Abstract
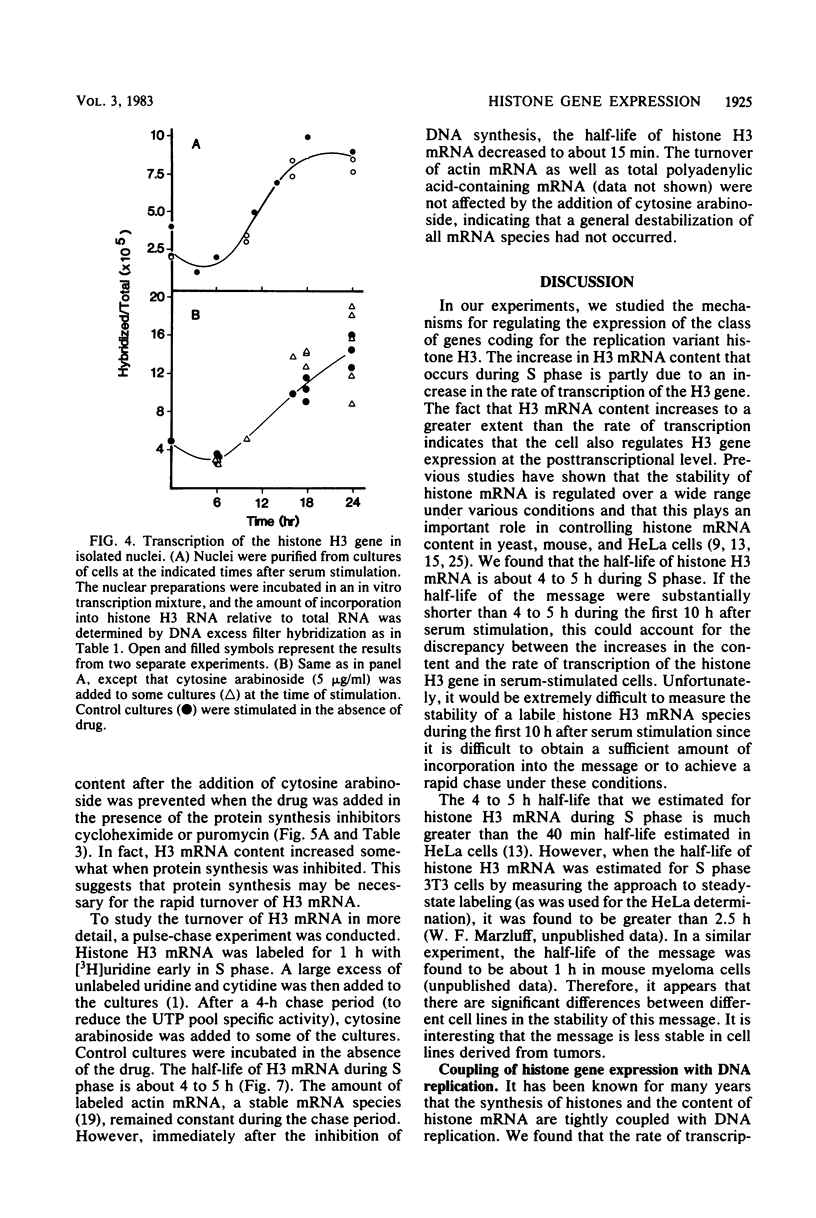
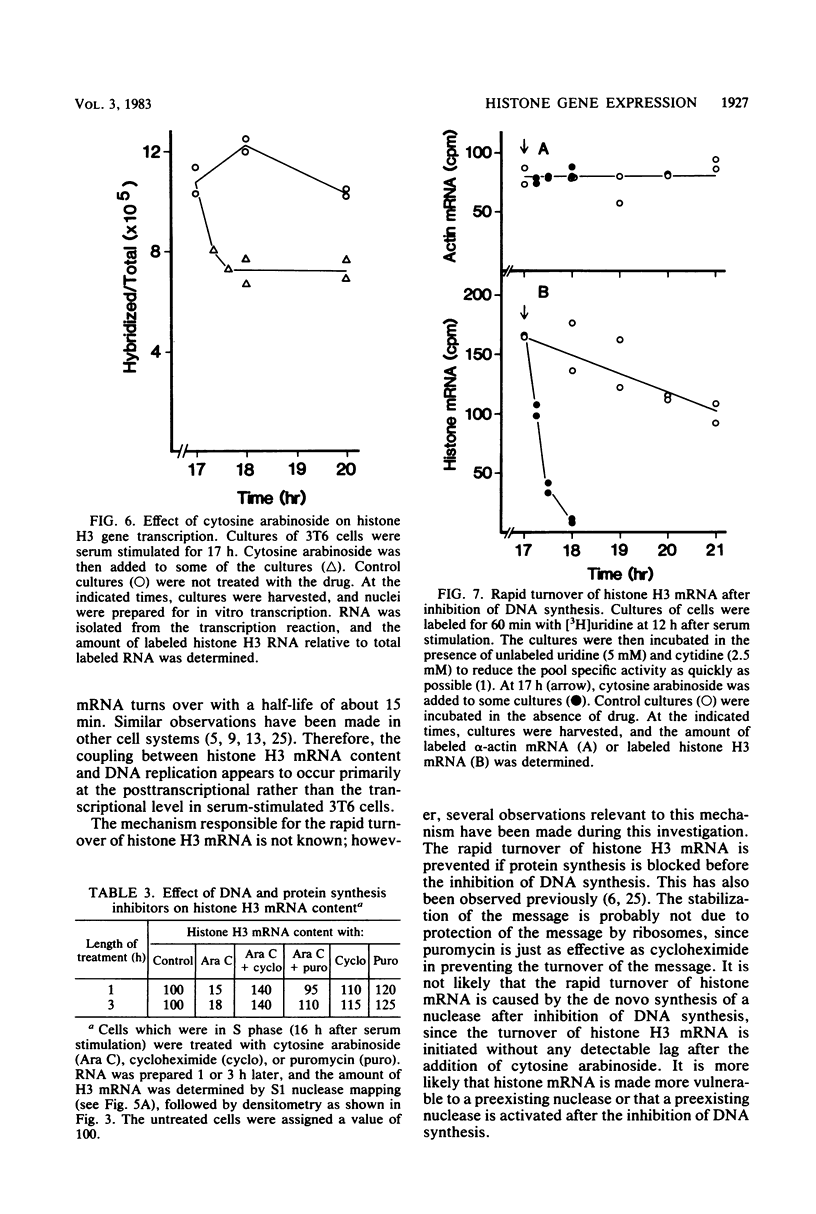
We measured the content and metabolism of histone mRNA in mouse 3T6 fibroblasts during a serum-induced transition from the resting to growing state. The content of several histone H3 and H2b mRNAs was measured by an S1 nuclease procedure. All of these increase in parallel by a factor of about 50 during S phase. However, the rate of H3 gene transcription increased only fivefold during this period, as determined in an in vitro transcription assay. This suggests that histone mRNA content is also controlled at the posttranscriptional level. When resting cells were serum stimulated in the presence of cytosine arabinoside, the rate of H3 gene transcription increased to about the same extent as that in control-stimulated cells. However, cytoplasmic H3 mRNA content increased only five to seven-fold. The half-life of H3 mRNA during S phase was about 4 to 5 h. When cytosine arabinoside was added to cells in the S phase, the half-life of the message decreased to about 15 min. The rapid turnover of H3 mRNA was prevented when the drug was added in the presence of cycloheximide or puromycin. The rate of H3 gene transcription decreased by only 35% after treatment with cytosine arabinoside. These results suggest that H3 gene transcription is not tightly coupled to DNA replication but is controlled temporally during the resting to growing transition. However, there is a correlation between the rate of DNA synthesis and the stability of histone H3 mRNA.
Full text
PDF

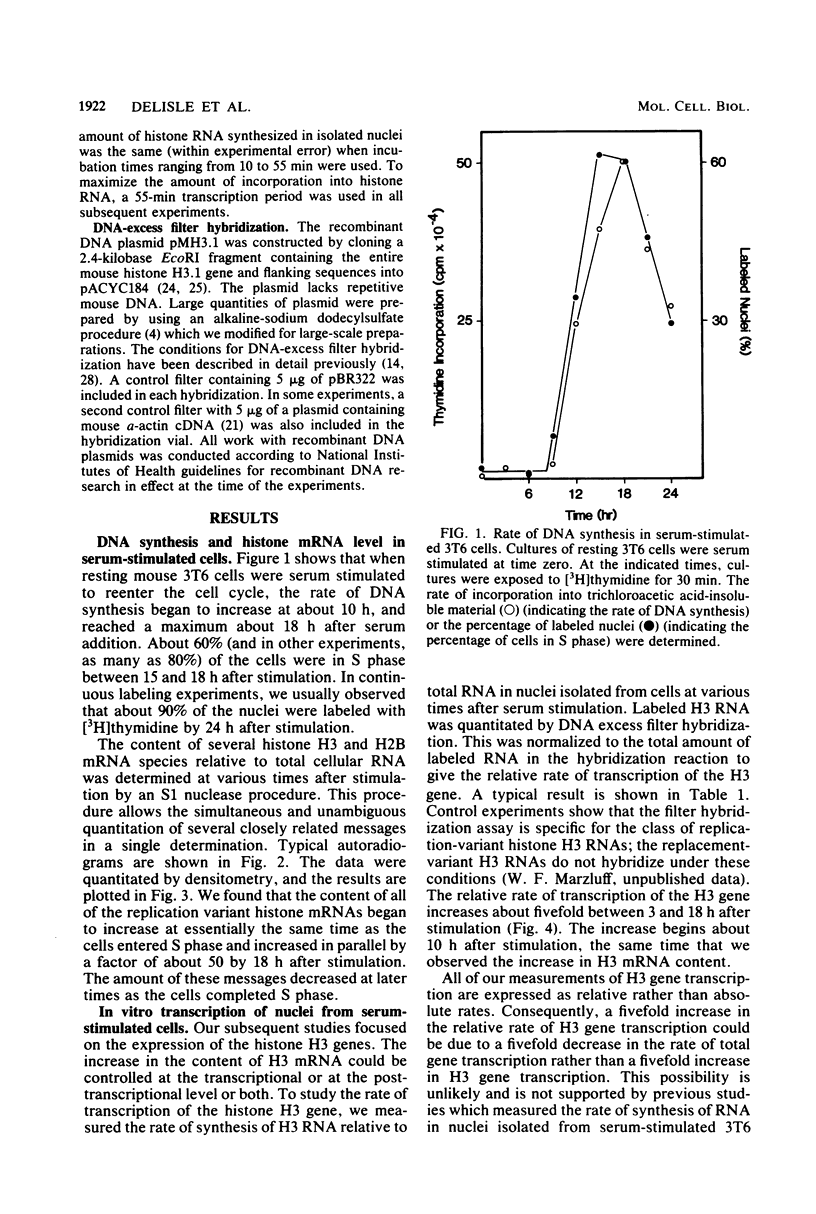
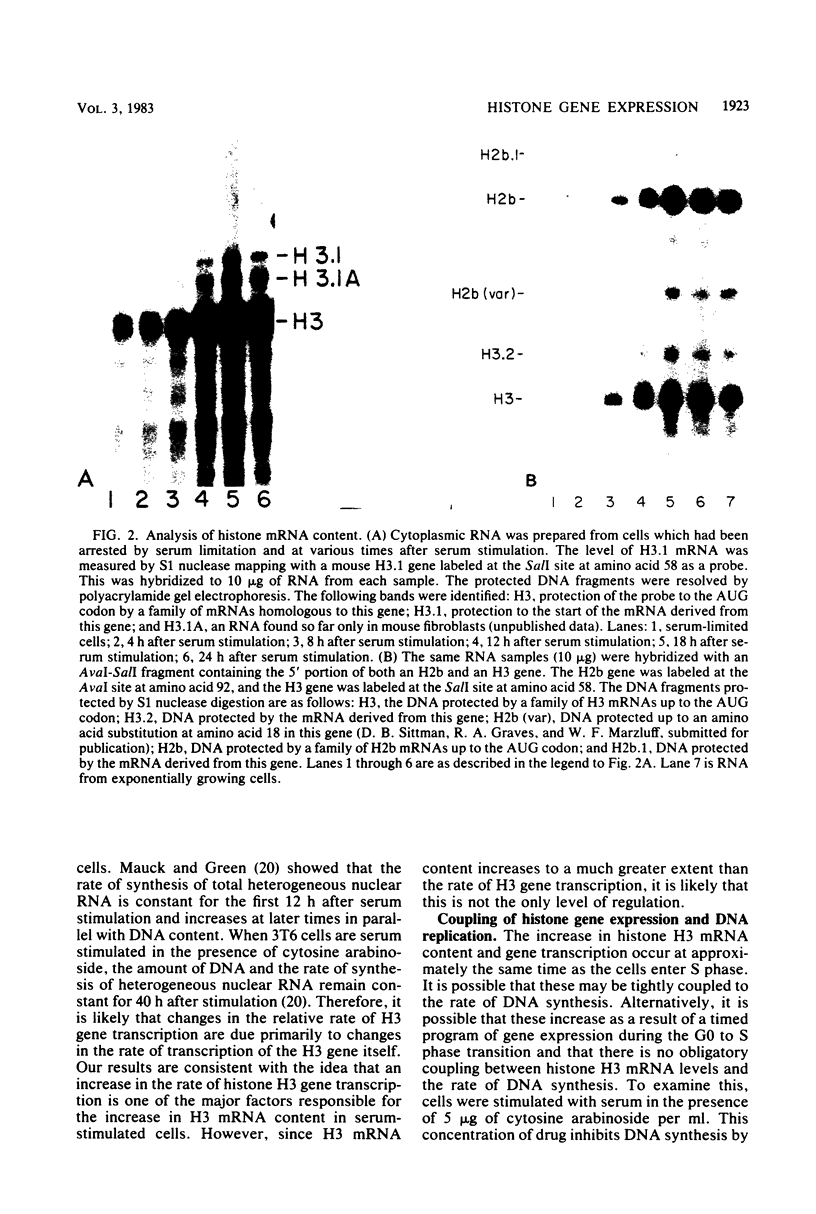
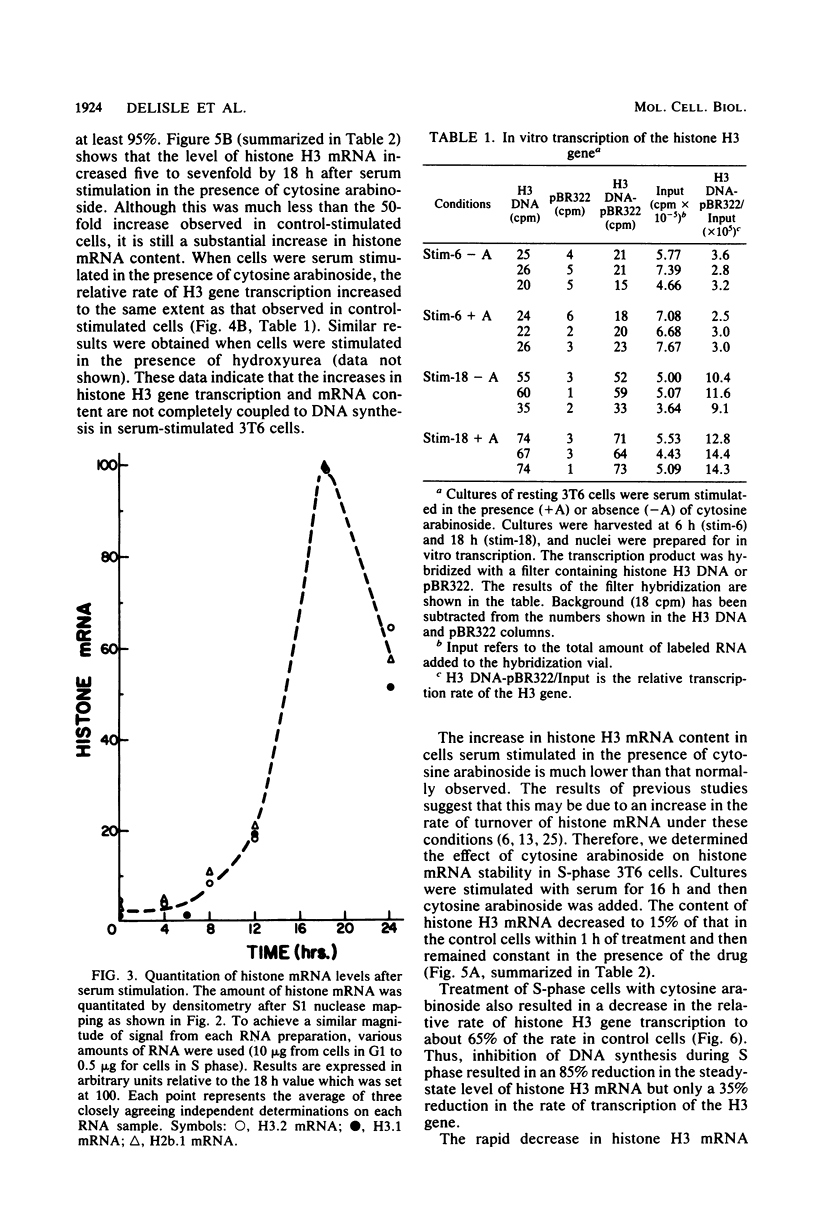



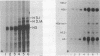
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Quantitation of elongating form A and B RNA polymerases in chick oviduct nuclei and effects of estradiol. Cell. 1976 Mar;7(3):455–465. doi: 10.1016/0092-8674(76)90176-8. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Gallwitz D. Kinetics of inactivation of histone mRNA in the cytoplasm after inhibition of DNA replication in synchronised HeLa cells. Nature. 1975 Sep 18;257(5523):247–248. doi: 10.1038/257247a0. [DOI] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Coffino P. G1 and S phase mammalian cells synthesize histones at equivalent rates. Cell. 1980 Aug;21(1):195–204. doi: 10.1016/0092-8674(80)90127-0. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. The metabolism of histone fractions. IV. Synthesis of histones during the G1-phase of the mammalian life cycle. Arch Biochem Biophys. 1972 Feb;148(2):633–641. doi: 10.1016/0003-9861(72)90182-8. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Wiedemann L. M. Regulation of dihydrofolate reductase gene expression in mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):397–306. doi: 10.1002/jcp.1040970314. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Rao L. G., Muench A. J. Regulation of thymidine kinase enzyme level in serum-stimulated mouse 3T6 fibroblasts. Exp Cell Res. 1982 Mar;138(1):79–85. doi: 10.1016/0014-4827(82)90093-3. [DOI] [PubMed] [Google Scholar]
- Kessler-Icekson G., Singer R. H., Yaffe D. The capacity of polyadenylated RNA from myogenic cells treated with actinomycin D to direct protein synthesis in a cell-free system. Eur J Biochem. 1978 Aug 1;88(2):403–410. doi: 10.1111/j.1432-1033.1978.tb12462.x. [DOI] [PubMed] [Google Scholar]
- Mauck J. C., Green H. Regulation of RNA synthesis in fibroblasts during transition from resting to growing state. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2819–2822. doi: 10.1073/pnas.70.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Chiu I. M., Pan C. J., Cohn R. H., Kedes L. H., Marzluff W. F. Isolation of two clusters of mouse histone genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4078–4082. doi: 10.1073/pnas.78.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. S., Johnson L. F. Regulation of dihydrofolate reductase gene transcription in methotrexate-resistant mouse fibroblasts. J Cell Physiol. 1982 Feb;110(2):183–189. doi: 10.1002/jcp.1041100212. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]