Abstract
Introduction
Progesterone (P4) and its product, 5α-pregnan-3α-ol-20-one (3α,5α-THP), act in the midbrain ventral tegmental area (VTA) to alter motivated behaviors, such as mating, and motor and anxiety behavior. Of interest is whether 3α,5α-THP formation requires the pregnane xenobiotic receptor (PXR), which is expressed in the midbrain of rats.
Aim
The role of PXR in the midbrain for 3α,5α-THP formation, which precedes modulation of motivated behaviors, was investigated.
Methods
Rats had estrous cycle phase determined and were assessed when they were in diestrus or proestrus. Diestrous and proestrous rats were infused with control or anti-sense oligodeoxyribonucleotides (AS-ODNs) targeted against PXR to the VTA.
Main Outcome Measures
In pilot studies, PXR gene and protein expression in the midbrain was determined with quantitative RT-PCR and western blotting, respectively. Diestrous and proestrous rats infused with control or AS-ODNs to the VTA were tested for anxiety (open field, plus maze), social (social interaction) and sexual (paced mating) behavior. Expression of PXR in the midbrain was verified with Western blotting. Plasma estradiol, P4, dihydroprogesterone (DHP), and 3α,5α-THP levels, and brain P4, DHP, and 3α,5α-THP levels, were measured. We predicted that proestrous rats infused with PXR AS-ODNs would have decreased anti-anxiety, social, and sexual behavior, lower midbrain expression of PXR, and lower midbrain levels of 3α,5α-THP, compared to controls.
Results
Results supported the hypothesis that formation of 3α,5α-THP requires PXR, and may be important for motivated behaviors. PXR AS-ODN, compared to control, infusions to the VTA reduced PXR expression and 3α,5α-THP levels in the midbrain, and attenuated sexual receptivity of proestrous rats.
Conclusions
Knockdown of PXR in the midbrain reduces 3α,5α-THP levels and sexual receptivity of proestrous rats. Thus, PXR in the midbrain may be required for the observed increase in 3α-5α-THP during proestrus, which has subsequent effects on motivated, reproductive behaviors.
Keywords: Allopregnanolone, Lordosis, Progesterone, Neurosteroids, Mating, Receptivity
Introduction
A focus on the functional role of progesterone (P4) has been on its pregnancy-maintaining effects; however, it is clear that P4, and its products, have effects beyond pregnancy for motivated behaviors, encompassing social interaction, mating, and other reproduction-relevant behaviors. One brain target of progestogens, such as P4 and 5α-pregnan-3α-ol-20-one (3α,5α-THP), is the midbrain VTA. Natural elevations of P4 and 3α,5α-THP across the estrous cycle coincide with increases in motivated behaviors, such as mating and reproduction-relevant behaviors (e.g. social interaction, anxiety), during proestrus [1–7]. Moreover, the effects of exogenous administration of progestogens support the role that they play for motivation. For example, administration of 3α,5α-THP to ovariectomized rats enhances mating, pro-social behavior, and anti-anxiety-like responses [4]. Results from other animal models corroborate these findings that progestogens can alter motivated processes. For instance, 3α,5α-THP produces a place preference [2,8,9] and rats will preferentially self-administer 3α,5α-THP over water [10]. Progestogens have effects to amplify motivational processes, such as those observed with cocaine. In support, proestrous rats have greater responses to cocaine than do diestrous rats [11]. Among ovariectomized rats, P4 produces sex-dependent effects on behavioral responses to cocaine [12,13] and sequential administration of estradiol (E2) and P4 increases cocaine self-administration and tends to increase behavioral sensitivity to cocaine [14]. Thus, progestogens play a role in motivated behaviors.
Determining the source of 3α,5α-THP is critical to investigating the role and mechanisms of 3α,5α-THP for anxiety, social, and reproductive processes. 3α,5α-THP is formed via activation of a metabolic and/or biosynthetic pathway. For example, in the VTA, ovarian P4 is readily metabolized to dihydroprogesterone (DHP) by 5α-reductase and DHP is converted to 3α,5α-THP by 3α-hydroxysteroid dehydrogenase. Blocking P4’s metabolism to 3α,5α-THP pharmacologically, or with knockout mouse models, inhibits progestogens’ facilitating effects on reproductive, social, and anti-anxiety behaviors, and 3α,5α-THP-replacement can produce the opposite effects [1,15–18]. For female rats, engaging in mating promotes biosynthesis, or neurosteroidogenesis, of 3α,5α-THP from the precursor, cholesterol in the midbrain VTA, as well as regions important for reward, emotion, and cognition (i.e. striatum, hippocampus, and cortex) [4,16,19]. The 18-kDa translocator protein (TSPO; formerly known as the peripheral-type benzodiazepine receptor) is essential for neurosteroidogenesis in that it transports cholesterol to cytochrome P450-dependent side chain cleavage enzymes (P450scc) [20–22]. The steroidogenic acute regulatory (StAR) protein is important for stimulating this action, but the precise mechanisms are unknown. Inhibiting formation of 3α,5α-THP through any of these metabolic pathways in the VTA attenuates these neuroendocrine and behavioral effects [1,16,18,23]. Thus, the effects of 3α,5α-THP on reproductive, social and anxiety behaviors occur following its generation from the metabolism of ovarian P4, which infiltrates the VTA, as well as from de novo synthesis from cholesterol within the brain.
It is generally accepted that progestogens are synthesized in the brain and peripheral nerves, but other factors that may regulate steroid biosynthesis are poorly understood. The Pregnane Xenobiotic Receptor (PXR), a promiscuous nuclear receptor, binds 3α,5α-THP and can mediate transcription of cytochrome (CYP) enzymes [24–26]. CYP enzymes are involved in biosynthesis of steroids. The rodent PXR is analogous to the Steroid and Xenobiotic Receptor (SXR) in humans, also referred to as the human-PXR. Whether behavioral and/or neuroendocrine variations in midbrain 3α,5α-THP occur concomitant with, and/or require, PXR is of interest.
Aims and Hypotheses
Our hypothesis is that formation of 3α,5α-THP requires PXR in the midbrain. First, we determined that there is expression of PXR gene and protein in the proestrous rat midbrain with quantitative reverse transcriptase polymerase chain reaction (qPCR) and Western blotting, respectively. Second, to determine whether PXR may underlie some of 3α,5α-THP’s actions in the midbrain VTA, diestrous and proestrous rats were administered placebo control or PXR AS-ODNs via infusions to the midbrain VTA. Following infusions to the midbrain, motivated and socially-relevant behaviors (open field, elevated plus maze, social interaction, and paced mating) of rats were assessed. For the open field and elevated plus maze, measures of anti-anxiety behavior were determined as a function of motor behavior in these tasks. Social behavior was assessed in the social interaction task and reproductive behavior was assessed in the paced mating task. Among behaviorally-tested rats administered control and PXR AS-ODN infusions, expression of PXR in the midbrain was verified with western blotting, plasma levels of E2, P4, DHP, and 3α,5α-THP were measured, and brain (cortex, hippocampus, striatum, hypothalamus, and midbrain) levels of P4, DHP, and 3α,5α-THP were determined.
Methods and Main Outcome Measures
These methods utilizing live animals (surgery, drug manipulations, behavioral testing, euthanasia) were approved by the Institutional Animal Care and Use Committee at The University at XXXX and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Experimental Overview
In pilot experiments to assess suitability of PXR AS-ODNs for knocking down expression of PXR in the midbrain, PXR mRNA and protein expression in the midbrain VTA was determined among diestrous and proestrous rats using qPCR (n=2–4/group) and Western blotting (n=5/group). PXR mRNA expression was determined in the midbrain VTA among diestrous and proestrous rats infused with PXR AS-ODN (diestrous n=2, proestrous n=2), compared to vehicle infusions (diestrous n=4, proestrous n=4), using qPCR. Typical western blotting methods were utilized to assess PXR protein expression in the midbrain of proestrous and diestrous rats. As described in the Results, rats administered AS-ODNs to the VTA had lower expression (as indicated as a fold-change from control) than did those administered vehicle; however, there was large variability in these groups. Data investigating PXR protein levels were more robust. These pilot results substantiated assessing PXR protein expression with western blotting in this experiment. As such, behavior, hormone levels, and PXR protein expression of diestrous and proestrous rats administered control or PXR AS-ODN infusions to the VTA are described herein.
Animal Housing
Adult (55–60 days old), Long-Evans female rats (N=88) were bred in the Life Sciences Laboratory Animal Care Facility at The University at Albany-SUNY (original stock obtained from Taconic, Germantown, NY, USA). Rats were group-housed (n=3–5/cage) in polycarbonate cages with woodchip bedding (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1 °C) and were maintained on a 12:12 h reversed light cycle (lights off at 08:00 am). Rats had continuous access to Purina Rat Chow and tap water in their home cages.
Estrous Cycle
On each day during the experiment (between 8:00 and 10:00 am), rats were “cycled”, in that vaginal epithelium of each rat was collected and examined under a light microscope for presence of different cell types that predominant in each stage of the estrous cycle. Rats were cycled through two normal estrous cycles (4–5 day cycle) prior to testing. Rats were tested on either proestrus (epithelium characterized by nucleated cells, 4–5 days after the previous occurrence) or diestrus (heterogeneous cell types in vaginal epithelium for two consecutive days, which transpired 4–5 days after the previous 2-days of diestrous).
Surgical Protocol
Some rats (n=78, which does not include the 10 cycling rats that were utilized in the pilot western blotting experiment) were stereotaxically implanted, under xylazine (12 mg/kg) and ketamine (80 mg/kg) anesthesia, with bilateral guide cannulae aimed at the VTA (from bregma: AP = −5.3, ML = 0.4, DV = −7.0, as per [27]). Guide cannulae consisted of 23-gauge stainless steel needles, with tips sanded smooth, with 30-gauge stainless steel removable inserts. Rats recovered from surgery for an average of 10 days. After surgery, rats were evaluated daily for their ability to right themselves and cage-climb, presence of proper muscle tone, and reflexive responses to hindlimb extension. Rats were also evaluated for weight gain after surgery. Only rats that passed these neurological evaluations and gained weight following surgery until behavioral testing commenced were continued in the experiment.
Infusion Condition
Proestrous rats were randomly-assigned to be administered 1 µl control (n=20) or PXR AS-ODN (n=13; 5’CTTGCGGAAGGGGCACCTCA3’; 250 ng/1 µl) infusions and diestrous rats were randomly-assigned to be infused with control (n=17) or PXR AS-ODN (n=16) infusions. Rats were infused with vehicle control or PXR AS-ODNs 0, 24 and 44 hours before behavioral testing. Because effects of AS-ODNs can be transient, rats were administered three infusions at these time points before testing. The timing of infusions of AS-ODNs in the present study (at 0, 24, and 44 hours before testing) was based upon pilot studies using this technique and assessing reproductive behavior and evidence of reduction in PXR expression [28]. As well, the procedure aimed to have PXR knocked down before and during behavioral testing. Rats were only tested in one occasion, a half-hour after the last infusion. ODN chimeras were synthesized (capped oligos with remaining links unmodified) and desalted by Invitrogen Life Technologies (Carlsbad, CA). The vehicle for AS-ODN infusions was sterile saline. There were no differences were noted in rats that were administered saline vehicle or scrambled antisense ODN (5’CTCCGAAACGGACATCTGA3’) as control infusions. As such, rats tested in these conditions were combined to form one control group. Irrespective of condition, all rats were infused with the same volume of infusate. For infusions, rats were gently held in one hand, resting on a table in the behavioral testing area. An infusion needle attached to PE tubing and a 5 µl Hamilton syringe, both of which were filled with infusate, was carefully placed inside one cannula of the experimental rat. The experimenter depressed the syringe slowly (1 µl/minute) and the infusion needle remained inside the cannula 1 minute. The process was then repeated for each rat’s second cannula. Although it was not possible to directly determine the spread of the infusate in the present experiment, previous investigations have determined that such midbrain VTA infusions spread approximately 1 mm in all directions from infusion site and do not extend beyond the midbrain. Moreover, we have shown that infusions of 3α,5α-THP using the infusion methods described here to the substantia nigra also diffused approximately 1 mm in all directions and did not impinge upon the VTA [29].
Behavioral Testing
Behavioral data were collected from all rats except those used solely for PXR expression (n=22 from pilot experiment). Behavioral data were collected simultaneously using the Any-maze behavioral assessment computer program (for open field, elevated plus maze, and social interaction) or a digital video camera (for paced mating) and by experimenters. There is greater than 95% concordance between data “hand-collected” by experimenters and those data automatically recorded by Any-Maze (Stoelting Inc., Wood Lawn, IL). Any-Maze can be programmed to automatically record all possible indices in each task and digitally records a video of the performance of rats in each test. Rats were tested sequentially in the following tasks, in the order listed, using previously reported methods: open field [3,16], elevated plus maze [3,16], social interaction [3,16], and paced mating task [2,16,28]. Each cohort of rats was subjected to this entire battery of tasks.
Open Field
The open field (76 × 57 × 35 cm) has a 48-square grid floor (6 × 8 squares, 9.5 cm/side), with 24 peripheral, 16 central, and 8 inner squares. Rats are placed in the open field, observed for 5 mins, while the number of entries made to the peripheral, central, and inner 8 squares (summed for total) is recorded [3,16]. The number of inner 8 square entries made by experimental groups compared to controls is utilized as an index of anti-anxiety behavior, while the total number of entries made by experimental groups compared to controls is an index of total motor behavior. Both of these measures in the open field are reported here as is the percentage of inner 8 entries to central entries.
Elevated Plus Maze
The elevated plus maze consists of 2 arms (49 cm long, 10 cm wide), enclosed by walls 30 cm high (a.k.a. closed arms), and 2 exposed arms (a.k.a. open arms). The arms of the apparatus are elevated 50 cm off of the ground. Rats are placed at the junction of the open and closed arms. The number of entries, and amount of time spent on the open (compared to closed) arms, are recorded for 5 min [3,16]. The number of entries made, and time spent, in the open arms in experimental groups compared to controls is reported here as indices of anti-anxiety-like behavior. The percentage of open arm entries to total arm entries and the number of total arm entries are reported as general activity measures in the maze. The indices in the closed arms are not reported to avoid redundancy in the report as rats are either deemed to be in the open or closed arms.
Social Interaction
The experimental subject and a conspecific rat (same-species, ovariectomized female) are placed in opposite corners of the open field. Time spent by the experimental rat engaging in social interaction (crawling over and under partner, sniffing of partner, following with contact, anogenital investigation, tumbling, and grooming) with the conspecific is recorded for 5 mins [3,16]. During this task, other performance measures, such as aggressive behavior (e.g. boxing, tail rattling, fighting, vocalizing), are recorded. These types of aggressive responses were not observed among any of the experimental subjects in this study.
Paced Mating
A previously described protocol for paced mating was utilized [2,16,28]. The female’s pacing of male’s contacts for an ejaculatory series is evaluated in a chamber with a partition, which divides the chamber equally. Males are relegated to one side. The timing of female’s entries and exits to the male-side of the chamber, through a small hole, are recorded, as are mounts, intromissions, and ejaculations by the male, so that return latencies, percent exits, inter-mount intervals, inter-intromission intervals, post-ejaculatory intervals, and frequency of each type of contact can be calculated. Proceptive (incidence of hopping, darting, and ear wiggling per total number of mounts), receptive (lordosis quotients, which is the percentage of total number of lordotic responses per total number of sexual contacts by the male, and lordosis ratings, which are the intensity of lordosis responses) aggression/rejection (aggression quotients; per total number of mounts) behaviors are recorded. Results from the most robust measures in the paced mating task (proceptivity, lordosis, and aggression quotients and lordosis ratings) are reported.
Tissue Collection
All rats were rapidly decapitated. For rats that were behaviorally tested, this occurred immediately after testing. Whole brain and trunk blood were collected to measure steroid hormone levels in brain and plasma. Brains were immediately placed on dry ice and then stored at −80 degrees C until dissections. For dissections, whole brains were briefly thawed on ice. From the whole brain, midbrain, hippocampus, striatum, and prefrontal cortex were dissected out. Dissected brain tissues were homogenized in 1ml ddH2O and 100 µg of midbrain tissue was pipetted off and stored at −80 degrees C for later western blot analyses.
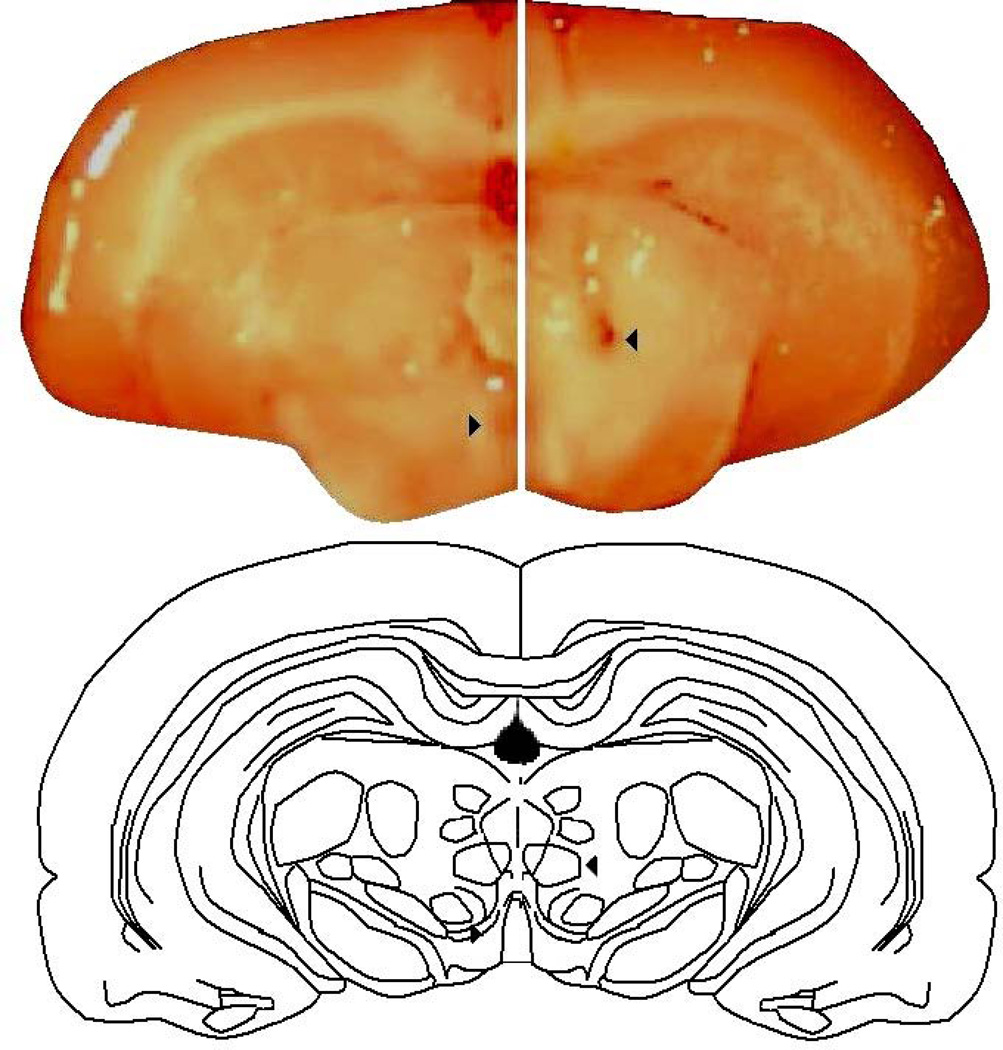
During dissections, it was noted whether rats had accurate placement of infusions to the VTA. Figure 1 depicts a picture and drawing of correct and missed site infusions. Briefly, slices were made posterior and anterior of the midbrain region so that infusion site could be verified. From the ventral plane, the optic chiasm and pontine regions were utilized as posterior and anterior borders for the gross dissection of the midbrain, respectively. The lateral borders of the midbrain dissection were approximately 1.5 mm from midline of the brain and the dorsal border was below the cerebral aqueduct. Rats with missed infusion placement (n=20), based upon visual inspection, were not included in statistical analyses. Although missed control infusions did not produce robust differences in the pattern of behavior compared to intra-VTA infusions as did missed AS-ODN infusions, only data from rats with infusions that could be verified to be aimed at the midbrain VTA were included. As such, behavioral effects, PXR protein expression determined by western blotting, and hormone levels in the midbrain determined by radioimmunoassay of cycling rats administered control (diestrous n=11, proestrous n=13) or PXR AS-ODN (diestrous n=13, proestrous n=9) infusions to the VTA are described in the Results.
Figure 1.
Brains were visually inspected during dissection to verify that infusions were to the midbrain (shown on left, indicated by arrowhead) or outside of the midbrain (shown on right, indicated by arrowhead). Data from 20 rats that had infusions outside of the midbrain were not included in statistical analyses.
qPCR methods
Standard qPCR methods were utilized on tissues from some rats. Tissues for qPCR were preserved in RNA later (Qiagen) to prevent degradation. Total RNA was isolated from tissue using the Qiagen RNeasy Micro Kit (Valencia, CA) according to the manufacturer’s protocol. Reverse transcription was carried out using Oligo(dT)20 and the Superscript III First-Strand Synthesis System for RT-PCR from Invitrogen (Carlsbad, CA). qPCR was performed using Bio-Rad SYBR Green Supermix (Hercules, CA) and the following gene-specific primers: β-actin forward (5’-GCT CGT CGT CGA CAA CGG CT-3’), β-actin reverse (5’-CAA ACA TGA TCT GGG TCA TCT TCT C-3’), PXR forward (5’-GCA TCC AGG ACA CGC ACC CC-3’), and PXR reverse (5’-GCC AGC ACG TCC CAC AGG CT-3’). Reactions were run on an Applied Biosystems 7900HT and analyzed using the comparative cycle time (DeltaDeltaCT) method (Applied Biosystems, Foster City, CA). DeltaDeltaCT values were normalized to the actin control and described as a percent change from control as an index of PXR mRNA expression.
Protein Expression/Western Blot Analyses methods
A standard western blotting protocol was employed to assess PXR protein in some tissues. The western protocol included the following sample preparation for loading: 2.5 µl of NuPAGE LDS (4×) sample buffer, 1 µl of NuPAGE Reducing Agent (10×), 6.5 µl of deionized water and 10 µl of homogenized midbrain protein in 1× sample buffer, which then were combined and loaded into NuPAGE Bis-Tris Mini Gels. Samples were run on NuPage 4–12% SDS Polyacrylamide gels with 1× MOPS running buffer with one lane reserved for the protein ladder and one for the positive control (liver homogenate, and/or heart homogenate). After electrophoresis, protein was transferred to nitrocellulose using NuPAGE Transfer buffer (1×). The blots were blocked in 3% BSA or 5% milk solution. Western blots were probed with an unconjugated antibody to PXR overnight at 4C, (Santa Cruz Biotechnology, Santa Cruz, CA; 1:3000) and with the secondary antibody Goat Anti-Mouse IG (H+L) Horseradish Peroxidase Conjugate (1:1250) for 1 hour on a shaker at room temperature. (Bio-rad Hercules, CA). Results were visualized using DuoLuX Chemiluminescent/Fluorescent Substrate Kit for Peroxidase (Vector Laboratories, Burlingame, CA) and imaging on a ChemiDoc XRS (Bio-rad, Hercules, CA) and analyzed using ImageJ software.
Radioimmunoassay for Steroid Hormones
Standard steroid extraction and radioimmunoassay techniques were employed to measure plasma and brain levels of E2, P4, DHP, and 3α,5α-THP [16]. Steroids were extracted from tissues. Briefly, E2, P4, DHP, and 3α,5α-THP were extracted from plasma with ether following incubation with water and 800 cpms of tritiated steroid. After snap-freezing twice, test tubes containing steroid and ether were evaporated to dryness in a heated savant vacuum centrifuge that removes organic solvents. Dried down tubes were then reconstituted with phosphate assay buffer to the original plasma volume. Progesterone, DHP, and 3α,5α-THP were extracted from midbrain, hippocampus, striatum, and prefrontal cortex following homogenization of tissue with a pestle in 50% MeOH and 1% acetic acid. Samples were then centrifuged at 3000 × g in a centrifuge at 4 degrees C. Supernatant was chromatographed on Sepak-cartridges equilibrated with 50% MeOH and 1% acetic acid. Steroids were eluted with 50% and 100% MeOH. Tubes were placed in a savant and 100% MeOH was removed. Samples were reconstituted by adding 300 µl assay buffer. Levels of steroids were determined with radioimmunoassay methods utilizing tritiated E2 (specific activity 51.3 Ci/mmol), P4 (specific activity 47.5 Ci/mmol), and 3α,5α-THP (specific activity 65.0 Ci/mmol) were from Perkin Elmer (Boston, MA) and specific antibodies to E2 (E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, CO), P4 (P#337, Dr. G.D. Niswender, Colorado State University), and 3α, 5α-THP (#921412–5; Dr. Robert Purdy, Veterans Medical Affairs, La Jolla, CA). Tritiated steroids and antibodies were added to tubes containing reconstituted samples and incubated. Binding was terminated with addition of ice cold charcoal buffer to each assay tube. Binding was then determined by standard liquid scintillation spectroscopy. Standard liquid scintillation methods were used with correction for recovery and efficiency of the scintillation counter. Concentrations were determined for each sample based upon concurrent standard curves run for each of the assays.
Statistical Analyses
In pilot experiments assessing PXR expression, descriptive statistics (mean ± sem) were utilized to demonstrate PXR mRNA and protein expression in the midbrain of diestrous and proestrous rats. Descriptive, rather than inferential, statistics are reported on here given the small number of observations per group (n=2–5). Multiple, two-way analyses of variances (ANOVAs) were utilized to test our hypothesis that manipulating PXR in the midbrain would alter progestogens and behavior of cycling rats, particularly among proestrous rats. To test this, we used two-way ANOVAs with 2 variables, each at 2 levels (estrous cycle phase (proestrous, diestrous) and PXR infusion condition (control, AS-ODN)) to determine effects on behavioral measures, endocrine levels, and PXR expression by Western blotting. The α level for statistical significance was reached when p ≤ 0.05. Fisher’s post hoc tests were used to examine group differences.
Results
Pilot experiment-PXR expression in the midbrain
PXR mRNA and protein were expressed in the midbrain of rats. Proestrous rats demonstrated a 23.7 ± 13.0 relative PXR expression change (relative to actin control) and diestrous rats demonstrated a 0.9 ± 0.5 relative PXR expression change in the midbrain. Proestrous rats and diestrous rats administered PXR AS-ODNs to the VTA had lower relative expression changes compared to proestrous rats administered control infusions (1.1 ± 0.9 and 9.9 ± 9.8, respectively); albeit, there was large variability in these results and both diestrous rats did not show a clear reduction in PXR expression following AS-ODN infusions. PXR protein was expressed in the midbrain of rats. Proestrous rats demonstrated a 2.8 ± 1.5 mean relative optical density of PXR (relative to actin bands) and diestrous rats demonstrated a 1.0 + 0.2 mean relative optical density of PXR in the midbrain.
Midbrain PXR protein expression following PXR AS-ODN infusions to the VTA
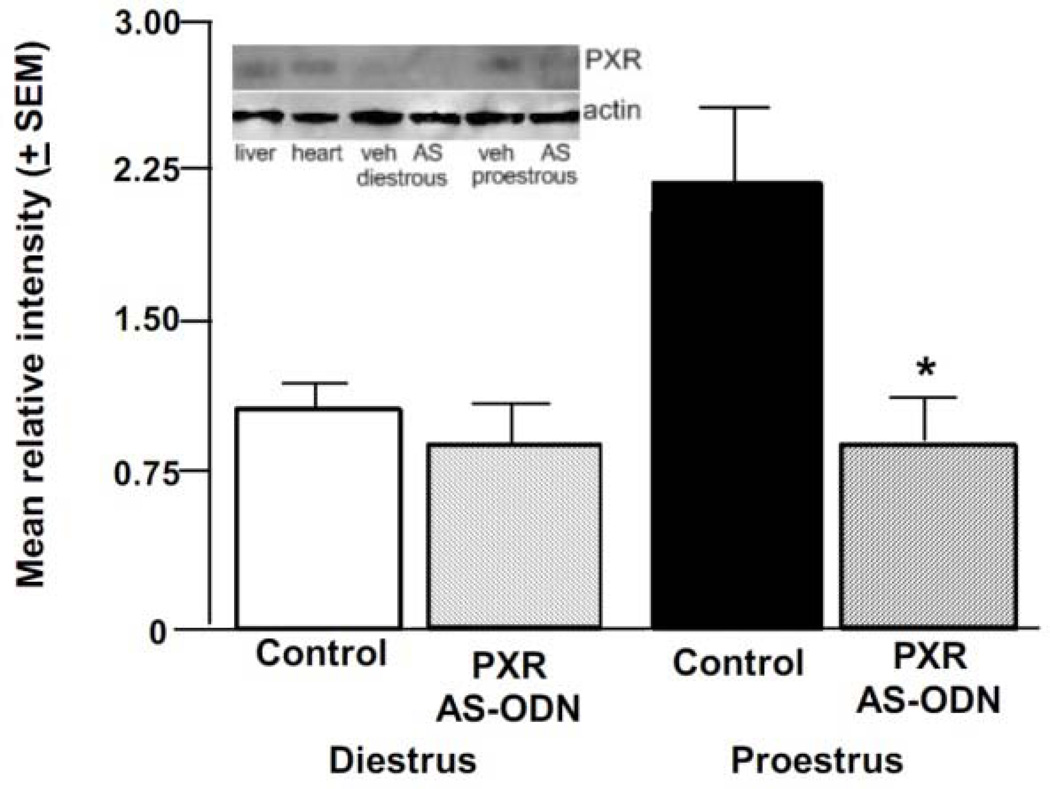
There was a significant interaction of cycle condition and infusion condition [F(1, 42) =7.31, p < 0.01] for mean relative density of PXR to actin control in the midbrain. Proestrous rats infused with PXR AS-ODNs, but not diestrous rats administered control or PXR AS-ODN infusions, had decreased PXR expression in the midbrain compared to proestrous rats (Figure 2).
Figure 2.
Depicts mean (+SEM) relative optical density of midbrain PXR bands to actin bands on the same blots of cycling rats administered vehicle control (diestrous n=11, proestrous n=13) or PXR AS-ODNs (diestrous n=13, proestrous n=9) infusions that were behaviorally-tested and had plasma and brain levels of steroids measured. Inset depicts representative picture of blot run for this experiment. In each blot, lanes were run with positive controls (liver and heart) and experimental conditions. * indicates a reduction due to PXR AS-ODN, compared to control, infusions of proestrous rats (P<0.05).
Open Field behavior following PXR AS-ODN infusions to the VTA
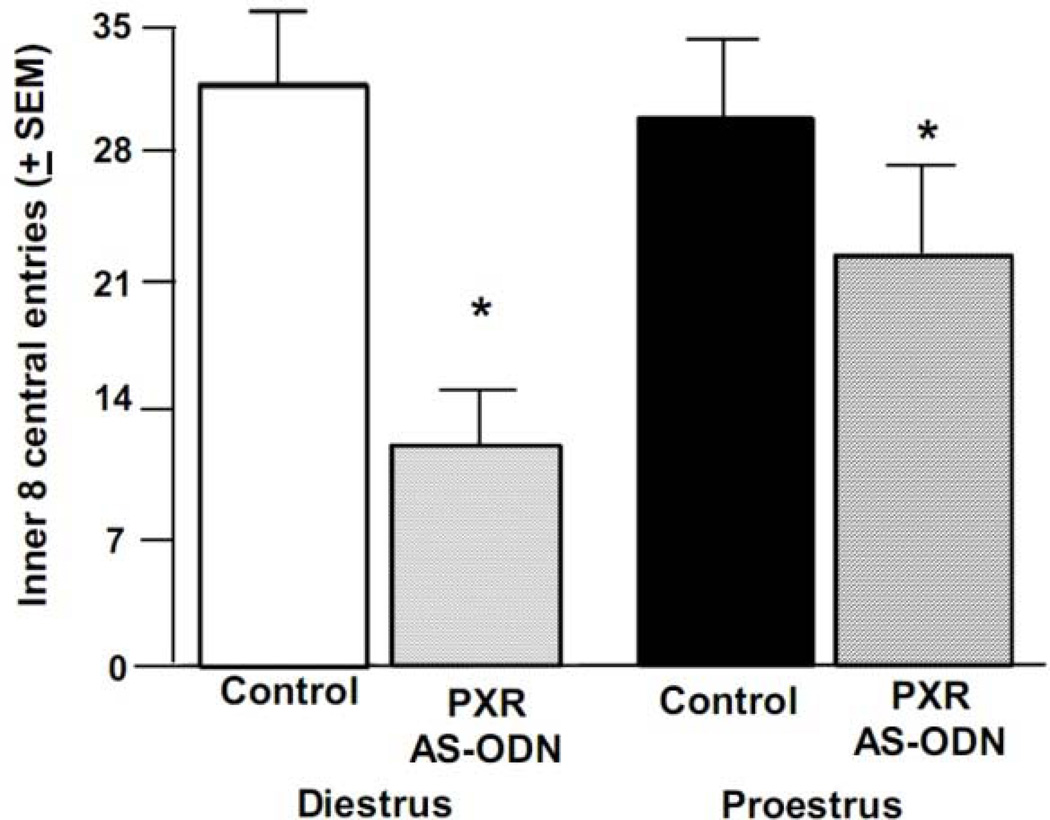
There was a significant difference for infusion condition [F(1, 42) = 7.09, p < 0.01] for behavior in the open field. Rats infused with PXR AS-ODNs made fewer inner 8 entries (index of anti-anxiety-like behavior) than did rats administered control infusions (Figure 3). There were no differences due to condition for total entries made in the open field (Table 1), but there was an effect of infusion condition for percentage of inner 8 to total entries in the open field [F(1, 42) = 4.40, p < 0.04]. When collapsing across cycle condition, compared to control infusions, PXR AS-ODN infusions reduced the percentage of inner 8 to total open field entries (Table 1).
Figure 3.
Depicts mean (+ SEM) entries to inner 8 squares in the open field of cycling rats administered vehicle control (diestrous n=11, proestrous n=13) or PXR AS-ODNs (diestrous n=13, proestrous n=9) infusions. * indicates a reduction due to PXR AS-ODN, compared to control, infusions (p<0.05).
Table 1.
Behavioral measures of diestrous or proestrous rats administered control or pregnane xenobiotic receptor (PXR) antisense oligodeoxynucleotide (AS-ODN) infusions to the midbrain ventral tegmental area.
| Behavioral Measures |
Condition | |||
|---|---|---|---|---|
| Diestrous control infusion to VTA |
Diestrous PXR AS-ODN infusion to VTA |
Proestrous control infusion to VTA |
Proestrous PXR AS-ODN infusion to VTA |
|
| Lordosis ratings | 0.4 ± 0.2 | 0.5 ± 0.3 | 1.5 ± 0.3 | 0.5 ± 0.3^ |
| Proceptivity quotients | 9.1 ± 9.1 | 16.7 ± 10.3 | 50.4 ± 10.1 | 8.3 ± 5.9^ |
| Aggression quotients | 41.1 ± 10.8 | 42.5 ± 9.9 | 42.3 ± 9.4 | 37.8 ± 12.7 |
| Open arm entries in the plus maze | 4.5 ± 1.2 | 2.2 ± 1.0* | 4.8 ± 1.0 | 2.6 ± 0.6* |
| Total arm entries in the plus maze | 16.1 ± 1.8 | 10.6 ± 1.7* | 14.5 ± 1.6 | 11.1 ± 1.5* |
| Percentage of open arm entries to total entries in the plus maze | 25.0 ± 5.4 | 15.9 ± 5.2 | 28.2 ± 4.7 | 23.6 ± 5.1 |
| Total open field entries | 385.2 ± 34.9 | 301.8 ± 22.3 | 343.2 ± 20.4 | 354.3 ± 106.7 |
| Percentage of open field inner 8 central entries to total entries | 7.9 ± 1.1 | 4.5 ± 0.8* | 8.3 ± 0.9 | 7.2 ± 1.6* |
indicates significant difference for PXR infusion condition compared to control infusions (but no effect of cycle phase for these results).
indicates significant interaction between estrous cycle and PXR infusion condition.
Elevated Plus Maze behavior following PXR AS-ODN infusions to the VTA
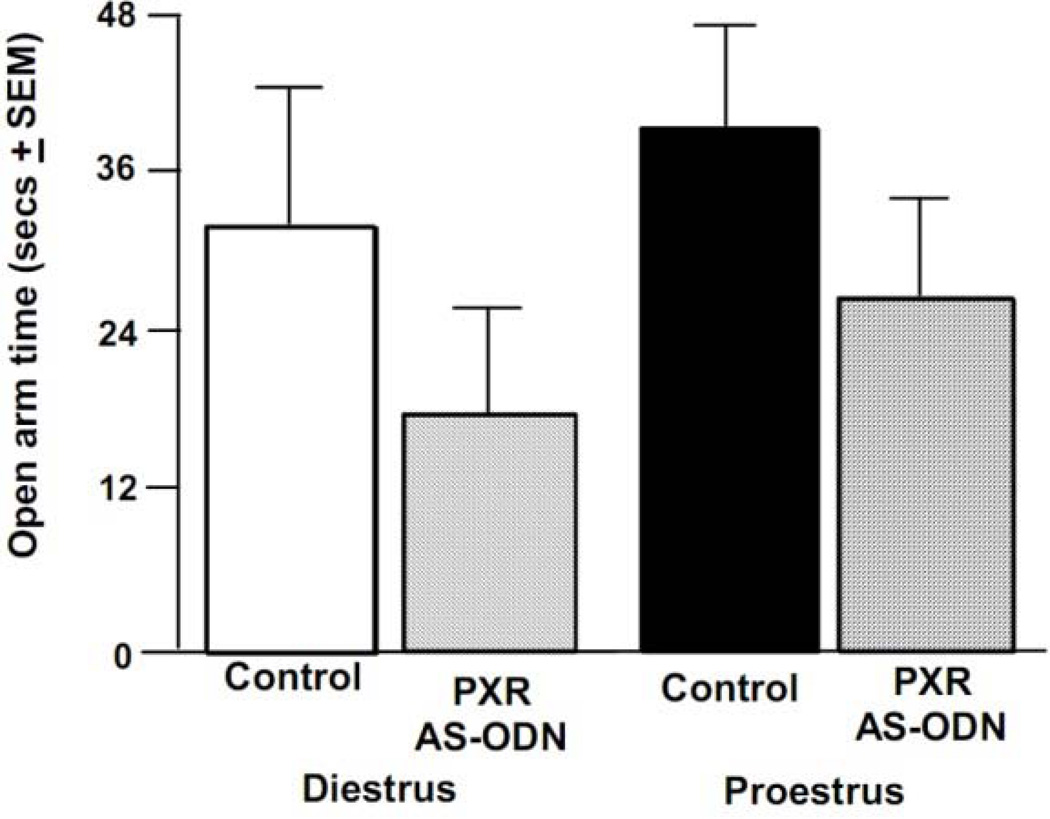
The effect of infusion condition for time spent the open arms of the elevated plus maze approached significance [F(1, 42) = 3.02, p < 0.09] (Figure 4), and there was a significant effect of infusion condition for open arm entries made [F(1, 42) = 4.65, p < 0.04; Table 1]. Rats infused with PXR AS-ODNs spent less time on the open arms of the plus maze and made fewer entries to the open arms than did rats administered control infusions. There were significant effects of infusion condition to alter total entries in the maze, such that rats administered PXR AS-ODNs made fewer total entries [F(1, 42) = 7.08, p < 0.01], but there were no differences in the percentage of open arm entries to total arm entries (Table 1).
Figure 4.
Depicts mean (+ SEM) open arm time in the elevated plus maze of cycling rats administered vehicle control (diestrous n=11, proestrous n=13) or PXR AS-ODNs (diestrous n=13, proestrous n=9) infusions.
Social interaction following PXR AS-ODN infusions to the VTA
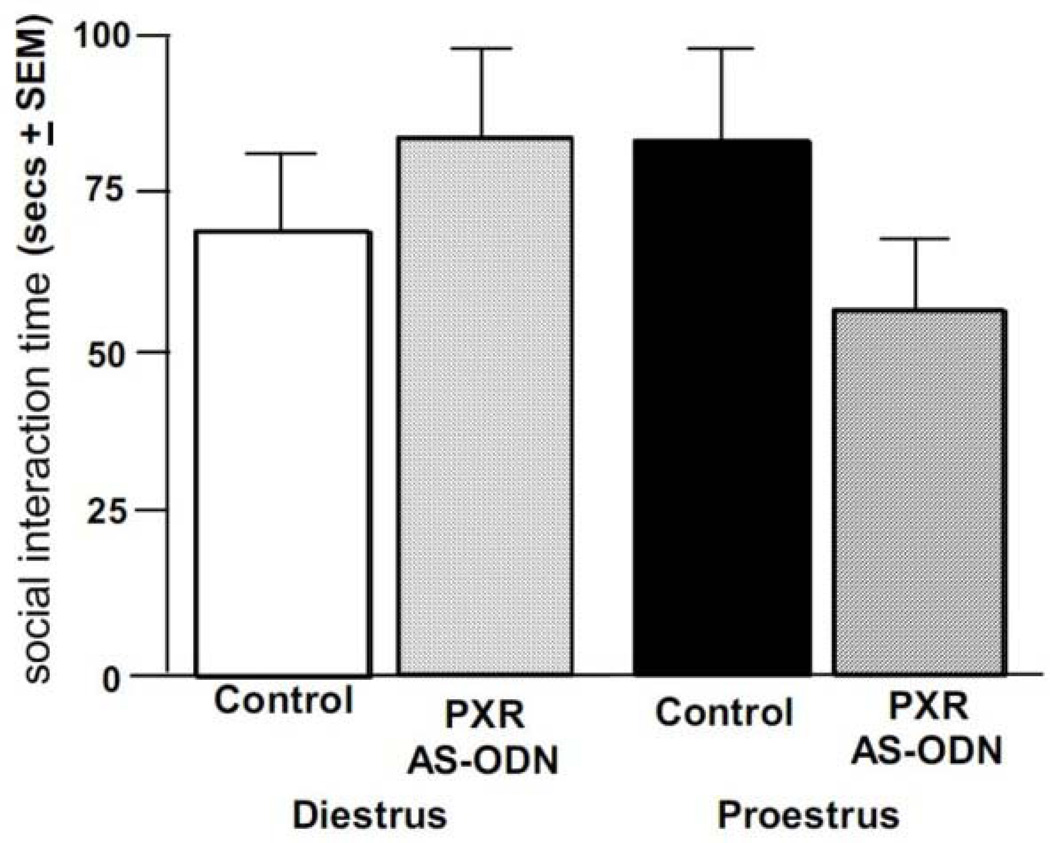
Proestrous rats spent less time interacting with a conspecific following infusions with PXR AS-ODN compared to rats administered control infusions, or diestrous rats administered control or AS-ODN infusions. This was a similar pattern of effects as observed for paced mating measures, but this interaction did not reach statistical significance (Figure 5).
Figure 5.
Depicts the mean (+S.E.M.) time spent in social interaction of cycling rats administered vehicle control (diestrous n=11, proestrous n=13) or PXR AS-ODNs (diestrous n=13, proestrous n=9) infusions.
Reproductive behavior following PXR AS-ODN infusions to the VTA
Lordosis
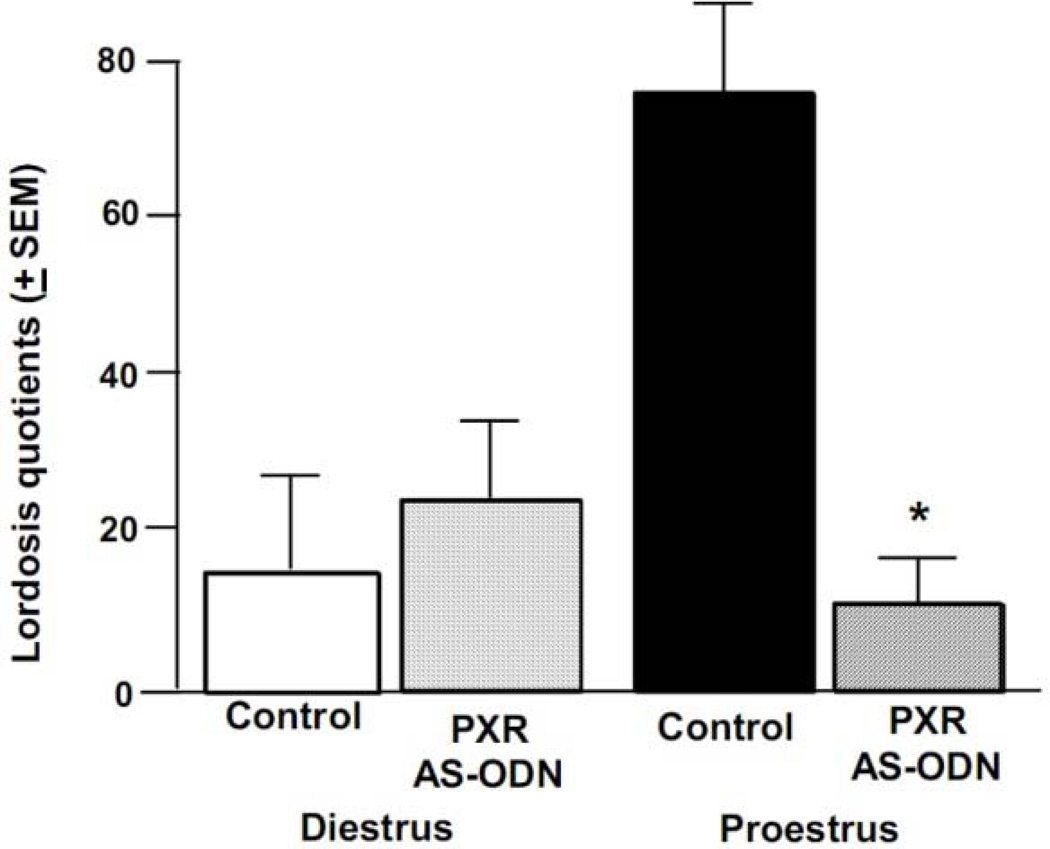
There was a significant interaction of cycle condition and infusion condition for lordosis quotients [F(1, 42) = 9.88, p < 0.01]. Proestrous rats infused with PXR AS-ODNs, but not diestrous rats administered control or PXR AS-ODN infusions, had lower lordosis quotients (percentage of lordosis responses following a mount by a sexually-experienced male), compared to proestrous rats administered control infusions (Figure 6). There was a significant interaction of cycle condition and infusion condition for lordosis ratings [F(1, 47) = 3.89, p < 0.05]. Proestrous rats infused with PXR AS-ODNs, but not diestrous rats administered control or PXR AS-ODN infusions, had lower lordosis ratings, compared to proestrous rats administered control infusions (Table 1).
Figure 6.
Depicts the mean (+S.E.M.) lordosis quotients of cycling rats administered vehicle control (diestrous n=11, proestrous n=13) or PXR AS-ODNs (diestrous n=13, proestrous n=9) infusions. * indicates a reduction due to PXR AS-ODN, compared to control, infusions among proestrous rats (P<0.05).
Proceptivity and Aggression
A similar pattern was observed for proceptivity quotients. There was a significant interaction of cycle condition and infusion condition for proceptivity quotients [F(1, 47) = 6.56, p < 0.01]. Proestrous rats infused with PXR AS-ODNs, but not diestrous rats administered control or PXR AS-ODN infusions, had lower proceptivity quotients, compared to proestrous rats administered control infusions (Table 1). There were no statistically significant effects for aggression quotients.
Midbrain 3a,5a-THP levels following PXR AS-ODN infusions to the VTA
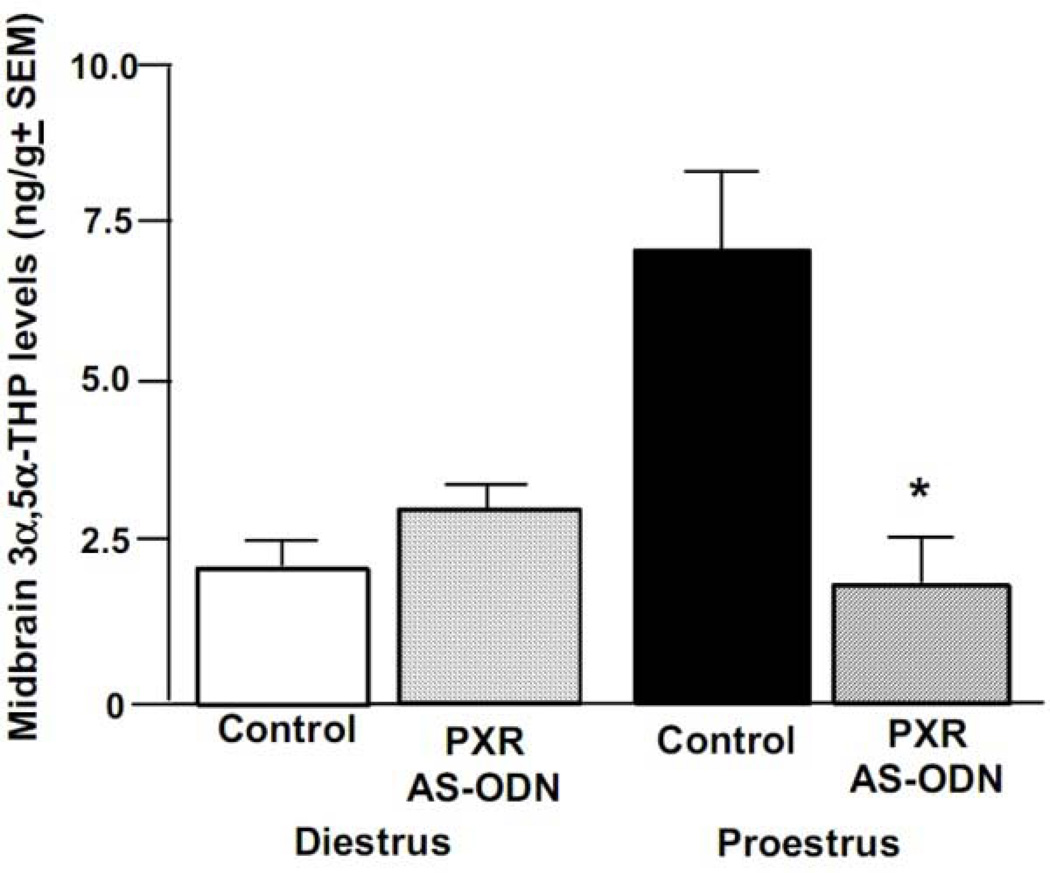
There was a significant interaction among cycle condition and infusion condition [F(1, 42) = 7.36, p < 0.01] for midbrain 3α,5α-THP levels. Proestrous rats infused with PXR AS-ODNs, but not diestrous rats administered control or PXR AS-ODN infusions, had lower levels of midbrain 3α,5α,-THP compared to proestrous rats administered control infusions (Figure 7). This same pattern of significant differences due to PXR AS-ODN infusions was not noted for the other progestogens examined in the midbrain or other regions, or for 3α,5α-THP levels in other regions, except for the striatum (see Table 2).
Figure 7.
Depicts mean (+SEM) 3α,5α-THP levels of cycling rats administered vehicle control (diestrous n=11, proestrous n=13) or PXR AS-ODNs (diestrous n=13, proestrous n=9) infusions. * indicates a reduction due to PXR AS-ODN, compared to control, infusions among proestrous rats (P<0.05).
Table 2.
Plasma levels of estradiol (E2) and plasma and brain levels of progesterone (P), dihydroprogesterone (DHP), and 5α-pregnan-3α-ol-20-one (3α,5α-THP) of diestrous or proestrous rats administered control or pregnane xenobiotic receptor (PXR) antisense oligodeoxynucleotide (AS-ODN) infusions to the midbrain ventral tegmental area. Please note that midbrain levels of 3α,5α-THP are included in Figure 7.
| Steroid levels | Condition | |||
|---|---|---|---|---|
| Diestrous control (n=11) |
Diestrous PXR AS-ODN (n=13) |
Proestrous Control (n=13) |
Proestrous PXR AS-ODN (n=9) |
|
| Plasma | ||||
| E2 (pg/ml) | 8.6 ± 3.9 | 13.7 ± 6.7 | 27.6 ± 9.1 | 21.6 ± 7.4 |
| P4 (ng/ml) | 13.5 ± 3.2 | 12.5 ± 3.5 | 8.6 ± 1.3 | 10.9 ± 3.3 |
| DHP (ng/ml) | 25.5 ± 2.1 | 34.9 ± 5.2 | 27.4 ± 4.5 | 22.9 ± 4.5 |
| 3α,5α-THP (ng/ml) | 55.3 ± 16.7 | 70.7 ± 17.8 | 71.5 ± 20.0 | 72.5 ± 14.5 |
| Cortex | ||||
| P4 (ng/mg) | 2.9 ± 0.5 | 3.9 ± 0.9 | 3.5 ± 0.6 | 4.0 ± 0.9 |
| DHP (ng/mg) | 15.7 ± 2.5 | 12.4 ± 2.2 | 14.0 ± 3.0 | 19.3 ± 3.8 |
| 3α,5α-THP (ng/mg) | 4.2 ± 1.4 | 4.9 ± 1.1 | 3.5 ± 0.7 | 6.9 ± 2.1 |
| Hippocampus | ||||
| P4 (ng/mg) | 3.2 ± 0.8 | 3.9 ± 0.7 | 4.1 ± 0.5 | 2.9 ± 0.7 |
| DHP (ng/mg) | 7.0 ± 1.4 | 9.4 ± 1.2 | 10.2 ± 1.6 | 8.6 ± 2.2 |
| 3α,5α-THP (ng/mg) | 4.7 ± 1.3 | 5.6 ± 1.7 | 21.9 ± 14.2 | 3.1 ± 1.0 |
| Striatum | ||||
| P4 (ng/mg) | 1.7 ± 0.5 | 2.3 ± 0.6 | 6.0 ± 1.8 | 1.2 ± 0.3^ |
| DHP (ng/mg) | 3.6 ± 0.8 | 4.1 ± 0.7 | 5.2 ± 0.6 | 3.8 ± 0.9 |
| 3α,5α-THP (ng/mg) | 2.1 ± 1.2 | 1.5 ± 0.7* | 3.7 ± 1.0 | 0.4 ± 0.1* |
| Hypothalamus | ||||
| P4 (ng/mg) | 5.3 ± 1.3 | 3.6 ± 1.1 | 3.7 ± 0.8 | 3.9 ± 1.0 |
| DHP (ng/mg) | 15.4 ± 2.9 | 11.1 ± 1.5 | 11.4 ± 1.8 | 10.7 ± 2.4 |
| 3α,5α-THP (ng/mg) | 11.4 ± 2.9 | 11.1 ± 6.2 | 13.0 ± 4.3 | 3.5 ± 0.7 |
| Midbrain | ||||
| P4 (ng/mg) | 12.2 ± 3.6 | 8.3 ± 1.6 | 13.1 ± 3.3 | 12.3 ± 2.7 |
| DHP (ng/mg) | 3.1 ± 0.5 | 5.8 ± 1.3 | 6.6 ± 1.0** | 6.4 ± 0.9** |
| 3α,5α-THP | Please see Figure 7. | |||
indicates significant difference compared to control infusions [F(1, 42) = 4.62, p < 0.04].
indicates significant difference compared to diestrous rats [F(1, 42) = 3.81, p < 0.05].
indicates significant interaction between estrous cycle and PXR infusion condition [F(1, 42) = 6.08, p < 0.02].
Discussion
The present results supported our hypothesis that manipulating PXR expression in the VTA via AS-ODN infusions influences 3α,5α-THP levels in the midbrain and behavior of proestrous rats. First, pilot studies indicated the expression of PXR mRNA and protein was determined in the midbrain of proestrous rats, and that VTA infusions of PXR AS-ODNs utilized in this study were effective in reducing PXR expression in the midbrain of proestrous rats. Compared to control infusions, infusions of PXR AS-ODNs to proestrous rats reduced expression of PXR protein in the midbrain, 3α,5α-THP levels in the midbrain, and sexual receptivity of proestrous rats, with modest effects on anti-anxiety-like behavior in the elevated plus maze and open field. There were few effects of PXR AS-ODNs, compared to control infusions, on midbrain PXR, 3α,5α-THP levels, or behavior of diestrous rats. These data demonstrate that knockdown of PXR expression in the midbrain of proestrous rats decreases 3α,5α-THP levels, specifically in the midbrain, concomitant with attenuating sexual receptivity. Thus, 3α-5α-THP’s effects in the midbrain for motivated, reproductively-relevant behaviors may require PXR for its formation.
The present study confirms and extends previous work on the importance of 3α,5α-THP formation for motivated and reproductive behaviors. Natural elevations in, and infusions to the VTA of, 3α,5α-THP enhance receptive and proceptive behaviors of rats [4,19,29–32]. Inhibiting TSPO, P450scc, 3βHSD, 5α-reductase, or 3βHSD activity in the VTA of proestrous or hormone-primed rats attenuates lordosis, but this behavior is rescued by increasing 3α,5α-THP levels in the VTA [1,2,18,33]. For instance, in one study, proestrous rats were infused with vehicle, an inhibitor of biosynthesis (PK11195), and/or an inhibitor of metabolism (indomethacin), followed by a second infusion of vehicle or an enhancer of biosynthesis (FGIN 1–27), before behavioral assessment [23]. Inhibition of 3α,5α-THP formation via biosynthesis or metabolism blockade decreased anti-anxiety behavior in the plus maze and open field, and sexual behavior, and midbrain levels of 3α,5α-THP. These effects could be reversed with infusions of FGIN 1–27 [23]. In the present study, we demonstrate that knocking down PXR similarly reduces the percentage of lordosis responses of proestrous rats as was shown in these prior studies using pharmacological blockade of enzymes likely to be downstream of PXR. Moreover, knocking down PXR has similar effects to reduce the intensity of the lordosis response, as measured by lordosis ratings, as well as proceptive behavior of proestrous rats (see Table 1). Notably, a similar pattern of effects was observed in the social interaction task, albeit these effects were more variable and less robust than measures in the mating task. PXR knockdown in the midbrain attenuated anti-anxiety behavior in the open field by reducing the number of inner 8 entries made. PXR AS-ODNs to the midbrain had modest effects for anti-anxiety behavior in the elevated plus maze, as demonstrated by a non-significant reduction in the time spent on the open arms and a significant reduction in the entries made to the open arms. Some of these effects in the open field and elevated plus maze may be related to reductions in motor activity following PXR AS-ODN infusions as there were smaller percentages of central or open arm entries to total entries, as well as reductions in central entries and open arm time/entries, in the open field and elevated plus maze, respectively. It may also be that these modest effects on anti-anxiety-like behavior in the elevated plus maze and open field are related to reductions in 3α,5α-THP being more robust in the midbrain, than in brain regions traditionally considered to be involved in anxiety-related behaviors, such as the hippocampus. Because rats were tested in a battery, it is unclear whether effects on reproductive behavior were secondary to changes in the other behavioral tasks assessed (social interaction, open field, and elevated plus maze). It can be argued that the most consistent effects of PXR knockdown in this study were the reduction of lordosis and midbrain 3α,5α-THP levels. Additionally, of interest is whether 3α,5α-THP replacement would attenuate these effects by rescuing the loss of 3α,5α-THP due to PXR knockdown. A related consideration in interpreting the present data is that PXR is a transcription factor and could have actions on other genes that may be related, or not, to 3α,5α-THP formation. Notably, others have demonstrated that ovarian expression of the CYP enzyme, P450scc, is elevated preceding, and coincident with, onset of sexual receptivity in rats, and declines in later stages of the estrous cycle [34]. In the present study, these prior results were extended with the observation that PXR expression in the midbrain was higher among rats in proestrus than in diestrus and this coincided with increased 3α,5α-THP levels in this region. Thus, these prior and present findings suggest that 3α,5α-THP formation, which may require PXR, in the midbrain VTA is important for appetitive (proceptive behavior), as well as consummatory (lordosis), aspects of mating behavior.
In addition to assessing the aforementioned functional effects, the present study extends what is known about the localization and function of PXR beyond liver metabolism. Indeed, PXR is most often studied in the liver, where it exists in relatively high concentrations [35], and differences in liver expression have been shown across pregnancy and the post-partum period of mice coincident with clear changes in P4 concentrations [36]. Beyond the liver, we have demonstrated in the present study among proestrous rats that PXR is expressed at the gene, RNA, and protein level in the midbrain, is a target for motivated behaviors and is involved in the reproductive circuit [1,12,35]. Other reports, using RT-PCR and Western blotting, have shown that PXR is expressed in the central nervous system. In support, PXR mRNA and protein is expressed in the rabbit cortex, midbrain, cerebellum [35], rat brain capillaries [37], and various regions of the human brain [38]. Higher gestational levels of 3α,5α-THP are associated with greater whole brain expression of PXR mRNA in offspring [36]. Moreover, in the present study, a cycle-dependent effect of PXR expression was revealed with rats in proestrous having higher expression than those in diestrous. The pilot results using qRT-PCR and Western blotting in and the Western blotting in behaviorally-tested rats confirmed what we have observed in a microarray study, using Affymetrix GeneChip Rat 230 2.0 arrays, assessing mRNA changes across proestrous mated rats and those that were not mated [30]. Although not specifically reported in this publication, we observed in these analyses that PXR mRNA was expressed in grossly-dissected out midbrain [30]. Of interest is the site-specificity for these effects. Pilot studies demonstrate cyclical differences in PXR expression in the midbrain. The effects of PXR AS-ODNs to the midbrain produced the clearest decrements in 3α,5α-THP formation; albeit, there is some indication of a similar pattern in the striatum and a more variable and non-significant pattern for hippocampal levels of 3α,5α-THP to be reduced in proestrous rats following knock down of PXR in the midbrain. A different pattern of effects was observed among rats that were administered PXR AS-ODNs infusions outside of the VTA. Indeed, these rats did not have lower midbrain PXR expression, lower levels of 3α,5α-THP in the midbrain, decreased anxiety-like behavior and sexual receptivity as was more clearly observed among rats administered PXR AS-ODNs infusions to the VTA. As these differences were most notable for 3α,5α-THP levels in the midbrain and a different pattern was observed for infusions outside of the midbrain, the data suggest site-specificity of the experimental effects of the PXR AS-ODNs in the midbrain where the infusions were aimed. Moreover, the reduction in PXR expression in the midbrain seems to be most evident in the area closest to the infusion site in the VTA [28]. Although previous systematic investigations of the diffusion of VTA infusions do not suggest spread beyond the midbrain [29], we do not know if midbrain VTA infusions utilized here spread to certain hypothalamic nuclei, such as mammillary, parabrachial or interpeduncular, which are near to VTA. There was a non-significant decrease in 3α-THP levels in the grossly-dissected out hypothalamus among proestrous rats after PXR AS-ODN infusions (from 13±4.3 in controls to 3.5±0.7 in PXR AS-ODN infused rats), which may suggest that there was spread of the AS-ODNs to the hypothalamus, or that midbrain manipulations alter hypothalamus levels of 3α,5α-THP. We are presently investigating these site-specific effects further in follow-up studies. Thus, these data substantiate future investigations of the functional effects of PXR in the midbrain of females over reproductive cycles.
Cycle-dependent effects of PXR and/or catalyzing enzyme expression indicate modulation via endogenous hormonal milieu. Progesterone plays an important role in modulating estrous cyclicity among rodents [39] and E2 also influences circadian timing of estrous cycle-promoting factors [40]. It is important to understand the extent to which estrogens and/or progestogens contribute to these effects involving PXR. Others have found that systemic administration of E2 to OVX/ADX rats enhances 3β-HSD activity and mRNA expression in hypothalamus [41]. 3α,5α-THP levels in the midbrain are particularly dynamic and change in response to ovarian secretion of E2 and P4, and with mating stimuli. In order for rodents to be sexually-receptive, ovarian secretion or exogenous administration of E2 is necessary [1,42,43]. E2 increases the formation of central progestogens, the activity of the 5α-reductase enzyme, and formation of 3α,5α-THP [44]. Further increases in receptive behaviors are produced by ovarian secretion of progestogens or exogenous administration of progestogens, which increases midbrain levels of 3α,5α-THP over that of diestrous, OVX, or OVX, E2-primed rodents [1]. Although the present study showing little to no effects of PXR manipulations to diestrous rats suggests that PXR may be involved in progestogens-, rather than E2-, facilitated lordosis, ongoing studies are systematically addressing this question.
Another question of interest is the influence of mating itself on midbrain PXR and 3α,5α-THP levels in the present study. As discussed above, enhancing 3α,5α-THP levels in the midbrain VTA facilitates sexual receptivity of rodents. Moreover, engaging in paced mating can enhance biosynthesis of 3α,5α-THP in midbrain of rats [32,45]. Such enhancement in 3α,5α-THP levels is not observed in standard mating paradigms. There are important consequences of this mating-induced enhancement, including improving fertility and fecundity [31] and, increases in 3α,5α-THP following mating in other regions (hippocampus, hypothalamus, cortex, striatum) that may underlie progestogens’ mediation of anxiety behavior and motivation [4,19,32,46,47]. In addition to these dynamic neuroendocrine changes and enhancement in pregnancy outcomes, paced mating can produce a conditioned place preference [2,48,49], which further supports progestogens’ role in motivated processes. Of interest in the present study is that the largest differences in progestogen concentrations outside the midbrain were those that occurred in the striatum for 3α,5α-THP. These data suggest that 3α,5α-THP and PXR may be factors in this well-known circuitry of the VTA and striatum for motivated behaviors. A follow-up question of interest would be the extent to which these effects are bidirectional, with PXR manipulations in the striatum altering 3α,5α-THP in the midbrain. Another interpretation of these data is that PXR in the brain alters peripheral steroid output which in turn affects brain levels of 3α,5α-THP, rather than a direct effect on 3α,5α-THP synthesis from cholesterol in the VTA. However, a lack of changes in peripheral levels of 3α,5α-THP and its precursors, P4 and DHP, in the present study, is not supportive of this notion.
Another consideration is the extent to which 3α,5α-THP may alter PXR. 3α,5α-THP is an endogenous positive activator of the PXR [36]. Enhancing gestational levels of 3α,5α-THP can increase whole brain expression of PXR mRNA in offspring [36]. With PXR knockdown in the present study, there was lower 3α,5α-THP production in the midbrain, but there should still be responses of rats to 3α,5α-THP administration to the midbrain. We are currently investigating this question. Moreover, a battery of tasks was utilized in this study, as we were interested in the extent to which manipulating PXR would alter these reproduction-related behaviors and 3α,5α-THP levels in the same subject. However, it can be difficult to interpret effects of manipulations on any one measure when such a battery of tasks is utilized. Future studies will investigate specific effects in each of these tasks for 3α,5α-THP synthesis. Indeed, the extent to which PXR contributes to mating-related biosynthesis of 3α,5α-THP in the VTA as well as other areas involved in motivated and reproduction-related behaviors, such as the striatum/nucleus accumbens, is of continued interest. Further investigation of downstream receptor targets of 3α,5α-THP is warranted. For example, clinical efficacy of flibanserin, a 5-HT1A agonist and 5-HT2A antagonist, in treating hypoactive sexual desire disorder among women, and other recent studies in female rodents and primates administered this compound, support a role of manipulating these serotonin receptors for receptivity [50–55]. Moreover, acute and repeated dosing of flibanserin to female rats had greater effects to increase levels of dopamine and norepinephrine, compared to serotonin, in areas such as the prefrontal cortex, nucleus accumbens and medial preoptic area [56]. How 3α,5a-THP, which has well-characterized effects through neurotransmitter targets, such as GABA, dopamine, and glutamate in the VTA for female rodent receptivity [1, 30], may potentially be involved in some of these actions of flibanserin, likely downstream of PXR, is an interesting question for future studies.
Conclusions
The present results support the notion that PXR, a promiscuous nuclear receptor, is localized to the midbrain VTA and is required for proestrous increases in 3α,5α-THP levels. In the midbrain VTA, 3α,5α-THP plays a role to influence motivated behaviors, such as mating. The functional effects of PXR in the midbrain are of continued interest and are currently being investigated further in the laboratory.
Acknowledgements
Assistance provided by Dr. Sridar Chittur, Daniel DaCosta, Ryan Keller, Amy Kohtz, Danielle Llaneza, Danielle Osborne, Jason Paris, and Jennifer Torgersen is appreciated.
Source of Funding: This research was supported by grants from the National Institute of Mental Health (MH0676980; RMH067698B).
Footnotes
Conflict of Interest: All authors report that they have no conflicts of interest (financial or otherwise) that would bias them to the outcome of these experiments.
References
- 1.Frye CA. Neurosteroids-From Basic Research to Clinical Perspectives. In: Rubin Robert T, Pfaff Donald W., editors. Hormones/Behavior Relations of Clinical Importance. San Diego: Academic Press; 2009. pp. 395–416. [Google Scholar]
- 2.Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- 3.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 4.Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Behav Brain Res. 2008;187:88–99. doi: 10.1016/j.bbr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holzbauer M. Physiological variations in the ovarian production of 5α-pregnane derivatives with sedative properties in the rat. J Steroid Biochem. 1975;6:1307–1310. doi: 10.1016/0022-4731(75)90357-x. [DOI] [PubMed] [Google Scholar]
- 6.Holzbauer M. Proceedings: Physiological aspects of the hypnotic properties of steriod hormones. Br J Pharmacol. 1976;56 382P-382P. [PMC free article] [PubMed] [Google Scholar]
- 7.Holzbauer M, Birmingham MK, De Nicola AF, Oliver JT. In vivo secretion of 3α-hydroxy-5α-pregnan-20-one, a potent anaesthetic steroid, by the adrenal gland of the rat. J Steroid Biochem. 1985;22:97–102. doi: 10.1016/0022-4731(85)90147-5. [DOI] [PubMed] [Google Scholar]
- 8.Finn DA, Phillips TJ, Okorn DM, Chester JA, Cunningham Cl. Rewarding effect of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in mice. Pharmacol Biochem Behav. 1997;56:261–264. doi: 10.1016/s0091-3057(96)00218-3. [DOI] [PubMed] [Google Scholar]
- 9.Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 10.Sinnott RS, Mark GP, Finn DA. Reinforcing effects of the neurosteroid allopregnanolone in rats. Pharmacol Biochem Behav. 2002;72:923–929. doi: 10.1016/s0091-3057(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 11.Kohtz AS, Paris JJ, Frye CA. Low doses of cocaine decrease, and high doses increase, anxiety-like behavior and brain progestogen levels among intact rats. Horm Behav. 2010;57:474–480. doi: 10.1016/j.yhbeh.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–219. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quiñones-Jenab V, Minerly AC, Niyomchia T, Akahvan A, Jenab S, Frye C. Progesterone and allopregnanolone are induced by cocaine in serum and brain tissues of male and female rats. Pharmacol Biochem Behav. 2008;89:292–297. doi: 10.1016/j.pbb.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Frye CA, Paris JJ, Rhodes ME. Exploratory, anti-anxiety, social, and sexual behaviors of rats in behavioral estrus is attenuated with inhibition of 3α,5α-THP formation in the midbrain ventral tegmental area. Behav Brain Res. 2008;193:269–276. doi: 10.1016/j.bbr.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mòdol L, Darbra S, Pallarès M. Neurosteroids infusion into the CA1 hippocampal region on exploration, anxiety-like behaviour and aversive learning. Behav Brain Res. 2011;222:223–229. doi: 10.1016/j.bbr.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 18.Petralia SM, Jahagirdar V, Frye CA. Inhibiting biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioural oestrus. J Neuroendocrinol. 2005;17:545–552. doi: 10.1111/j.1365-2826.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- 19.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Frye CA, Paris JJ. Progesterone turnover to its 5α-reduced metabolites in the ventral tegmental area of the midbrain is essential for initiating social and affective behavior and progesterone metabolism in female rats. J Endocrinol Invest. 2011;34:e188–e199. doi: 10.3275/7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kliewer SA, Lehmann JM, Milburn MV, Willson TM. The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways. Recent Prog Horm Res. 1999;54:345–367. [PubMed] [Google Scholar]
- 25.Lim YP, Huang JD. Interplay of pregnane X receptor with other nuclear receptors on gene regulation. Drug Metab Pharmacokinet. 2008;23:14–21. doi: 10.2133/dmpk.23.14. [DOI] [PubMed] [Google Scholar]
- 26.Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain. NY NY: Academic Press; 1986. [Google Scholar]
- 28.Frye CA, Paris JJ, Walf AA, Rusconi JC. Effects and mechanisms of 3α,5α,-THP on emotion, motivation, and reward functions involving pregnane xenobiotic receptor. Front Neurosci. 2011;5:136. doi: 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 30.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–913. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90:375–385. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- 32.Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83:336–347. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frye CA, Paris JJ, Rhodes ME. Increasing 3α,5α-THP following inhibition of neurosteroid biosynthesis in the ventral tegmental area reinstates anti-anxiety, social, and sexual behavior of naturally receptive rats. Reproduction. 2009;137:119–128. doi: 10.1530/REP-08-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lephart ED, Doody KJ, McPhaul MJ, Simpson ER. Inverse relationship between ovarian aromatase cytochrome P450 and 5α-reductase enzyme activities and mRNA levels during the estrous cycle in the rat. J Steroid Biochem Mol Biol. 1992;42:439–447. doi: 10.1016/0960-0760(92)90255-h. [DOI] [PubMed] [Google Scholar]
- 35.Marini S, Nannelli A, Sodini D, Dragoni S, Valoti M, Longo V, Gervasi PG. Expression, microsomal and mitochondrial activities of cytochrome P450 enzymes in brain regions from control and phenobarbital-treated rabbits. Life Sci. 2007;13:910–917. doi: 10.1016/j.lfs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Masuyama H, Hiramatsu Y, Mizutani Y, Inoshita H, Kudo T. The expression of pregnane X receptor and its target gene, cytochrome P450 3A1, in perinatal mouse. Mol Cell Endocrinol. 2001;172:47–56. doi: 10.1016/s0303-7207(00)00395-6. [DOI] [PubMed] [Google Scholar]
- 37.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 38.Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol. 2004;199:251–265. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Axelson JF, Gerall AA, Albers HE. Effect of progesterone on the estrous activity cycle of the rat. Physiol Behav. 1981;26:631–635. doi: 10.1016/0031-9384(81)90137-2. [DOI] [PubMed] [Google Scholar]
- 40.Moline ML, Albers HE, Moore-Ede MC. Estrogen modifies the circadian timing and amplitude of the luteinizing hormone surge in female hamsters exposed to short photoperiods. Biol Reprod. 1986;35:516–523. doi: 10.1095/biolreprod35.3.516. [DOI] [PubMed] [Google Scholar]
- 41.Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3β-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frye CA, Paris JJ, Rhodes ME. Estrogen is necessary for 5α-pregnan3α-ol-20-one (3α,5α-THP) infusion to the ventral tegmental area to facilitate social and sexual, but neither exploratory nor affective behavior of ovariectomized rats. Pharmacol Biochem Behav. 2008;91:261–270. doi: 10.1016/j.pbb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Steroid receptor levels in intact and ovariectomized estrogen-treated rats: an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981;33:158–165. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- 44.Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- 45.Walf AA, Paris JJ, Llaneza DC, Frye CA. I. Levels of 5α-reduced progesterone metabolite in the midbrain account for variability in reproductive behavior of middle-aged female rats. Brain Res. 2011;1379:137–148. doi: 10.1016/j.brainres.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camacho FJ, García-Horsman P, Paredes RG. Hormonal and testing conditions for the induction of conditioned place preference by paced mating. Horm Behav. 2009;56:410–415. doi: 10.1016/j.yhbeh.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm Behav. 1997;32:114–124. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- 48.González-Flores O, Camacho FJ, Domínguez-Salazar E, Ramírez-Orduna JM, Beyer C, Paredes RG. Progestins and place preference conditioning after paced mating. Horm Behav. 2004;46:151–157. doi: 10.1016/j.yhbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci. 1997;111:123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- 50.Aubert Y, Gustison ML, Gardner LA, Bohl MA, Lange JR, Allers KA, Sommer B, Datson NA, Abbott DH. Flibanserin and 8-OH-DPAT implicate serotonin in association between female marmoset monkey sexual behavior and changes in pair-bond quality. J Sex Med. 2012;9:694–707. doi: 10.1111/j.1743-6109.2011.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derogatis LR, Komer L, Katz M, Moreau M, Kimura T, Garcia M, Jr, Wunderlich G, Pyke R VIOLET Trial Investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserinin the VIOLET Study. J Sex Med. 2012;9:1074–1085. doi: 10.1111/j.1743-6109.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 52.Gelez H, Greggain-Mohr J, Pfaus JG, Allers KA, Giuliano F. Flibanserin Treatment Increases Appetitive Sexual Motivation in the Female Rat. J Sex Med. 2013 doi: 10.1111/jsm.12094. in press. [DOI] [PubMed] [Google Scholar]
- 53.Jayne C, Simon JA, Taylor LV, Kimura T, Lesko LM SUNFLOWER study investigators. Open-label extension study of flibanserin in women with hypoactive sexual desire disorder. J Sex Med. 2012;9:3180–3188. doi: 10.1111/j.1743-6109.2012.02942.x. [DOI] [PubMed] [Google Scholar]
- 54.Thorp J, Simon J, Dattani D, Taylor L, Kimura T, Garcia M, Jr, Lesko L, Pyke R DAISY trial investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserinin the DAISY study. J Sex Med. 2012;9:793–804. doi: 10.1111/j.1743-6109.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- 55.Stahl SM, Sommer B, Allers KA. Multifunctional pharmacology of flibanserin: possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011;8:15–27. doi: 10.1111/j.1743-6109.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- 56.Allers KA, Dremencov E, Ceci A, Flik G, Ferger B, Cremers TIFH, Ittrich C, Sommer B. Acute and repeated flibanserin administration in female rats modulates monoamines differentially across brain areas: A microdialysis study. J Sex Med. 2010;7:1757–1767. doi: 10.1111/j.1743-6109.2010.01763.x. [DOI] [PubMed] [Google Scholar]