Abstract
In the last decade, there has been an upsurge of interest in understanding the mechanisms of behavior change (MOBC) and effective behavioral interventions as a strategy to improve addiction-treatment efficacy. However, there remains considerable uncertainty about how treatment research should proceed to address the MOBC issue. In this article, we argue that limitations in the underlying models of addiction that inform behavioral treatment pose an obstacle to elucidating MOBC. We consider how advances in the cognitive neuroscience of addiction offer an alternative conceptual and methodological approach to studying the psychological processes that characterize addiction, and how such advances could inform treatment process research. In addition, we review neuroimaging studies that have tested aspects of neurocognitive theories as a strategy to inform addiction therapies and discuss future directions for transdisciplinary collaborations across cognitive neuroscience and MOBC research.
Keywords: neuroimaging, addiction treatment mechanisms of behavior change, treatment process
In the last decade, there has been an upsurge of interest in understanding the mechanisms of behavior change (MOBC) and effective psychosocial treatments as critical strategies to improve the treatment of addiction (Kazdin, 2007; Kazdin & Nock, 2003). The ascendancy of MOBC as a focus of treatment research is reflected in new initiatives at the U.S. National Institutes of Health (NIH), such as the Science of Behavior Change website (http://commonfund.nih.gov/behaviorchange), and in the addiction area via the MOBC program at the National Institute of Alcohol Abuse and Alcoholism (Huebner & Tonigan, 2007; NIAAA, 2009). The current interest in MOBC among addiction-treatment researchers largely reflects a recent and growing concern with the dominant approach to addiction treatment research: the randomized controlled clinical trial (RCT) and other methods subsumed under the label of the “stage” (Rounsaville, Carroll, & Onken, 2001) or “psychotherapy technology” models (Babor, 2008; Morgenstern & McKay, 2007). Stated succinctly, remarkable advances in the 1980s and 1990s led to the development of a number of effective behavioral treatments for addiction (Dutra et al., 2008; Miller & Wilbourne, 2002). However, existing behavioral interventions are only modestly effective and efforts in the last decade or so to improve their efficacy using a traditional RCT approach have not yielded the robust results that were expected (e.g., Anton et al., 2006; Morgenstern & McKay, 2007; Morgenstern et al., in press).
One of the unexpected but important lessons gleaned from several decades of addiction RCT research is that we have a more limited understanding of how effective behavioral treatments work than anticipated (e.g., Apodaca & Longabaugh, 2009; Morgenstern & Longabaugh, 2000). As a result, there is a general consensus that treatment research should focus more on mechanism issues, and that improved understanding of the mechanisms that underlie effective treatments will lead to improvements in treatment efficacy and the dissemination of evidence-based practices.
One of the challenges that confronts mechanism research is the development of a set of approaches and research methods that provide the field with a strong scientific foundation. Methods required to determine whether a behavioral treatment is effective are quite different than those needed to support a theory about how a treatment works. For example, the requirements to establish strong causal inference for a hypothesized mediator of treatment are different than those for establishing strong causal inference for the effect of the treatment itself. Clinical-trial methods for behavioral interventions were developed over several decades, beginning in the early 1980s. Mechanism research can be characterized as still in a formative phase of determining which conceptual frameworks, design considerations, data-analytic approaches, constructs, and measures will yield the best science.
Cognitive neuroscience approaches are increasingly being used to study the effects of psychotherapy across an array of psychiatric disorders. Cognitive neuroscience methods include functional brain imaging, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), to measure brain activity during the experimental manipulation of a specific cognitive process; structural brain imaging using MRI to examine the size, tissue composition, and connectivity of different brain regions that subserve specific cognitive functions; and lesion-behavior analyses that examine the effects of focal brain damage on dissociable cognitive functions. These modalities for measuring brain structure and function have been coupled with increasingly sophisticated cognitive psychology paradigms that allow for the ability to parse component cognitive processes of complex behaviors, along with psychophysiological measurements, such as electroencephalographic measures of brain electrical and autonomic indices of attention and emotional arousal.
Most behavioral interventions for addiction are hypothesized to work through changes in cognitive, affective, and learning processes, such as motivation, emotion regulation, cognitive reframing, and reward learning. Over the last 20 years, cognitive neuroscience has been focused on probing the cognitive mechanisms and neural substrates of precisely these cognitive and affective processes (Ochsner & Lieberman, 2001). Thus, cognitive neuroscience can offer a powerful approach to study MOBC. However, until very recently, the use of cognitive neuroscience methods to examine mechanisms in addiction treatment has been absent. By contrast, cognitive neuroscience is playing a more prominent role in the study of psychotherapy for depression and anxiety disorders (Carrig, Kolden, & Strauman, 2009; Clark & Beck, 2010).
The primary aim of this article is to examine how both the conceptual framework and methodological approaches of cognitive neuroscience can inform our understanding of the cognitive, behavioral, and affective processes that underlie effective behavioral treatments and successful self-change efforts in addiction. One of the main arguments we make in this paper is that recent research sheds new light on potential shortcomings in underlying models of addiction that inform our treatments, that is, the factors that maintain addiction and how treatments and self-change efforts remediate these factors. Mechanism research has appropriately identified limitations in clinical-trials methods and proposed innovative solutions to address these issues. However, there has been less consideration of potential shortcomings in the explanatory frameworks that undergird addiction treatments in light of advances in other areas of science.
Accordingly, in the first half of the paper we briefly describe the current focus of addiction MOBC research and an alternative perspective that calls attention to the conceptual underpinnings of addiction-treatment theories. We then describe key aspects of the new neurocognitive and neurobiological theories of addiction, with a focus on how these theories identify cognitive processes that might be the targets of treatments. In the second half of the paper, we briefly review neuroimaging studies that have tested aspects of these newer theories as a strategy to inform addiction treatment. We note that our discussion of the literature is not designed to be comprehensive, and throughout the paper, we direct the reader to reviews that more fully explicate the phenomena under discussion.
The Role of Treatment Theory in Mechanism Research
The impetus for current mechanism research derived from the unexpected inability of traditional clinical-trial research methods to support the hypothesized effects of most behavioral interventions for addiction, that is, how the interventions work. Results from Project MATCH illustrate this problem (Longabaugh & Wirtz, 2001). Despite strong methodological features, only a small number of critical hypotheses about treatment matching and causal chain analyses related to treatment mediation were supported. These results and others led researchers to conclude that traditional clinical-trial methods were limited in their ability to explain how treatments work, and that new approaches that focused specifically on the mechanism question were needed (cf. Babor, 2008; Bühringer, 2006; Orford, 2006; Morgenstern & McKay, 2007).
Important early contributions to mechanism research focused on defining new conceptual frameworks, definitional enhancements, and recommended improvements in research design. For example, conceptual and definitional considerations of terms such as “mediator,” “mechanism,” “active ingredient,” “causal chain,” “common change process” and so on received extensive consideration in the general psychotherapy (e.g., Doss, 2004; Kazdin, 2007; Wampold, 2001) and addiction (Huebner & Tonigan, 2007; Longabaugh & Wirtz, 2001; Moos, 2007) literatures. Another important topic has concerned methodological considerations of study-design features and data-analytic strategies used to assess mechanisms hypotheses in clinical trials (Kazdin & Noch, 2003; Longabaugh, McGill, Morgenstern & Huebner, in press; MacKinnon, 2008; Nock, 2007; Preacher & Hayes, 2008). These important contributions have led to new and more rigorous MOBC research for addiction, including new methods to assess within-session therapist–client interactions (Karno & Longabaugh, 2005; Moyers, Martin, Houck, Christopher, & Tonigan, 2009); new studies that experimentally manipulate hypothesized active treatment ingredients (Litt, Kadden, & Kabela-Cormier, 2009; Morgenstern & McKay, 2007; Morgenstern et al., in press; Walters, Vader, Harris, Field, & Jouriles, 2009); microlongitudinal analysis of mediators using ecological momentary assessment (Kranzler, Armeli, Feinn, & Tennen, 2004; Holt, Litt & Cooney, 2011), and better methods to strengthen inference using traditional treatment mediation designs (Kelly, McGill, & Stout, 2009; Kiluk, Nich, Babuscio, & Carroll, 2010; Mensinger, Lynch, TenHave, & McKay, 2007).
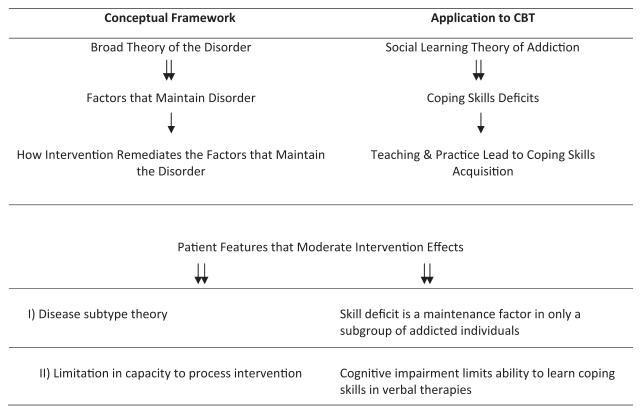
Although these contributions have advanced the field, we believe there has been an underappreciation of the role treatment theory plays in mechanism research and how limitations in these theories hamper our ability to explain how treatments work. Figure 1 provides a simplified logic model of fundamental assumptions that undergird addiction-behavioral interventions using cognitive–behavioral therapy (CBT) as an example. This framework presents a nested hierarchy between a broad theory of the disorder, a specific set of psychological factors that maintains the disorder, a theory of how the intervention works to alter these maintenance factors, and a set of patient characteristics that help define subtypes and clarify the limits of intervention effects. This simplified logic model defines the problem space or the hypothesized causal chains that are currently being tested in mechanism research (Longabaugh, & Wirtz, 2001).
Figure 1.
Hierarchical components of framework that undergirds current behavioral intervention research.
For example, social learning theory (SLT) is the conceptual framework that underlies CBT. According to this framework, deficits in cognitive and behavioral coping skills are the factors that maintain addictive disorders. CBT therefore targets deficits in coping skills via skills training, and this leads to skills acquisition and mastery, which promote abstinence. One example of a moderator hypothesis is that skill deficits maintain addiction in only certain groups of patients, whereas other patients have intact coping skills, but suffer from another deficit that maintains the disorder, such as low motivation to change. Thus, CBT would be more effective for the first subgroup than for the second. A second class of moderators concerns patient capacities needed to benefit from the treatment. For example, skills training assumes a certain level of cognitive ability needed to acquire skills, such that patients who are sufficiently cognitively impaired would not benefit from CBT. We limit moderator classes to these two for heuristic purposes, but note others could be posited.
Figure 1 highlights the connection between mechanism hypotheses and theories of addictive disorder characterization or pathophysiology. For purposes of clarity, we note that pathophysiology refers to the factors that maintain a disorder, whereas etiology refers to factors that contribute to its development. Although etiology is relevant to treatment concerns, pathophysiology is critical to treatment because pathophysiological factors maintain the disease state that treatments target and attempt to remediate. Some of these targets may be amenable to change using behavioral interventions, others to pharmacotherapies, and still other may be resistant to any intervention. During the last decade, there have been significant revisions to our theories regarding the pathophysiology of addiction and, more broadly, to that of all psychiatric disorders (Insel et al., 2010). In the case of addiction, revisions have largely been based on advances in cognitive science and neurobiology. Although newer theories of addiction build on earlier work, it is generally understood that they provide a novel account regarding the pathophysiological factors of addiction and the methods used to study these factors.
Treatment theories that underlie most effective behavioral addiction treatments, especially those that are most widely used, are based on theories of addictive disorders from the 1970s and 1980s. For example, the CBT framework described above derives from application of SLT to addiction (Marlatt & Gordon, 1985). Similarly, the theories that have guided mechanism research on MI (Motivational Interviewing) are largely based on the work of Janis and Mann (1977) and Orford (1985) regarding decision making, cognitive dissonance, and ambivalence. We note that calling attention to the limitations in the theories that underlie treatment does not imply that treatments are less effective. Indeed, one of the virtues of clinical-trial research is that we can demonstrate the efficacy of an intervention without needing to fully understand how it works. In addition, behavior therapies developed recently do incorporate new treatment theories (Bowen, Chalwa, & Marlatt, 2010). However, it is important to note that the explanatory models that underlie effective SUD (Substance Use Disorders) treatments may themselves be flawed and the application of newer theories and methods may lead to a better understanding of how treatments work.
As noted above, addiction-mechanism research has appropriately sought to develop approaches that improve on clinical-trial methods and enable the testing of mechanisms. However, innovations have largely been restricted to the development of new methods, which are then used to test existing treatment theories, as illustrated in the work cited above. We argue that method innovations are critical and necessary, but not sufficient to advance the field without also addressing limitations to treatment theories. Stated somewhat differently, mechanism research has appropriately identified how advances in experimental design (e.g., statistical methods to test mediation) can improve on the limitations of earlier methods, but has yet to fully appreciate how advances in our understanding of the nature of addiction and methods to study psychological processes can help address limitations in existing treatment theories, in order to build a strong foundation for mechanism science.
Contemporary Neurocognitive Theories of Addiction
Over the last two decades, discoveries in neurobiology, cognitive neuroscience, and other basic science disciplines have led to significant revisions to our understanding of addiction. Scientific study of addiction remains an active area of research. No single theory has emerged as correct and many questions remain to be answered. However, there are several common features across theories that appear well established. First, neurobiological or neurocognitive theories view addiction as characterized by deficits in one or more cognitive processes, such as learning, motivation, memory, attention, and decision making. In addition, these deficits are identified across multiple levels of analysis, including self-reported cognitions, behavioral measures of cognitive processes, neural circuits, and neurotransmitter systems. For example, one account views addiction as a deficit in reward learning characterized by a hyperactivation of brain-reward systems for addictive cues that is manifest in craving, compulsive behavior, alterations in subcortical brain circuitry, and changes in the neurotransmitter dopamine (Robinson & Berridge, 2000). Second, although much of the early debate among theorists focused on which cognitive processes, neural mechanisms, and neurotransmitter systems characterize the disorder, there has been an increasing recognition that addiction involves deficits in multiple cognitive processes and related neural and neurotransmitter systems, and that these deficits likely vary across individuals, stages of the disorder, or type of substance (Redish et al., 2008; Stacy & Wiers, 2010). Finally, many neurocognitive theories have proposed that addiction reflects an impairment in normal self-control processes, such that the salience of addictive cues is strengthened relative to natural reinforcers, while at the same time executive cognitive processes that normally control impulsive responding are weakened. This imbalance between overvalued drug rewards and weakened control systems maintains addiction (Bechara, 2005; Baler & Volkow, 2006; Curtin, McCarthy, Piper, & Baker, 2006: George & Koob, 2010; Stacy & Wiers, 2010).
In this section, we present a brief overview of one of these theories: Bechara and colleagues’ neurocognitive perspective on addiction (Bechara, 2005; Verdejo-García & Bechara, 2009). We select Bechara’s account because it integrates several different neurobiological theories of addiction, is a relatively accessible perspective for behavioral scientists, and it can be directly applied to the clinical phenomenology of addiction and its treatment.
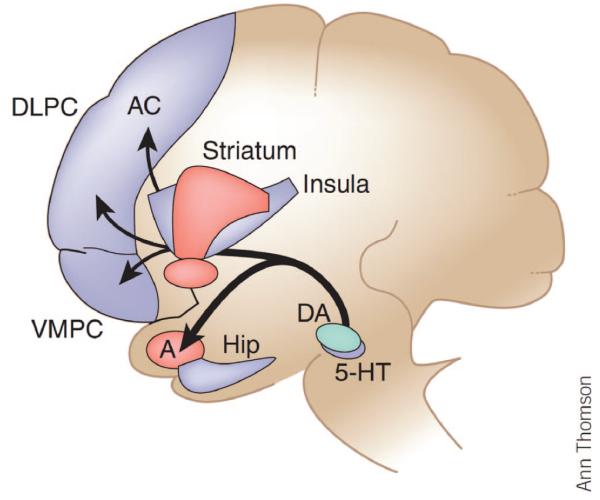
Bechara postulates that addiction is a disorder of judgment and decision making manifested in the impairment of the psychological processes and neural systems engaged by self-control or will-power (see Figure 2). Adaptive decision making is subserved by the smooth interaction between two separate neural systems: (a) an impulsive system for signaling pain and pleasure of immediate prospects of an option, subserved by the amygdala, the nucleus accumbens, and the mesotelencephalic dopamine (DA) system and (a) a reflective system for signaling pain and pleasure of long-term prospects, subserved by distributed networks within the prefrontal cortex.
Figure 2.
A schematic diagram illustrating key structures belonging to the impulsive system (red) and the reflective system (blue). An emergent dominant pattern of affective signaling can modulate activity of several components of the impulsive and reflective systems. These include regions involved in (a) representing patterns of affective states (e.g., the insula and somatosensory cortices); (b) triggering of affective states, e.g., amygdala (A) and ventromedial prefrontal cortex (VMPFC); (c) memory, impulse, and attention control (e.g., lateral orbitofrontal, inferior frontal gyrus, and dorsolateral prefrontal (DLPC), hippocampus (Hip) and anterior cingulate (AC); and (d) behavioral actions (e.g., striatum and supplementary motor area). 5-HT = serotonin; DA = dopamine. Reprinted by permission Macmillan Publishers Ltd. from “Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective,” by Antoine Bechara, 2005, Nature Neuroscience, 8, p. 1459. Copyright 2005 by Nature Publishing Group.
In the impulsive system, the amygdala recognizes the immediate sources of pleasure or satisfaction of homeostatic needs (e.g., withdrawal states) within the environmental features of a stimulus without accessing higher order thought processes. It then translates this recognition into a representation of immediate reward value or saliency and, through its connections with the nucleus accumbens, the DA system, and subcortical regions (such as the hypothalamus), which coordinate bodily homeostatic functions, triggers a pattern of behavioral (e.g., reward seeking), visceral (e.g., increased heart rate) and cognitive reactions (e.g., attention deployment), promoting the obtainment of the immediately available rewards or the avoidance of the immediately obtainable punishments. This process is largely involuntary, automatic, nonconscious, and associational in nature.
The reflective system is responsible for higher order mental operations and is controlled by neural systems in the prefrontal cortex (PFC). These operations include executive functions such as attention, working memory, cognitive control, planning, response inhibition, reward-reversal learning, and self-aware processing. These functions coordinate strategic action plans for goal pursuit, and inhibit prepotent responses or impulses that would impede the attainment of goals. These operations are relatively slow, controlled, and effortful, and are based on symbolic representations and operations that must be maintained within working memory.
According to Bechara’s model, both the impulsive system and the reflective system operate through the deployment of bodily states, called somatic markers, that serve to affectively label various options for behavior in terms of their immediate and longer-term negative and positive consequences. In this way, decision-making processes that involve uncertain rewards and punishments are inherently tied to the body and to emotional experience. This model has particular relevance to addictions, where drugs of abuse exert highly salient effects upon the body that may become integrated into the brain’s representation of what is pleasurable or punishing about using the drug, as well as the long-term consequences of using the drug (Naqvi & Bechara, 2010).
In the course of experience, individuals encounter many situations in which the immediate prospect of an action is in conflict with its long-term outcome. For example, the temptation to eat a delicious dessert may conflict with a long-term plan to lose weight. Similar to other dual-system theorists (e.g., Hofman, Friese, & Strack, 2009; Mead, Alquist, & Baumeister, 2010), Bechara postulates that the outcome of this conflict, one in which immediate and long-term prospects are at odds, will depend on the interaction of the reflective and impulsive systems. Final decisions are determined by the relative strength of value associated with immediate versus long-term prospects. In cases where the long-term outcome of an action is judged as more rewarding or painful than the immediate outcome, the long-term perspective will win out. Otherwise, the immediate prospect predominates and decisions are based on a default short-term horizon.
Although the capacity to maintain self-control vacillates as a normal part of living, in addiction there is a fundamental break-down or impairment in the cognitive processes and neural systems associated with self-control, leading to a tendency to favor immediate over long-term outcomes, even in the face of threats to survival. According to Bechara (2005), impairments that maintain addiction may occur in either or both reflective and impulsive systems.
Reflective-System Impairment
Bechara (2005) postulated that addiction may be maintained by deficits in two reflective-system domains: decision making and cognitive control. Bechara’s perspective on decision making deficits and addiction derives from research on patients with lesions in the ventromedial prefrontal cortex (VMPFC). VMPFC-lesion patients are characterized by obliviousness to the long-term consequences of their decisions, an inability to learn from mistakes, a lack of emotional responses to social stimuli, and a lack of insight into their problems. At the same time, they have intact intelligence, memory, and other complex cognitive functions. Traditional neuropsychological tests have notably failed to detect these abnormalities, despite the obvious problems VMPFC-lesion patients have in judgment and social functioning. Bechara developed a laboratory task, the Iowa Gambling Task (IGT), which was designed to simulate real-life decisions under conditions of uncertainty, risk, and reward. In the IGT, participants make choices by picking cards from four decks, which are either advantageous or disadvantageous in the long term. Subjects do not know at the start of the task which decks lead to which outcomes. Through feedback, normal subjects quickly learn to select advantageous decks, but those with VMPFC lesions persist in disadvantageous choices, despite rising losses. They also fail to deploy somatic markers, indexed by autonomic responses, when they are in the process of making their choices (Verdejo-García & Bechara, 2009).
Bechara (2005) noted the similarity between addicted individuals and VMPFC-lesion patients and examined these in a series of studies comparing normal controls, VMPFC-lesion patients, and individuals with substance dependence (SDI; Bechara, 2005; Verdejo-García, & Bechara, 2009). Overall, these studies found that one subgroup of SDIs had similar profiles to VMPFC-lesion patients on the IGT and other decision-making tasks, including faulty affective responses in the presence of long-term choices and overall poor performance on the IGT. Another subgroup of SDI had low performance on the IGT, but normal affective responding, and a third SDI subgroup appeared similar to normal controls on decision making tasks. Based on these findings, Bechara postulated that addicted individuals might differ on the nature of cognitive and neural impairment by subgroup; that faulty decision making characterized a large majority, but not all addicted individuals; and that a specific subgroup of addicted individuals had similar impairments to VMPFC-lesion patients, characterized by a myopia with regard to future consequences as well as other impairments in affective processing.
Bechara (2005) postulated that impairment in cognitive-control processes may also maintain addictive behaviors. Cognitive-control processes are critical to the flexible selection and implementation of tasks in pursuit of higher order goals, such as ongoing monitoring of task performance and inhibition of unwanted responses. Cognitive-control processes that have been investigated in addiction include attention control, inhibitory control, and behavioral monitoring (Garavan & Hester, 2007). These processes are subserved by neural circuits in the dorsolateral prefrontal cortex (DLPFC), lateral orbitofrontal cortex (LOFC), and the anterior cingulate cortex (ACC). Similar to his views on decision making, Bechara postulates that deficits in cognitive control likely vary across addicted individuals, with some having intact cognitive-control processes and others having deficits in one or more areas, such as attention control or response inhibition.
Impulsive-System Impairment
Bechara (2005) postulated that addiction is maintained by hyperactivation of the impulsive system, such that drug cues take on increased motivational value or incentive salience relative to other natural reinforcers (Robinson & Berridge, 2003). The heightened incentive salience of drug cues overwhelms normal executive-control processes, leading to a persistent selection of behaviors favoring immediate over long-term outcomes. Incentive-salience theories hypothesize that repeated drug use in vulnerable individuals changes the neural circuits and cells that normally regulate the attribution of incentive salience to stimuli. This “neuroadaptation” renders brain circuits sensitized in a way that results in pathological attribution of incentive salience to drugs and drug cues. This sensitized state persists long after cessation of drug use.
Bechara’s (2005) account references incentive salience theory (Robinson & Berridge, 2003), but it is important to note that other neurobiological theories postulate deficits in neural systems related to habit learning (Everitt et al., 2008) and the relief of negative affect and stress (Koob & LeMoal, 2008). However, as noted above, these three neuroadaptational theories may not be incompatible, but represent different impulsive-system deficits across different individuals, stages of addiction, or substances (cf. George & Koob, 2010; Redish et al., 2008).
Cognitive Neuroscience Approach to Subtypes
Bechara suggests a novel approach to subtyping based on cognitive neuroscience. Specifically, addiction might vary based on the specific deficit in cognitive processes and associated neural systems that maintain the disorder. For example, some addicted individuals may have intact reflective systems or executive-cognitive functions, but have a deficit in reward learning associated with incentive salience. By contrast, other addicted individuals may have multiple impulsive and reflective system deficits including, VMPFC impairment. This subtyping approach has clear implications for prognosis and treatment selection. For example, addicted individuals with intact reflective-system function may be able to change on their own, whereas those with VMPFC impairment may be vulnerable to chronic relapse, despite repeated treatment. Bechara and others (Baler & Volkow, 2006: George & Koob, 2010) have proposed that this approach could be used to develop targeted pharmacotherapies based on neurotransmitter abnormalities associated with cognitive-process deficits. In addition, very recently Gladwin, Figner, Crone, & Wiers (2011) have proposed a similar approach to cognitive therapies focused on attention control and working memory.
Summary and Implications for MOBC-Addiction Research
Overall, advances in cognitive neuroscience, among other areas of basic science, have contributed to the development of novel theories regarding the factors that maintain addiction. Self-control of all appetitive behaviors is determined by the interaction between a reflective system subserving flexible, goal-directed behaviors and an impulsive system subserving behavioral, autonomic, and cognitive responses to appetitive cues that are rapidly deployed, relatively inflexible and occur largely outside of awareness. Addiction is characterized by impairment in the smooth interaction between reflective and impulsive systems, as evidenced by studies examining goal-directed behavior, explicit cognition, implicit cognition, and the underlying functioning neural systems. Addiction-related impairments may occur in a number of different components of the reflective system, including those involved in judgment, attention control, inhibition of preprogrammed responses, and performance monitoring. Similarly, impairment in impulsive-system processes may lead to a radical disruption of normal reward processes, such that drug cues take on exaggerated valuations that eclipse natural rewards, such as food or social interactions.
We note that there are a number of important common assumptions that underlie cognitive neuroscience perspectives on addiction and earlier theories that undergird behavioral treatments. First, both perspectives identify deficits in cognitive processes as the factors that define the disorder, rather than solely biological factors or descriptive features, such as those represented in Diagnostic Statistical Manual of Mental Disorders (4th ed., text rev., DSM–IV; American Psychiatric Association, 2000). In addition, both perspectives hypothesize subtypes based on cognitive-process deficits. Moreover, both perspectives frame addiction in the context of self-regulation failures (Kanfer, 1986). At the same time, neurocognitive theories represent a significant scientific advance over earlier SLT and other models regarding the pathophysiology of addiction, and thus, offer a new perspective to guide treatment research.
The application of cognitive neuroscience perspectives could advance mechanism research in at least two important ways. First, cognitive neuroscience offers a more precise, differentiated, informative, and sometimes novel view of cognitive processes and their relationship to addiction. As such, this perspective can lead to novel approaches to testing treatment-theory mediation and treatment matching. We briefly illustrate this point using impulsivity as an example. Impulsive responding, the tendency to select immediate rewards over longer term goals, is a critical treatment target of all addiction-behavior therapies. Over the last decade, translational research has shown that impulsivity is not a single construct, but has multiple components (Dick et al., 2010). For example, impulsivity may represent a deficit in reward valuation, such that drug cues are experienced as excessively rewarding; a deficit in control systems associated with the inability to inhibit prepotent motor responses; or a deficit in reversal learning, such that reward cues are not devalued based on contingent reinforcement leading to inflexible and perseverative drug use (Garavan, 2011).
This distinction may have important implications for treatment matching research. For example, MI may be differentially more effective for individuals who excessively value drugs, but have intact control systems because MI primarily targets altering the balance between pros and cons of drug use. By contrast, CBT may be more effective for those with response-inhibition deficits to drug cues via its focus on enhancing coping in high-risk situations. MI and CBT may not be effective for those with deficits in reversal learning. However, being able to prospectively identify nonresponders to treatment would have an important advantage in reducing error variance and adding precision to matching hypotheses and subsequent data analyses. This example is speculative, although it illustrates how greater precision in our ability to understand and measure the cognitive-process deficits that maintain addiction can lead to novel and clinically important approaches to guide mechanism research.
Second, cognitive neuroscience employs a multilevel approach to assess cognitive processes typically including self-report measures, laboratory tasks to assess implicit cognitions, and neuroimaging to probe neural circuits. Over the last decade, there has been a growing awareness that cognitive processes that are the target of behavioral treatments like motivation, coping, or emotion regulation are themselves complex and multifaceted constructs, thus, not likely to be accurately captured using relatively simple measures. In addition, addiction is likely maintained by implicit cognitions or processes that occur outside of awareness (Wiers & Stacy, 2006). Thus, the use of self-report measures alone may be inherently limited in capturing treatment effects. We note that the use of multimodal approaches to study cognitive processes is not new to mechanism research. For example, much of the early research that tested coping as a mediator of CBT employed laboratory as well as self-report measures (Monti et al., 1993; Carroll, Nich, Frankforter, Bisighini, 1999). However, recent mediation studies have migrated away from such multilevel approaches (Apodaca & Longabaugh, 2009).
Cognitive neuroscience can advance mechanism research, but we believe its ability to do so will require a thoughtful and iterative integration across clinical and basic science perspectives that would characterize the best forms of translational research. One impediment to integration is the assumption that brain mechanisms and other biological processes have primacy as the causal factors that define disease or facilitate recovery. Newer heuristics, such as the one proposed by the Research Domain Criteria (RDoC) project clearly reject this assumption in favor of a framework that views causal influences as multidirectional across levels of analysis, including neural circuits, behavior, experience, and social relations (Insel et al., 2010; Sanislow et al., 2010). Thus, one formidable challenge for future integrative efforts will be characterizing the reciprocal causal influences of behavioral interventions across interpersonal, cognitive, and neural domains, because factors in each domain influence treatment process and outcome. In addition, though the pathophysiology of addiction helps identify treatment targets, it does not explain how cognitive deficits are resolved either by treatment or natural recovery. As illustrated in the next section, we are still in the very early stages of developing a neurocognitive model of recovery.
Neuroimaging Research on Addiction Treatment
This section provides a brief review of neuroimaging studies that have tested aspects of neurocognitive theories of addiction as a strategy to inform addiction-behavioral treatments. Two types of studies were included: (a) Those that examined whether cognitive and related neural-process deficits assessed prior to treatment predict responses to behavioral treatments and (b) studies that examined the effects of behavioral treatments on neural circuits by examining those circuits before and after treatment. Our review has two purposes. First, we provide a brief overview of the current research in this emerging field, including the nature and types of studies that have been conducted and their findings. Second, we describe several studies in some detail to illustrate how concepts introduced in earlier sections have been tested and their potential informative value to mechanism research. Consistent with these goals, the review is not designed to be a comprehensive critique of the literature.
Relapse-Predictor Studies
The top section of Table 1 lists studies that used neuroimaging to test whether neural deficits associated with addiction predict response to behavioral treatments. One study, Martinez et al., (2011) examined relapse predictors and treatment effects and, as a result, is listed in the bottom section of the table. Relapse-predictor studies are grouped based on the imaging paradigm and method. Examination of Table 1 indicates the nascent stage of research in this area. Only 10 predictor studies were identified, and only four of these had sample sizes of more than 30 participants. In addition, studies were heterogeneous in terms of imaging paradigms, type of patients, substances, treatments, and outcome indicators. For example, the only studies that examined the same diagnostic group of patients using the same imaging method and paradigm were the three studies that examined alcohol-dependent patients using structural MRI and morphometry. These limitations make it difficult to draw definitive conclusions across the literature, but the studies illustrate how neuroimaging has been used in this context and its potential value to inform MOBC.
Table 1.
Neuroimaging Studies of Addiction Treatment: Relapse Predictors and Treatment Effects on Neurocircuitry
| Author (date) | Imaging paradigm | Sample | Outcome | Finding |
|---|---|---|---|---|
| Relapse Predictorsa | ||||
| Cardenas et al. (2011) | Structural MRI morphometry | 75 alcoholics | Abstinence: | Structural abnormalities in related systems predicts relapse |
| Rando et al. (2011) | Structural MRI morphometry | 40 controls 45 alcoholics |
8 months Time to relapse: 90 days |
Gray-matter reduction in frontal medial clusters predicts relapse |
| Wrase et al. (2008) | Structural MRI morphometry | 50 controls 51 alcoholics |
Relapse to heavy drinking: 6 months |
Decrease in amygdala volume predicts relapse |
| Kosten et al. (2006) | fMRI Cue-induced activation | 52 controls 12 cocaine-dependent subjects |
Abstinence: 10 weeks | Relapse associated with greater activation in posterior cingulate |
| Grusser et al. (2004) | fMRI Cue-induced activation | 10 alcoholics | Relapse to heavy drinking: 3 months |
Relapse associated with greater activation in bilateral ACC, MPFC, striatum |
| Janes et al. (2010) | fMRI smoking Stroop | 21 Nicotine-dependent women |
Abstinent from smoking: 8 weeks |
Activation and functional connectivity findings suggest relapsers have impaired top down control of reward regulation |
| Brewer et al. (2008) | fMRI color–word Stroop | 20 cocaine-dependent subjects |
Longest period of abstinence: 8 weeks |
Abstinence associated with activation in right putamen, left MPFC, left PCC |
| Paulus et al. (2005) | fMRI Decision-making task | 46 Methamphetamine- dependent men |
Abstinence at 12 months | Relapsers showed less activation in brain network associated with decision making |
| Ghitza et al. (2010) | PET mu-opioid receptor binding (MOR) |
25 subjects with cocaine-use disorders |
Percent of positive urines— longest duration abstinence: 24 weeks |
Elevated MOR binding associated with reward sensitivity predicts worse outcome |
| Treatment studies | ||||
| Vollstadt-Klein et al. (2011) | fMRI Cue-activation pre and post treatment |
30 alcohol Dep. randomized to cue exposure(CET) or usual care (UC) |
fMRI cue activation at end of treatment at 21 days |
After treatment CET showed less activation than UC in left ventral striatum |
| DeVito et al. (in press) | fMRI Color-interference Stroop pre-and posttreatment |
12 subjects with substance-use disorder receiving CBT 12 healthy controls |
fMRI stroop at 8 weeks | Patients showed decreased bold signals in regions associated with cognitive control |
| Martinez et al. (2011) | PET striatal dopamine signaling pre- and posttreatment |
25 cocaine-dependent subjects receiving 24 weeks community- reinforcement approach (CRA) |
1. Abstinence at 11 weeks 2. PET 11C-raclopride at 11 weeks |
1. Abstainers had higher BPND and dopamine release 2. no change pre to mid treatment in BPND |
Note.
The Martinez et al. (2011) study examined relapse predictors and treatment effects. In order to avoid duplication, it is listed under Treatment studies.
The most robust set of relapse-predictor studies used structural MRI and morphometric analysis to examine structural characteristics of gray matter (GM) and white matter (WM) in cortical and subcortical brain areas among alcohol-dependent participants. These structural characteristics included volume, surface area, and thickness, depending on the study and brain region under investigation. Wrase and colleagues (2008) conducted the first study to test whether alterations in what they referred to as “the brain-reward system (BRS)” predicted relapse. In an earlier study, this group (Makris, Gasic, et al., 2008) hypothesized that critical components of the mesocorticolimbic system central to the processing of reward stimuli would be altered in participants with chronic alcoholism. This hypothesis is consistent with the neurocognitive theories of addiction described above. The BRS included subcortical regions such as the amygdala, hippocampus, and ventral striatum as well as cortical areas responsible for executive function such as the DLPFC, occipital frontal cortex (OFC), the anterior cingulate cortex (ACC), and the insula. Similar to the Bechara model (2005), Wrase, Makris and colleagues (2008) considered the BRS to be an interconnected, multifunctional network associated with reward processes as well as motivation, evaluation of long-term prospects, impulsivity and inhibition. In an initial study comparing chronic alcoholics to normal controls, Makris, Oscar-Berman and colleagues (2008) found decreased total reward-network volume in the alcoholics. Reduction was especially pronounced in the right DLPFC, right ACC, right anterior insula, and left amygdala.
In a follow-up study, Wrase et al. (2008) examined whether alterations in the BRS predicted relapse. Participants were 51 alcohol-dependent patients attending an inpatient detoxification program. Participants were imaged approximately one week after admission and were followed for six months. Controls were also recruited: 52 matched healthy participants, but given our focus on relapse predictors, we limit the discussion of findings to the clinical sample. Results indicated that relapsers to heavy drinking had significantly reduced basolateral amygdala volume when compared with abstainers. Relapsers had about 10% less amygdala volume than abstainers. In addition, reduced amygdala volume was associated with greater craving. Amygdala activation in humans is associated with assessment of positive and negative stimuli and valuation of reward representations. Alcoholics with amygdala-volume alterations display decision-making deficits in a simulated gambling task (Fein et al., 2006), similar to the impairments Bechara found among individuals with substance-use disorder (Bechara, 2005). In addition, animals with basolateral amygdala lesions lack response flexibility during unconditioned-stimulus devaluation, suggesting that amygdala dysfuction may be related to an inability to adaptively revalue stimuli that are no longer associated with rewards (Koob, 2006).
Cardenas and colleagues conducted a separate set of studies examining whether alterations in BRS predicted relapse (Cardenas et al., 2011; Durazzo et al., 2011). Participants were 75 alcohol-dependent patients enrolled in a U.S. Veterans Affairs medical center outpatient, substance-abuse treatment program. Participants were imaged approximately one week after admission and followed for eight months. Forty matched controls were also recruited. The two studies used the same sample, but somewhat different approaches to assess morphometric changes. Results were similar across studies. Relapsers relative to abstainers had significantly smaller total BRS volume. The greatest morphological differences were observed in the OFC, an area that overlaps with the VMPFC. The OFC together with the ACC are involved in decision making, especially with regard to choices between rewarding and punishing stimuli. Specifically, these regions process the reward value or salience of environmental stimuli, assess the future consequences of the individual’s actions, and inhibit impulsive behavior. Impaired functioning of the OFC is hypothesized as part of many neurobiological theories of addiction (Baler & Volkow, 2006; Bechara, 2005; Koob, 2006).
Rando and colleagues (2011) tested whether smaller frontal gray matter volume was predictive of relapse in 45 alcohol-dependent participants completing inpatient treatment. Participants were imaged after one month of abstinence and followed for 90 days after treatment. After controlling for demographics, and past and current drinking, smaller gray-matter volume in the medial frontal cluster and the posterior occipital cluster were predictive of shorter time to relapse and heavy drinking during relapse. Medial frontal cortex, which includes the ACC, is associated with cognitive control. Findings are consistent with those of Garavan and Hester (2007; reviewed above), indicating impairment in adaptive pursuit of higher order goals including poor response inhibition in addiction.
Overall, findings from these studies provide consistent support for models suggesting addiction is characterized by deficits in impulsive and reflective systems related to the processing of rewards. In two of the studies, comorbid drug dependence and other major DSM–IV (APA, 2000) Axis I disorders were excluded. Thus, findings can be attributed to alcohol dependence. In addition, the studies support the idea that neural deficits may define subtypes of addiction that fail to respond to behavioral treatment. For example, findings from Wrase et al. (2008) and Cardenas et al. (2011) suggest that a subgroup of chronic alcoholics may have difficulty learning to revalue the salience of alcohol cues, a key element of all behavioral treatments for addiction.
Findings from the study by Martinez and colleagues (2011) using PET help to elucidate a similar subgroup phenomenon among cocaine-addicted individuals in treatment. Prior to outpatient, community-reinforcement-approach (CRA) treatment with monetary vouchers, 25 cocaine-dependent participants underwent two PET scans using 11C-raclopride before and after administration of a stimulant to measure striatal DA–receptor binding and presynaptic DA release. In an earlier study, Martinez and colleagues (2004) found that cocaine abusers with low stimulant-induced DA release were more likely to choose cocaine over money in a cocaine administration study. This finding suggested that low-DA transmission might predict inability to respond to contingent reinforcement. Participants were followed during the 24 weeks of treatment and were scanned again at Week 12. In addition, a matched group of 25 healthy controls was also studied. Treatment outcomes tended to be bimodal, with some participants demonstrating good outcome (responders, n = 10) and others demonstrating poor outcome (nonresponders, n = 15). PET scans at baseline indicated that responders had higher DA–receptor binding and DA release than nonresponders. Consistent with other studies, these findings suggest that DA signaling in the limbic striatum may be critical to learning to shift between competing reinforcers such that among participants with low DA transmission, a preservative, habitual behavior is produced, even in the presence of an alternative reward of greater value.
Though studies differ with regard to methods and populations, a common finding (Martinez et al., 2011; Wrase et al., 2008; Cardenas et al., 2011) is that a subgroup of addicted individuals may lack the ability to benefit from strategies employed by behavior therapies; specifically those designed to alter the salience or valuation of addictive stimuli and, as a consequence, this subgroup fails to respond to treatment. The Martinez et al. study (2011) is especially strong from a design perspective, because it demonstrates a specific link between a neural deficit (low DA signaling) and the inability to benefit from a demonstrated effective treatment: contingent reinforcement.
It is important to note the limitations of the findings described above. For example, despite using similar samples and methods, the three morphometric studies yielded different findings regarding the specific brain areas that differentiated relapsers and abstainers. In addition, chronic alcoholics show recovery from gray matter volume atrophy after a period of sustained abstinence (Mon, Delucchi, Durazzo, Gazdzinski, & Meyerhoff, 2011) and this factor was not examined in the three studies. Thus, additional research is clearly needed to elucidate this area.
Nevertheless, the four studies described above illustrate how neuroimaging could advance our understanding of behavioral treatments, if future studies are able to identify specific neural deficits that are associated with psychological processes related to behavioral treatments that predict outcome. Future studies will be most informative if the neural deficits investigated are linked directly to the hypothesized effects of the behavioral treatment, similar to the Martinez et al. study (2011). For example, a study examining whether impaired judgment and related OFC deficits predict poor outcomes in MI would test the link between an important therapy component of MI, increasing the salience of future negative consequences of substance use and a deficit in the capacity to appropriately assign affective value to future events. Although such a predictor study would not fully test the mechanisms of MI, it would support the hypothesis that MI’s mechanism of action is related to changing the salience of negative future events in the same way early predictor studies, which found coping skills deficits predicted poor outcome in SUD treatment, set the stage for mechanism research on improvements in coping skills as a mechanism for CBT (Morgenstern & Longabaugh, 2000).
Treatment-Effect Studies
In the framework presented in Figure 1, the critical role for treatment research is to determine whether factors associated with maintaining addiction are malleable and what interventions, either behavioral or pharmacological, can remediate the specific factor(s) that maintain addiction. To date, there is little behavioral intervention research that tests hypotheses derived from neurocognitive theories of addiction. Two broad types of behavioral treatment research could be conducted. The first type would be to develop new behavioral treatments that target specific deficits identified by neurocognitive theories. Although it is beyond the scope of this article to elaborate on this new direction, we note efforts in the area of improving one aspect of cognitive control, attentional bias to alcohol cues through the development of attentional bias retraining (Wiers, Eberl, Rinck, Becker, & Lindenmeyer, 2011). In addition, Bickel and colleagues are in the early phases of testing whether improving working memory capacity might lead to a greater ability to attend to future consequences and thereby improve decision making and reduce impulsivity among those dependent on drugs (Bickel, Yi, Landes, Hill & Baxter, 2011).
The second type of research involves examining whether effective behavioral interventions work through changing specific psychological processes and their underlying neural circuits, a concern that is central to MOBC research. We were able to identify only three published studies that tested some form of this hypothesis (see bottom of Table 1). All studies were published in 2011. Vollstädt-Klein and colleagues (2011) examined the effects of cue exposure training (CET) on changes on neural mesolimbic activation using fMRI. Abstinent alcohol-dependent participants were randomly assigned to CET (n = 15) or a control group (n = 15). All participants received supportive outpatient treatment. CET consisted of nine sessions over 3 weeks. Cue-induced fMRI activation to alcohol (vs. neutral) cues was measured pre and post treatment. Subjective craving during cue presentation was also assessed. The study hypothesized that reductions in cue activation would be especially notable in the ventral and dorsal striatum. Results indicated that CET relative to control showed a greater reduction in alcohol cue activation in the ventral and dorsal striatum, the ACC, the precentral gyrus, the insula, and several frontal regions. There were no differences in conditions on subjective craving. The authors interpret the findings as confirming the hypotheses, but note a relatively small decrease in striatum activation relative to the decrease in activation in frontal areas. The study did not examine drinking outcomes.
DeVito and colleagues (2011) examined whether SUD participants receiving a course of behavior therapy would show improvement in cognitive control, response inhibition, and reward-related learning. Participants were 12 individuals with a mix of SUD diagnoses who were part of a larger treatment study. Participants received either treatment-as-usual (TAU) or TAU and computer-assisted CBT. Participants were imaged prior to and at the end of treatment (8 weeks) using an event-related, fMRI Stroop color–word interference task. In addition, healthy participants (n = 12) were recruited and performed the same task under test-retest conditions. Drinking outcomes were not reported. The patient group showed improved behavioral performance and decreased Blood Oxygen Dependent Level (BOLD) activation in frontal regions following treatment. Compared with the healthy controls, patients showed a greater activation decrease in the subthalamic nucleus (STN). The authors note that findings only partially support the hypotheses. Although the STN has been identified in some studies as a region involved in response inhibition, its function is not fully understood. In addition, as the authors note, one would expect greater cognitive control to be associated with greater activation in STN, not a decrease in activation. The authors offer a potential explanation for the seemingly contradictory pattern of activation and note the need for further study.
The Vollstädt-Klein et al. (2011) and DeVito et al. (in press) studies are the first two published studies that use fMRI to test the impact of an addiction behavioral intervention on neural functioning. The studies tested aspects of the neurocognitive model of addiction. Vollstädt-Klein et al. tested whether CET led to a reduction in activation of impulsive or limbic system processes in the striatum. DeVito et al. tested whether a generic behavioral intervention led to an increase in reflective system processes associated with greater cognitive control and increased response inhibition. In addition, each study included a control group to address confounds related to test–retest. Although study findings generally supported the hypotheses, each study had significant methodological weaknesses that affected internal validity, including small sample sizes and designs that did not compare a clearly effective treatment to a weaker control condition.
In addition, the two studies had significant design limitations relative to paradigms now being used to test the neural effects of behavioral treatments for depression and anxiety disorders using fMRI (Carrig et al., 2009; Ritchey, Dolcos, Eddington, Strauman, & Cabeza, 2011). Specifically, most studies begin by testing behavioral interventions with strong evidence for efficacy, rather than relatively weak treatments such as CET or TAU. In addition, studies typically use normal control groups in addition to a clinical sample to establish normative values on key dependent measures of neural processing. Predictor hypotheses unique to the study treatment are formulated and symptom-outcome analyses, as well as changes on neural and other psychological process measures are integrated into the design as a strategy to explore patterns of treatment response.
The Martinez et al. study (2011) used a modified version of this design to test the impact of CRA on neural functioning. In the section above, we described the relapse-predictor component of the study. However, relapse prediction was part of a broader set of hypotheses. Specifically, Martinez included a matched control sample (n = 25). In addition, clinical participants returned for a second PET session after 12 weeks of treatment. Control participants were included in order to demonstrate that the same pattern of abnormal neurochemistry regarding DA identified in early studies would be found in this sample. In addition, the study hypothesized that clinical participants who responded to the treatment would show normalization of DA transmission, thus, indicating that the treatment reduced the factor thought to maintain addiction. Results indicated that clinical participants relative to the control subjects had blunted striatal DA signaling. Fifteen of the 25 clinical participants returned for the second PET session. Among the nine responders there were no significant differences in PET results across the two tests. However, treatment responders did not differ from the control subjects before treatment, suggesting that presynaptic DA was largely intact in the responders to begin with. Overall, the major finding of the study was that low DA transmission predicts poor response to CRT, but the study provides limited insight on how CRA works to reduce cocaine use.
Summary of Treatment Studies
Overall, we are just now beginning to conduct empirical studies that use a cognitive neuroscience framework to test the MOBC of effective addiction behavioral interventions. The three treatment effects studies are useful in illustrating how neurocognitive theories and imaging methods can be applied to test behavioral interventions effects. Methodological weaknesses limit the ability of the studies to provide substantive conclusions and serve to illustrate the preliminary nature of this research direction as well as the challenges of mounting informative studies that combine MOBC behavioral treatment and neuroscience addiction perspectives. The Martinez et al. study (2011) employed the most common design used in prior neuroimaging studies examining behavior therapy in other mental health disorders (Linden, 2006). This design examines whether a neural deficit thought to characterize the disorder differentiates clinical and normal controls, whether the deficit predicts treatment outcome, and whether among treatment responders the deficit improves and approaches levels in normal controls.
Although this design does not address whether the treatment has unique effects relative to other treatments, when well-conceived and implemented, it can provide novel knowledge about MOBC. For example, a recent study of CBT for depression using an emotional processing task and fMRI found that greater activation in vmPFC, an area associated with emotion regulation, predicted better outcome (Ritchey et al., 2011). In addition, greater strength of response to negative stimuli also predicted better outcome. Finally, responders showed normalization in emotion regulation processes and more robust response to positively valenced stimuli relative to nonresponders. Findings were interpreted as consistent with an emotion regulation perspective on CBT (Ochsner & Gross, 2008). Specifically, patients with greater pretreatment neural capacity for emotion regulation and a tendency to respond with stronger negative bias do best with CBT. In addition, CBT appears to improve emotion regulation such that responses to emotional stimuli among responders were similar to normal controls.
Discussion: Informing Future Mechanism Studies
One important issue raised by the review of empirical studies is how best to design future imaging studies so they inform addiction MOBC. Future studies would benefit from the incorporation of stronger design features that are now standard in clinical-trial research: larger samples, examination of demonstrated effective behavioral treatments, careful ascertainment of samples, and stronger methods to test mediation. In addition, neuroimaging has strengths and limitations as a tool to advance mechanism research and these need to be understood by treatment researchers. Two important considerations involve the methodological features of neuroimaging studies and how neuroimaging findings are used to build mechanistic theories of cognition and behavior.
Although fMRI and and structural MRI have provided important insights into the neural and cognitive processes underlying addiction and behavior change, these methods involve limitations related to reproducibility. Some of these limitations are specific to brain imaging and relate to variations in methods used to acquire or analyze functional and structure data. For example, there are several different methods for segmenting gray and white matter, including both automated and manual techniques. Differences in functional imaging pulse sequences and acquisition protocols may increase or decrease the sensitivity of fMRI to detect activity in particular brain regions. These technique-specific limitations necessitate caution when making statements about the robustness or reproducibility of imaging findings. At the same time, there are a number of limitations on the reproducibility of brain imaging that are general to any endeavor utilizing behavioral probes to address specific cognitive functions. For example, subtle variations in a behavioral paradigm meant to probe a particular cognitive process may lead to significant differences in results between studies; or there may be changes between pre- and posttreatment brain activity that are due to habituation, as opposed to specific treatment effects. These measurement-related sources of variability may confound more clinically relevant differences, such as choice of clinical population, and so forth, limiting the ability to make conclusions about mechanisms of behavior change. Together, this points to the need for greater standardization of methods across laboratories, for accurate reporting of methods, and for attention to the subtleties of psychological experimental design.
In the field of cognitive neuroscience, neuroimaging plays a critical role in the development of mechanistic explanations of cognitive processes. Briefly stated, mechanism exploration involves the iterative development of multilevel models that link (a) measures of behavior, experiential and physiological responses, (b) descriptions of information processing mechanisms, and (c) their neural substrates (cf. Ochsner & Gross, 2008). This multilevel process allows inferences at the behavioral level of analysis to constrain thinking at the neural level of analysis and vice versa. This bidirectional, across-level analytic process allows for a more complete and deeper understanding of cognition and contributes to the development of mechanistic models that specify the components and subcomponents of a process and how these parts inter-relate to explain its operative characteristics (Bechtel & Wright, 2009; Darden, 2006). Neuroimaging has been especially critical in advancing understanding of the relationship between cognition and emotion, a critical area for MOBC research (cf. Ochsner & Lieberman, 2001). In addition, it is important to note that neuroimaging research needs to be integrated with other cognitive and neuroscience methods as well as theories drawn from animal models in constructing mechanistic accounts of psychological processes (Poldrack, Wagner, Cacioppo, Bernston, & Nusbaum, 2008). Viewed from this perspective, neuroimaging findings in isolation are no more valuable than findings from any other dependent measure for MOBC research and have no special claim on causality. By contrast, neuroimaging has unique informative value when used in the context of strong theory-driven research designed to test how behavioral interventions remediate impairments in neurocognitive processes that are hypothesized to maintain addiction (see Figure 1).
Thus, a critical step for improving future MOBC studies is articulating a conceptual and multilevel methodological framework that connects the cognitive process impairments that maintain addiction to the hypothesized action of effective behavioral treatments. The development of such frameworks is just beginning (Feldstein Ewing, Filbey, Hendershot, McEachern, & Hutchison, 2011; Hölzel et al., 2011; Kober et al., 2010; Potenza, Sofuoglu, Carroll, & Rounsaville, 2011) and represents an important challenge for future research. Despite rapid advances, considerable uncertainty remains about the nature of psychological and neural impairments that characterize addiction, what biomarkers reliably index these impairments, and whether impairments characterize addiction in general or specific subgroups. Future studies need to carefully consider these issues. For example, cognitive control impairment indexed by the color-word Stroop task is limited in its ability to capture impairments in reward processing that are now viewed as core to addiction. By contrast, the Martinez et al. (2011) study provides a stronger test of a hypothesized neural impairment that characterizes addiction: low striatal DA transmission as measured by PET (Volkow, Baler, & Goldstein, 2011).
In summary, future research will benefit from a better and more informed integration of cognitive neuroscience and addiction treatment research knowledge. Specifically, greater specificity is needed in the formulation of hypotheses that link treatment mechanisms to neurocognitive deficits associated with maintaining addiction. Neurocognitive methods including experimental psychology and imaging paradigms should have demonstrated reliability and validity in the population of interest. Finally, rigorous methods well known in testing mechanisms in clinical-trial research should be employed.
Limitations
This article has a number of limitations. Because we attempted to connect several disparate and relatively complex research topics, we omitted details that normally would be part of a more focused review and subsumed phenomena under a single rubric that, in other contexts, might merit separate classification and discussion. For example, we discussed addiction as a unitary disorder, rather than distinguishing between different forms of addiction, including different classes of substances. Similarly, we did not discuss specific cognitive processes such as decision making, reward learning or cognitive control in any depth. In addition, our review of specific topics was necessarily limited and intended to be illustrative of critical ideas.
Conclusion
In the last decade, there has been an upsurge of interest in understanding the MOBC of effective addiction behavioral interventions as a critical strategy to improve treatment efficacy. Although considerable progress had been made, the MOBC field is still in a formative phase in determining how best to build a strong foundation that will advance mechanisms science (Longabaugh et al., in press). In this article, we have highlighted the critical role treatment theory plays in the search for mechanisms. Rapid advances in the basic sciences have led to important revisions in our understanding of the pathophysiology of addiction. Advances in our understanding of the factors that maintain addiction, including novel hypotheses about subtypes, have important implications for guiding future treatment research, especially in the area of MOBC (Potenza et al., 2011). The treatment theories that undergird most effective behavioral interventions have yet to be revised in light of these recent advances.
The cognitive neuroscience of addiction offers a novel conceptual and methodological framework to study the psychological processes that characterize addiction and, as such, can serve as a useful platform to guide mechanism research. Importantly, cognitive neuroscience shares many core assumptions of behavioral therapies, including the primary role of cognitive processes and self-regulation impairments in maintaining addiction. These shared assumptions are likely to facilitate translational research efforts.
At the same time, there are formidable challenges to link the clinical MOBC model with cognitive neuroscience models of addiction. Each discipline has its own unique set of assumptions, technical language, siloed research expertise, and methodological complexities. As such, programmatic research that bridges these areas will require a sustained transdisciplinary and science-team approach. In addition, the field of cognitive neuroscience and its application to addiction is a relatively new and rapidly evolving area, in which conceptualizations and methodologies are constantly being revised and updated. However, as the review of the empirical literature indicates, the field is sufficiently mature in such a way that relapse-predictor and treatment-effect studies are beginning to generate new insights into potential moderators of behavioral treatment. Thus, there is good reason to anticipate that studies with stronger design features will lead to important advances in elucidating MOBC and, ultimately, to improvements in treatment efficacy.
Contributor Information
Jon Morgenstern, Department of Psychiatry, Columbia University.
Nasir H. Naqvi, Department of Psychiatry, Columbia University
Robert Debellis, Department of Psychiatry, Columbia University.
Hans C. Breiter, Department of Psychiatry, Harvard Medical School and Northwestern University
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 2000. text rev. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. Journal of the American Medical Association: Journal of the American Medical Association. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. doi:10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Apodaca TR, Longabaugh R. Mechanisms of change in motivational interviewing: A review and preliminary evaluation of the evidence. Addiction. 2009;104:705–715. doi: 10.1111/j.1360-0443.2009.02527.x. doi:10.1111/j.1360-0443.2009.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF. Treatment for persons with substance use disorders: Mediators, moderators, and the need for a new research approach. International Journal of Methods in Psychiatric Research. 2008;17:S45–S49. doi: 10.1002/mpr.248. doi:10.1002/mpr.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. doi:10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of will-power to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. doi:10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechtel W, Wright C. What is psychological explanation? In: Calvo P, Symons J, editors. The Routledge companion to philosophy of psychology. Routledge; London, England: 2009. pp. 113–130. [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. doi:10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Marlatt GA. Mindfulness-based relapse prevention for addictive behaviors: A clinician’s guide. Guilford Press; New York, NY: 2010. [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. doi:10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühringer G. Allocating treatment options to patient profiles: Clinical art or science? Addiction. 2006;101:646–652. doi: 10.1111/j.1360-0443.2006.01366.x. doi:10.1111/j.1360-0443.2006.01366.x. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biological Psychiatry. 2011;70:561–567. doi: 10.1016/j.biopsych.2011.04.003. doi:10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrig MM, Kolden GG, Strauman TJ. Using functional magnetic resonance imaging in psychotherapy research: A brief introduction to concepts, methods, and task selection. Psychotherapy Research. 2009;19:409–417. doi: 10.1080/10503300902735864. doi:10.1080/10503300902735864. [DOI] [PubMed] [Google Scholar]
- Carroll K, Nich C, Frankforter T, Bisighini R. Do patients change in the ways we intend? Assessing acquisition of coping skills among cocaine-dependent patients. Psychological Assessment. 1999;11:77–85. doi:10.1037/1040-3590.11.1.77. [Google Scholar]
- Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends in Cognitive Sciences. 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. doi:10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Stacy A, Wiers R, editors. Handbook of implicit cognition and addiction. Sage Publications; Thousand Oaks, CA: 2006. pp. 233–250. doi: 10.4135/9781412976237.n16. [Google Scholar]
- Darden L. Reasoning in biological discoveries: Essays on mechanisms, interfield relations, and anomaly resolution. Cambridge University Press; West Nyack, NY: 2006. doi:10.1017/CBO9780511498442. [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence. 2012;122:228–235. doi: 10.1016/j.drugalcdep.2011.10.002. doi:10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Review: Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. doi:10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss BD. Changing the way we study change in psychotherapy. Clinical Psychology: Science and Practice. 2004;11:368–386. doi:10.1093/clipsy.bph094. [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: Relationships to relapse and extended abstinence. Alcoholism: Clinical and Experimental Research. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. doi:10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. The American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. doi:10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society: B. Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. doi:10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics whodemonstrate impairment on a simulated gambling task. NeuroImage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. doi:10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Hendershot CS, McEachern AD, Hutchison KE. Proposed model of the neurobiological mechanisms underlying psychosocial alcohol interventions: The example of motivational interviewing. Journal of Studies on Alcohol and Drugs. 2011;72:903–916. doi: 10.15288/jsad.2011.72.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H. Impulsivity and addiction. In: Adinoff B, Stein E, editors. Neuroimaging in Addiction. John Wiley & Sons; New York, NY: 2011. pp. 157–176. doi:10.1002/9781119998938.ch7. [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychology Review. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. doi:10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. doi:10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Preston KL, Epstein DH, Kuwabara H, Endres CJ, Bencherif B, Gorelick DA. Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biological Psychiatry. 2010;68:697–703. doi: 10.1016/j.biopsych.2010.05.003. doi:10.1016/j.biopsych.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, Figner B, Crone EA, Wiers RW. Addiction, adolescence, and the integration of control and motivation. Developmental Cognitive Neuroscience. 2011;1:364–376. doi: 10.1016/j.dcn.2011.06.008. doi:10.1016/j.dcn.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. doi:10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspectives on Psychological Science. 2009;4:162–176. doi: 10.1111/j.1745-6924.2009.01116.x. doi:10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Holt LJ, Litt MD, Cooney NL. Prospective analysis of early lapse to drinking and smoking among individuals in concurrent alcohol and tobacco treatment. Psychology of Addictive Behaviors. 2012;26:561–572. doi: 10.1037/a0026039. doi:10.1037/a0026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6:537–559. doi: 10.1177/1745691611419671. doi:10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Huebner RB, Tonigan JS. The search for mechanisms of behavior change in evidence-based behavioral treatments for alcohol use disorders: Overview. Alcoholism: Clinical and Experimental Research. 2007;31:1s–3s. doi: 10.1111/j.1530-0277.2007.00487.x. doi:10.1111/j.1530-0277.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. doi:10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert BN, Garvey MA, Heinssen RK, Pine DS, Quinn KJ, Wang PS. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. doi:10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick BD, Chuzi S, Pachas G, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. doi:10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis IL, Mann L. Decision making: A psychological analysis of conflict, choice, and commitment. Free Press; New York, NY: 1977. [Google Scholar]
- Kanfer FH. Implications of a self-regulation model of therapy for treatment of addictive behaviors. In: Miller WE, Heather N, editors. Treating addictive behaviors: Processes of change. Plenum Press; New York, NY: 1986. pp. 29–47. [Google Scholar]
- Karno MP, Longabaugh R. Less directiveness by therapists improves drinking outcomes of reactant clients in alcoholism treatment. Journal of Consulting and Clinical Psychology. 2005;73:262–267. doi: 10.1037/0022-006X.73.2.262. doi: 10.1037/0022-006X.73.2.262. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Nock MK. Delineating mechanisms of change in child and adolescent therapy: Methodological issues and research recommendations. Journal of Child Psychology and Psychiatry. 2003;44:1116–1129. doi: 10.1111/1469-7610.00195. doi:10.1111/1469-7610.00195. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Magill M, Stout RL. How do people recover from alcohol dependence? A systematic review of the research on mechanisms of behavior change in Alcoholics Anonymous. Addiction Research & Theory. 2009;17:236–259. doi:10.1080/16066350902770458. [Google Scholar]
- Kiluk BD, Nich C, Babuscio T, Carroll KM. Quality versus quantity: Acquisition of coping skills following computerized cognitive-behavioral therapy for substance use disorders. Addiction. 2010;105:2120–2127. doi: 10.1111/j.1360-0443.2010.03076.x. doi:10.1111/j.1360-0443.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. PNAS: Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. doi:10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: A neuroadaptational view relevant for diagnosis. Addiction. 2006;101:23–30. doi: 10.1111/j.1360-0443.2006.01586.x. doi:10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. doi:10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. doi:10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Feinn R, Tennen H. Targeted naltrexone treatment moderates the relations between mood and drinking behavior among problem drinkers. Journal of Consulting and Clinical Psychology. 2004;72:317–327. doi: 10.1037/0022-006X.72.2.317. doi:10.1037/0022-006X.72.2.317. [DOI] [PubMed] [Google Scholar]
- Linden DEJ. How psychotherapy changes the brain—the contribution of functional neuroimaging. Molecular Psychiatry. 2006;11:528–538. doi: 10.1038/sj.mp.4001816. doi:10.1038/sj.mp.4001816. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Kabela-Cormier E. Individualized assessment and treatment program for alcohol dependence: Results of an initial study to train coping skills. Addiction. 2009;104:1837–1838. doi: 10.1111/j.1360-0443.2009.02693.x. doi: 10.1111/j.1360-0443.2009.02693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh R, Wirtz PW. Matching hypotheses. In: Longabaugh R, Wirtz PW, Mattson ME, Myers J, editors. Project MATCH hypotheses: Results and causal chain analyses. Vol. 8. U.S. Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2001. pp. 4–17. [Google Scholar]
- Longabaugh R, McGill M, Morgenstern J, Huebner R, McCrady BSE. Addictions: A comprehensive guidebook. 2nd ed. Oxford University Press; New York, NY: Mechanisms of change in behavioral treatments for addictions. in press. [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Lawrence Erlbaum Associates; Hillsdale, NJ: 2008. [Google Scholar]
- Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Breiter HC. Cortical thickness abnormalities in cocaine addiction-a reflection of both drug use and a pre-existing disposition to drug abuse. Neuron. 2008;60:174–188. doi: 10.1016/j.neuron.2008.08.011. doi:10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. doi:10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: Maintenance strategies in addictive behavior change. Guilford; New York, NY: 1985. [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: Relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. doi:10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Nunes E. Imaging dopamine transmission in cocaine dependence: Link between neurochemistry and response to treatment. The American Journal of Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. doi:10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead NL, Alquist JL, Baumeister RF. Ego depletion and the limited resource model of self-control. Self-Control in Society, Mind, and Brain. 2010;1:375–389. doi:10.1093/acprof:oso/9780195391381.003.0020. [Google Scholar]
- Mensinger JL, Lynch KG, TenHave TR, McKay JR. Mediators of telephone-based continuing care for alcohol and cocaine dependence. Journal of Consulting and Clinical Psychology. 2007;75:775–784. doi: 10.1037/0022-006X.75.5.775. doi:10.1037/0022-006X.75.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Wilbourne PL. Mesa Grande: A methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97:265–277. doi: 10.1046/j.1360-0443.2002.00019.x. doi:10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Mon A, Delucchi K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. A mathematical formula for prediction of gray and white matter volume recovery in abstinent alcohol-dependent individuals. Psychiatry Research. 2011 Nov 30;194:198–204. doi: 10.1016/j.pscychresns.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Abrams DB, Zwick WR, Binkoff JA, Munroe SM, Cooney NL. Development of a behavior analytically derived alcohol-specific role-play assessment. Journal of Studies on Alcohol. 1993;54:710–721. doi: 10.15288/jsa.1993.54.710. [DOI] [PubMed] [Google Scholar]
- Moos RH. Theory-based processes that promote remission of substance use disorders. Clinical Psychology Review. 2007;27:537–551. doi: 10.1016/j.cpr.2006.12.006. doi:10.1016/j.cpr.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Kuerbis A, Amrhein P, Lynch K, McKay J. Motivational interviewing: A pilot test of active ingredients and mechanisms of change. Psychology of Addictive Behaviors. doi: 10.1037/a0029674. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Kuerbis AN, Chen AC, Kahler CW, Bux DA, Jr., Kranzler HR. A randomized clinical trial of naltrexone and behavioral therapy for problem drinking men who have sex with men. Journal of Consulting and Clinical Psychology. doi: 10.1037/a0028615. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Longabaugh R. Cognitive-behavioral treatment for alcohol dependence: A review of evidence for its hypothesized mechanisms of action. Addiction. 2000;95:1475–1490. doi: 10.1046/j.1360-0443.2000.951014753.x. doi:10.1046/j.1360-0443.2000.951014753.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, McKay J. Rethinking the paradigms that inform behavioral treatment research for substance use disorders. Addiction. 2007;102:1377–1389. doi: 10.1111/j.1360-0443.2007.01882.x. doi:10.1111/j.1360-0443.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Houck JM, Christopher PJ, Tonigan JS. From in-session behaviors to drinking outcomes: A causal chain for motivational interviewing. Journal of Consulting and Clinical Psychology. 2009;77:1113–1124. doi: 10.1037/a0017189. doi:10.1037/a0017189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Structure & Function. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. doi:10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK. Conceptual and design essentials for evaluating mechanisms of change. Alcoholism: Clinical and Experimental Research. 2007;31:4s–12s. doi: 10.1111/j.1530-0277.2007.00488.x. doi:10.1111/j.1530-0277.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. doi:10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. American Psychologist. 2001;56:717–734. doi:10.1037/0003-066X.56.9.717. [PubMed] [Google Scholar]
- Orford J. Excessive appetites: A psychological view of addiction. 1st ed. Wiley Press; Chichester, England: 1985. [Google Scholar]
- Orford J. Is treatment matching dead? Comments on Bühringer. Addiction. 2006;101:653–654. doi: 10.1111/j.1360-0443.2006.01390.x. 2006. doi:10.1111/j.1360-0443.2006.01390.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. doi:10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Cacioppo JT, Berntson GG, Nusbaum HC. Neuroimaging as a new tool in the toolbox of psychological science. Current Directions in Psychological Science. 2008;17:62–67. doi:10.1111/j.1467-8721.2008.00550.x. [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of Behavioral and Pharmacological Treatments for Addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. doi:10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. doi:10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rando K, Hong K-I, Bhagwagar Z, Li C-SR, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: A prospective study. The American Journal of Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. doi:10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: Vulnerabilities in the decision process. Behavioral and Brain Sciences. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. Journal of Psychiatric Research. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. doi:10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95:91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. doi:10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clinical Psychology: Science and Practice. 2001;8:133–142. doi:10.1093/clipsy.8.2.133. [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. doi:10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: A tool for explaining paradoxical behavior. Annual Review of Clinical Psychology. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. doi:10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism Director’s report on institute activities to the 122nd meeting of NIAAA. 2009 Sep; Retrieved from http://www.niaaa.nih.gov/about-niaaa/our-work/advisory-council/directors-reports-council/niaaa-directors-report-institute-2.
- Verdejo-García A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. doi:10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ. Addiction: Pulling at the neural threads of social behaviors. Neuron. 2011;69:599–602. doi: 10.1016/j.neuron.2011.01.027. doi: 10.1016/j.neuron.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: A randomized trial. Biological Psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. doi:10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Walters ST, Vader AM, Harris TR, Field C, Jouriles EN. Dismantling motivational interviewing and feedback for college drinkers: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2009;77:64–73. doi: 10.1037/a0014472. doi:10.1037/a0014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampold B. The great psychotherapy debate: Models, methods, and findings. Lawrence Erlbaum; Hillsdale, NJ: 2001. [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychological Science. 2011;22:490–497. doi: 10.1177/0956797611400615. doi:10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Sage Publications; Thousand Oaks, CA: 2006. [Google Scholar]
- Wrase J, Makris N, Braus D, Mann K, Smolka M, Kennedy D, Albaugh M. Amygdala volume associated with alcohol abuse relapse and craving. The American Journal of Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. doi:10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]