Abstract
In advanced cirrhosis, impaired function is caused by intrinsic damage to the native liver cells and from the abnormal microenvironment in which the cells reside. The extent to which each plays a role in liver failure and regeneration is unknown. To examine this issue, hepatocytes from cirrhotic and age-matched control rats were isolated, characterized, and transplanted into the livers of noncirrhotic hosts whose livers permit extensive repopulation with donor cells. Primary hepatocytes derived from livers with advanced cirrhosis and compensated function maintained metabolic activity and the ability to secrete liver-specific proteins, whereas hepatocytes derived from cirrhotic livers with decompensated function failed to maintain metabolic or secretory activity. Telomere studies and transcriptomic analysis of hepatocytes recovered from progressively worsening cirrhotic livers suggest that hepatocytes from irreversibly failing livers show signs of replicative senescence and express genes that simultaneously drive both proliferation and apoptosis, with a later effect on metabolism, all under the control of a central cluster of regulatory genes, including nuclear factor κB and hepatocyte nuclear factor 4α. Cells from cirrhotic and control livers engrafted equally well, but those from animals with cirrhosis and failing livers showed little initial evidence of proliferative capacity or function. Both, however, recovered more than 2 months after transplantation, indicating that either mature hepatocytes or a subpopulation of adult stem cells are capable of full recovery in severe cirrhosis.
Conclusion
Transplantation studies indicate that the state of the host microenvironment is critical to the regenerative potential of hepatocytes, and that a change in the extracellular matrix can lead to regeneration and restoration of function by cells derived from livers with end-stage organ failure.
Expansion and altered composition of the extracellular matrix as a result of collagen deposition is a common response to injury and plays a major role in chronic heart, liver, and kidney failure. Understanding the extent to which reversal of this process can lead to functional organ recovery is a critical issue, as numerous interventions have been proposed to improve fibrosis and presumably reverse organ failure.1–4 The unique capacity of hepatic parenchymal cells to undergo extensive proliferation in response to injury makes the liver an ideal organ to study cellular regeneration in acquired chronic disease.
In the liver, expansion of the extracellular matrix with capillarization of the sinusoidal endothelium and loss of fenestrae results in cirrhosis with production of regenerative hepatic nodules, portal hypertension, loss of hepatocytes, and liver failure.5 Loss of significant hepatocyte mass does not routinely produce hepatic failure, because the liver is capable of normal function with less than half its normal complement of hepatocytes.6,7 Thus, the cause of organ failure in cirrhosis is not well understood. Impaired hepatic function results from intrinsic damage to the native liver cells and from the abnormal microenvironment in which they reside.8–14 Because collagen deposition and vascular changes in cirrhosis can be extensive before there is functional hepatic decompensation, it is not clear to what extent each plays a role or at what point these factors tip parenchymal cell function toward organ failure. Mito et al.15 attempted to address the role of the microenvironment in hepatic failure by transplanting hepatocytes from the livers of patients with cirrhosis back into their own spleens to reverse decompensated liver disease. If it is possible to recover the function of parenchymal cells from a cirrhotic liver by changing the microenvironment, it may be possible to restore hepatic function in the cirrhotic liver by reversing hepatic structural abnormalities, and individual cells derived from some cirrhotic livers might prove to be useful as an untapped source of transplantable cells for the treatment of patients with liver-based metabolic disorders, where the liver microenvironment is intact.
Here, we demonstrate that primary cells derived from cirrhotic livers with decompensated function exhibit severe alterations in gene expression and defects in proliferative capacity and function directly after isolation, but engraft normally in a noncirrhotic microenvironment. Transplanted cells recover their capacity to expand and function when removed from the cirrhotic microenvironment, but normalization of hepatocyte function occurs only after a period of months.
Materials and Methods
Induction of Liver Cirrhosis
Liver cirrhosis was induced as described beginning in 4-week-old inbred male Lewis rats, weighing 100−130 g, using Phenobarbital (Sigma, St. Louis, MO) and carbon tetrachloride (CCl4, Sigma).16 Specific details are provided in the Supporting Information.
Hepatocyte Transplantation
Four-week-old male inbred Nagase analbuminemic rats (weighing approximately 100−130g) were treated with two doses of 30 mg/kg retrorsine, a pyrrolizidine alkaloid that inhibits hepatocyte proliferation,17–19 given 2 weeks apart via intraperitoneal injection. Four weeks after the last injection, a 70% partial hepatectomy was performed to induce donor hepatocyte proliferation. Hepatectomy was performed via ligation of the median and left lateral lobes of the liver. Animals (five per group) then underwent transplantation via the spleen with primary hepatocytes isolated from normal or cirrhotic rat livers or received intrasplenic injection of 0.1 mL Dulbecco’s modified Eagle’s medium as a control. Cyclosporine was given to control rejection by daily intramuscular injection at 15 mg/kg body weight. Five million cells for each transplantation procedure were washed, resuspended in 0.1 mL of phosphate-buffered saline, and injected into the splenic pulp over 30 seconds using a 27-gauge needle. Primary hepatocytes injected into the spleens of recipient noncirrhotic rodents are known to migrate and engraft into the liver parenchyma. Hepatocytes from four donor sources were used for these studies: 6− and 9-month-old control naїve Lewis rats (control); 6-month-old Lewis rats treated for 14 weeks with phenobarbital and CCl4 to induce cirrhosis with normal liver function (cirrhosis without liver failure); and 9-month-old Lewis rats treated with at least 26 weeks of phenobarbital and CCl4 to induce stable cirrhosis-induced (Child-Pugh class C) liver failure (cirrhosis with chronic liver failure).
Microarray Expression Analysis
All RNA samples were analyzed using an Agilent Bioanalyzer Lab-on-a-Chip Nano 6000 chip to determine the integrity and concentration of the samples. Only samples passing this quality control step with a mass ratio of the 28S to 18S RNA peaks of ≥2.0 were used for expression analysis. Twenty micrograms of total RNA was indirectly labeled using amino-allyl deoxyuridine triphosphate and an anchored oligo(dT)20 to prime reverse-transcription. Fluorescent label (CyDye, Amersham Biosciences) was coupled to the complementary DNA (cDNA) and hybridized to the PancChip version 5.0 13K cDNA microarray.20 For each array hybridization, labeled liver RNA from one animal from a study group was hybridized with an RNA sample from an animal in the age-matched control group labeled with a different CyDye. For each array hybridization, a dye-swap hybridization was performed, such that for each data point, two of the biological replicates were hybridized as test (Cy5, red) versus control (Cy3, green) and the other two biological samples were hybridized as control (Cy5) versus test (Cy3). The replicate dye-swap analysis reduces the impact of dye bias or other labeling artifacts on the ratio of gene expression at a given data point. The median intensities of each spot on the array were measured by an Agilent Scanner using GenePix version 5 software, and the ratio of expression for each element on the array was calculated in terms of M (log2 (red/green)) and A ((log2 (red) + log2 (green))/2)). The data were normalized by the print tip lowess method using the Statistical Microarray Analysis package in the software package R.21 For statistical analysis, the genes were identified as differentially expressed using the Patterns from Gene Expression package (PaGE version 5.0) as described.22 Two-class, unpaired data tests were also performed to specifically identify genes that were differentially expressed by more than 1.5-fold when comparing the different data points. Microarray data can be found on GEO, the public web site, at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?cc=GSE22977.
The values of fold change across the three comparisons were used to perform a hierarchical clustering analysis using Euclidean distance and the average agglomeration method.23 This procedure assigned each expressed gene to a unique cluster; these clusters were then classified according to their dynamics of change over time. Each gene cluster was subjected to a core analysis via Ingenuity Pathway Analysis (IPA, Ingenuity Systems), using the fold change difference between compensated and decompensated cirrhosis, for an assessment of the signaling pathways, molecular networks, and biological processes most significantly perturbed by the genes expressed per cluster with progression of cirrhosis to the decompensated state. IPA is based on a manually curated database of interactions among genes and gene products and can impute the presence of a given gene in a network from the expression pattern based on this interaction database. The gene networks generated by this analysis were scored by IPA to rank according to the degree of relevance to the set of genes present in our cluster.
Additional methods are presented in the Supporting Information.
Results
Induction of Cirrhosis and Characterization of Cells Isolated from Control and Cirrhotic Livers
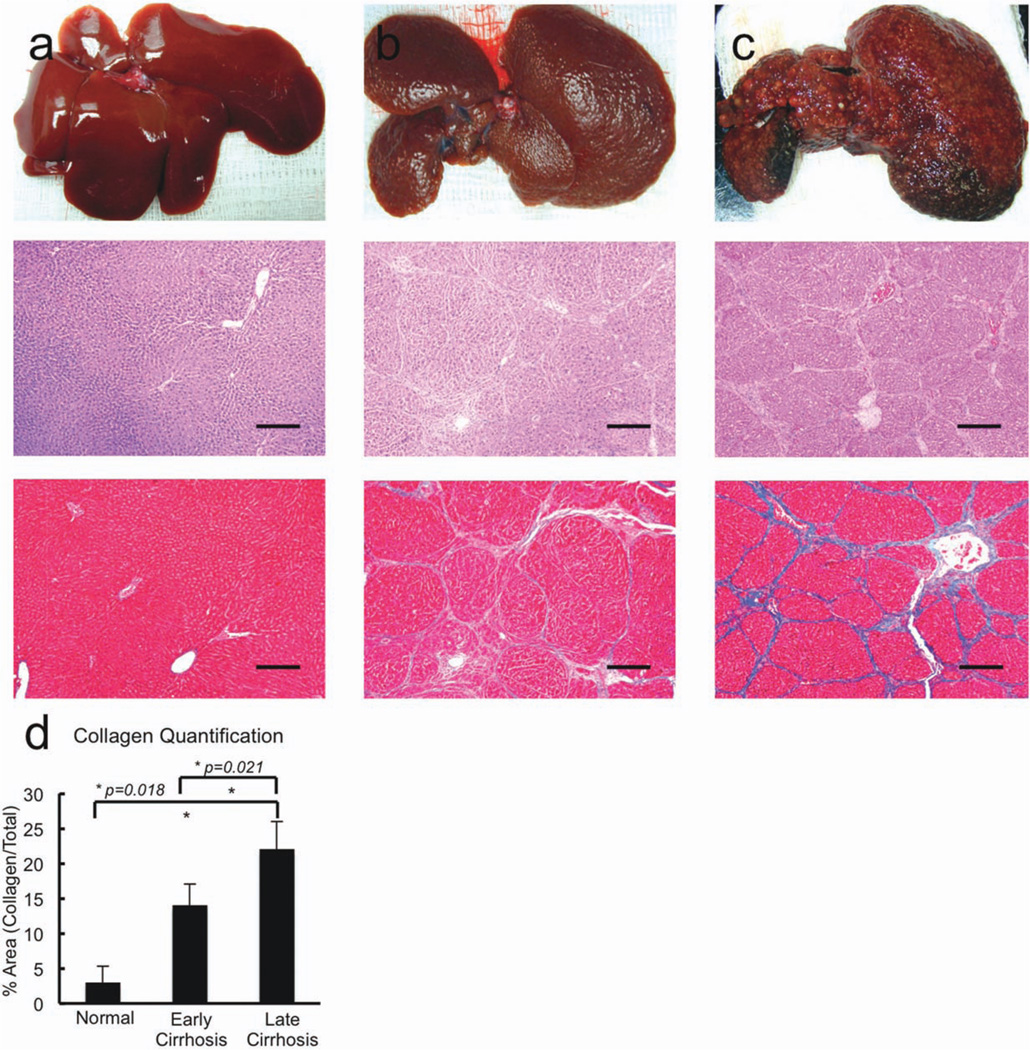
Hepatocytes were isolated from the livers of Lewis rats at two different stages of cirrhosis and from age-matched controls. Animals treated with 14 weeks of CCl4 had normal liver function (compensated cirrhosis) with bilirubin levels of <0.1 mg/dL, albumin levels of 3.4− 3.6 g/dL, prothrombin times of 13−14 seconds, and hepatic encephalopathy scores of 15, representing normal behavior.24 Animals that received 26−28 weeks of CCl4, however, had decompensated liver function with bilirubin levels of 0.4 ± 0.2 mg/dL, albumin levels of 2.2 ± 0.4 g/dL, prothrombin times of 21 ± 1.4 seconds, and hepatic encephalopathy scores of 8 ± 0.7. All assessments were performed at least 4 weeks after the last administration of CCl4 to eliminate the effects of acute CCl4 intoxication. Cirrhotic livers contained numerous regenerating nodules on gross inspection. Histologic analysis documented nodular regenerative hyperplasia and cirrhosis in both groups of animals, though fibrosis was quantitatively slightly more extensive in animals that received the greater amount of CCl4 (Fig. 1).
Fig. 1.
Gross and histologic changes associated with CCl4-induced liver injury. (a) Examination of normal control livers, (b) early cirrhotic livers from animals treated with 14 weeks of CCl4, and (c) advanced cirrhotic livers from animals treated with 26–28 weeks of CCl4 were examined via gross examination (upper panels), hematoxylin and eosin (middle panels; bar = 250 µm), and Masson’s trichrome stain (lower panels; bar = 250 µm). Cirrhotic livers contained numerous regenerating nodules on gross inspection. Histologic analysis documented nodular regenerative hyperplasia and cirrhosis in both groups of animals although fibrosis and (d) collagen deposition was more extensive in animals that received the greater amount of CCL4.
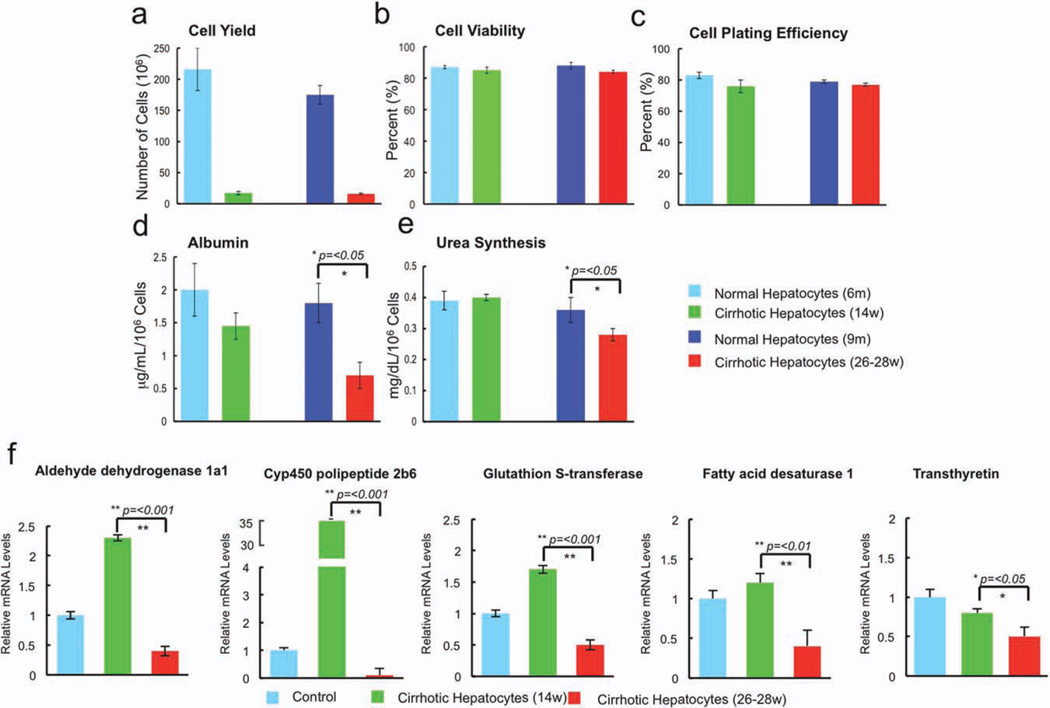
The yield of cells recovered by collagenase digestion from cirrhotic livers was significantly lower than that recovered from age-matched controls, and was approximately 5% of that recovered from control livers (Fig. 2a), but hepatocyte viability and plating efficiency were not statistically different among groups (Fig. 2b,c). As shown in Fig 2d and 2e, hepatocytes derived from control rats and rats with compensated cirrhosis secreted equal amounts of albumin and urea, whereas hepatocytes from the livers of cirrhotic rats with liver failure secreted significantly less of each (P < 0.05, decompensated cirrhosis versus compensated cirrhosis and age matched controls). Thus, directly after isolation, hepatocytes derived from the livers of cirrhotic rats with liver failure functioned less well in vitro than those derived from all other donor groups. A cohort of liver-specific genes (ADH1a1, CYP4502b9, GST, fatty acid desaturase-1, and transthyretin) was examined via quantitative polymerase chain reaction (qPCR) and confirmed significant down-regulation of CYP450 and metabolic enzyme gene expression in hepatocytes derived from the livers of rats with decompensated cirrhosis (Fig 2f).
Fig. 2.
Yield after isolation and functional characteristics of cells recovered from normal control livers, early cirrhotic livers, and advanced cir-rhotic livers. (a) The yield of cells recovered by collagenase digestion from cirrhotic livers was significantly lower than that recovered from age-matched controls and was approximately 5% of that recovered from control livers. (b,c) Cell viability (b) and cell plating efficiency (c) were not statistically different among groups. (d,e) Hepatocytes derived from control rats and rats with compensated cirrhosis secreted equal amounts of albumin (d) and urea (e), whereas hepatocytes from the livers of cirrhotic rats with liver failure secreted significantly less of each (P < 0.05). (f) A cohort of liver-specific genes was examined using qPCR and documented up-regulation in early cirrhosis followed by significant down-regulation (compared with control) in late cirrhosis of CYP450 and metabolic enzyme gene expression in hepatocytes derived from the livers of rats with decompensated cirrhosis: ADH1a1, CYP4502b9, GST, FADS1, and transthyretin.
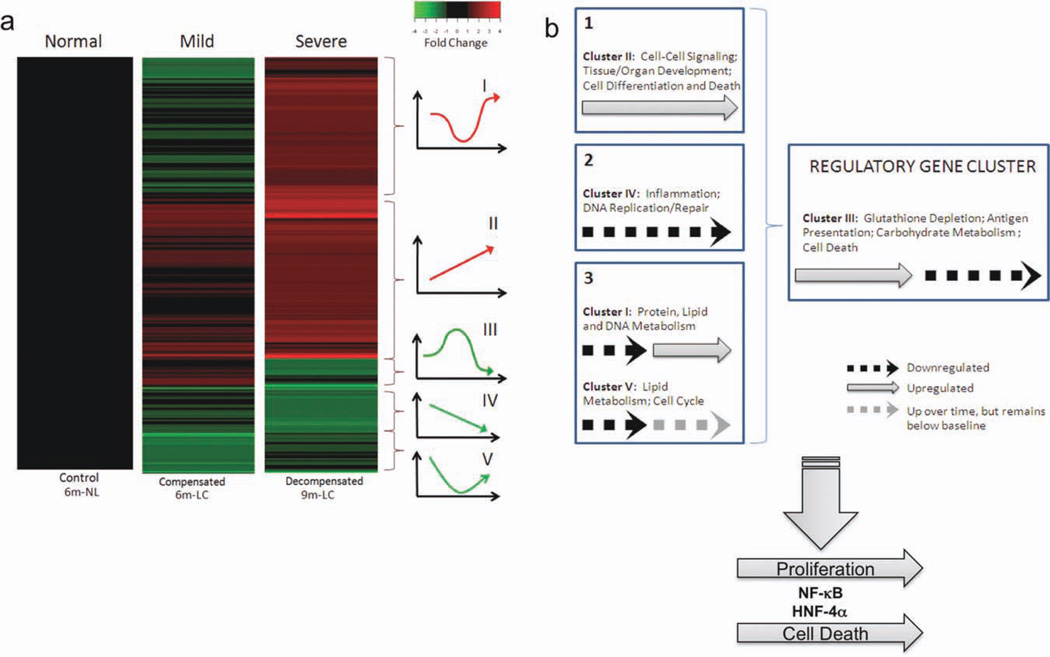
We then used DNA microarray analysis to study the gene expression profile of the hepatocytes recovered from cirrhotic livers with compensated and decompensated liver function versus age-matched controls. As shown in Fig. 3a, hierarchical clustering of the microarray data revealed five major dynamic patterns associated with progressive changes in degree of cirrhosis and liver dysfunction. Each expressed gene is assigned to a unique cluster, and therefore, to one of these five dynamic patterns. Cluster III, which consists of 60 genes, shows up-regulation in early cirrhosis, followed by down-regulation (compared with control) in late cirrhosis (Fig. 3a). Genes included in this cluster are those for aldehyde dehydrogenase (ALDH1a1), cytochrome P450 (CYP2d6, CYP2a2), glutathione S-transferase (GSTM1, GSTM4, GSTM5), fatty acid desaturase-1 (FADS1), and transthyretin (TTR) (Supporting Fig. 1). These results concur with the qPCR results shown in Fig. 2f.
Fig. 3.
DNA microarray analysis of messenger RNA from hepatocytes recovered from normal control, early cirrhotic, and advanced cirrhotic livers. (a) Hierarchical clustering of differentially expressed genes from hepatocytes isolated from livers with compensated and decompensated cirrhosis demonstrated significant gene expression differences among groups depending on the extent of cirrhosis from which the hepatocytes were derived. (b) Schematic representing hypotheses regarding liver cirrhosis, as gleaned from gene expression data. The DNA microarray results suggest that the irreversibly cirrhotic liver is expressing genes that simultaneously drive both proliferation and apoptosis, with a later effect on metabolism (clusters I, II, IV, and V ) , under the control of a central cluster of regulatory genes (cluster III). This regulatory process involves the actions of NF-κB and HNF-4α.
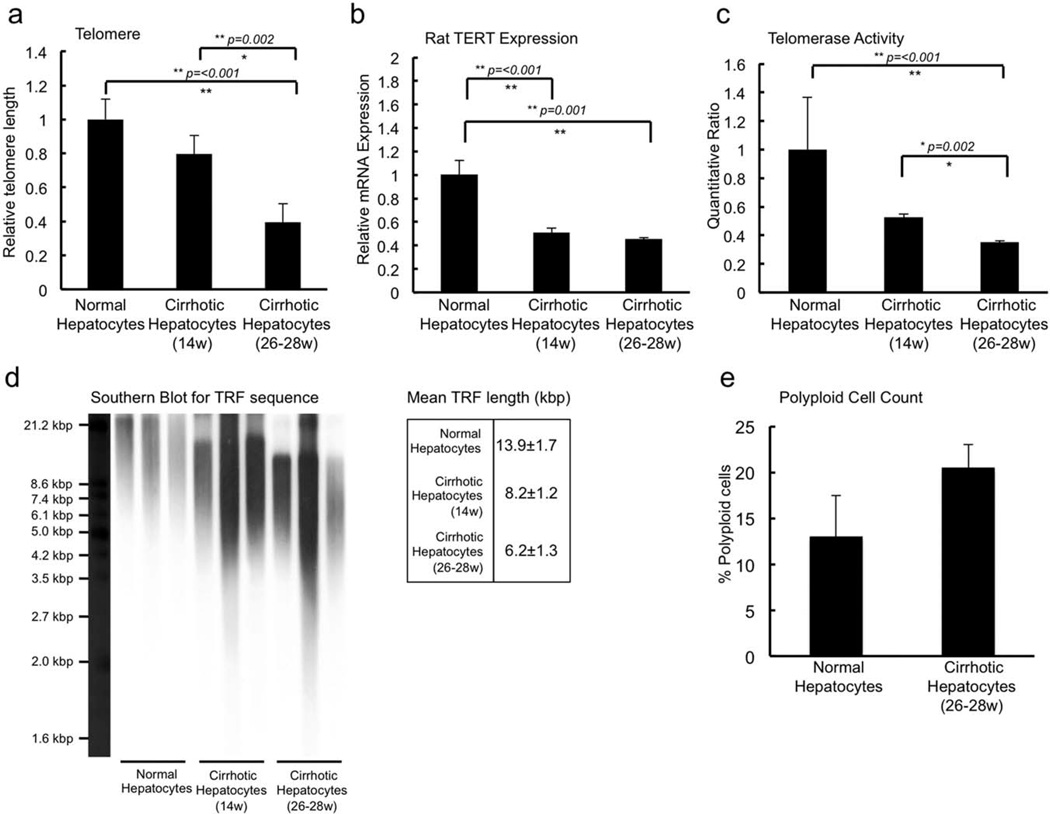
Performing a core analysis in IPA on each of the five clusters, nuclear factor κB (NF-κB) was found to be a central node in the most highly active networks generated by the genes in each cluster (Supporting Fig. 2). The expression of the gene for NF-κB itself was in cluster V, where it is seen to be slightly down-regulated as cirrhosis progresses from compensated to decompensated. Because NF-κB is assigned to only cluster V, in all other clusters predominant signaling pathways generated by IPA for the genes present were able to impute the NF-κB complex as a central node. These analyses indicate activation of signals promoting proliferation and regeneration, apoptosis, and cell death. These gene expression changes are most likely mediated by inflammation and oxidative stress, and are associated with progressive loss of gene expression representing worsening of metabolic function. Fig. 3b summarizes our inferences from these data, in which we suggest that clusters I, II, IV, and V are regulated by genes in cluster III (see Discussion). In addition, there was a progressive loss of telomerase activity, an increase in polyploidy, and a critical shortening of telomere length (Fig. 4), indicating replicative senescence as cirrhosis led to decompensated liver function. HNF4-α was also found to be a central node in networks of expressed genes in each of the five cluster patterns identified (Supporting Fig. 3). The expression of HNF4-α expression progressively fell with worsening liver function, regulating function as seen in two of the highly ranked networks generated by the genes in cluster IV, indicating dedifferentiation of hepatocytes. Because HNF-4α is present only in cluster IV, it was imputed as a node in the networks generated by IPA for the genes present in all other clusters. Thus, hepatocytes derived from livers with progressive worsening cirrhosis appeared to be undergoing replicative senescence and dedifferentiation. This finding is further supported by studies showing that inhibitor of κB phosphorylation changes significantly, as expected, with severity of cirrhosis (Supporting Fig. 4a).
Fig. 4.
Assessment of markers for senescence in hepatocytes recovered from normal control, early cirrhotic, and advanced cirrhotic livers. (a,b) qPCR for telomere length (a) and rat telomerase expression (b) showed progressive loss of activity with progression of cirrhosis. These data were confirmed by direct assessment of functional telomerase activity (c), and Southern blot analysis (d) demonstrated critical shortening of telomere length in hepatocytes derived from end-stage cirrhotic livers. These studies indicate the occurrence of replicative senescence, because cirrhosis leads to decompensated liver function. (e) An increase in polyploidy was also noted.
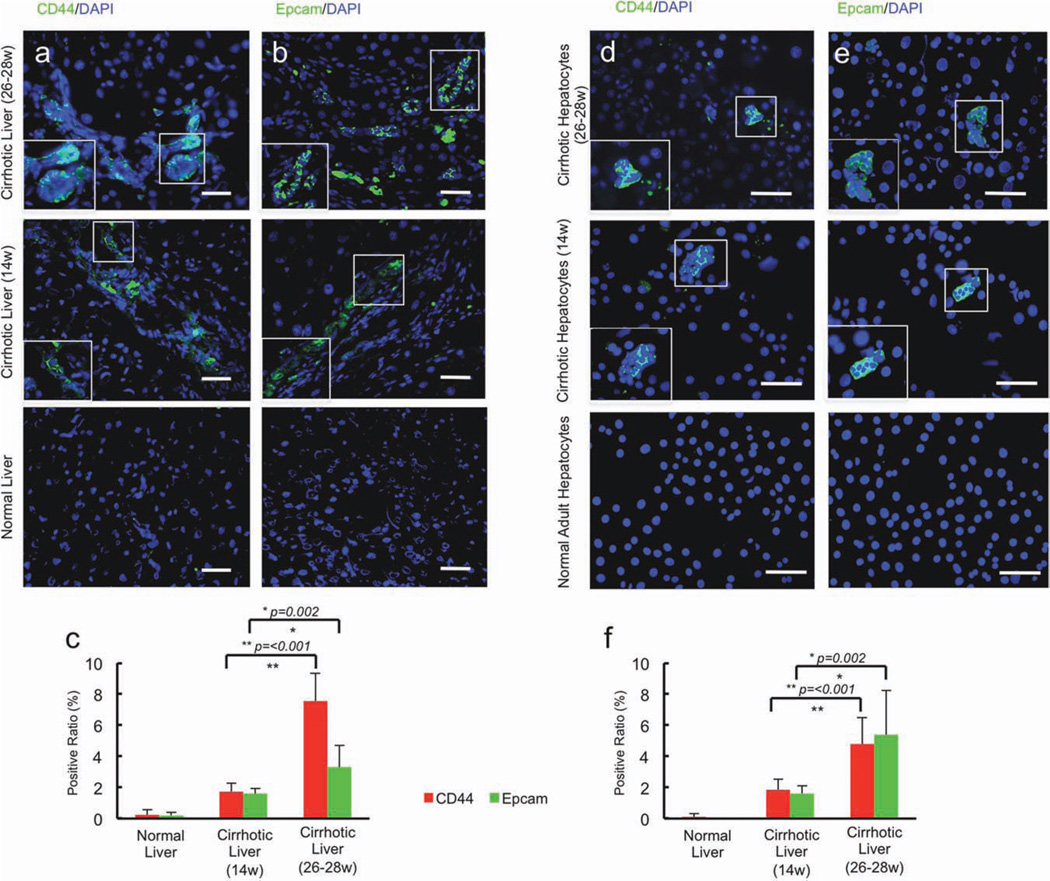
To further characterize the cells isolated from these livers, we examined whether worsening cirrhosis generated liver progenitor cells. As cirrhosis progressed there was an associated increase in the percentage of cells expressing alpha fetoprotein (data not shown), and putative liver progenitor cell markers CD44 and Epcam in liver sections (Fig. 5a–c). A nearly identical percentage of the cells isolated from cirrhotic livers expressed each of the marker proteins found in liver sections (Fig. 5d–f), indicating that the distribution of cell phenotypes derived from cirrhotic livers after isolation most likely represented that found in intact livers even though the cell yield following collagenase digestion from these livers was significantly lower than that obtained following digestion of control livers.
Fig. 5.
Induction of hepatic progenitor cells in progressively worsening cirrhosis. (a–c) As cirrhosis progressed, there was an associated increase in the percentage of cells expressing putative liver progenitor cell markers (a) CD44 and (b) Epcam in liver sections bar = 100 µm. **P ≤ 0.05. Hepatocytes isolated from control, early cirrhotic, and end-stage cirrhotic livers, which were used in transplantation studies, were similarly characterized for the presence of putative liver progenitor cells. (d–f) A nearly identical percentage of the cells isolated from cirrhotic livers expressed CD44 (d) and Epcam bar = 100 µm (e) in liver sections. These data indicate that the distribution of cell phenotypes derived from cirrhotic livers after isolation most likely represented that found in intact livers even though the cell yield after collagenase digestion was significantly lower than that obtained after digestion of control livers.
Transplantation of Isolated Hepatocytes
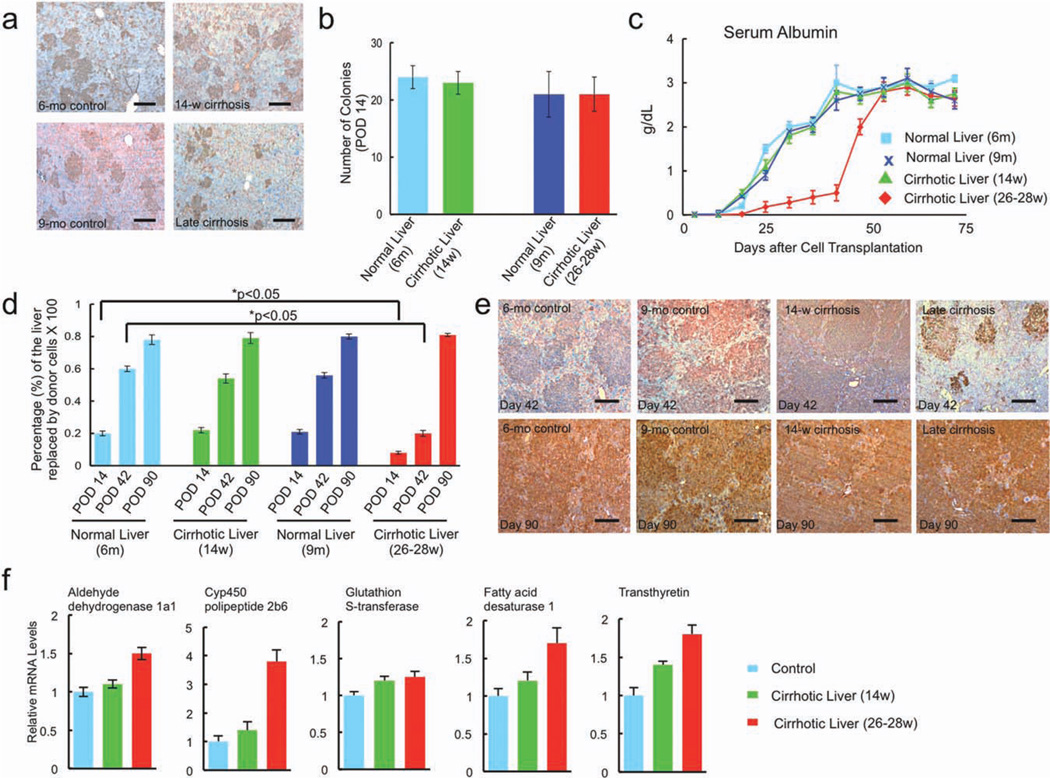
To examine the extent to which the impaired function and the altered gene expression associated with isolated cells derived from cirrhotic livers is affected by their microenvironment, cells from the livers of cirrhotic and age-matched controls were transplanted into the livers of Nagase analbuminemic rats. Recipients were treated with retrorsine and underwent partial hepatectomy, a combination of interventions that allows a selective long-term survival and repopulation advantage to the engrafted donor hepatocytes. Fourteen days after transplantation, donor cells from cirrhotic and age-matched control livers appeared to engraft with equal capacity. The albumin-expressing hepatocyte colonies were relatively small in size, as expected, and their numbers were not significantly different among groups (ranging from 21 to 24 engrafted donor colonies per low-power field), although 26- to 28-week cirrhotic hepatocyte clusters were smaller in size than the control and 14-week cirrhotic hepatocyte clusters (Fig 6a,b).
Fig. 6.
Repopulation of retrorsine-treated Nagase analbuminemic rat livers by donor hepatocytes. (a) Fourteen days after transplantation, donor cells from age-matched control, early cirrhotic, and advanced cirrhotic livers appeared to engraft with equal capacity bar = 150 µm. The albumin-expressing hepatocyte colonies were relatively small in size, as expected, and their numbers (b) were not significantly different among groups. (c) Early after transplantation, serum albumin levels in rats that received cells derived from donors with early cirrhosis and controls were significantly higher than in rats that received cells from cirrhotic rats with liver failure. The serum albumin levels in recipients with cell transplants from failing cirrhotic livers, however, recovered their capacity to expand and release albumin approximately 2 months after engraftment in noncir-rhotic livers. (d) Fourteen days after transplantation, there was a small difference in the percentage of the liver replaced by donor hepatocytes from control and early cirrhotic livers compared with that replaced by donor hepatocytes from failing cirrhotic livers (P < 0.05). (e) By posttransplantation day 42, there was considerable expansion of transplanted hepatocytes derived from controls and livers with early cirrhosis with coalescence of hepatocyte colonies, whereas expansion of transplanted hepatocytes recovered from failing cirrhotic livers was significantly less. By posttransplantation day 90, however, approximately 80% of the liver of all recipient rats was replaced by albumin-producing hepatocytes, independent of the source of the donor cells. Expression levels of previously measured liver-specific genes were assessed to determine whether recovered donor hepatocytes normalize function after transplantation. (f) qPCR demonstrated essentially no difference in ADH1a1, CYP4502b9, GST, FADS1, and transthyretin in the livers of repopulated animals irrespective of the source of donor cells used for transplantation.
Because native Nagase rat hepatocytes produce no albumin, serum albumin level was used to noninvasively assess the extent to which engrafted donor hepatocytes function and expand in the noncirrhotic environment. As shown in Fig. 6c, early after transplantation serum albumin levels in rats that received cells derived from donors with early cirrhosis and age-matched controls were significantly higher than in rats that received cells from cirrhotic rats with liver failure, but by posttransplantation day 49, the difference was dramatic (3 ± 0.7 g/dL versus 0.5 ± 0.5 g/dL, controls and early cirrhosis versus failing cirrhotic livers) (P < 0.05). The serum albumin levels in recipients transplanted with cells from failing cirrhotic livers, however, recovered their capacity to expand and release albumin approximately 2 months after engraftment in noncirrhotic livers. By posttransplantation day 63, there was statistically no difference in the levels of serum albumin among recipient transplantation groups.
Liver sections from recipients (two animals per group) were examined at various times after transplantation for albumin-expressing donor cells. As shown in Fig 6d, 14 days after transplantation there was a small difference in the percentage of the liver replaced by donor cells from control and early cirrhotic livers compared with that replaced by donor cells from failing cirrhotic livers (P < 0.05), but by posttransplantation day 42, there was considerable expansion of transplanted cells derived from controls and livers with early cirrhosis (57 ± 10% repopulation) with coalescence of hepatocyte colonies, whereas expansion of transplanted cells recovered from failing cirrhotic livers was significantly less (22 ± 3% repopulation) (P < 0.05, control and early cirrhosis versus failing cirrhotic livers). By posttransplantation day 90, however, approximately 80% of the liver of all recipient rats was replaced by albumin-producing hepatocytes, independent of the source of the donor cells (Fig 6e). To confirm that recovered donor hepatocytes ultimately normalize function 90 days after transplantation, we examined expression levels of the previously measured liver-specific genes (ADH1a1, CYP4502b9, GST, FADS1, and transthyretin). As shown in Fig. 6f, qPCR demonstrated essentially no difference in the expression of hepatocyte-specific enzymes in the livers of repopulated animals irrespective of the source of donor cells used for transplantation. In addition, the repopulating cells derived from advanced cirrhotic livers also regained telomerase activity and telomere length (Supporting Fig. 4b).
Discussion
Chronic tissue injury mediated by ischemia, autoimmunity, or numerous other processes results in interstitial fibrosis and collagen deposition that can produce impaired parenchymal cell function and organ failure. This process has been documented in ischemic and hypertensive heart disease, diabetic nephropathy, chronic pancreatitis, and cirrhosis of the liver,2,4,25 and results from both direct injury to tissue-specific parenchymal and nonparenchymal cells and interaction with a severely altered extracellular matrix. Here we showed that cells derived from failing cirrhotic livers have significant gene expression abnormalities and intrinsic defects in function after isolation, and contain a subpopulation of cells with characteristics consistent with hepatic progenitor cells. The isolated cells engraft without difficulty in a noncirrhotic hepatic microenvironment where the intrinsic defects in hepatocyte function and proliferation capacity recover over a period of months.
The extent to which the hepatocellular injury associated with end-stage liver cirrhosis is reversible has not been examined extensively. Our studies indicate that resolution of collagen deposition, vascular abnormalities, and fibrosis with restoration of the liver microenvironment may be able to restore hepatocyte function in end-stage cirrhosis. This issue is critical because interventions in animals have been shown to improve hepatic fibrosis and reverse cirrhosis.1,26–28 Another potential consequence of this work might be that injured and modestly functioning organs may serve as an untapped source of cells that could potentially be used for cell therapy in some settings.
The composition of the extracellular matrix is known to change during the development of cirrhosis, and the changes have been shown to inhibit hepatocyte regeneration and promote collagen deposition.29–33 Our studies demonstrate that hepatocyte expansion in response to partial hepatectomy is held in check for a period of months even after recovered cells from end-stage livers are transplanted in a noncirrhotic hepatic microenvironment. The mechanism by which parenchymal cell recovery may occur is difficult to know, as simple reversal of hepatocyte injury would not require months for repair.
Because the cells isolated from failing cirrhotic livers are not a homogeneous population, it is not possible to unequivocally determine the extent to which such complex signals are active in individual adult hepatocytes or whether an induced progenitor population is responsible for regeneration. Although decompensated cirrhotic livers contain a subpopulation of cells with characteristics consistent with hepatic progenitor cells, the presence of these cells may not fully account for the difference in repopulation kinetics of the cells obtained from 14-week versus 26- to 28-week cirrhotic livers. In fact, the heterogeneity of the cell population seems to be evident in the repopulation data. The number of expanding hepatocyte clusters per low-power field was the same for control and cirrhotic hepatocytes at 2 weeks after cell transplantation, but the size of the clusters was smaller with 26- to 28-week cirrhotic hepatocytes versus control or 14-week cirrhotic hepatocytes. Thus, the transplanted cells from the 26- to 28-week cirrhotic livers seem to already be proliferating 2 weeks after transplantation, though at a slower rate. In addition, Fig. 6 shows that engrafted 26- to 28-week cirrhotic hepatocytes are expressing albumin at that time, presumably when the progenitors cells, which are present in extremely low numbers, would not be generating albumin and before they would have had time to differentiate or begin expanding.
The results of our DNA microarray analysis suggest an apparent paradox, namely that the irreversibly cirrhotic liver is expressing a transcriptomic program of both proliferation and apoptosis, along with increased metabolism. We interpret this gene expression profile, along with the qPCR analysis and the functional data, to indicate that individual hepatocytes are severely affected by expansion and alteration of the surrounding extracellular matrix and conversion of the discontinuous sinusoidal lining into a continuous one. On the path to irreversible cirrhosis, we believe that chronic injury, in this case mediated by prolonged exposure to CCl4, initially sends two normally mutually exclusive messages to the hepatocyte: signals to proliferate simultaneously with signals to die. This dual signal appears to be mediated via NF-κB, a stress-sensitive transcription factor that regulates the balance between apoptosis on the one hand and inflammation (as a means of communicating cellular stress) on the other. In the face of these competing and confusing signals, we hypothesize that hepatocytes eventually can neither proliferate nor die, and that this process is regulated by HNF-4α. Normal hepatocyte turnover is impeded, and this stasis leads to a reduced number of functioning hepatocytes. Early in cirrhosis, hepatocyte metabolic functions are elevated by up-regulation of multiple networks of metabolism-related genes; however, this compensatory response can be maintained only to a certain point, beyond which hepatocytes can no longer support the elevated demand and subsequently fail. Cluster III contains genes that span all of the above processes, and it is tempting to speculate that this gene cluster serves a key regulatory role. Our hypothesis is supported by the expression pattern of this cluster, namely the initial increase in gene expression followed by a decrease below the baseline expression levels. We interpret this expression pattern to suggest a continuous attempt at maintenance of hepatic homeostasis driven by genes in cluster III.
Indeed, the loss of HNF-4α expression, activation of numerous networks involving NF-κB,30,34–38 loss of telomerase, and critical shortening of telomeres strongly indicate that worsening cirrhosis leads to replicative senescence of hepatocytes. Whether this process in cirrhosis is reversible is not known. Changes in the microenvironment may result in loss of polarity, marked alterations in tight intracellular junctions, and other structural receptor-mediated cell–cell communication processes that could take months to recover.10,33 As previously noted, it is not clear whether the majority of engrafted hepatocytes undergo such a repair process or whether recovery and repopulation is mediated by a small population of surviving stem-like cells that eventually expand to competitively replace the host Nagase rat liver cells. Arguments can be made for either possibility. Hepatocyte dedifferentiation has been shown to be reversible with changes in the composition of the extracellular matrix.29,33 However, the time from engraftment to recovery of proliferation capacity and function is consistent with activation of progenitor cells that need to differentiate into functional hepatic cells. This process takes time and does not occur consistently in a diseased liver.39 One interpretation of the data might be that hepatocytes from decompensated cirrhotic livers initially engraft and begin to repopulate the liver, but that these cells gradually undergo apoptosis and the progenitor cells, which are not readily detectable during the initial engraftment, later take over and repopulate the liver.
Regardless of the source of the regenerating cell population, long-term correction of cirrhosis by hepatocyte transplantation may be possible only following serious modification of the environment into which the cells engraft as the extracellular hepatic matrix may interfere with the function and expansion potential of the newly engrafted cells. This concept has support from the results of rodent studies wherein correction of hepatic failure and prolonged survival in end-stage cirrhosis after hepatocyte transplantation using syngeneic cells has been demonstrated to last for only a few months.16
In conclusion, we have demonstrated for the first time that parenchymal cells recovered from end-stage cirrhotic livers have the capacity to engraft, proliferate, and resume normal hepatic function when placed in a noncirrhotic liver environment. Although Sirma et al.40 have shown that human telomerase reverse-transcriptase is activated in hepatocytes during liver regeneration, our studies were performed in rodents and will need to be repeated with human hepatocytes derived from end-stage cirrhotic livers to confirm that the same process occurs in human hepatocytes. These studies provide important information on potential causes of organ dysfunction after chronic injury and insights into potential therapies that might be effective in treating chronic diseases associated with alteration and thickening of the extracellular matrix.
Supplementary Material
Acknowledgment
Supported by National Institutes of Health Grants DK48794 (to I. J. F.), DK083556 (to A. S.-G.), and DK072146-05 (to Y.V.).
We thank Maria Bond for technical assistance with manuscript preparation.
Abbreviations
- CCl4
carbon tetrachloride
- cDNA
complementary DNA
- HNF-4α
hepatocyte nuclear factor 4α
- NF-κB
nuclear factor κB
- qPCR
quantitative polymerase chain reaction
Footnotes
Potential conflict of interest: Dr. Kaestner consults for Johnson & Johnson.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeisberg M, Kalluri R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr Nephrol. 2008;23:1395–1398. doi: 10.1007/s00467-008-0818-x. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 4.Du XJ, Xu Q, Lekgabe E, Gao XM, Kiriazis H, Moore XL, et al. Reversal of cardiac fibrosis and related dysfunction by relaxin. Ann N Y Acad Sci. 2009;1160:278–284. doi: 10.1111/j.1749-6632.2008.03780.x. [DOI] [PubMed] [Google Scholar]
- 5.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pack GT, Islami AH, Hubbard JC, Brasfield RD. Regeneration of human liver after major hepatectomy. Surgery. 1962;52:617–623. [PubMed] [Google Scholar]
- 7.Lin TY, Lee CS, Chen CC, Liau KY, Lin WS. Regeneration of human liver after hepatic lobectomy studied by repeated liver scanning and repeated needle biopsy. Ann Surg. 1979;190:48–53. doi: 10.1097/00000658-197907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Hernandez A, Martinez J. The role of capillarization in hepatic failure: studies in carbon tetrachloride-induced cirrhosis. Hepatology. 1991;14:864–874. doi: 10.1002/hep.1840140519. [DOI] [PubMed] [Google Scholar]
- 9.Reichen J, Hoilien C, Le M, Jones RH. Decreased uptake of taurocholate and ouabain by hepatocytes isolated from cirrhotic rat liver. Hepatology. 1987;7:67–70. doi: 10.1002/hep.1840070115. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984;51:57–74. [PubMed] [Google Scholar]
- 11.Pessayre D, Lebrec D, Descatoire V, Peignoux M, Benhamou JP. Mechanism for reduced drug clearance in patients with cirrhosis. Gastroenterology. 1978;74:566–571. [PubMed] [Google Scholar]
- 12.Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008;48:1932–1941. doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–1354. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- 14.Vaubourdolle M, Gufflet V, Guechot J, Ballet F, Jaillon P, Giboudeau J, et al. Evidence of the intact hepatocyte theory in alcoholic cirrhosis. Scand J Gastroenterol. 1989;24:467–474. doi: 10.3109/00365528909093076. [DOI] [PubMed] [Google Scholar]
- 15.Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplantat Proc. 1992;24:3052–3053. [PubMed] [Google Scholar]
- 16.Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM, et al. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31:851–857. doi: 10.1053/he.2000.5636. [DOI] [PubMed] [Google Scholar]
- 17.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson JE. Effects of the pyrrolizidine alkaloid, lasiocarpine N-oxide, on nuclear and cell division in the liver rats. J Pathol Bacteriol. 1965;89:153–171. doi: 10.1002/path.1700890117. [DOI] [PubMed] [Google Scholar]
- 19.Jago MV. The development of the hepatic megalocytosis of chronic pyrrolizidine alkaloid poisoning. Am J Pathol. 1969;56:405–421. [PMC free article] [PubMed] [Google Scholar]
- 20.White P, Brestelli JE, Kaestner KH, Greenbaum LE. Identification of transcriptional networks during liver regeneration. J Biol Chem. 2005;280:3715–3722. doi: 10.1074/jbc.M410844200. [DOI] [PubMed] [Google Scholar]
- 21.Dudoit S, Fridlyand J. A prediction-based resampling method for estimating the number of clusters in a dataset. Genome Biol. 2002;3:RESEARCH0036. doi: 10.1186/gb-2002-3-7-research0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manduchi E, Grant GR, McKenzie SE, Overton GC, Surrey S, Stoeckert CJ., Jr Generation of patterns from gene expression data by assigning confidence to differentially expressed genes. Bioinformatics. 2000;16:685–698. doi: 10.1093/bioinformatics/16.8.685. [DOI] [PubMed] [Google Scholar]
- 23.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bures JBO, Huston JP. Techniques and Basic Experiments for the Study of Brain and behavior. New York, NY: Elsevier; 1976. Innate and motivated behavior; pp. 37–45. [Google Scholar]
- 25.DeLeve LD. Hepatic microvasculature in liver injury. Semin Liver Dis. 2007;27:390–400. doi: 10.1055/s-2007-991515. [DOI] [PubMed] [Google Scholar]
- 26.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 27.Wasmuth HE, Lammert F, Zaldivar MM, Weiskirchen R, Hellerbrand C, Scholten D, et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology. 2009;137:309–319. doi: 10.1053/j.gastro.2009.03.053. 319.e301-e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 29.Godoy P, Hengstler JG, Ilkavets I, Meyer C, Bachmann A, Muller A, et al. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology. 2009;49:2031–2043. doi: 10.1002/hep.22880. [DOI] [PubMed] [Google Scholar]
- 30.Helenius M, Makelainen L, Salminen A. Attenuation of NF-kappaB signaling response to UVB light during cellular senescence. Exp Cell Res. 1999;248:194–202. doi: 10.1006/excr.1999.4393. [DOI] [PubMed] [Google Scholar]
- 31.Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47–49. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 32.Kallis YN, Robson AJ, Fallowfield JA, Thomas HC, Alison MR, Wright NA, et al. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut. 2011;60:525–533. doi: 10.1136/gut.2010.224436. [DOI] [PubMed] [Google Scholar]
- 33.Talamini MA, Kappus B, Hubbard A. Repolarization of hepatocytes in culture. Hepatology. 1997;25:167–172. doi: 10.1002/hep.510250131. [DOI] [PubMed] [Google Scholar]
- 34.Batsi C, Markopoulou S, Vartholomatos G, Georgiou I, Kanavaros P, Gorgoulis VG, et al. Chronic NF-kappaB activation delays RasV12-induced premature senescence of human fibroblasts by suppressing the DNA damage checkpoint response. Mech Ageing Dev. 2009;130:409–419. doi: 10.1016/j.mad.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bu DX, Johansson ME, Ren J, Xu DW, Johnson FB, Edfeldt K, et al. Nuclear factor {kappa}B-mediated transactivation of telomerase prevents intimal smooth muscle cell from replicative senescence during vascular repair. Arterioscler Thromb Vasc Biol. 30:2604–2610. doi: 10.1161/ATVBAHA.110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy K, Mansfield L, Mackay A, Benvenuti S, Ismail S, Arora P, et al. Transcriptional networks and cellular senescence in human mammary fibroblasts. Mol Biol Cell. 2005;16:943–953. doi: 10.1091/mbc.E04-05-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helenius M, Hanninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318:603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helenius M, Kyrylenko S, Vehvilainen P, Salminen A. Characterization of aging-associated up-regulation of constitutive nuclear factor-kappa B binding activity. Antioxid Redox Signal. 2001;3:147–156. doi: 10.1089/152308601750100669. [DOI] [PubMed] [Google Scholar]
- 39.Dolle L, Best J, Mei J, Al Battah F, Reynaert H, van Grunsven LA, et al. The quest for liver progenitor cells: a practical point of view. J Hepatol. 52:117–129. doi: 10.1016/j.jhep.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Sirma H, Kumar M, Meena JK, Witt B, Weise JM, Lechel A, et al. The promoter of human telomerase reverse transcriptase is activated during liver regeneration and hepatocyte proliferation. Gastroenterology. 2011;141:326–337. doi: 10.1053/j.gastro.2011.03.047. 337.e321-e323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.