo (193, 268). Other workers have found relaxation of arterioles in the brain, mouse aorta and isolated human and rat coronary arteries (45, 125, 173, 242, 243) as well as a significant increase in muscle blood flow and respiration rate at rest and exercise (119). A biphasic change in vascular tone has been reported in isolated arterioles, in which the initial constriction turned into dilation, dependent on the concentration of H2O2 (64). It has also been reported that the vascular response to H2O2 depends on the diameter of arterial vessels (205), and on the age of the experimental animals (245). Regarding the mechanism of the vasoactive action of hydrogen peroxide, it is assumed that H2O2 is an endothelium derived hyperpolarizing factor (EDHF) that causes vascular relaxation by opening K+ channels and then by hyperpolarizing the membrane of vascular smooth muscle (42, 254). The low content of catalase in muscle tissue suggests that H2O2 is removed from the tissue by uptake into the blood, where the content of catalase is high. This makes the H2O2 signal relatively slow compared with the time scale of transients in demand and supply, associated with the onset of muscle contractions.
A parallel line of investigations of EDHFs was conducted during the last decade which examined the role of the gas signaling molecule hydrogen sulfide, H2S (174). There is good evidence to suggest that H2S is an EDHF, because it produces all the relevant physiological effects attributed to such a factor (201). The concern is that the necessary physiological effects were obtained at unrealistic concentrations of H2S, 100 µM, corresponding to a sublethal dose (209). It is surprising that these two lines of EDHF studies do not intersect, although the presence of H2O2 and H2S in the same volume inevitably leads to a specific reaction of detoxification of hydrogen sulfide, which ends with the formation of water and neutral sulfur (43, 120). If neutralization of toxic H2S is an important function of H2O2 produced from superoxide anion, then it may explain the need for high concentrations of hydrogen sulfide to obtain a vasomotor response and the biphasic character of that response.
NO/O2− CONTROL OF LOCAL CIRCULATION: CELL CIRCUIT
A large body of experimental and theoretical results support the concept of local regulation of microvascular tone, based on the interaction of the two complementary radicals NO and O2− (7, 17, 32, 62, 156, 171, 187, 272–274, 307, 310). The direct determinant of local blood flow is the state of the NO and O2− system rather than by-products of their interaction, which may act as metabolic vasodilators in the traditional scheme of hypoxic metabolic feedback. In order to construct a regulatory “cell circuit” (9), based only on NO/O2− interactions, we must consider revising the basic principle of local blood flow regulation and abandon the traditional model of proportional negative feedback control via elusive vasodilating metabolites produced by parenchymal cells.
In the virtual experiment discussed earlier, we replaced muscle fibers with glass fibers (to keep the interstitial volume unchanged), and found that a constant NO flux through the interstitial space from endothelial cells to the smooth muscle cells of the arterioles determines their relaxed state. Since the endothelium in arterioles is normally under a stable supply of oxygen and carbohydrates, so long as the NO production rate is steady and constant, arterioles are dilated and local flow is high. In the absence of influence from the parenchymal cells, the normal state of the arterioles is vasodilation; in other words, the arterioles function as a normally open gate valve. In this thought experiment, returning the resting muscle fibers to their places would change the status of the microcirculation to a state of low perfusion, corresponding to a typical minimal flow for resting muscle, limited by constricted arterioles. That indicates a neutralization of the NO flux by superoxide, produced mainly by skeletal muscle fibers under conditions of sufficient supply with oxygen and glucose.
The sufficiency condition can be defined as the rate of oxygen and substrate delivery equal to or even higher than their rate of consumption by mitochondria. At rest, glycolysis in muscle fibers provides a high level of cytosolic NADH and NADPH supply to NAD(P)H oxidase to produce superoxide into a relatively small interstitial space. Thus, in resting muscle the metabolic resources of the cell are partly used to regulate the necessary level of blood flow, which is ultimately determined by the low level of mitochondrial respiration. Similarly to the action of the float valve regulator of the fluid level in a tank (Fig. 2), when the oxygen and substrate supply to cells reaches some threshold level, the cells (predominantly parenchymal cells) produce a high level of superoxide into the interstitium, leading to a decrease in the vasodilator signal NO, thereby reducing blood flow to avoid excessive tissue oxygenation.
Released ADP in contracting muscle stimulates the resynthesis of ATP that requires increased flow of electrons from NADH, including the confiscation of reducing equivalents from the cytosol. Under this condition the export of mitochondrial citrate is stopped and the production of NADPH in the cytosol has to be interrupted. Thus, the activated mitochondria do not export NADPH to the cytosol and instead import NADH from the cytosol. Low levels of substrate for NAD(P)H oxidase in the cytosol decrease the yield of superoxide into the interstitium to a low level, which corresponds to the high level for the nitric oxide signal, dilation of arterioles and high local blood flow.
As a result of diffusion resistance and increased respiration, a steep PO2 gradient is formed in the perivascular space and PO2 in the muscle fibers is reduced. This is another limiter of superoxide production by NAD(P)H oxidase. In this model, the exercising muscle does not spend cellular resources on maintaining hyperemia via production of signaling metabolites. The cost for permanent endothelial NO production is compensated for by direct contact of these cells with an abundance of resources in the arterial blood. Note that the signal for increased perfusion is activated by muscle contraction, and not only by low oxygen concentration, whereas the simultaneous deficit of oxygen and glucose delivery alone may cause similar effects.
Bang-bang model: performance
To construct the cell circuit only well-known components are used: 1) the three types of cells that form a local interacting set – endothelium, vascular smooth muscle cells and muscle fibers; 2) four key enzymes – eNOS, sGC, NAD(P)H oxidase and ecSOD; 3) two complementary rapidly diffusing messengers – NO and O2−; and 4) metabolites – O2, glucose and lactate. Relatively slow external modulators, such as angiotensin II, are not considered for events on this time scale.
The behavior of the various components of this regulatory cell circuit can also be described under the assumption of Boolean logic, with two states – “High” and “Low” – and logical functions that determine this design of the regulatory network (see Table 1). The permanent signal generated by the local pool of endothelial cells is the flux of nitric oxide, which reports the availability of a sufficient amount of glucose and oxygen, [(NO) = (Glucose) AND (O2)], in the blood. Since an arteriole is a normally open valve, the normal value of the local blood flow is “High.” The level of superoxide in the interstitium can be presented as a function [(O2−) = (Glucose) AND (O2)] in parenchymal cells, or [(O2−) = (NADH) OR (NADPH)] AND (O2)]. A “High” level of interstitial superoxide serves as the logical operation NOT for the vasodilating signal of nitric oxide (Table 1), thus reducing local blood flow. When the production of O2− becomes lower than the rate of its dismutation by ecSOD in the interstitium, the signal state of nitric oxide in the interstitium is “High,” which corresponds to the dilation of arterioles.
Table 1.
States of the bang-bang regulatory system (presented as HIGH and LOW)
| State of muscle | Local Blood Flow |
VO2 | ISF PO2 | ISF O2− | ISF NO |
|---|---|---|---|---|---|
| Exercise | High | High | Low | Low | High |
| Rest | Low | Low | High | High | Low |
In contracting skeletal muscle low production of interstitial superoxide leads to high levels of nitric oxide in the wall of the arterioles, their dilation and increased local blood flow. At rest, a low metabolic rate increases local PO2 and superoxide production, which neutralizes nitric oxide, resulting in constriction of arterioles and decrease in blood flow.
The principle of operation of the proposed regulating cell circuit can be demonstrated by analogy with a simple float regulator (Fig. 2). This is a bang-bang regulator with negative feedback that closes the inflow when the preset level of fluid is reached. In our analogy it is the level of reducing agents and oxygen in the cytoplasm, depending on the balance between inflow of nutrients and O2 with the blood (gate valve) and their outflow, determined by the rate of consumption in mitochondria (bottom valve). When that level is low, the inflow gate valve (arterioles) is passively opened by the weight (labeled NO, Fig. 2), which provides a permanent opening force. When the inflow of supply materials exceeds their outflow, the level of fluid in tank (reducing agents and oxygen) rises to the set point, the float (O2− flux produced by NAD(P)H oxidase) closes the gate valve (arterioles) by neutralizing the opening force of NO, thus reducing the local blood flow. In this model, the inflow increased by a passive mechanism, while the upper limit of the oxygen and glucose supply is actively regulated.
Interaction between the components of the regulator can be demonstrated in more detail, using the analogy and symbolism of electronic circuits (Figs. 3 and 4). Compartmentalization of superoxide inside the narrow interstitial space makes the interstitium a key element in determining the state of the signaling radicals in intercellular communications. It is important to note that the input signal of glucose and oxygen availability in blood is mediated in the endothelium through cytosolic NAD(P)H. When the blood supply is sufficient, a constant flux of NO flows from endothelial cells (ECs) to vascular smooth muscle cells (VSMCs) (i.e., from eNOS to sGC) through the local interstitial space (Fig. 3). This flux is modulated by injection into the interstitium of superoxide produced by all types of cells present, but with the dominant contribution coming from the parenchymal cells. Thus, with respect to NO flux the interstitium acts as a chemical gate (Fig. 3), because the combination of NO diffusion and its reaction with O2− in the interstitial fluid significantly reduces the concentration of NO in the target VSMCs. Theoretical analysis of NO gradients, based on the rate of superoxide production in the interstitium, has been conducted by several authors (37, 40, 53, 158) who have shown a high sensitivity of NO output to variation of the input O2− signal; i.e., the system behaves as a high gain interstitial chemical transistor (Fig. 3). Furthermore, the interstitial space behaves as a gate for superoxide diffusion from NAD(P)H oxidase to ecSOD, so the interstitium should be presented as two interrelated gates for each signaling radical (Fig. 4).
Figure 3.
The flux of nitric oxide, continuously produced by microvascular endothelial cells, is transported by diffusion to smooth muscle cells in the walls of arterioles. The interstitial concentration and distance of NO propagation depends on the presence of superoxide injected into the extracellular space by NAD(P)H oxidase. Thus the interstitial space represents a chemical gate, which controls the NO flux to the smooth muscle cells of arterioles via emission of superoxide mainly by parenchymal cells. Graphically, this process can be presented as a kind of transistor where the source is endothelial eNOS, the drain is sGC of the VSMCs and the interstitium is a gate controlled by the concentration of superoxide. Similarly, the flow of superoxide to ecSOD is modulated by NO concentration in the interstitium, and the interstitium also can be represented as a gate for superoxide, controlled by the interstitial concentration of NO. This concept is based on the results of mathematical modeling for the propagation of NO and superoxide in a diffusion/reaction system (37, 40, 158).
Figure 4.
Hypothetical logical network (cell circuit) controlling local blood flow has three levels: microvessels, cells and interstitial space. Three types of cells are involved in the regulation of the local supply of oxygen and substrates: vascular smooth muscle cells (VSMC), endothelial cells (EC) and skeletal muscle cells (Skeletal MC). Basically, the signaling involves four enzymes: eNOS, NAD(P)H oxidase, sGC and ecSOD.
Under normal conditions in resting muscle, with enough glucose (Glu) and oxygen in the arterial blood, the endothelium gets these materials without limitation. The constitutive enzyme eNOS produces a constant NO flux using oxygen, cytosolic NADPH and L-arginine, which is recyclable. Thus, in this diagram eNOS is represented as a logical AND gate, which has a “High” output state if both input levels (Glu and O2) are “High.” When the level of oxygen or glucose in blood is “Low,” the NO output is “Low.” If the levels of the input signals (Glu and O2) are “High,” and the level of interstitial superoxide is “Low.” the flux of NO through interstitium reaches sGC in the VSMCs, the arteriolar gate is open, and local blood flow is “High.”
The flux of glucose and oxygen from the blood to the muscle fibers is limited by the diffusion resistance of the vascular wall, interstitium and the cells. At rest, with an adequate supply of glucose and oxygen, the level of reducing agents in the sarcoplasm is high and also the oxygen tension on the surface of muscle fibers is high. In this case NAD(P)H oxidase, located in sarcolemma, also works as an AND gate, producing a “High” level of superoxide in the interstitial space. This leads to the closure of the left interstitial NO gate and, consequently, to the “Low” level of local blood flow, corresponding to the state of resting muscle. In this cell circuit the pool of ecSOD actively reduces the concentration of superoxide in the interstitium, setting the set-point for the upper level of oxygen and glucose supply in resting muscle.
When the mitochondria in the muscle fibers are activated (switches at the mitochondria box are connected) the reducing agents are sequestered from the cytosol by mitochondria and oxygen tension on the cell surface is decreased due to increased consumption and diffusion limitations. “Low” levels of the input signals of NAD(P)H oxidase lead to “Low” levels of superoxide output. The activity of ecSOD in the interstitium and a “High” level of NO accelerate the transition process, so the left NO gate opens, while the right one (Superoxide gate) closes; the sGC output is “High,” dilating the arterioles and setting the blood flow “High.”
The endothelium of arterioles and capillaries is supplied by glucose and oxygen from the arterial blood with practically no diffusion limitations (Fig. 4). Glucose is transported across the endothelial cell membrane and then through the capillary wall via GLUT1 transporters, expressed at the highest level in endothelial cells (208). In heart 76% of GLUT1 is localized in the endothelium and only 24% in cardiac myocytes (66). The basal rate of glucose transport catalyzed by GLUT1 is high and independent of insulin (47).
Uptake of oxygen and glucose by the skeletal muscle fiber is limited by the diffusion resistance of the vessel wall, interstitium and the myocytes themselves. Additional resistance to glucose flux into muscle fibers is created by the rate-limiting transporter GLUT4 localized in the sarcolemma. GLUT4 is the predominant glucose transporter expressed in adipose tissue, skeletal muscle and heart muscle (66, 208, 275, 294, 295). The resistance offered by GLUT4 transporters to glucose flux is determined by their density in the cell membrane, which is regulated by translocation of GLUT4 to the sarcolemma under the influence of insulin, hypoxia and exercise (163, 229). In the absence of insulin, 65% of the resistance to transport of glucose is accounted for by the GLUT transporters. With an excess of insulin, the contribution of the membrane transporter to the overall resistance is reduced to 5%, the extracellular resistance accounts for 55% and the remaining 40% of the resistance is due to the phosphorylation of glucose. During exercise the extracellular portion of resistance to glucose flux is reduced to 5%, the membrane transporter accounts for 30% of the resistance and the remaining 65% of the resistance is associated with the phosphorylation of glucose (294, 295). Thus, the resistance to glucose flux into the muscle cell, shown in Fig. 4, is a multi-component variable, depending on the functional state of the muscle and the organism.
In resting muscle cells the supply rate of oxygen and glucose is high enough for cytosolic NAD(P)H production and for a high yield of superoxide into the interstitial compartment. The relatively small interstitial volume allows an effective concentration of superoxide to be reached rapidly. It is important to note that both types of cells, endothelial and striated muscle, function as logical AND gates, producing high outputs of NO and O2− when the states on the inputs, glucose and oxygen, are “High” (Fig. 4). Thus, the interstitium and the two fluxes of complementary radicals (two transistors in Fig. 4) form a logical latch with the apparent property of bistability, or the ability to exist in either of two stable states. The high level of superoxide sets the NO signal at VSMCs at a “Low” level, which leads to a “Low” level of arteriolar diameter and local blood flow. This mode corresponds to the resting state of a skeletal muscle.
During steady-state exercise (Fig. 4, switches at Mitochondria box are connected) the fall of superoxide production by sarcolemmal NAD(P)H oxidase is caused directly due to muscle fiber contractions which promote the import of reducing equivalents from the cytosol to the mitochondria. The decrease of PO2 on the surface of striated muscle fibers is a secondary consideration, although its contribution to the reduction of superoxide production is not in doubt.
Thus, the regulatory signal is not the tissue PO2 when reduced below some standard level, but rather the functional state of the cells, caused by an insufficient supply of substrates and oxygen to muscle cells. In that case, there is no need for a specific oxygen sensor, set for a standard value of tissue PO2, below which the cells release the hypothetical metabolic vasodilators. The increase of mitochondrial respiration is sufficient to siphon off the cytosolic reducing equivalents and cause extracellular superoxide production to fall to the rate of its dismutation by ecSOD. The presence of a highly active ecSOD in the interstitium creates asymmetry in the interstitial NO/O2− latch gate, speeding up the transition to a “High” level for NO and accompanying high blood flow in the capillaries. In regard to the flux of superoxide, emitted into the small interstitial volume, ecSOD acts as a “bleeder,” preventing integration of the O2− signal and improving the dynamic characteristics of the regulator. Ultimately ecSOD activity in the interstitium may influence a set point for blood flow in resting muscle, and indirectly the corresponding PO2 in the interstitium. However, low levels of ecSOD in rat tissues show that this enzyme is not a necessary element of local blood flow regulation.
The dynamic behavior of the bang-bang controller is determined by the threshold and width of the dead-band, which are set by the activity of the enzymes and the relative volume of the interstitium. In addition, there are internal positive feedbacks supporting bistability of the latch switching between the states of high and low blood flow. A high level of blood flow in the contracting muscles stimulates the production of NO by increased shear stress on the endothelium, thus stabilizing the vasodilation. In resting muscle the excess of muscle blood flow causes an increase in interstitial PO2, thus increasing superoxide production by NAD(P)H oxidase, which blocks the conductivity of the NO gate in the cell circuit. External disturbances such as the administration of an NO donor or SOD mimetic should promote vasodilation and elevate the PO2 in the muscle at rest. The administration of an excessive amount of glucose and oxygen should open the right superoxide gate, which will cause the closing of the left NO gate and arteriolar vasoconstriction. Conversely, insulin-induced hypoglycemia produces hyperemia in skeletal muscle (115) by decreased production of superoxide, in accordance with the proposed model. Similarly, other external influences on the regulatory cell circuit can be interpreted, such as long-term administration of an antagonist of angiotensin II receptors used mainly to treat high blood pressure. Suppression of the angiotensin II-stimulated production of superoxide by NAD(P)H oxidase promotes a shift to the opening of the left NO gate and reduces arteriolar tone.
Bang-bang model: applications and interpretations
Transitions between the rest and exercise states in muscle
On a shorter time scale the transitions between rest and exercise in a muscle (i.e., changing the states of the latch in Fig. 4) are not instantaneous and detailed study of the transients in interstitial concentrations of oxygen, NO, O2− and products of their interactions can provide information about the cellular circuitry of the controller. It is also necessary to make testable predictions of the controller’s behavior under different experimental conditions.
At the onset of contractions in a previously resting muscle the activation of glycolysis leads to an excess of lactate production and a shift in the cytosolic redox balance toward the direction of increasing availability of NADH and superoxide production (188). During this time, the NO signal is blocked, thereby delaying the development of arteriolar dilation. That process lasts until the activated mitochondria begin recycling the NADH to NAD+, and PO2 on the surface of muscle fibers decreases. Superoxide production drops to a low level allowing the NO level to become high and the arterioles are dilated. That sequence of events can explain the observed latency in the development of hyperemia reported previously by Gorczynski and Duling (100, 101). More recent studies using a single muscle fiber showed a similar response: about 10–15 seconds after the first onset of muscle contraction, intracellular PO2 remained at the resting level, and then started to decline (146). The latency in activation of mitochondria after the first onset of contraction in a single muscle fiber has also been reported (95). A similar time delay has been reported in microvascular PO2 in skeletal muscle in situ (27, 219).
Considering this phenomenon, the latency is not a propagation delay for the signal carried by a metabolic vasodilator, but rather a built-in muscle cell timer that delays the drop of peripheral resistance for a time period needed to increase heart rate and blood pressure via the exercise pressor reflex induced mainly from contraction of fast-twitch fibers (215).
The termination of muscle contraction and the transition back to the resting state generate the most informative transients in variables characterizing the controller in its active process of regulation. At this moment the respiration rate of mitochondria is still high, and therefore the interstitial PO2 and superoxide are at their “Low” states. The endothelium is in direct contact with blood and the rate of NO production is high due to the elevated shear stress on the endothelium, so that the recovery of interstitial PO2 can be faster than the restoration of the basal respiration rate.
The increase of interstitial PO2 and decrease of the respiration rate will approach a threshold level corresponding to a sufficient cell supply of oxygen and substrate, producing an increased yield of superoxide to the interstitium which will switch the controller into a low blood flow state. The rapid rise of PO2 by the post-contraction hyperemia produces a positive feedback that accelerates the transition back to the resting state of muscle and the microcirculation. Another positive feedback is the flow-stimulated increase of NO production by the endothelium at the onset of active hyperemia. These two opposing positive feedbacks characterize the bistability of the flow regulator, so that the appearance of a gradual response of muscle blood flow is rather due to the stochastic effect of switching multiple elementary regulators throughout the tissue.
Reactive hyperemia
In the metabolic theory of local blood flow regulation, reactive and active hyperemias are similar in the mechanisms of their formation: the basis of both reactions is cellular hypoxia that evokes the production and accumulation of metabolic vasodilators and then the relaxation of VSMCs in arterioles. In our model, reactive hyperemia is different from active hyperemia in that, after a period of time following blood flow arrest, the endothelium and other cells experience limitations in metabolic resources, and are unable to effectively produce NO. Therefore the behavior of the system during reperfusion is determined by the duration of the ischemia (41, 134, 175) and the presence of oxygen in the blood (80). In this case, reactive hyperemia caused by widespread arrest of blood flow may be different from reactive hyperemia caused by localized tissue compression with a supra-systolic pressure. Reperfusion with oxygenated blood primarily provides the supply for endothelial cells, which restore NO production and support vasodilation, while the production of O2− in striated muscle fibers is delayed by the time required to restore high-energy phosphates by the mitochondria. Only after the restoration of high-energy phosphates can the resumption of superoxide production lead to recovery from reactive hyperemia. In the absence of oxygen in the blood, recovery from reactive hyperemia does not occur (80), a result which is consistent with the proposed model.
Capillary density
It is worthwhile to investigate the effect of morphological correlations on the balance of NO/O2− and the behavior of the local blood flow controller. It is known that the spatial density of microvessels in oxidative muscles is higher than in glycolytic muscles (28, 83, 225). Accordingly, the volume share of endothelial tissue is greater in oxidative muscle fibers, which can account for the higher specific production NO in this tissue.
Volumes of distribution for mediators
For the sake of simplicity, the diagram (Fig. 4) does not display one type of important functional element of the regulator, which is the capacity or volume of distribution for the radical mediators. Nitric oxide is distributed in almost the entire tissue volume. The volume of distribution for extracellular superoxide, however, is an order of magnitude smaller than that for NO. That fact makes the response time of the superoxide signal much shorter than that of the much more stable signal of NO. The ratio of these volumes may also affect the stability of the regulation and control the width of the dead-band of the switching between on/off states. Changes of these volumes under physiological and pathological conditions due to age, edema, etc. can affect the performance of the local regulation of blood flow.
NO measurements in tissue
The dynamic nature of interstitial NO/O2−, related to the current status of the latch (Fig. 4), may clarify the difficulties experienced in determining the "real physiological NO concentration in vivo" (53, 108). The concentration of NO is a dynamic variable, which is low in resting muscle due to its neutralization by superoxide. Measurement of the NO concentration with fluorescent probes in that case cannot be successful because of the relatively slow reaction of the probes with NO, compared with the NO reaction with superoxide. However, based on the proposed model, we can expect a sharp increase in the detection of NO in muscle during its transition from the resting to the contracting state. Detection of superoxide with fluorescent probes is even more difficult because of the presence of two highly reactive competitors for the probe, NO and ecSOD.
Oxidative stress
Local blood flow and interstitial PO2 in resting muscle can change in response to the experimental impact of changes in the controller parameters (Fig. 4). For instance, the predicted response to inhibition of eNOS or scavenging NO is commonly known; it is excessive vasoconstriction and reduced tissue PO2. The opposite reaction is observed during the administration of NO or its donors: excessive vasodilation and increased PO2 in the tissue. The same effect can be obtained by impacting the opposite component of the interstitial latch, related to the status of the superoxide level (Fig. 4). In the proposed model, the administration of nitric oxide donors to address myocardial ischemia or hypertension should not be accompanied with the infusion of glucose and oxygen inhalation. An experimental decrease in ecSOD activity through an inhibitor would cause the endothelium-dependent contraction of the VSMCs (290), while an increase of this activity using the antioxidant Tempol would reduce arteriolar tone (300), resulting in increased tissue oxygenation. It must be emphasized that the production of superoxide in tissue is not a consequence of “oxidative stress,” but is rather a signal preventing excessive tissue oxygenation and the damaging effects of oxygen. Enthusiasm for the clinical application of various “antioxidants” should be balanced with an understanding of their adverse effects on local blood flow regulation.
Conclusion
"For every problem there is a solution which is simple, clean and wrong" (Henry Louis Mencken). This well-known aphorism might be well suited to the metabolic theory of local blood flow regulation, which has dominated cardiovascular physiology for over a century, due in part to its exceptional clarity and simplicity. However, it is time to admit that the metabolic theory of flow regulation, using proportional feedback by producing vasodilator metabolites in response to a lack of oxygen, does not have quantitative experimental support and is ineffective for solving the problems of practical medicine. For example, in the case of coronary vasospasm (angina pectoris), a physician would not wait for sufficient accumulation of metabolic vasodilators, but rather would immediately administer a donor of nitric oxide, which relaxes blood vessels. Despite the great pharmacological potential of hypothetical metabolically-linked vasodilators, the search for such agents has been abandoned in favor of drugs which successfully control the tone of the microvasculature via NO signaling pathways. During the last decade researchers recognized the importance of physiological radicals in cell-to-cell communication. A significant amount of evidence has been accumulated that a complementary pair of radicals, NO and O2−, provide the signaling mechanism involved in physiological and pathological processes related to communication between microvessels and the parenchyma in organs.
We propose a model of local regulation of blood flow, providing matching supply and demand in tissue associated with changing functional and metabolic activity of a muscle. We assume that this model works in tissues other than muscles (possibly with some modification in the lungs); however, we are focused on muscles, because their activity can be accurately controlled in experiments, and because cardiac muscle is critical to the general health and life of an organism. In the metabolic theory the error signal for negative feedback control is generated by parenchymal cells in response to a lack of oxygen. Thus, in order to maintain arteriolar vasodilation for prolonged active hyperemia, the parenchymal cells must reside in an extended hypoxic condition. In other words, the goal of regulation, i.e., the elimination of hypoxia in contracting muscle, cannot be achieved in principle. In contrast to this theory, we propose a model that actively controls the upper level of the supply rate for oxygen and glucose under resting conditions, so that the constriction of arterioles works as a level control valve to prevent cells from being exposed to an excessive supply of oxygen and substrates. This “gate valve” opens passively in response to increased activity of skeletal muscle fibers and it is actively closed at rest, forming a simple and effective circuit of bang-bang regulation. The proposed regulator employs only well known enzymes and small molecules, so there is no need to search for elusive metabolites. It does not require a special “device” for proportional control, as does the metabolic theory, and it is optimal in the sense of regulatory speed.
This regulation is physiologically justified because it integrates the vascular response and the metabolic state of parenchymal cells, i.e., their supply of oxygen and substrates. The state of cell satiety is a signal to restrict delivery of oxygen and substrates, thereby saving cost to the organism for the circulation and respiration and ensuring for the well being of tissue cells. The proposed regulator is not aimed at maintaining a standard PO2 in the tissue, so in different tissues and under different functional states the PO2 can vary. The behavior of the controller is in good agreement with experimental data on the change of muscle blood flow in various physiological conditions. The model opens an approach to study the roles of lactate, glucose and blockers of the angiotensin II pathway in local regulation through long-term influences on the external elements of the NO/O2− cell circuit. A better understanding of the importance of the balance between signaling radicals in the interstitium may help to dampen enthusiasm for the daily use of "antioxidants" aimed at the prevention of aging and a wide range of diseases. Analysis of the functional disturbances in the elements and the topology of the controller cell circuit can provide insight into the development of pathological conditions and interventions for their correction. We are aware that the proposed model also looks simple and clear, but we hope that efforts focused on its experimental verification will help to break the stalemate of the metabolic theory of local blood flow regulation.
PERSPECTIVES.
The study of metabolic regulation of local blood flow has led to a century-long effort to identify specific hypoxia-induced metabolites responsible for active and reactive hyperemia. We propose a model of active vasoregulation in skeletal muscle which comes into effect when the supply of oxygen and glucose exceeds the demand, leading to increased production of superoxide by membrane NADPH oxidase into the interstitial space and subsequent neutralization of the interstitial NO. This conceptual model can be generalized to other organs such as myocardium and brain. We expect that future research to investigate the validity of this model should lead to a better understanding of deficiencies associated with matching blood flow and O2 supply to altered O2 demand, as expressed in various pathological states, such as hypertension and diabetes, and in aging.
ACKNOWLEDGEMENTS
We thank the reviewers for their thoughtful comments and suggestions which led to substantial clarification of the content and strengthening of the arguments of this article. The work was supported by grant R01 HL18292 from the National Heart, Lung and Blood Institute.
REFERENCES
- 1.Afanas'ev I. Interplay between superoxide and nitric oxide in aging and diseases. Biogerontology. 2004;5:267–270. doi: 10.1023/B:BGEN.0000038047.96106.ad. [DOI] [PubMed] [Google Scholar]
- 2.Afanas'ev I. ROS and RNS signaling in heart disorders: could antioxidant treatment be successful? Oxid Med Cell Longev. 2011;2011:293769. doi: 10.1155/2011/293769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afanas'ev I. Signaling of reactive oxygen and nitrogen species in Diabetes mellitus. Oxid Med Cell Longev. 2010;3:361–373. doi: 10.4161/oxim.3.6.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afanas'ev IB. On mechanism of superoxide signaling under physiological and pathophysiological conditions. Med Hypotheses. 2005;64:127–129. doi: 10.1016/j.mehy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Afanas'ev IB. Signaling functions of free radicals superoxide & nitric oxide under physiological & pathological conditions. Mol Biotechnol. 2007;37:2–4. doi: 10.1007/s12033-007-0056-7. [DOI] [PubMed] [Google Scholar]
- 6.Afanas'ev IB. Signaling Mechanisms of Oxygen and Nitrogen Free Radicals. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- 7.Ahmeda AF, Johns EJ. The regulation of blood perfusion in the renal cortex and medulla by reactive oxygen species and nitric oxide in the anaesthetised rat. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2011.02346.x. [DOI] [PubMed] [Google Scholar]
- 8.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alon U. Simplicity in biology. Nature. 2007;446:497. doi: 10.1038/446497a. [DOI] [PubMed] [Google Scholar]
- 10.Altura BM. Chemical and humoral regulation of blood flow through the precapillary sphincter. Microvasc Res. 1971;3:361–384. doi: 10.1016/0026-2862(71)90039-2. [DOI] [PubMed] [Google Scholar]
- 11.Andrews AM, Jaron D, Buerk DG, Kirby PL, Barbee KA. Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide. 2011;23:335–342. doi: 10.1016/j.niox.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andries LJ, Brutsaert DL, Sys SU. Nonuniformity of endothelial constitutive nitric oxide synthase distribution in cardiac endothelium. Circ Res. 1998;82:195–203. doi: 10.1161/01.res.82.2.195. [DOI] [PubMed] [Google Scholar]
- 13.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 14.Arkin A, Fink EB. The influence of an oxidizing substance (sodium iodoxybenzoate) on the catalase value of the blood and tissues. J Infect Dis. 1918;22:515–522. [Google Scholar]
- 15.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babior BM. Oxygen-dependent microbial killing by phagocytes (second of two parts) N Engl J Med. 1978;298:721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- 17.Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H1404–H1410. doi: 10.1152/ajpheart.00235.2003. [DOI] [PubMed] [Google Scholar]
- 18.Barcroft H. An enquiry into the nature of the mediator of the vasodilatation in skeletal muscle in exercise and during circulatory arrest. J Physiol. 1972;222:99P–118P. doi: 10.1113/jphysiol.1972.sp009826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barron JT, Gu L, Parrillo JE. Malate-aspartate shuttle, cytoplasmic NADH redox potential, and energetics in vascular smooth muscle. J Mol Cell Cardiol. 1998;30:1571–1579. doi: 10.1006/jmcc.1998.0722. [DOI] [PubMed] [Google Scholar]
- 20.Barron JT, Gu L, Parrillo JE. NADH/NAD redox state of cytoplasmic glycolytic compartments in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2000;279:H2872–H2878. doi: 10.1152/ajpheart.2000.279.6.H2872. [DOI] [PubMed] [Google Scholar]
- 21.Basini G, Grasselli F, Bianco F, Tirelli M, Tamanini C. Effect of reduced oxygen tension on reactive oxygen species production and activity of antioxidant enzymes in swine granulosa cells. Biofactors. 2004;20:61–69. doi: 10.1002/biof.5520200201. [DOI] [PubMed] [Google Scholar]
- 22.Baudry N, Laemmel E, Vicaut E. In vivo reactive oxygen species production induced by ischemia in muscle arterioles of mice: involvement of xanthine oxidase and mitochondria. Am J Physiol Heart Circ Physiol. 2008;294:H821–H828. doi: 10.1152/ajpheart.00378.2007. [DOI] [PubMed] [Google Scholar]
- 23.Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JS. The Physiological and Pathological Chemistry of Nitric Oxide. In: Jack Lancaster J, editor. Nitric Oxide. San Diego, CA: Academic Press, Inc.; 1996. pp. 1–82. [Google Scholar]
- 25.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 26.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 27.Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol. 2002;133:229–239. doi: 10.1016/s1569-9048(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 28.Bennett RA, Pittman RN, Sullivan SM. Capillary spatial pattern and muscle fiber geometry in three hamster striated muscles. Am J Physiol. 1991;260:H579–H585. doi: 10.1152/ajpheart.1991.260.2.H579. [DOI] [PubMed] [Google Scholar]
- 29.Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol. 1997;272:H2693–H2700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- 30.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 31.Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101:2206–2212. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- 32.Bharadwaj LA, Prasad K. Mechanism of superoxide-anion induced modulation ov vascular tone. International Journal of Angiology. 2002;11:23–29. [Google Scholar]
- 33.Bissel CC. A history of automatic control. In: Nof SY, editor. Springer Handbook of Automation. Berlin-Heidelberg: Springer-Verlag; 2009. pp. 53–70. [Google Scholar]
- 34.Boron WF, Boulpaep EL. Medical Physiology (updated edition) New Heaven: Elsvier Saunders; 2005. [Google Scholar]
- 35.Broughton BR, Donald JA. Nitric oxide regulation of the central aortae of the toad Bufo marinus occurs independently of the endothelium. J Exp Biol. 2002;205:3093–3100. doi: 10.1242/jeb.205.19.3093. [DOI] [PubMed] [Google Scholar]
- 36.Bucher T, Brauser B, Conze A, Klein F, Langguth O, Sies H. State of oxidation-reduction and state of binding in the cytosolic NADH-system as disclosed by equilibration with extracellular lactate-pyruvate in hemoglobin-free perfused rat liver. Eur J Biochem. 1972;27:301–317. doi: 10.1111/j.1432-1033.1972.tb01840.x. [DOI] [PubMed] [Google Scholar]
- 37.Buerk DG. Mathematical modeling of the interaction between oxygen, nitric oxide and superoxide. Adv Exp Med Biol. 2009;645:7–12. doi: 10.1007/978-0-387-85998-9_2. [DOI] [PubMed] [Google Scholar]
- 38.Buerk DG. Nitric oxide regulation of microvascular oxygen. Antioxid Redox Signal. 2007;9:829–843. doi: 10.1089/ars.2007.1551. [DOI] [PubMed] [Google Scholar]
- 39.Buerk DG, Barbee KA, Jaron D. Modeling O-dependent effects of nitrite reductase activity in blood and tissue on coupled NO and O transport around arterioles. Adv Exp Med Biol. 2011;701:271–276. doi: 10.1007/978-1-4419-7756-4_36. [DOI] [PubMed] [Google Scholar]
- 40.Buerk DG, Lamkin-Kennard K, Jaron D. Modeling the influence of superoxide dismutase on superoxide and nitric oxide interactions, including reversible inhibition of oxygen consumption. Free Radic Biol Med. 2003;34:1488–1503. doi: 10.1016/s0891-5849(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 41.Burton KS, Johnson PC. Reactive hyperemia in individual capillaries of skeletal muscle. Am J Physiol. 1972;223:517–524. doi: 10.1152/ajplegacy.1972.223.3.517. [DOI] [PubMed] [Google Scholar]
- 42.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 43.Cadena F, Peters RW. Evaluation of chemical oxidizers for hydrogen sulfide control. J Water Pollut Control Fed. 1988;60:1259–1263. [Google Scholar]
- 44.Cannon WB. The wisdom of the body. New York: W.W. Norton & Co., inc.; 1932. [Google Scholar]
- 45.Capettini LS, Cortes SF, Gomes MA, Silva GA, Pesquero JL, Lopes MJ, Teixeira MM, Lemos VS. Neuronal nitric oxide synthase-derived hydrogen peroxide is a major endothelium-dependent relaxing factor. Am J Physiol Heart Circ Physiol. 2008;295:H2503–H2511. doi: 10.1152/ajpheart.00731.2008. [DOI] [PubMed] [Google Scholar]
- 46.Carlsson LM, Marklund SL, Edlund T. The rat extracellular superoxide dismutase dimer is converted to a tetramer by the exchange of a single amino acid. Proc Natl Acad Sci U S A. 1996;93:5219–5222. doi: 10.1073/pnas.93.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab. 2009;297:E836–E848. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol. 2011;111:1527–1538. doi: 10.1152/japplphysiol.00895.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakravarthy U, Stitt AW, McNally J, Bailie JR, Hoey EM, Duprex P. Nitric oxide synthase activity and expression in retinal capillary endothelial cells and pericytes. Curr Eye Res. 1995;14:285–294. doi: 10.3109/02713689509033528. [DOI] [PubMed] [Google Scholar]
- 50.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- 52.Chandel NS, Schumacker PT. Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol. 2000;88:1880–1889. doi: 10.1152/jappl.2000.88.5.1880. [DOI] [PubMed] [Google Scholar]
- 53.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–1198. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Buerk DG, Barbee KA, Jaron D. A model of NO/O2 transport in capillary-perfused tissue containing an arteriole and venule pair. Ann Biomed Eng. 2007;35:517–529. doi: 10.1007/s10439-006-9236-z. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Gill PS, Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol. 2005;289:F749–F753. doi: 10.1152/ajprenal.00115.2005. [DOI] [PubMed] [Google Scholar]
- 56.Cieslar J, Huang MT, Dobson GP. Tissue spaces in rat heart, liver, and skeletal muscle in vivo. Am J Physiol. 1998;275:R1530–R1536. doi: 10.1152/ajpregu.1998.275.5.R1530. [DOI] [PubMed] [Google Scholar]
- 57.Clanton TL. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol. 2007;102:2379–2388. doi: 10.1152/japplphysiol.01298.2006. [DOI] [PubMed] [Google Scholar]
- 58.Clark ER, Clark EL. B. The relation of the "Rouget" cells to capillary contractility. Am J Anat. 1925;35:265–282. [Google Scholar]
- 59.Clark ER, Clark EL. A. The development of adventitial (Rouget) cells on the blood capillaries of amphibian larvae. Am J Anat. 1925;35:239–268. [Google Scholar]
- 60.Clifford PS. Local control of blood flow. Adv Physiol Educ. 2011;35:5–15. doi: 10.1152/advan.00074.2010. [DOI] [PubMed] [Google Scholar]
- 61.Copp SW, Hirai DM, Ferguson SK, Musch TI, Poole DC. Role of neuronal nitric oxide synthase in modulating microvascular and contractile function in rat skeletal muscle. Microcirculation. 2011;18:501–511. doi: 10.1111/j.1549-8719.2011.00111.x. [DOI] [PubMed] [Google Scholar]
- 62.Cosentino F, Sill JC, Katusic ZS. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension. 1994;23:229–235. doi: 10.1161/01.hyp.23.2.229. [DOI] [PubMed] [Google Scholar]
- 63.Crawford DG, Fairchild HM, Guyton AC. Oxygen lack as a possible cause of reactive hyperemia. Am J Physiol. 1959;197:613–616. doi: 10.1152/ajplegacy.1959.197.3.613. [DOI] [PubMed] [Google Scholar]
- 64.Cseko C, Bagi Z, Koller A. Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. J Appl Physiol. 2004;97:1130–1137. doi: 10.1152/japplphysiol.00106.2004. [DOI] [PubMed] [Google Scholar]
- 65.D'Agostino DP, Putnam RW, Dean JB. Superoxide (*O2− ) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J Neurophysiol. 2007;98:1030–1041. doi: 10.1152/jn.01003.2006. [DOI] [PubMed] [Google Scholar]
- 66.Davey KA, Garlick PB, Warley A, Southworth R. Immunogold labeling study of the distribution of GLUT-1 and GLUT-4 in cardiac tissue following stimulation by insulin or ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H2009–H2019. doi: 10.1152/ajpheart.00663.2006. [DOI] [PubMed] [Google Scholar]
- 67.Deisseroth A, Dounce AL. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970;50:319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 68.Demchenko IT, Boso AE, Bennett PB, Whorton AR, Piantadosi CA. Hyperbaric oxygen reduces cerebral blood flow by inactivating nitric oxide. Nitric Oxide. 2000;4:597–608. doi: 10.1006/niox.2000.0313. [DOI] [PubMed] [Google Scholar]
- 69.Demchenko IT, Boso AE, O'Neill TJ, Bennett PB, Piantadosi CA. Nitric oxide and cerebral blood flow responses to hyperbaric oxygen. J Appl Physiol. 2000;88:1381–1389. doi: 10.1152/jappl.2000.88.4.1381. [DOI] [PubMed] [Google Scholar]
- 70.Demchenko IT, Gutsaeva DR, Moskvin AN, Zhilyaev SY. Involvement of extracellular superoxide dismutase in regulating brain blood flow. Neurosci Behav Physiol. 2010;40:173–178. doi: 10.1007/s11055-009-9240-5. [DOI] [PubMed] [Google Scholar]
- 71.Deonikar P, Kavdia M. A computational model for nitric oxide, nitrite and nitrate biotransport in the microcirculation: effect of reduced nitric oxide consumption by red blood cells and blood velocity. Microvasc Res. 2010;80:464–476. doi: 10.1016/j.mvr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Didion SP, Faraci FM. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2002;282:H688–H695. doi: 10.1152/ajpheart.00576.2001. [DOI] [PubMed] [Google Scholar]
- 73.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 74.Duling BR. Microvascular responses to alterations in oxygen tension. Circ Res. 1972;31:481–489. doi: 10.1161/01.res.31.4.481. [DOI] [PubMed] [Google Scholar]
- 75.Duling BR. Oxygen sensitivity of vascular smooth muscle. II. In vivo studies. Am J Physiol. 1974;227:42–49. doi: 10.1152/ajplegacy.1974.227.1.42. [DOI] [PubMed] [Google Scholar]
- 76.Duling BR, Pittman RN. Oxygen tension: dependent or independent variable in local control of blood flow? Fed Proc. 1975;34:2012–2019. [PubMed] [Google Scholar]
- 77.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 78.Elliott WH, Elliott DC. Biochemistry and Molecular Biology. Oxford, New York: Oxford University Press; 1997. [Google Scholar]
- 79.Elliott WH, Elliott DC. Biochemistry and Molecular Biology. Oxford University Press; 2009. [Google Scholar]
- 80.Fairchild HM, Ross J, Guyton AC. Failure of recovery from reactive hyperemia in the absence of oxygen. Am J Physiol. 1966;210:490–492. doi: 10.1152/ajplegacy.1966.210.3.490. [DOI] [PubMed] [Google Scholar]
- 81.Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 82.Feletou M, Kohler R, Vanhoutte PM. Nitric oxide: Orchestrator of endothelium-dependent responses. Ann Med. 2012;44:694–716. doi: 10.3109/07853890.2011.585658. [DOI] [PubMed] [Google Scholar]
- 83.Folkow B, Hakicka HD. A comparison between "red" and "white" muscle with respect to blood supply, capillary surface area and oxygen uptake during reat and exercise. Microvascular Research. 1968;1:1–14. [Google Scholar]
- 84.Forman HJ, Fridovich I. Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys. 1973;158:396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- 85.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 87.Fridovich I. Superoxide anion radical (O2−.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 88.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 89.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fulton GP, Lutz BR. The Neuro-Motor Mechanism of the Small Blood Vessels of the Frog. Science. 1940;92:223–224. doi: 10.1126/science.92.2384.223. [DOI] [PubMed] [Google Scholar]
- 91.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999;19:235–251. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- 92.Furchgott RF. Nitric oxide: from basic research on isolated blood vessels to clinical relevance in diabetes. An R Acad Nac Med (Madr) 1998;115:317–331. [PubMed] [Google Scholar]
- 93.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- 94.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 95.Gandra PG, Nogueira L, Hogan MC. Mitochondrial activation at the onset of contractions in isolated myofibres during successive contractile periods. J Physiol. 2012;590:3597–3609. doi: 10.1113/jphysiol.2012.232405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gentry RM, Johnson PC. Reactive hyperemia in arterioles and capillaries of frog skeletal muscle following microocclusion. Circ Res. 1972;31:953–965. doi: 10.1161/01.res.31.6.953. [DOI] [PubMed] [Google Scholar]
- 97.Gnaiger E, Steinlechner-Maran R, Mendez G, Eberl T, Margreiter R. Control of mitochondrial and cellular respiration by oxygen. J Bioenerg Biomembr. 1995;27:583–596. doi: 10.1007/BF02111656. [DOI] [PubMed] [Google Scholar]
- 98.Golub AS, Pittman RN. Oxygen dependence of respiration in rat spinotrapezius muscle in situ. Am J Physiol Heart Circ Physiol. 2012;303:H47–H56. doi: 10.1152/ajpheart.00131.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Golubew A. Beitrage zur Kenntniss des Baues und der Entwicklungsgeschichte der CApillargefasse des Frosches. Arch f mikr Anatom. 1869;5 [Google Scholar]
- 100.Gorczynski RJ, Duling BR. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol. 1978;235:H505–H515. doi: 10.1152/ajpheart.1978.235.5.H505. [DOI] [PubMed] [Google Scholar]
- 101.Gorczynski RJ, Klitzman B, Duling BR. Interrelations between contracting striated muscle and precapillary microvessels. Am J Physiol. 1978;235:H494–H504. doi: 10.1152/ajpheart.1978.235.5.H494. [DOI] [PubMed] [Google Scholar]
- 102.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 103.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 104.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 105.Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol. 1972;34:13–46. doi: 10.1146/annurev.ph.34.030172.000305. [DOI] [PubMed] [Google Scholar]
- 106.Haldane JS. The New Physiology. Science. 1916;44:619–631. doi: 10.1126/science.44.1140.619. [DOI] [PubMed] [Google Scholar]
- 107.Haldane JS. The physlosophy of a biologist. London: Oxford University Press; 1935. [Google Scholar]
- 108.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology. Philadelphia, PA: Saunders/Elsevier; 2011. [Google Scholar]
- 110.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 111.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 112.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 113.Harris PD, Longnecker DE. Significance of precapillary sphincter activity for microcirculatory function. Microvasc Res. 1971;3:385–395. doi: 10.1016/0026-2862(71)90040-9. [DOI] [PubMed] [Google Scholar]
- 114.Hart Y, Antebi YE, Mayo AE, Friedman N, Alon U. Design principles of cell circuits with paradoxical components. Proc Natl Acad Sci U S A. 2012;109:8346–8351. doi: 10.1073/pnas.1117475109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hickner RC, Ungerstedt U, Henriksson J. Regulation of skeletal muscle blood flow during acute insulin-induced hypoglycemia in the rat. Diabetes. 1994;43:1340–1344. doi: 10.2337/diab.43.11.1340. [DOI] [PubMed] [Google Scholar]
- 116.Hill AV, Long CN, Lupton H. Muscular exercise, Lactic acid and the Supply and Utilisation of Oxygen. Proc R Soc Lond B. 1924;97:155–176. [Google Scholar]
- 117.Hill DK. Oxygen tension and the respiration of resting frog's muscle. J Physiol. 1948;107:479–495. doi: 10.1113/jphysiol.1948.sp004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hilton SM, Hudlicka O, Marshall JM. Possible mediators of functional hyperaemia in skeletal muscle. J Physiol. 1978;282:131–147. doi: 10.1113/jphysiol.1978.sp012453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirai DM, Copp SW, Schwagerl PJ, Musch TI, Poole DC. Acute effects of hydrogen peroxide on skeletal muscle microvascular oxygenation from rest to contractions. J Appl Physiol. 2011;110:1290–1298. doi: 10.1152/japplphysiol.01489.2010. [DOI] [PubMed] [Google Scholar]
- 120.Hoffmann MR. Kinetics and mechanism of oxidation of hydrogen sulfide by hydrogen peroxide in acidic solution. Environmental science and technology. 1977;11:61–66. [Google Scholar]
- 121.Honig CR, Myers HA. Evaluation of possible adrenergic and cholinergic mechanisms in sympathetic vasodilation. Fed Proc. 1968;27:1379–1383. [PubMed] [Google Scholar]
- 122.Hudlicka O, Zweifach BW, Tyler KR. Capillary recruitment and flow velocity in skeletal muscle after contractions. Microvasc Res. 1982;23:201–213. doi: 10.1016/0026-2862(82)90065-6. [DOI] [PubMed] [Google Scholar]
- 123.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 124.Ignarro LJ. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989;3:31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- 125.Iida Y, Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- 126.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal. 2011;15:2477–2486. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol. 2007;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 128.Jackson WF, Duling BR. The oxygen sensitivity of hamster cheek pouch arterioles. In vitro and in situ studies. Circ Res. 1983;53:515–525. doi: 10.1161/01.res.53.4.515. [DOI] [PubMed] [Google Scholar]
- 129.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 130.Jenkins RR, Tengi J. Catalase activity in skeletal muscle of varying fibre types. Experientia. 1981;37:67–68. doi: 10.1007/BF01965573. [DOI] [PubMed] [Google Scholar]
- 131.Ji LL, Dillon D, Wu E. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physiol. 1990;258:R918–R923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- 132.Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59:483–495. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- 133.Johnson PC. Renaissance in the microcirculation. Circ Res. 1972;31:817–823. doi: 10.1161/01.res.31.6.817. [DOI] [PubMed] [Google Scholar]
- 134.Johnson PC, Burton KS, Henrich H, Henrich U. Effect of occlusion duration on reactive hyperemia in sartorius muscle capillaries. Am J Physiol. 1976;230:715–719. doi: 10.1152/ajplegacy.1976.230.3.715. [DOI] [PubMed] [Google Scholar]
- 135.Jones DP, Kennedy FG. Analysis of intracellular oxygenation of isolated adult cardiac myocytes. Am J Physiol. 1986;250:C384–C390. doi: 10.1152/ajpcell.1986.250.3.C384. [DOI] [PubMed] [Google Scholar]
- 136.Jones RD, Berne RM. Vasodilation in skeletal muscle. Am J Physiol. 1963;204:461–466. doi: 10.1152/ajplegacy.1963.204.3.461. [DOI] [PubMed] [Google Scholar]
- 137.Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol. 2007;583:855–860. doi: 10.1113/jphysiol.2007.135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 139.Karlsson K, Marklund SL. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988;255:223–228. [PMC free article] [PubMed] [Google Scholar]
- 140.Karlsson K, Marklund SL. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987;242:55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- 142.Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol. 2004;97:293–301. doi: 10.1152/japplphysiol.00049.2004. [DOI] [PubMed] [Google Scholar]
- 143.Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin- based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282:H2245–H2253. doi: 10.1152/ajpheart.00972.2001. [DOI] [PubMed] [Google Scholar]
- 144.Kempner W. Effect of oxygen tension on cellular metabolism. J Cell Comp Physiol. 1937;10:339–363. [Google Scholar]
- 145.Kennedy FG, Jones DP. Oxygen dependence of mitochondrial function in isolated rat cardiac myocytes. Am J Physiol. 1986;250:C374–C383. doi: 10.1152/ajpcell.1986.250.3.C374. [DOI] [PubMed] [Google Scholar]
- 146.Kindig CA, Howlett RA, Hogan MC. Effect of extracellular PO2 on the fall in intracellular PO2 in contracting single myocytes. J Appl Physiol. 2003;94:1964–1970. doi: 10.1152/japplphysiol.00893.2002. [DOI] [PubMed] [Google Scholar]
- 147.Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 148.Klabunde RE, Laughlin MH, Armstrong RB. Systemic adenosine deaminase administration does not reduce active hyperemia in running rats. J Appl Physiol. 1988;64:108–114. doi: 10.1152/jappl.1988.64.1.108. [DOI] [PubMed] [Google Scholar]
- 149.Kleniewska P, Piechota A, Skibska B, Goraca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 2012;60:277–294. doi: 10.1007/s00005-012-0176-z. [DOI] [PubMed] [Google Scholar]
- 150.Klitzman B, Damon DN, Gorczynski RJ, Duling BR. Augmented tissue oxygen supply during striated muscle contraction in the hamster. Relative contributions of capillary recruitment, functional dilation, and reduced tissue PO2. Circ Res. 1982;51:711–721. doi: 10.1161/01.res.51.6.711. [DOI] [PubMed] [Google Scholar]
- 151.Krogh A. The anatomy and physiology of capillaries. New Haven: Yale University Press; 1922. [Google Scholar]
- 152.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol. 1919;52:457–474. doi: 10.1113/jphysiol.1919.sp001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Krogh A, Harrop GA, Rehberg PB. Studies on the physiology of capillaries: III. The innervation of the blood vessels in the hind legs of the frog. J Physiol. 1922;56:179–189. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62:505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 155.Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension. 2011;58:650–656. doi: 10.1161/HYPERTENSIONAHA.111.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 158.Lancaster JR., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- 160.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 161.Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol. 1990;68:2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- 162.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 164.Lewis FL. Applied Optimal Control and Estimation. Texas Instruments Inc.; 1992. [Google Scholar]
- 165.Lewis RS, Deen WM. Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions. Chem Res Toxicol. 1994;7:568–574. doi: 10.1021/tx00040a013. [DOI] [PubMed] [Google Scholar]
- 166.Lewis T GR. Observation upon reactive hyperemia in man. Heart. 1926;12:73–120. [Google Scholar]
- 167.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 168.Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci. 2009;14:4809–4814. doi: 10.2741/3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lindbom L, Tuma RF, Arfors KE. Blood flow in the rabbit tenuissimus muscle. Influence of preparative procedures for intravital microscopic observation. Acta Physiol Scand. 1982;114:121–127. doi: 10.1111/j.1748-1716.1982.tb06960.x. [DOI] [PubMed] [Google Scholar]
- 170.Lindbom L, Tuma RF, Arfors KE. Influence of oxygen on perfused capillary density and capillary red cell velocity in rabbit skeletal muscle. Microvasc Res. 1980;19:197–208. doi: 10.1016/0026-2862(80)90040-0. [DOI] [PubMed] [Google Scholar]
- 171.Liochev SI, Fridovich I. Superoxide and nitric oxide: consequences of varying rates of production and consumption: a theoretical treatment. Free Radic Biol Med. 2002;33:137–141. doi: 10.1016/s0891-5849(02)00864-x. [DOI] [PubMed] [Google Scholar]
- 172.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 173.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu YH, Lu M, Hu LF, Wong PT, Webb GD, Bian JS. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal. 2012;17:141–185. doi: 10.1089/ars.2011.4005. [DOI] [PubMed] [Google Scholar]
- 175.Lombard JH, Duling BR. Multiple mechanisms of reactive hyperemia in arterioles of the hamster cheek pouch. Am J Physiol. 1981;241:H748–H755. doi: 10.1152/ajpheart.1981.241.5.H748. [DOI] [PubMed] [Google Scholar]
- 176.Longmuir IS. Respiration rate of rat-liver cells at low oxygen concentrations. Biochem J. 1957;65:378–382. doi: 10.1042/bj0650378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lundby C, Calbet JA, Robach P. The response of human skeletal muscle tissue to hypoxia. Cell Mol Life Sci. 2009;66:3615–3623. doi: 10.1007/s00018-009-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lustgarten MS, Bhattacharya A, Muller FL, Jang YC, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. Biochem Biophys Res Commun. 2012;422:515–521. doi: 10.1016/j.bbrc.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res. 2010;342:325–339. doi: 10.1007/s00441-010-1060-y. [DOI] [PubMed] [Google Scholar]
- 180.Marklund SL, Holme E, Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982;126:41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- 181.Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- 182.Mathieu-Costello O, Hepple RT. Muscle structural capacity for oxygen flux from capillary to fiber mitochondria. Exerc Sport Sci Rev. 2002;30:80–84. doi: 10.1097/00003677-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 183.Matouk CC, Marsden PA. Epigenetic regulation of vascular endothelial gene expression. Circ Res. 2008;102:873–887. doi: 10.1161/CIRCRESAHA.107.171025. [DOI] [PubMed] [Google Scholar]
- 184.Maxwell JC. On governors. Proceedings of the Royal Society. 1867;16:270–283. [Google Scholar]
- 185.McCuskey RS. Sphincters in the microvascular system. Microvasc Res. 1971;3:428–433. doi: 10.1016/0026-2862(71)90045-8. [DOI] [PubMed] [Google Scholar]
- 186.Melikian N, Seddon MD, Casadei B, Chowienczyk PJ, Shah AM. Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc Med. 2009;19:256–262. doi: 10.1016/j.tcm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mian KB, Martin W. Differential sensitivity of basal and acetylcholine-stimulated activity of nitric oxide to destruction by superoxide anion in rat aorta. Br J Pharmacol. 1995;115:993–1000. doi: 10.1111/j.1476-5381.1995.tb15909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mohazzab HK, Kaminski PM, Wolin MS. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997;96:614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- 189.Moller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem. 2005;280:8850–8854. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 190.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(Suppl 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 192.Moncada S, Palmer RM, Gryglewski RJ. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986;83:9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moreno JM, Rodriguez Gomez I, Wangensteen R, Perez-Abud R, Duarte J, Osuna A, Vargas F. Mechanisms of hydrogen peroxide-induced vasoconstriction in the isolated perfused rat kidney. J Physiol Pharmacol. 2010;61:325–332. [PubMed] [Google Scholar]
- 194.Munzel T, Heitzer T, Harrison DG. The physiology and pathophysiology of the nitric oxide/superoxide system. Herz. 1997;22:158–172. doi: 10.1007/BF03044353. [DOI] [PubMed] [Google Scholar]
- 195.Munzel T, Hink U, Heitzer T, Meinertz T. Role for NADPH/NADH oxidase in the modulation of vascular tone. Ann N Y Acad Sci. 1999;874:386–400. doi: 10.1111/j.1749-6632.1999.tb09253.x. [DOI] [PubMed] [Google Scholar]
- 196.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci Rep. 1999;19:133–154. doi: 10.1023/a:1020265417394. [DOI] [PubMed] [Google Scholar]
- 197.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nystrom T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Murrant CL, Sarelius IH. Coupling of muscle metabolism and muscle blood flow in capillary units during contraction. Acta Physiol Scand. 2000;168:531–541. doi: 10.1046/j.1365-201x.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 199.Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Regul Integr Comp Physiol. 2002;282:R969–R978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- 200.Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper's Illustrated Biochemistry. New York, Chicago: Lange Medical Books / McGraw-Hill; 2003. [Google Scholar]
- 201.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin. II. NADPH Oxidase, and Redox Signaling in the Vasculature. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nossaman BD, Bivalacqua TJ, Champion HC, Baber SR, Kadowitz PJ. Analysis of vasodilator responses to peroxynitrite in the hindlimb vascular bed of the cat. J Cardiovasc Pharmacol. 2007;50:358–366. doi: 10.1097/FJC.0b013e31811242cd. [DOI] [PubMed] [Google Scholar]
- 204.Nossaman BD, Dabisch PA, Liles JT, Baber SR, Champion HC, Kaye AD, Feng CJ, Anwar M, Bivalacqua TJ, Santiago JA, De Witt BJ, Kadowitz PJ. Peroxynitrite does not impair pulmonary and systemic vascular responses. J Appl Physiol. 2004;96:455–462. doi: 10.1152/japplphysiol.01159.2002. [DOI] [PubMed] [Google Scholar]
- 205.Nowicki PT, Flavahan S, Hassanain H, Mitra S, Holland S, Goldschmidt-Clermont PJ, Flavahan NA. Redox signaling of the arteriolar myogenic response. Circ Res. 2001;89:114–116. doi: 10.1161/hh1401.094367. [DOI] [PubMed] [Google Scholar]
- 206.Nuutinen EM, Wilson DF, Erecinska M. Mitochondrial oxidative phosphorylation: tissue oxygen sensor for regulation of coronary flow. Adv Exp Med Biol. 1984;169:351–357. doi: 10.1007/978-1-4684-1188-1_30. [DOI] [PubMed] [Google Scholar]
- 207.Olmsted JMD. Claude Bernard Physiologist. New York, London: Harper & Brotherst; 1938. [Google Scholar]
- 208.Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- 209.Olson KR. Hydrogen sulfide as an oxygen sensor. Clin Chem Lab Med. 2012:1–10. doi: 10.1515/cclm-2012-0551. [DOI] [PubMed] [Google Scholar]
- 210.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 211.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 212.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 213.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 214.Peterson DA, Peterson DC, Archer SL, Weir EK. Superoxide dismutase: an antireductant enzyme? Biochem Soc Trans. 1993;21:88S. doi: 10.1042/bst021088s. [DOI] [PubMed] [Google Scholar]
- 215.Petrofsky JS, Lind AR. The blood pressure response during isometric exercise in fast and slow twitch skeletal muscle in the cat. Eur J Appl Physiol Occup Physiol. 1980;44:223–230. doi: 10.1007/BF00421621. [DOI] [PubMed] [Google Scholar]
- 216.Picard M, Hepple RT, Burelle Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol. 2012;302:C629–C641. doi: 10.1152/ajpcell.00368.2011. [DOI] [PubMed] [Google Scholar]
- 217.Pittman RN, Duling BR. Oxygen sensitivity of vascular smooth muscle. I. In vitro studies. Microvasc Res. 1973;6:202–211. doi: 10.1016/0026-2862(73)90020-4. [DOI] [PubMed] [Google Scholar]
- 218.Poole DC, Brown MD, Hudlicka O. Counterpoint: There is not capillary recruitment in active skeletal muscle during exercise. J Appl Physiol. 2008;104:891–893. doi: 10.1152/japplphysiol.00779.2007a. discussion 893–894. [DOI] [PubMed] [Google Scholar]
- 219.Poole DC, Copp SW, Hirai DM, Musch TI. Dynamics of muscle microcirculatory and blood-myocyte O(2) flux during contractions. Acta Physiol (Oxf) 2011;202:293–310. doi: 10.1111/j.1748-1716.2010.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Prewitt RL, Johnson PC. The effect of oxygen on arteriolar red cell velocity and capillary density in the rat cremaster muscle. Microvasc Res. 1976;12:59–70. doi: 10.1016/0026-2862(76)90007-8. [DOI] [PubMed] [Google Scholar]
- 221.Pries AR, Heide J, Ley K, Klotz KF, Gaehtgens P. Effect of oxygen tension on regulation of arteriolar diameter in skeletal muscle in situ. Microvasc Res. 1995;49:289–299. doi: 10.1006/mvre.1995.1025. [DOI] [PubMed] [Google Scholar]
- 222.Proctor KG, Duling BR. Adenosine and free-flow functional hyperemia in striated muscle. Am J Physiol. 1982;242:H688–H697. doi: 10.1152/ajpheart.1982.242.4.H688. [DOI] [PubMed] [Google Scholar]
- 223.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 225.Reis DJ, Wooten GF. The relationship of blood flow to myoglobin, capillary density, and twitch characteristics in red and white skeletal muscle in cat. J Physiol. 1970;210:121–135. doi: 10.1113/jphysiol.1970.sp009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 227.Richmond KN, Burnite S, Lynch RM. Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. Am J Physiol. 1997;273:C1613–C1622. doi: 10.1152/ajpcell.1997.273.5.C1613. [DOI] [PubMed] [Google Scholar]
- 228.Richmond KN, Shonat RD, Lynch RM, Johnson PC. Critical PO(2) of skeletal muscle in vivo. Am J Physiol. 1999;277:H1831–H1840. doi: 10.1152/ajpheart.1999.277.5.H1831. [DOI] [PubMed] [Google Scholar]
- 229.Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60:145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rodriguez-Puyol M, Griera-Merino M, Perez-Rivero G, Diez-Marques ML, Ruiz-Torres MP, Rodriguez-Puyol D. Angiotensin II induces a rapid and transient increase of reactive oxygen species. Antioxid Redox Signal. 2002;4:869–875. doi: 10.1089/152308602762197407. [DOI] [PubMed] [Google Scholar]
- 231.Ronson RS, Nakamura M, Vinten-Johansen J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc Res. 1999;44:47–59. doi: 10.1016/s0008-6363(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 232.Rosen GM, Freeman BA. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984;81:7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ross JM, Fairchild HM, Weldy J, Guyton AC. Autoregulation of blood flow by oxygen lack. Am J Physiol. 1962;202:21–24. doi: 10.1152/ajplegacy.1962.202.1.21. [DOI] [PubMed] [Google Scholar]
- 234.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876-2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- 235.Roy CS, Brown JG. The Blood-Pressure and its Variations in the Arterioles, Capillaries and Smaller Veins. J Physiol. 1880;2:323–446 321. doi: 10.1113/jphysiol.1880.sp000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890;11:85–158 117. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 238.Rubanyi GM, Vanhoutte PM. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986;250:H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- 239.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 240.Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem. 1990;265:15392–15402. [PubMed] [Google Scholar]
- 241.Sahlin K. NADH and NADPH in human skeletal muscle at rest and during ischaemia. Clin Physiol. 1983;3:477–485. doi: 10.1111/j.1475-097x.1983.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 242.Saitoh S, Kiyooka T, Rocic P, Rogers PA, Zhang C, Swafford A, Dick GM, Viswanathan C, Park Y, Chilian WM. Redox-dependent coronary metabolic dilation. Am J Physiol Heart Circ Physiol. 2007;293:H3720–H3725. doi: 10.1152/ajpheart.00436.2007. [DOI] [PubMed] [Google Scholar]
- 243.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 244.Salgo MG, Bermudez E, Squadrito GL, Pryor WA. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes [corrected] Arch Biochem Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- 245.Samora JB, Frisbee JC, Boegehold MA. Hydrogen peroxide emerges as a regulator of tone in skeletal muscle arterioles during juvenile growth. Microcirculation. 2008;15:151–161. doi: 10.1080/10739680701508497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sandison JC. Contraction of blood vessels and observation on the circulation in the transparent chamber in the rabbit's ear. The Anatomical Record. 1932;54:105–127. [Google Scholar]
- 247.Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (Oxf) 2010;199:349–365. doi: 10.1111/j.1748-1716.2010.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Scandurra FM, Gnaiger E. Cell respiration under hypoxia: facts and artefacts in mitochondrial oxygen kinetics. Adv Exp Med Biol. 2010;662:7–25. doi: 10.1007/978-1-4419-1241-1_2. [DOI] [PubMed] [Google Scholar]
- 249.Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol. 1999;277:H1167–H1177. doi: 10.1152/ajpheart.1999.277.3.H1167. [DOI] [PubMed] [Google Scholar]
- 250.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 253.Sheff MF, Zacks SI. Interstitial space of mouse skeletal muscle. J Physiol. 1982;328:507–519. doi: 10.1113/jphysiol.1982.sp014280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch. 2010;459:915–922. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- 255.Shingu M, Yoshioka K, Nobunaga M, Yoshida K. Human vascular smooth muscle cells and endothelial cells lack catalase activity and are susceptible to hydrogen peroxide. Inflammation. 1985;9:309–320. doi: 10.1007/BF00916279. [DOI] [PubMed] [Google Scholar]
- 256.Smith CH. Wallace's Unfinished Business. Wiley Periodicals, Inc Complexity. 2004;10:25–32. [Google Scholar]
- 257.Sparks HV., Jr Learning the regulation of peripheral blood flow. Am J Physiol. 1999;277:S164–S173. doi: 10.1152/advances.1999.277.6.S164. [DOI] [PubMed] [Google Scholar]
- 258.Squadrito GL, Pryor WA. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem Biol Interact. 1995;96:203–206. doi: 10.1016/0009-2797(94)03591-u. [DOI] [PubMed] [Google Scholar]
- 259.Steinlechner-Maran R, Eberl T, Kunc M, Margreiter R, Gnaiger E. Oxygen dependence of respiration in coupled and uncoupled endothelial cells. Am J Physiol. 1996;271:C2053–C2061. doi: 10.1152/ajpcell.1996.271.6.C2053. [DOI] [PubMed] [Google Scholar]
- 260.Stone JR. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch Biochem Biophys. 2004;422:119–124. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 261.Stone JR, Maki JL, Collins T. Basal and hydrogen peroxide stimulated sites of phosphorylation in heterogeneous nuclear ribonucleoprotein C1/C2. Biochemistry. 2003;42:1301–1308. doi: 10.1021/bi0268091. [DOI] [PubMed] [Google Scholar]
- 262.Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 263.Stricker S. Untersuchungen uber die capillaren Blutgefasse der Nickhaut des Frosches. Sitzungsber d Wiener Acad d Wissensch. 1868;51 [Google Scholar]
- 264.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 265.Sullivan SM, Johnson PC. Effect of oxygen on arteriolar dimensions and blood flow in cat sartorius muscle. Am J Physiol. 1981;241:H547–H556. doi: 10.1152/ajpheart.1981.241.4.H547. [DOI] [PubMed] [Google Scholar]
- 266.Sullivan SM, Johnson PC. Effect of oxygen on blood flow autoregulation in cat sartorius muscle. Am J Physiol. 1981;241:H807–H815. doi: 10.1152/ajpheart.1981.241.6.H807. [DOI] [PubMed] [Google Scholar]
- 267.Sullivan SM, Pittman RN. Relationship between mitochondrial volume density and capillarity in hamster muscles. Am J Physiol. 1987;252:H149–H155. doi: 10.1152/ajpheart.1987.252.1.H149. [DOI] [PubMed] [Google Scholar]
- 268.Suvorava T, Lauer N, Kumpf S, Jacob R, Meyer W, Kojda G. Endogenous vascular hydrogen peroxide regulates arteriolar tension in vivo. Circulation. 2005;112:2487–2495. doi: 10.1161/CIRCULATIONAHA.105.543157. [DOI] [PubMed] [Google Scholar]
- 269.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 270.Tanaka Y, Yamaki F, Koike K, Toro L. New insights into the intracellular mechanisms by which PGI2 analogues elicit vascular relaxation: cyclic AMP-independent, Gs-protein mediated-activation of MaxiK channel. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:257–265. doi: 10.2174/1568016043356273. [DOI] [PubMed] [Google Scholar]
- 271.Tarchanoff JF. Beobachtungen uber contractile Elemente in der Blut und Lymph Capillaren. Pflugers Arch. 1874;9 [Google Scholar]
- 272.Taverne YJ, de Beer VJ, Hoogteijling BA, Juni RP, Moens AL, Duncker DJ, Merkus D. Nitroso-redox balance in control of coronary vasomotor tone. J Appl Physiol. 2012;112:1644–1652. doi: 10.1152/japplphysiol.00479.2011. [DOI] [PubMed] [Google Scholar]
- 273.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, Hussain SP, Harris CC, DeGraff W, Roberts DD, Mitchell JB, Wink DA. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006;281:25984–25993. doi: 10.1074/jbc.M602242200. [DOI] [PubMed] [Google Scholar]
- 275.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Titchener EB. Experimental Psychology, a Manual of Laboratory Practice: Volume 1, Quantitative Experiments, Part II. Instructors's Manual. New York: Macmillan; 1918. [Google Scholar]
- 277.Trajanovska S, Donald JA. Endothelial nitric oxide synthase in the amphibian, Xenopus tropicalis. Comp Biochem Physiol B Biochem Mol Biol. 2011;158:274–281. doi: 10.1016/j.cbpb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 278.Tsoukias NM. Nitric oxide bioavailability in the microcirculation: insights from mathematical models. Microcirculation. 2008;15:813–834. doi: 10.1080/10739680802010070. [DOI] [PubMed] [Google Scholar]
- 279.Tsypkin YZ. Relay control systems. New York: Cambridge University Press; 1984. [Google Scholar]
- 280.Tuma RF, Lindbom L, Arfors KE. The effect of tissue oxygen tension on reactive hyperemia in skeletal muscle. Bibl Anat. 1977:425–428. [PubMed] [Google Scholar]
- 281.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 282.Tune JD, Richmond KN, Gorman MW, Feigl EO. K(ATP)(+) channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2001;280:H868–H875. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- 283.Uppu RM, Nossaman BD, Greco AJ, Fokin A, Murthy SN, Fonseca VA, Kadowitz PJ. Cardiovascular effects of peroxynitrite. Clin Exp Pharmacol Physiol. 2007;34:933–937. doi: 10.1111/j.1440-1681.2007.04641.x. [DOI] [PubMed] [Google Scholar]
- 284.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 286.Vanderkooi JM, Erecinska M, Silver IA. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol. 1991;260:C1131–C1150. doi: 10.1152/ajpcell.1991.260.6.C1131. [DOI] [PubMed] [Google Scholar]
- 287.Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 288.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998;274:H1705–H1714. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 289.Veal E, Day A. Hydrogen peroxide as a signaling molecule. Antioxid Redox Signal. 2011;15:147–151. doi: 10.1089/ars.2011.3968. [DOI] [PubMed] [Google Scholar]
- 290.Wambi-Kiesse CO, Katusic ZS. Inhibition of copper/zinc superoxide dismutase impairs NO.-mediated endothelium-dependent relaxations. Am J Physiol. 1999;276:H1043–H1048. doi: 10.1152/ajpheart.1999.276.3.H1043. [DOI] [PubMed] [Google Scholar]
- 291.Wang Y, El-Zaru MR, Surks HK, Mendelsohn ME. Formin homology domain protein (FHOD1) is a cyclic GMP-dependent protein kinase I-binding protein and substrate in vascular smooth muscle cells. J Biol Chem. 2004;279:24420–24426. doi: 10.1074/jbc.M313823200. [DOI] [PubMed] [Google Scholar]
- 292.Ward JP. Oxygen sensors in context. Biochim Biophys Acta. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 293.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 294.Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296:E11–E21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol. 2011;214:254–262. doi: 10.1242/jeb.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985;57:781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]
- 297.Welch WJ. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension. 2008;52:51–56. doi: 10.1161/HYPERTENSIONAHA.107.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS One. 2010;5:e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wiedeman MP, Tuma RF, Mayrovitz HN. Defining the precapillary sphincter. Microvasc Res. 1976;12:71–75. doi: 10.1016/0026-2862(76)90008-x. [DOI] [PubMed] [Google Scholar]
- 300.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wilson DF. Quantifying the role of oxygen pressure in tissue function. Am J Physiol Heart Circ Physiol. 2008;294:H11–H13. doi: 10.1152/ajpheart.01293.2007. [DOI] [PubMed] [Google Scholar]
- 302.Wilson DF, Owen CS, Erecinska M. Quantitative dependence of mitochondrial oxidative phosphorylation on oxygen concentration: a mathematical model. Arch Biochem Biophys. 1979;195:494–504. doi: 10.1016/0003-9861(79)90376-x. [DOI] [PubMed] [Google Scholar]
- 303.Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem. 1988;263:2712–2718. [PubMed] [Google Scholar]
- 304.Wolin MS, Ahmad M, Gao Q, Gupte SA. Cytosolic NAD(P)H regulation of redox signaling and vascular oxygen sensing. Antioxid Redox Signal. 2007;9:671–678. doi: 10.1089/ars.2007.1559. [DOI] [PubMed] [Google Scholar]
- 305.Wolin MS, Ahmad M, Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol. 2005;289:L159–L173. doi: 10.1152/ajplung.00060.2005. [DOI] [PubMed] [Google Scholar]
- 306.Wolin MS, Burke-Wolin TM, Mohazzab HK. Roles for NAD(P)H oxidases and reactive oxygen species in vascular oxygen sensing mechanisms. Respir Physiol. 1999;115:229–238. doi: 10.1016/s0034-5687(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 307.Wolin MS, Cherry PD, Rodenburg JM, Messina EJ, Kaley G. Methylene blue inhibits vasodilation of skeletal muscle arterioles to acetylcholine and nitric oxide via the extracellular generation of superoxide anion. J Pharmacol Exp Ther. 1990;254:872–876. [PubMed] [Google Scholar]
- 308.Yan MS, Matouk CC, Marsden PA. Epigenetics of the vascular endothelium. J Appl Physiol. 2010;109:916–926. doi: 10.1152/japplphysiol.00131.2010. [DOI] [PubMed] [Google Scholar]
- 309.Zacharia IG, Deen WM. Diffusivity and solubility of nitric oxide in water and saline. Ann Biomed Eng. 2005;33:214–222. doi: 10.1007/s10439-005-8980-9. [DOI] [PubMed] [Google Scholar]
- 310.Zhilyaev SY, Moskvin AN, Platonova TF, Gutsaeva DR, Churilina IV, Demchenko IT. Hyperoxic vasoconstriction in the brain is mediated by inactivation of nitric oxide by superoxide anions. Neurosci Behav Physiol. 2003;33:783–787. doi: 10.1023/a:1025145331149. [DOI] [PubMed] [Google Scholar]
- 311.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem. 2011;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zuo L, Christofi FL, Wright VP, Liu CY, Merola AJ, Berliner LJ, Clanton TL. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:C1058–C1066. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]
- 314.Zuo L, Clanton TL. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C207–C216. doi: 10.1152/ajpcell.00449.2004. [DOI] [PubMed] [Google Scholar]
- 315.Zuo L, Nogueira L, Hogan MC. Reactive oxygen species formation during tetanic contractions in single isolated Xenopus myofibers. J Appl Physiol. 2011;111:898–904. doi: 10.1152/japplphysiol.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zweifach BW. Landis Award acceptance speech. Microvasc Res. 1971;3:345–353. doi: 10.1016/0026-2862(71)90037-9. 1971. [DOI] [PubMed] [Google Scholar]
- 317.Zweifach BW. The character and distribution of the blood capillaries. Anat Rec. 1939;73:475–495. [Google Scholar]
- 318.Zweifach BW, Lee RE, Hyman C, Chambers R. Omental Circulation in Morphinized Dogs Subjected to Graded Hemorrhage. Ann Surg. 1944;120:232–250. doi: 10.1097/00000658-194408000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]