Abstract
Objectives
To test the hypothesis that pubertal peak height velocity (PHV) in cystic fibrosis (CF) has improved and is influenced by pre-pubertal growth and genetic potential.
Study design
PHV from 1862 children born in 1984–87 and documented in the 1986–2008 US CF Foundation Registry was determined by statistical modeling and classified into normal, delayed (2-SD > average age), attenuated (magnitude < 5th percentile), or both (D&A). Genetic potential for height was estimated by parental stature.
Results
PHV averaged 8.4 cm/y at age 14.0 y in boys and 7.0 cm/y at age 12.1 y in girls, ~6 mo delay and ~15% reduction compared with healthy children. PHV was normal in 60%, delayed in 9%, attenuated in 21% and D&A in 5%. Patients with delayed PHV reached similar adult height percentile (boys: 34th, girls: 46th) to those with normal PHV (boys: 33rd, girls: 34th); both were significantly taller than the attenuated (boys: 11th, girls: 19th) and D&A PHV subgroups (boys: 8th, girls: 14th). Pancreatic sufficient patients had taller pre-pubertal and adult heights but similar PHV compared with pancreatic insufficient or meconium ileus patients. Adjusting for genetic potential reduced adult height percentiles more in boys (25th to 16th) than girls (28th to 24th). Height at age 7 y, PHV age and magnitude, and parental stature significantly predicted adult height.
Conclusions
Pubertal PHV has improved in children with CF born after mid 1980s compared with older cohorts but remains below normal. Suboptimal pre-pubertal and pubertal growth led to adult height below genetic potential in CF.
Keywords: growth, linear growth, nutritional status, puberty, adolescents, height, height velocity, peak height velocity, genetic potential
Adolescence is a critical period of accelerated height growth. Children with chronic diseases that increase nutritional requirements such as cystic fibrosis (CF) are at high risk for impaired pubertal growth (1). Results from previous studies (2–11) confirmed the clinical observation that children with CF had delayed and attenuated pubertal growth compared with healthy children. However, the majority of these studies were conducted in the 1980–90’s using data from children with CF born prior to 1970s (2–8) and few were from the US (2, 4–5, 11).
With advances in new therapies, such as enteric-coated pancreatic enzymes (12–14) and comprehensive nutrition management that emphasizes high-calorie, high-fat diet and growth monitoring (15–20), pubertal growth in children with CF born after 1980s may have improved, although recent studies still report impaired pubertal growth (9–11). Most importantly, critical factors of pubertal growth such as pre-pubertal growth and genetic potential for height (21, 22) have not been carefully evaluated.
The scarcity of studies of pubertal growth in CF is likely attributable to the lack of longitudinal and frequent height data available throughout adolescence and the difficulties in accurately determining the age and magnitude of peak height velocity (PHV). The former is required in order to capture non-linear and seasonal variation in height velocity (HV). The latter is best achieved by using appropriate statistical methods to avoid errors in HV interpolated or extrapolated from adjacent height measurements. Hence, we conducted the present study by utilizing the US CF Foundation (CFF) Registry (23) and a novel semi-parametric growth curve model (24) to estimate PHV from ~1800 children born after mid 1980s, a period coinciding with increasingly emphasized nutritional care, to test the hypothesis that pubertal PHV in children with CF has improved and is influenced by pre-pubertal growth and genetic potential.
METHODS
The CFF Registry documents the diagnosis and follow-up evaluations of patients with CF seen at accredited centers in the US(23). Height data were reported annually before 1993 and quarterly after 1994. Therefore, patients born in 1984–87 would have quarterly height data from 1994 (at age 7–10 y) and reached adulthood by 2008, the most recent year of CFF Registry data available for this study. Of the 4198 born in 1984–87, 309 died, 951 were lost to follow-up before age 18 y, and 1076 had <3 height measurements per year during age 10–18 years. The remaining 1862 patients were included. This study population did not differ significantly from those excluded from the analysis on sex (boys: 52.9% vs. 52.6%, p = 0.85) and pre-pubertal height percentile at age 7 y (22nd vs. 23rd, p = 0.20). The study protocol was approved by the human subjects committee at the University of Wisconsin – Madison.
Growth Curve Modeling to Identify Peak Height Velocity (PHV)
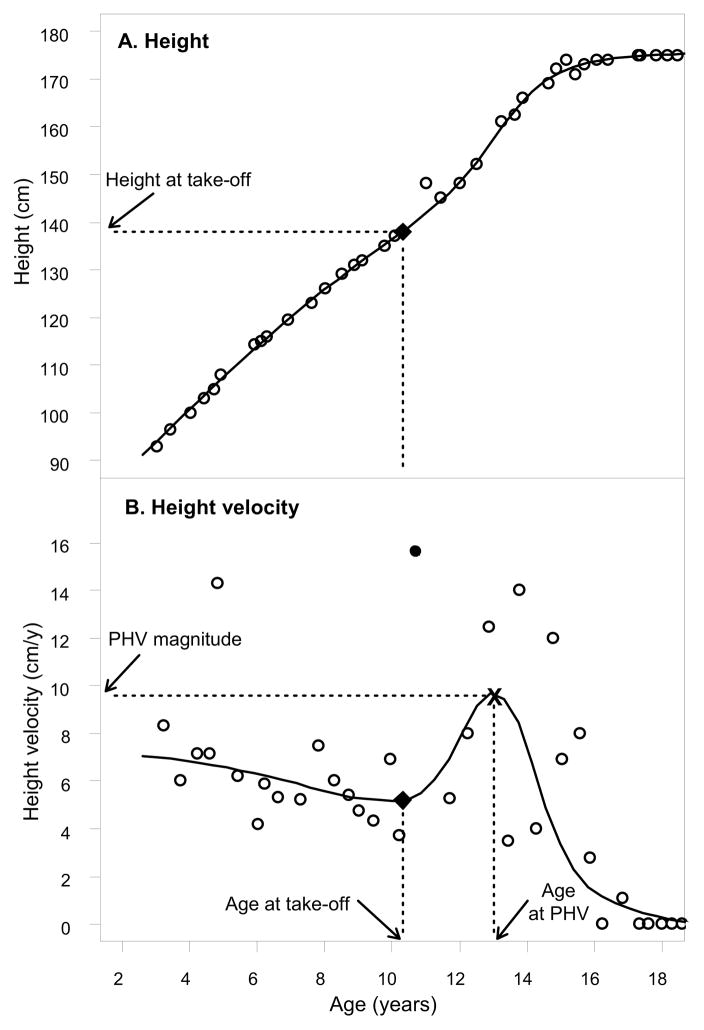
A semi-parametric shape-invariant model developed by Lindstrom was used (24). Conceptually, this method assumes that all individuals of the same sex have a common shape for their age versus height curve, which is estimated using data from all children by a non-linear mixed effects model with regression spline that has 2 continuous analytical derivatives. Each child’s individual height curve is then determined by shifting and scaling this common curve to obtain the best fit for his/her data. Once an individual’s height curve is fitted, the calculated first derivatives of this curve are used to determine the HV curve. Using this approach, 4 measurements characterizing pubertal growth for each child are identified: age at take-off, height at take-off, age at PHV, and magnitude of PHV (Figure 1).
Figure 1.
An example illustrating A, height curve of a child with CF and B, height velocity curve fitted by the semi-parametric shape-invariant model (24). Point “x” denotes the PHV magnitude derived from this modeling. Point “●”, the largest Y-axis value of all open circles, denotes PHV magnitude calculated from 2 adjacent heights.
Defining Delayed and Attenuated Peak Height Velocity (PHV)
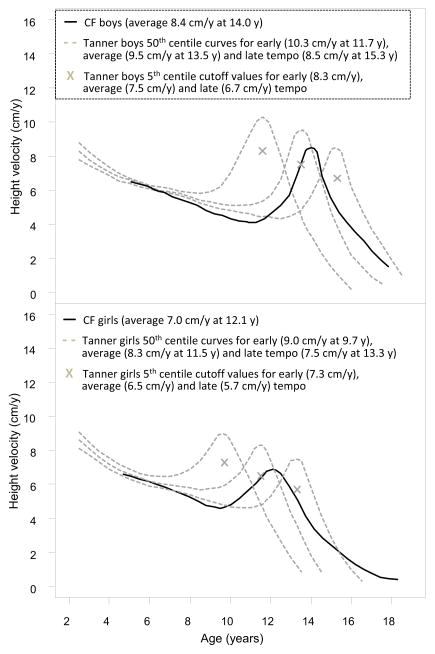
Longitudinal standards of PHV for North American children developed by Tanner and Davies (25) were used to define normal PHV because they provide reference values for children with different growth tempo (Figure 2). Delayed PHV was defined as PHV age at 2 SD later than average, namely, after 15.3 y in boys and 13.3 y in girls; attenuated PHV was defined as PHV magnitude below the 5th percentile (Figure 2). Using these criteria, PHV was classified into normal (PHV neither delayed nor attenuated), delayed (PHV delayed but not attenuated), attenuated (PHV attenuated but not delayed), and delayed and attenuated (D&A).
Figure 2.
Height velocity curves for boys and girl with CF compared with those for healthy children from Tanner’s reference population (25).
CF Phenotypes and Pre-pubertal Nutritional Status
Meconium ileus (MI) was retrieved from the CFF Registry. Because more than one-quarter of patients (28.7%) were not genotyped, pancreatic insufficiency (PI) and pancreatic sufficiency (PS) were defined by whether or not pancreatic enzymes were used, although this approach may have misclassified ~10% of patients as PI rather than PS because fecal elastase-1 was not available to define pancreatic functional status (26). Growth at age 7 y was used to reflect pre-pubertal nutritional status, as the age of PHV take-off in healthy girls with early PHV was 6.5 y (25) and none of the children with CF in our study entered PHV before age 7 years. Growth at age 7 y was indicated by height and body mass index (BMI) z-scores and percentiles, calculated using the 2000 CDC growth charts (27).
Adjusting for Genetic Potential for Height
The genetic potential for height was estimated from parental stature using the Himes method (28) validated for CF (21). This method does not directly predict the child’s genetic potential for height but eliminates the influence of tall and short parental stature by generating an “adjusted height” that represents the child’s height as if his/her parents had average stature (28). The following steps are used to calculate Himes adjusted height: 1) calculate mid-parental height, 2) find the Himes adjustment value (28) based on the child’s sex, age, height and mid-parent height, and 3) apply the adjustment value to the child’s height to obtain adjusted height.
Of the 1862 patients, 269 (14.4%) had self-reported parental height data documented in the CFF Registry. This subsample did not differ significantly from those without parental height data on sex (boys: 53.2% vs. 54.0%, p = 0.81), height percentile at age 7 y (23rd vs. 24th, p = 0.81) and adult height percentile (the measurement closest to age 21 y; 27th vs. 28th, p = 0.64). Himes adjusted heights at age 7 y and 21 y were calculated as described above (28). Percentiles and z-scores of parental heights, unadjusted and Himes adjusted adult heights were calculated using the 2000 CDC references at age 20 y (27).
Statistical Analyses
Shape invariant modeling of height and HV curves by the Lindstrom method (24) was performed by using the R nlme package (http://www.r-project.org). SAS (version 9.13, SAS Institute, Inc, Cary, NC) was used for all other analyses. Student t-test was used for two-group comparisons of continuous variables. Fisher Least Square Difference method was used for multiple-group comparisons of continuous variables. Chi-square or Fisher exact test was used to compare categorical variables. Multiple regression analysis was used to assess the effects of various predictors on PHV magnitude and adult height.
RESULTS
HV curve and Pubertal PHV Pattern
Figure 1 shows the height and HV curve of a child with CF fitted by the shape invariant model (24). This method yields more accurate PHV magnitude because it uses a smooth estimate from the fitted curve rather than that calculated from 2 adjacent heights (point “•”, the largest Y-axis value of all open circles).
Figure 2 shows the overall common shape of HV curves. Compared with healthy children (25), PHV in children with CF occurred later (0.5 years later in boys and 0.6 years later in girls) with reduced magnitude (1.1 cm less in boys and 1.3 cm in girls). Results from fitting individual curves revealed that PHV was normal in 60.3%, delayed in 9.4%, attenuated in 20.8% and D&A in 5.3%. The remaining 4.2% had HV curves that were flat, decreasing, or increasing; therefore PHV could not be ascertained. Proportionately more boys had normal PHV than girls, p = 0.002.
Pre-pubertal Growth, Pubertal PHV, and Adult Height by PHV Subgroups
Table I presents all growth measures for patients with identifiable PHV. In boys with CF with normal PHV, height at PHV take-off was similar to that at age 7 y and PHV magnitude was near normal (9.2 cm/y versus 9.5 cm/y; Figure 2), therefore, adult height percentile was maintained above the pre-pubertal value.
Table I.
Pre-pubertal heights, pubertal peak height velocities (PHV) and adult heights by PHV subgroups
| Peak height velocity (PHV) subgroups
|
||||
|---|---|---|---|---|
| Normal | Delayed (D) | Attenuated (A) | D&A | |
| Boys: | ||||
| N | 637 | 100 | 175 | 48 |
| Pre-pubertal growth at age 7 y | ||||
| Height percentile | 27 ± 1.1a | 16 ± 2.0b | 26 ± 2.0a | 11 ± 2.7b |
| BMI percentile | 47 ± 11 | 47 ± 2.6 | 53 ± 2.0 | 43 ± 4.0 |
| Pubertal PHV | ||||
| Age at take-off, y | 10.7 ± 0.04c | 12.9 ± 0.09b | 11.0 ± 0.11c | 13.4 ± 0.1a |
| Height at take-off, cm | 138.0 ± 0.3b | 144.4 ± 0.8a | 138.2 ± 0.7b | 143.9 ± 1.2a |
| Height at take-off, percentile | 26 ± 1.0a | 7 ± 0.1b | 21 ± 1.8a | 3 ± 1.2c |
| Age at PHV, y | 13.7 ± 0.04c | 15.9 ± 0.05b | 13.7 ± 0.11c | 16.2 ± 0.1a |
| PHV magnitude, cm/y | 9.2 ± 0.04a | 8.2 ± 0.10b | 6.7 ± 0.06c | 5.7 ± 0.1d |
| Total gain, take-off to age 21, cm | 35.6 ± 0.3a | 29.5 ± 0.6b | 29.9 ± 0.6b | 23.0 ± 0.9c |
| Adult height at age 21 y | ||||
| Centimeter | 173.6 ± 0.3a | 173.8 ± 0.7a | 168.1 ± 0.6b | 166.8 ± 1.0b |
| Percentile | 33 ± 1.1a | 34 ± 0.4a | 11 ± 1.7b | 8 ± 2.7b |
| Girls: | ||||
| N | 486 | 75 | 212 | 50 |
| Pre-pubertal growth at age 7 y | ||||
| Height percentile | 24 ± 1.1a | 17 ± 2.9ab | 20 ± 1.5ab | 16 ± 3.3b |
| BMI percentile | 41 ± 1.2a | 33 ± 2.7ab | 41 ± 1.9a | 25 ± 4.0b |
| Pubertal PHV | ||||
| Age at take-off, y | 8.9 ± 0.04d | 11.0 ± 0.10b | 9.7 ± 0.06c | 12.4 ± 0.20a |
| Height at take-off, cm | 128.2 ± 0.3d | 136.4 ± 1.0b | 131.3 ± 0.5c | 139.6 ± 1.1a |
| Height at take-off, percentile | 24 ± 1.1a | 12 ± 2.2b | 20 ± 1.4a | 4 ± 1.8c |
| Age at PHV, y | 11.6 ± 0.04d | 13.8 ± 0.06b | 12.0 ± 0.06c | 14.7 ± 0.20a |
| PHV magnitude, cm/y | 7.8 ± 0.05a | 6.8 ± 0.10b | 5.7 ± 0.04c | 4.3 ± 0.21d |
| Total gain, take-off to age 21, cm | 32.4 ± 0.3a | 26.1 ± 0.7b | 26.3 ± 0.4b | 17.0 ± 1.0c |
| Adult height at age 21 y | ||||
| Centimeter | 160.6 ± 0.3b | 162.6 ± 0.8a | 157.6 ± 0.4c | 156.6 ± 0.9c |
| Percentile | 34 ± 1.3b | 46 ± 3.4a | 19 ± 1.7c | 15 ± 3.4c |
Values are mean ± SE. Comparisons among 4 PHV subgroups were assessed by Fisher Least Square method and values superscripted with different letters within the same row were significantly different at p< 0.05.
In boys with CF with delayed PHV, height percentile at age 7 y was low, which decreased further at PHV take-off (as average healthy children enter PHV ~2 years earlier); yet absolute height at take-off was ~6 cm higher than the normal subgroup (due to the additional ~2 y of pre-pubertal growth). PHV magnitude and total gain from take-off to age 21 y were also low, but compensated by the longer duration of pre-pubertal growth, resulting in similar adult height compared with the normal subgroup. In other words, boys with CF with delayed but not attenuated PHV are similar to those with normal PHV except their PHV is shifted ~2 years later.
In boys with CF with attenuated PHV, height percentile at age 7 y and PHV take-off were similar to the normal subgroup but PHV magnitude and total gain from take-off to age 21 y were significantly reduced. Consequently, adult height percentile (11th) was below that of age 7 y and was significantly lower than the normal and delayed subgroups. In the D&A subgroup, height percentile at age 7 y was very low and the reductions in PHV magnitude and total gain from take-off to age 21 y were more pronounced, resulting in the lowest adult height percentile (8th).
In girls with CF, the catch-up improvement from pre-pubertal height at age 7 y to adult height percentile in the delayed subgroup (17th to 46th) was greater than boys (16th to 34th; Table I). In addition, adult height percentiles in girls with CF with attenuated or D&A were close to height at age 7 y and were significantly better than boys with attenuated or D&A, p = 0.002.
Pre-pubertal Height and Adult Height Adjusted for Genetic Potential
Table II presents data from the 269 patients with parental heights. On average, children with CF were shorter than healthy children at ages 7 years and 21 years, and their parents had average heights. Hence, Himes adjusted heights were lower than unadjusted heights. For example, at age 21 years of age, boys with CF were 6.6 cm shorter than their fathers and girls with CF were 3.7 cm shorter than their mothers. Applying Himes adjustments increased these differences to 9.2 cm in boys with CF and 4.4 cm in girls with CF. Consistent with these results, 80% of boys with CF and 77% of girls with CF had adult heights below their average parental height percentiles; these percentages were significantly higher in those with attenuated and D&A PHV with those with normal or delayed PHV.
Table II.
Heights adjusted for genetic potential for 269 children with CF who had parental height data
| All patients | By peak height velocity (PHV)
|
||
|---|---|---|---|
| Normal+Delayed(D)* | Attenuated(A)+D&Aŧ | ||
| N (%) | 269 | 195 (72.5%) | 74 (27.5%) |
| Boys: N (%) | 143 | 111 (77.6%) | 32 (22.4%) |
| Parental heights | |||
| Father, cm (percentile) | 178.7 ± 0.6 (60 ± 2.5) | 179.1 ± 0.7 (63 ± 2.8) | 177.2 ± 1.5 (52 ± 5.5) |
| Mother, cm (percentile) | 164.7 ± 0.6 (58 ± 2.5) | 164.8 ± 0.7 (59 ± 2.9) | 164.3 ± 1.2 (56 ± 5.3) |
| Average parental percentile | 59 ± 1.9 | 61 ± 2.1 | 55 ± 4.2 |
| CF Children’s heights | |||
| Height at age 7 y | |||
| Unadjusted percentile | 25 ± 2.3 | 27 ± 2.8 | 21 ± 4.6 |
| Himes adjusted4 percentile | 18 ± 2.0 | 19 ± 2.3 | 17 ± 4.1 |
| Pubertal PHV, cm/y | 8.5 ± 0.13 | 9.1 ± 0.10 | 6.5 ± 0.16 |
| Adult height at age 21 y | |||
| Unadjusted, cm (percentile) | 172.1 ± 0.7 (25 ± 2.4) | 173.4 ± 0.7a (32 ± 2.8a) | 167.4 ± 1.5b (9 ± 4.1b) |
| Himes adjusted, cm (percentile) | 169.5 ± 0.6 (16 ± 1.9) | 170.6 ± 0.7a (20 ± 2.3a) | 165.9 ± 1.4b (7 ± 3.2b) |
| Unadjusted percentile < average parental height percentile, n (%) | 115 (80.4%) | 85 (76.6%b) | 30 (93.8%a) |
| Girls: N (%) | 126 | 84 (66.7%) | 42 (33.3%) |
| Parental heights | |||
| Father, cm (percentile) | 177.9 ± 0.8 (56 ± 2.8) | 178.2 ± 1.0a (57 ± 3.3a) | 177.2 ± 1.5a (54 ± 5.1b) |
| Mother, cm (percentile) | 163.2 ± 0.7 (49 ± 2.8) | 163.4 ± 0.9a (49 ± 3.4a) | 162.8 ± 1.1a (49 ± 4.8b) |
| Average parental percentile | 53 ± 2.1 | 53 ± 2.5 | 52 ± 3.9 |
| CF Children’s heights | |||
| Height at age 7 y | |||
| Unadjusted percentile | 22 ± 2.2 | 23 ± 2.7 | 21 ± 3.7 |
| Himes adjusted4 percentile | 19 ± 2.0 | 19 ± 2.5 | 19 ± 3.2 |
| Pubertal PHV, cm/y | 7.1 ± 0.14 | 7.9 ± 0.12 | 5.5 ± 0.19 |
| Adult height at age 21 y | |||
| Unadjusted, cm (percentile) | 159.5 ± 0.6 (28 ± 2.4) | 160.4 ± 0.7a (33 ± 3.1a) | 157.6 ± 0.9b (19 ± 3.6b) |
| Himes adjusted, cm (percentile) | 158.8 ± 0.5 (24 ± 2.1) | 159.6 ± 0.6a (29 ± 2.8a) | 157.2 ± 0.7b (17 ± 2.8b) |
| Unadjusted percentile < average parental height percentile, n (%) | 97 (77.0%) | 60 (71.4%b) | 37 (88.1%a) |
Normal: N=98 (70%) in boys, N=74 (59%) in girls; Delayed: N=13 (9%) in boys, N=10 (8%) in girls.
Attenuated: N=28 (20%) in boys, N=36 (29%) in girls; D&A: N=4 (3%) in boys, N=5 (8%) in girls.
Values are mean ± SE. Comparisons between “Normal + Delayed” and “Delayed + D&A” was assessed by t-test and values superscripted with different letters within the same row are significantly different at p < 0.05).
The reduction in adult height percentile after Himes adjustment was greater in boys with CF than girls with CF (p = 0.015). Therefore, although unadjusted adult height percentile was not significantly different between boys and girls with CF (25th vs. 28th, p = 0.60), adjusted adult height percentile was significantly lower in boys with CF than girls with CF (16th vs. 24th, p = 0.011).
Factors associated with PHV magnitude and adult height
Univariate analyses revealed that height percentiles at age 7 y and adult heights were higher in PS compared with patients with PI and MI but PHV age and the magnitude did not differ significantly among the 3 phenotypes (Table III; available at www.jpeds.com). Nevertheless, more patients with PS (70%) had normal PHV compared with PI and MI combined (60%), p = 0.013.
Table III.
Pre-pubertal heights, pubertal PHV, and adult heights by CF phenotype
| All patients | By CF phenotype
|
|||
|---|---|---|---|---|
| Meconium Ileus | Pancreatic insufficiency | Pancreatic sufficiency | ||
| N (%) | 1862 | 417 (22.4%) | 1352 (72.6%) | 93 (5.0%) |
| Boys: N (%) | 985 | 220 (22.3%) | 712 (72.3%) | 53 (5.4%) |
| Pre-pubertal growth at age 7 y | ||||
| Height percentile | 25 ± 0.8 | 25 ± 1.8 | 24 ± 1.0 | 31 ± 3.9 |
| BMI percentile | 48 ± 0.9 | 44 ± 1.8 | 49 ± 1.0 | 48 ± 4.1 |
| Pubertal PHV | ||||
| Age at PHV, y | 14.0 ± 0.04 | 14.1 ± 0.09 | 14.0 ± 0.05 | 14.0 ± 0.12 |
| PHV magnitude, cm/y | 8.4 ± 0.05 | 8.4 ± 0.11 | 8.4 ± 0.06 | 8.7 ± 0.19 |
| Adult height at age 21 y | ||||
| Centimeter | 172.3 ± 0.3 | 172.1 ± 0.6b | 172.2 ± 0.3b | 174.3 ± 1.1a |
| Percentile | 26 ± 0.9 | 25 ± 2.0b | 26 ± 1.0b | 36 ± 4.3a |
| Girls: N (%) | 877 | 197 (22.5%) | 640 (73.0%) | 40 (4.6%) |
| Pre-pubertal growth at age 7 y | ||||
| Height percentile | 21 ± 0.8 | 17 ± 1.6b | 21 ± 1.0b | 32 ± 4.1a |
| BMI percentile | 39 ± 0.9 | 37 ± 1.8 | 39 ± 1.0 | 42 ± 4.4 |
| Pubertal PHV | ||||
| Age at PHV, y | 12.1 ± 0.04 | 12.1 ± 0.09 | 12.1 ± 0.05 | 12.0 ± 0.14 |
| PHV magnitude, cm/y | 7.0 ± 0.05 | 6.9 ± 0.10 | 7.0 ± 0.06 | 7.1 ± 0.30 |
| Adult height at age 21 y | ||||
| Centimeter | 160.0 ± 0.2 | 159.2 ± 0.5b | 159.6 ± 0.3b | 161.9 ± 1.0a |
| Percentile | 28 ± 1.0 | 26 ± 1.9b | 28 ± 1.1b | 42 ± 4.9a |
Values are mean ± SE. Comparisons among 3 phenotypes were assessed by Fisher Least Square method and values superscripted with different letters within the same row were significantly different at p < 0.05.
Subsequent analyses by multiple regression indicated that the significant effect of PS on adult height became insignificant when pre-pubertal height at age 7 and PHV were included in the model (Table IV; available at www.jpeds.com). On the other hand, PHV age, PHV magnitude and pre-pubertal height at age 7 were found to be stronger predictors of adult height (all with p < 0.001). Specifically, later PHV was associated with smaller magnitude of PHV but greater adult height, and larger PHV magnitude was associated with greater adult height after adjusting for PHV age.
Table IV.
Factors associated with peak height velocity (PHV) and adult height
| PHV magnitude (cm/y)
|
Adult height at age 21 y (z-score)
|
|||
|---|---|---|---|---|
| Multiple regression models | coefficient | p-value | coefficient | p-value |
| Boys: | ||||
| CF phenotype* | 0.42 | 0.26 | ||
| PI (vs. MI) | −0.03 | 0.78 | 0.07 | 0.20 |
| PS (vs. PI) | 0.28 | 0.19 | 0.08 | 0.38 |
| PS (vs. MI) | 0.25 | 0.28 | 0.15 | 0.15 |
| Pre-pubertal growth | ||||
| Height z-score at age 7 | 0.11 | 0.020 | 0.71 | < 0.001 |
| BMI z-score at age 7 | −0.17 | 0.001 | −0.003 | 0.17 |
| PHV | ||||
| Age (years) | −0.37 | < 0.001 | 0.25 | < 0.001 |
| Magnitude (cm/year) | NA | NA | 0.29 | < 0.001 |
| Girls: | ||||
| CF phenotype* | 0.42 | 0.30 | ||
| PI (vs. MI) | 0.13 | 0.23 | −0.07 | 0.21 |
| PS (vs. PI) | 0.07 | 0.73 | 0.11 | 0.29 |
| PS (vs. MI) | 0.02 | 0.37 | 0.05 | 0.69 |
| Pre-pubertal growth | ||||
| Height z-score at age 7 | −0.04 | 0.41 | 0.75 | < 0.001 |
| BMI z-score at age 7 | −0.05 | 0.28 | −0.37 | 0.13 |
| PHV | ||||
| Age (years) | −0.59 | < 0.001 | 0.28 | < 0.001 |
| Magnitude (cm/year) | NA | NA | 0.29 | < 0.001 |
MI: meconium ileus, PI: pancreatic insufficiency, PS: pancreatic sufficiency.
The effect of pre-pubertal growth also varied with growth indicators and sex (Table IV). In boys with CF, height at age 7 years had a significant positive effect on PHV magnitude and adult height, and BMI at age 7 years had a negative effect which was significant for PHV magnitude but not for adult height. In girls with CF, only height at age 7 years was significantly associated with adult height.
In the sub-population with parental heights, average parental percentile did not have a significant effect on PHV magnitude (p > 0.10), but was a very strong predictor of adult height (p = 0.001 in both boys and girls with CF).
DISCUSSION
With the use of a novel semi-parametric growth curve model (24) that can accommodate missing data, irregular measurement intervals and patterns of growth that may not be well fitted by parametric models (29, 30) used in previous studies (5, 10), our study generated new and important findings on pubertal growth in the CF population. First, we demonstrated that children with CF born in mid-1980s still experience delayed and attenuated pubertal PHV compared with healthy children, but the delay and attenuation were less severe compared with those reported in previous US studies (2, 4–5). For example, Byard studied 230 children born prior to the mid-1970’s and followed in a single CF center, and reported PHV of 7.9 cm/y at age 14.7 y (5), 0.5 cm lower and 0.7 y later than boys with CF in our study population. A consistent trend but smaller differences were observed in girls with CF (ie, 0.2 cm lower and 0.4 y later; 6.8 cm/y at age 12.5 y) than our study population. The improved pubertal PHV in our study cohort may be attributable to improved nutrient absorption through the use of enteric-coated pancreatic enzymes (12–15) as well as greater emphasis on increasing energy intake from high-fat diet and multi-disciplinary team approach to CF care recommended by CFF clinical practice guidelines in the last two decades (16–17, 20, 31).
Second, our study provided new estimates of the prevalence of impaired pubertal PHV in the US CF population; 23% of boys with CF and 30% of girls with CF had impaired PHV magnitude that was below the 5th percentile of healthy children (25). Of those with impaired PHV, boys with CF had a greater reduction in PHV magnitude and lower adult height percentiles than girls with CF, a finding consistent with Byard’s observation (5). Another related, novel finding of our study is that about 10% of children with CF experienced delayed but not attenuated PHV. These children should be considered having normal pubertal growth because their slightly lower PHV magnitude is a typical physiological phenomenon for late maturers (25) and is compensated by longer pre-pubertal growth. This allowed them to achieve a similar adult height compared with children with CF who had average onset and magnitude of PHV. These findings are supported by our multiple regression analyses that demonstrated inverse associations between PHV age and magnitude, which concurs with normal physiology of stature growth that children entering puberty early have a larger PHV magnitude compared with those entering puberty late (25), but the latter had longer period of pre-pubertal growth prior to PHV take-off, resulting in comparable adult heights in early and late maturers.
Third, despite improved pubertal PHV, our study showed that adult heights of children with CF remained lower than healthy children’s, and the majority (80% of boys and 70% of girls) did not reach their genetic potential (ie, below the average parental height percentile). Adult heights in our study cohort were comparable with those previously reported for US and Italian patients (5, 10) but differed from a British study (9) in which 52% were reported to achieve population norms and genetic potential. The latter study (9) had a very small sample size and included only well-nourished patients; therefore, their findings may not be representative of the CF population.
With regard to factors influencing pubertal growth and adult height, our study uncovered new associations and sex differences. Pre-pubertal height at age 7 y was found to be a strong determinant of adult height in both sexes. This finding implies that it is critical to target nutritional care to achieve optimal pre-pubertal growth, thereby maximizing the likelihood of attaining a greater adult height. This emphasis is particularly important in boys with CF, as our study showed that height at age 7 y also had a significant positive effect on pubertal PHV magnitude in boys with CF. As we demonstrated previously, the greatest likelihood of achieving optimal growth at age 6 y is through maximizing weight gain during the first two years of life (32); this in turn, depends on sustained high intakes of energy and essential nutrients such as essential fatty acids beginning in early infancy (33) after diagnosis through newborn screening. Improving nutrition and growth is now possible for all infants born with CF in the US, as a new era began with nationwide implementation of CF newborn screening in 2010 (34, 35).
In our analysis, patients with PS did not have greater PHV magnitudes but had taller adult heights than patients with PI and MI. However, the significant, positive association between PS and adult height observed in the univariate analysis became insignificant in multiple regression analysis. This observation suggests that the advantage of mild gastrointestinal phenotype on adult height is mediated primarily through better pre-pubertal height rather than greater pubertal growth. This is an encouraging finding because it implies that the negative impact of more severe CF phenotypes on long-term growth is “modifiable” through attaining better pre-pubertal growth that can be achieved by early diagnosis through newborn screening coupled with aggressive therapy. It is also possible that the relationship between pancreatic functional status and growth is influenced by other factors, such as modifier genes, which were found to contribute to BMI variability in pre-pubertal children with CF (36).
Our study has several limitations. First, the accuracy of height data documented in the CFF Registry cannot be ascertained because they were measured in routine clinical settings, although our previous study demonstrated that the quality of growth data available from the CFF Registry is comparable with that obtained by research protocols (37). Second, the sample size for parental heights was small relative to the entire population and therefore the findings related to genetic potential on pubertal height velocity need to be confirmed in future studies with larger sample sizes. Third, we were not able to investigate the relationships of PHV timing and magnitude to puberty development (i.e., sexual maturation) because the latter were not available from the CFF Registry.
In conclusion, despite major improvements in nutritional management during the past 2–3 decades, the challenges to patients with CF to achieve normal pubertal growth acceleration remain daunting unless CF clinicians set the goal to achieve maximal growth early in life and continue with unwavering effort to maintain adequate growth through pre-adolescence. Of equal importance is to initiate routine height velocity monitoring during adolescence, which warrants recommendation to the current clinical practice guidelines for CF (17, 20, 31).
Acknowledgments
The authors thank Dr Preston W. Campbell (Cystic Fibrosis Foundation) for providing the registry data and Dr Philip Farrell (University of Wisconsin – Madison) for reviewing the manuscript and commenting on pubertal growth and its implications on clinical care of patients with CF.
Supported by the National Institutes of Health (R01DK072126).
List of Abbreviations
- CF
cystic fibrosis
- D&A
delayed and attenuated
- HV
height velocity
- MI
meconium ileus
- PHV
peak height velocity
- PI
pancreatic insufficiency
- PS
pancreatic sufficiency
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landon C, Rosenfeld RG. Short stature and pubertal delay in cystic fibrosis. Pediatrician. 1987;14:253–60. [PubMed] [Google Scholar]
- 2.Landon C, Rosenfeld RG. Short stature and pubertal delay in male adolescents with cystic fibrosis. Androgen treatment. Am J Dis Child. 1984;138:388–91. doi: 10.1001/archpedi.1984.02140420054017. [DOI] [PubMed] [Google Scholar]
- 3.Barkhouse LB, Fahey J, Gillespie CT, Cole DE. Quantitating the effect of cystic fibrosis on linear growth by mathematical modeling of longitudinal growth curves. Growth Dev Aging. 1989;53:185–90. [PubMed] [Google Scholar]
- 4.Byard PJ. Relationship between clinical parameters and linear growth in children with cystic fibrosis. Am J Hum Biol. 1989;1:719–25. doi: 10.1002/ajhb.1310010609. [DOI] [PubMed] [Google Scholar]
- 5.Byard PJ. The adolescent growth spurt in children with cystic fibrosis. Ann Hum Biol. 1994;21:229–40. doi: 10.1080/03014469400003242. [DOI] [PubMed] [Google Scholar]
- 6.Haeusler G, Frisch H, Waldhor T, Gotz M. Perspectives of longitudinal growth in cystic fibrosis from birth to adult age. Eur J Pediatr. 1994;153:158–63. doi: 10.1007/BF01958975. [DOI] [PubMed] [Google Scholar]
- 7.Johannesson M, Gottlieb C, Hjelte L. Delayed puberty in girls with cystic fibrosis despite good clinical status. Pediatrics. 1997;99:29–34. doi: 10.1542/peds.99.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Laursen EM, Koch C, Petersen JH, Muller J. Secular changes in anthropometric data in cystic fibrosis patients. Acta Paediatrica. 1999;88:169–74. doi: 10.1080/08035259950170349. [DOI] [PubMed] [Google Scholar]
- 9.Aswani N, Taylor CJ, McGaw J, Pickering M, Rigby AS. Pubertal growth and development in cystic fibrosis: a retrospective review. Acta Paediatrica. 2003;92:1029–1032. [PubMed] [Google Scholar]
- 10.Assael BM, Casazza G, Iansa P, Volpi S, Milani S. Growth and long-term lung function in cystic fibrosis: A longitudinal study of patients diagnosed by neonatal screening. Pediatr pulmonol. 2009;44:209–15. doi: 10.1002/ppul.21001. [DOI] [PubMed] [Google Scholar]
- 11.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.08.040. epub ahead of print ( http://dx.doi.org/10.1016/j.jpeds.2012.08.40) [DOI] [PubMed]
- 12.Carroccio A, Pardo F, Montalto G, Japichino L, Iacono G, Collura M, et al. Effectiveness of enteric-coated preparations on nutritional parameters in cystic fibrosis. A long-term study. Digestion. 1988;41:201–6. doi: 10.1159/000199788. [DOI] [PubMed] [Google Scholar]
- 13.Brady MS, Rickard K, Yu PL, Eigen H. Effectiveness of enteric coated pancreatic enzymes given before meals in reducing steatorrhea in children with cystic fibrosis. J Am Diet Assoc. 1992;92:813–7. [PubMed] [Google Scholar]
- 14.Francisco MP, Wagner MH, Sherman JM, Theriaque D, Bowser E, Novak DA. Ranitidine and omeprazole as adjuvant therapy to pancrelipase to improve fat absorption in patients with cystic fibrosis. J Pediatr Gastroenterol & Nutr. 2002;35:79–83. doi: 10.1097/00005176-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Corey M, McLaughlin FJ, Williams M, Levinson H. A comparison of survival, growth and pulmonary function in patients with cystic fibrosis in Toronto and Boston. J Clin Epidemiol. 1988;41:583–91. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey BW, Farrell PM, Pencharz P Consensus Committee. Nutritional assessment and management in cystic fibrosis. Am J Clin Nutr. 1992;55:108–16. doi: 10.1093/ajcn/55.1.108. [DOI] [PubMed] [Google Scholar]
- 17.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol & Nutr. 2002;35:246–59. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Lai HJ. Comparison of body mass index percentile and percentage of ideal body weight for screening malnutrition in children with cystic fibrosis. Am J Clin Nutr. 2004;80:982–91. doi: 10.1093/ajcn/80.4.982. [DOI] [PubMed] [Google Scholar]
- 19.Lai HJ, Shoff SM. Classification of malnutrition in cystic fibrosis: implications on evaluating and benchmarking clinical practices. Am J Clin Nutr. 2008;88:161–166. doi: 10.1093/ajcn/88.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Shoff SM, Lai HJ. Incorporating genetic potential when evaluating stature in children with cystic fibrosis. J Cyst Fibros. 2010;9:135–142. doi: 10.1016/j.jcf.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai HJ. Classification of nutritional status in patients with cystic fibrosis. Curr Opin Pulm Med. 2006;12:422–427. doi: 10.1097/01.mcp.0000245709.66762.f9. [DOI] [PubMed] [Google Scholar]
- 23.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom MJ. Self-Modeling with Random Shift and Scale Parameters and a free knot spline shape function. Stat Med. 1995;14:2009–2021. doi: 10.1002/sim.4780141807. [DOI] [PubMed] [Google Scholar]
- 25.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–29. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 26.Baker SS, Borowitz D, Duffy L, Fitzpatrick L, Gyamfi J, Baker RD. Pancreatic enzyme therapy and clinical outcomes in patients with cystic fibrosis. J Pediatr. 2005;146:189–193. doi: 10.1016/j.jpeds.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital & Health Statistics Series. 2002;11:1–190. [PubMed] [Google Scholar]
- 28.Himes JH, Roche AF, Thissen D, Moore WM. Parent-specific adjustments for evaluation of recumbent length and stature of children. Pediatrics. 1985;75:304–313. [PubMed] [Google Scholar]
- 29.Preece MA, Baines MJ. A new family of mathematical models describing the human growth curve. Ann Hum Biol. 1978;5:1–24. doi: 10.1080/03014467800002601. [DOI] [PubMed] [Google Scholar]
- 30.Jolicoeur P, Pontier J, Pernin MO, Sempe M. A lifetime asymptotic growth curve for human height. Biometrics. 1988;44:995–1003. [PubMed] [Google Scholar]
- 31.Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkamp G, Heijerman HG, et al. Nutrition in patients with cystic fibrosis: a European Consensus. J Cystic Fibrosis. 2002;1:51–75. doi: 10.1016/s1569-1993(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 32.Lai HJ, Shoff SM, Farrell PM the Wisconsin CF Neonatal Screening Group. Recovery of birth weight z-score within two years of diagnosis is positively associated with pulmonary status at age six years in children with cystic fibrosis. Pediatrics. 2009;123:714–722. doi: 10.1542/peds.2007-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoff SM, Ahn H, Davis LA, Lai HJ, Wisconsin CF Neonatal Screening Group. Temporal associations between energy intake, plasma linoleic acid and growth improvement in response to treatment initiation after diagnosis of cystic fibrosis. Pediatrics. 2006;117:391–400. doi: 10.1542/peds.2004-2832. [DOI] [PubMed] [Google Scholar]
- 34.Rock MJ, Levy H, Zaleski C, Farrell PM. Factors accounting for a missed diagnosis of cystic fibrosis after newborn screening. Pediatric Pulmonolol. 2011;46:1166–74. doi: 10.1002/ppul.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Therrell BL, Hannon WH, Hoffman G, Ojodu J, Farrell PM. Immunoreactive trypsinogen (IRT) as a biomarker for cystic fibrosis: challenges in newborn dried blood spot screening. Mol Genet Metab. 2012;106:1–6. doi: 10.1016/j.ymgme.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Bradley GM, Blackman SM, Watson CP, Doshi VK, Cutting G. Genetic modifiers of nutritional status in cystic fibrosis. Am J Clin Nutr. 2012 doi: 10.3945/ajcn.112.943406. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai HC, FitzSimmons SC, Allen DB, Kosorok MR, Rosenstein BJ, Campbell PW, et al. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. New Engl J Med. 2000;342:851–9. doi: 10.1056/NEJM200003233421204. [DOI] [PubMed] [Google Scholar]