Abstract
Toll-like receptor 9 (TLR9) recognizes microbial DNA containing unmethylated cytosyl guanosyl (CpG) sequences, induces innate immune responses, and facilitates antigen-specific adaptive immunity. Recent studies report that in addition to stimulating innate immunity, TLR9 ligands induce apoptosis of TLR9 expressing cancer cells. To understand the mechanism of TLR9-induced apoptosis, we compared the effects of CpG containing oligodeoxynucleotides (CpG ODN) on a mouse B-cell lymphoma line, CH27, with those on mouse splenic B cells. CpG ODN inhibited constitutive proliferation and induced apoptosis in the CH27 B-cell lymphoma line. In contrast, CpG ODN-treated primary B cells were stimulated to proliferate and were rescued from spontaneous apoptosis. The induction of apoptosis required the ODNs to contain the CpG motif and the expression of TLR9 in lymphoma B cells. A decrease in Bcl-xl expression and an increase in Fas and Fas ligand expression accompanied lymphoma B-cell apoptosis. Treatment with the Fas ligand-neutralizing antibody inhibited CpG ODN-induced apoptosis. CpG ODN triggered a transient NF-κB activation in the B-cell lymphoma cell line, which constitutively expresses a high level of c-Myc, while CpG ODN induced sustained increases in NF-κB activation and c-Myc expression in primary B cells. Furthermore, an NF-κB inhibitor inhibited the proliferation of the CH27 B-cell lymphoma line. Our data suggest that the differential responses of lymphoma and primary B cells to CpG ODN are the result of differences in NF-κB activation. The impaired NF-κB activation in the CpG ODN-treated B-cell lymphoma cell line alters the balance between NF-κB and c-Myc, which induces Fas/Fas ligand-dependent apoptosis.
Keywords: apoptosis, lymphoma B cells, NF-κB, Toll-like receptor
Introduction
Toll-like receptors (TLRs) belong to a family of pattern-recognition receptors1 that recognize pathogen-associated molecular patterns, such as bacterial lipopolysaccharides (LPS) and DNAs. TLRs trigger innate immune responses, which play a crucial role in limiting the early replication and spread of pathogens during infection. TLRs also cause the activation of antigen presenting cells, which leads to the induction of adaptive immune responses that provide long-lasting protection.2
Toll-like receptor 9 (TLR9) recognizes unmethylated cytosyl guanosyl (CpG) sequences that are present at a higher frequency in the genomes of prokaryotes than that of eukaryotes.3,4 TLR9 is primarily expressed by B cells and plasmacytoid dendritic cells (pDCs) in humans.5 In mice, TLR9 is expressed by macrophages and myeloid dendritic cells (mDCs) in addition to B cells and pDCs.6 TLR9 binds its ligand in endocytic vesicles and recruits the adaptor protein MyD88,7 leading to the formation of TLR-MyD88-IRAK4-IRAK1-TRAF6 signaling complexes.8 IRAK1 recruits and phosphorylates tumor necrosis factor receptor-associated factor 6 (TRAF6).9 TRAF6 activates tumor growth factor β-activated protein kinase 1 (TAK1),10 which activates IκB kinase complex (IκK) and leads to the activation of nuclear factor (NF)-κB. Treating B cells with CpG oligodeoxynucleotides (ODN) causes the upregulation of several transcription factors, including NF-κB, c-Myc, activating protein 1 (AP-1), and the cAMP-responsive element-binding protein (CREB),11,12 resulting in a strong Th-1-biased immune response with secretion of pro-inflammatory cytokines, such as IL-6 and IL-12.13
CpG DNA-induced signaling rescues mature naive B cells from spontaneous apoptosis and promotes polyclonal B-cell proliferation in culture.14 This anti-apoptotic effect is attributed to TLR9-induced sustained NF-κB activation and increases in the protein levels of c-Myc and the anti-apoptosis protein Bcl-xl.14,15,16
In contrast to its anti-apoptotic function, signaling through TLRs has also been shown to induce apoptosis. Bacterial lipoproteins, LPS and dsRNA, which signal through TLR2, TLR4 and TLR3, respectively, have been shown to induce apoptosis, especially in prestimulated cells.17,18,19 TLR-mediated apoptosis of prestimulated cells has been proposed as a crucial feedback mechanism that downregulates TLR-induced immune cell activation and prevents overactive inflammatory responses that may lead to autoimmune diseases and other disease pathologies, such as sepsis and airway inflammation.20 However, the mechanism underlying TLR-induced apoptosis remains to be elucidated.
Recently, studies on the immunological functions of TLR9 discovered the potential use of CpG ODN as a therapeutic agent for cancer and autoimmune diseases.21 It has been reported that CpG ODN alone or in combination with other therapies, such as chemotherapy and monoclonal antibody (mAb), induced tumor regression of lung and skin malignancies.21 In murine models of T-cell lymphoma and cervical carcinoma, CpG DNA treatment led to tumor regression as the result of T cell- or NK cell-dependent lysis of tumor cells.22,23 B-cell lymphomas are unique in that they can express TLR9 and directly respond to CpG DNA. Recent reports showed that CpG ODN sensitizes B-cell chronic lymphocytic leukemia (B-CLL) cells to apoptotic triggers.24,25 These studies suggest that TLR9 ligands have dual effects on TLR9-expressing cancer cells: stimulating anti-cancer immune responses and inducing apoptosis of cancer cells.
To examine the mechanism by which TLR9 induces apoptosis in cancer B cells, we compared the effects of CpG ODN on the CH27 mouse B-cell lymphoma cell line with those on mouse splenic B cells. We showed that CpG ODN induces apoptosis in the B-cell lymphoma line, while stimulating proliferation and inhibiting spontaneous apoptosis of naive splenic B cells in culture. By studying signaling molecules downstream of TLR9, we discovered that CpG ODN treatment induced an aberrant NF-κB activation and c-Myc expression, a downregulation of the anti-apoptotic protein Bcl-xl, and an upregulation of Fas and Fas ligand in the B-cell lymphoma line, triggering multiple interrelated mechanisms that lead to apoptosis.
Materials and methods
Cells, oligodeoxynucleotides and reagents
Purified phosphorothioate-modified ODNs were purchased from Integrated DNA Technologies (Coralville, IA, USA). A CpG ODN sequence (1826) that is optimal for activating mouse B cells was used: 5′-TCCATGACGTTCCTGACGTT-3′. A GpC control ODN was also used: 5′-TCCATGAGCTTCCTGAGCTT-3′. Polymyxin B (Sigma, St Louis, MO, USA) was used to eliminate the possibility of LPS contamination in the ODNs. No significant effect of polymyxin B treatment was observed (data not shown).
The mouse B-cell lymphoma line, CH27, was generated and characterized by Haughton et al.26 and is an H-2k IgM+ cell line. CH27 cells were cultured in DMEM, containing 15% fetal bovine serum, and were supplemented as described previously.27 TLR9-negative CH27 cells were identified by cloning CH27 cells by limiting dilution and screening for clones that lack tlr9 mRNA using reverse transcription polymerase chain reaction (RT-PCR).
Mononuclear cells were isolated from the spleens of BALB/c and C57BL/6 mice (Charles River Laboratories, Inc., Frederick, MD, USA) by Ficoll (Sigma-Aldrich, St Louis, MO, USA) density gradient centrifugation. B cells were isolated by T-cell depletion using anti-Thy 1.2 antibody (BD Bioscience, San Diego, CA, USA) and guinea pig complement (Rockland Immunochemicals Inc., Gilbertsville, PA, USA). The resulting cells were panned to remove monocytes and dendritic cells. All experiments involving animals have been reviewed and proved by the Institution Animal Care and Use Committee at University of Maryland (R-07-41 and R-10-87).
RT-PCR analyses of tlr9 mRNA
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to purify RNA from two CH27 clones and splenic B and T cells as recommended by the manufacturer. cDNA was generated from this RNA using SuperScript II reverse transcriptase (Invitrogen). Tlr9 mRNA levels were assessed by PCR amplification with tlr9 specific primers and Taq DNA polymerase (Invitrogen) and the following cycling conditions: 94 °C for 30 s, 55 °C for 30 s and 68 °C for 1 min for 25 cycles. The β-tubulin gene was amplified as a control using the following cycling conditions: 94 °C for 30 s, 56 °C for 30 s and 68 °C for 1 min for 25 cycles. The primers specific for tlr9 were 5′-GCACAGGAGCGGTGAAGGT-3′ and 5′-GCAGGGGTGCTCAGTGGAG-3′, and the β-tubulin-specific primers are 5′-TGGAATCCTGTGG CATCCA-3′ and ′-TAACAGTCCGCCTAGAA GCA-3′ (Integrated DNA Technologies).
Cell proliferation assay
CH27 B-cell lymphoma (1×105 cells/ml) or splenic B cells (5×105 cells/ml) from BALB/c or C57BL/6 mice were treated for 66 h with varying concentrations of CpG ODN, control GpC ODN, Escherichia coli LPS (Sigma-Aldrich), the NF-κB inhibitor 6-amino-4-(4-phenoxyphenylethyl amino)quinazoline,28,29 phorbol-12-myristate-13-acetate (PMA), ionomycin, or PMA plus ionomycin (EMD Chemicals, Billerica, MA, USA) in the presence of CpG or GpC ODN (7 µg/ml). [3H]-thymidine (1 µCi; MP Biomedicals, Irvine, CA, USA) was added to each well during the last 18 h of incubation. Cells were harvested, and cell-associated radioactivity was measured using a scintillation counter.
Apoptosis assay
CH27 B-cell lymphoma cells (1×105 cells/ml) and splenic B cells (4×105 cells/ml) were incubated with or without 1 or 10 µg/ml GpC or CpG ODN for 24 or 48 h. Apoptotic and necrotic cells were stained using an apoptosis detection kit (Invitrogen), as recommended by the manufacturer, and analyzed using a flow cytometer (FACSCanto; BD Bioscience, San Jose, CA, USA). To neutralize Fas ligand, cells were incubated with anti-Fas ligand mAb (10 µg/ml) (MFL4; BioLegend, San Diego, CA, USA) or an isotype control antibody (Armenian Hamster IgG; BioLegend) in the presence of 1 or 10 µg/ml GpC or CpG ODN for 48 h followed by apoptosis analysis.
TLR9 transfection
TLR9 negative CH27 cells were transfected with pUNO-mTLR9 (InvivoGen, San Diego, CA, USA) by electroporation using a Nucleofection kit (Lonza, Walkersville, MD, USA). After 24 h, the cells were incubated with 1 or 10 µg/ml CpG ODN for 48 h and stained with Alexa Fluor 488-labeled Annexin V (Invitrogen). After fixation and permeabilization, cells were stained with an anti-mouse TLR9 antibody (IMAGENEX, San Diego, CA, USA) and analyzed using a flow cytometer.
Surface expression of Fas and Fas ligand by flow cytometry
CH27 cells (1×105 cells/ml) were incubated with medium alone or 10 µg/ml ODNs at 37 °C for 48 h and were stained with anti-mouse FAS (CD95) antibody (BD Bioscience) plus an Alexa Fluor 405 conjugated secondary antibody (Invitrogen) or a PE-conjugated anti-mouse Fas ligand (CD178) antibody (BD Bioscience).
Assessing NF-κB translocation into the nucleus by immunofluorescence microscopy
Cells (1×106/ml) were incubated with 7 µg/ml ODNs at 37 °C for varying lengths of time, washed with DMEM containing 6 mg/ml bovine serum albumin, and adhered to poly-ℓ-lysine-coated slides (Sigma) for 40 min at 4 °C. Cells were fixed and permeabilized with cold methanol and were incubated with the mAb that was specific for the p65 subunit of NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by an incubation with an Alexa Fluor 546-conjugated secondary antibody (Invitrogen). Cells were also incubated with SYTO-Green (Invitrogen) for 20 min to label the nucleus. Cells were fixed, mounted with Gel Mount (Biomeda, Foster City, CA, USA), and analyzed by confocal microscopy (LSM 510 Zeiss). Control experiments where the primary antibody was omitted showed no significant staining. The immunofluorescence data were quantified by counting cells in five random fields from each slide for each time point from three independent experiments. The number of cells showing translocation of the p65 subunit to the nucleus was counted and expressed as a percentage of the total number of cells in the images.
Western blot analysis
CH27 cells (2×106 cells/ml) and splenic B cells (5×106 cells/ml) were incubated with medium alone, 10 µg/ml ODNs or 1 µg/ml LPS at 37 °C for varying amounts of time. Cells were harvested and lysed in 1% Nonidet P-40 lysis buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 1 mM EGTA, 1 mM Na3VO4, 50 mM NaF and protease-inhibitor cocktail) at 4 °C. Equal amounts of lysates were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and western blotting. Blots were probed with anti-cleaved caspase-3, anti-Bcl-2, anti-Bcl-xl, anti-Bax (Cell Signaling Technology, Danvers, MA, USA), anti-IκBα (Santa Cruz Biotechnology) or anti-c-Myc (Millipore, Bedford, MA, USA) antibodies followed by horseradish peroxidase-conjugated secondary antibodies. The blots were then stripped and reprobed with anti-tubulin antibody (Sigma) and a horseradish peroxidase-conjugated secondary antibody as a loading control.
Statistical analysis
The significance of differences was assessed using Student's t-test for independent population mean values.
Results
CpG ODN inhibits the proliferation of CH27 B-cell lymphoma cells
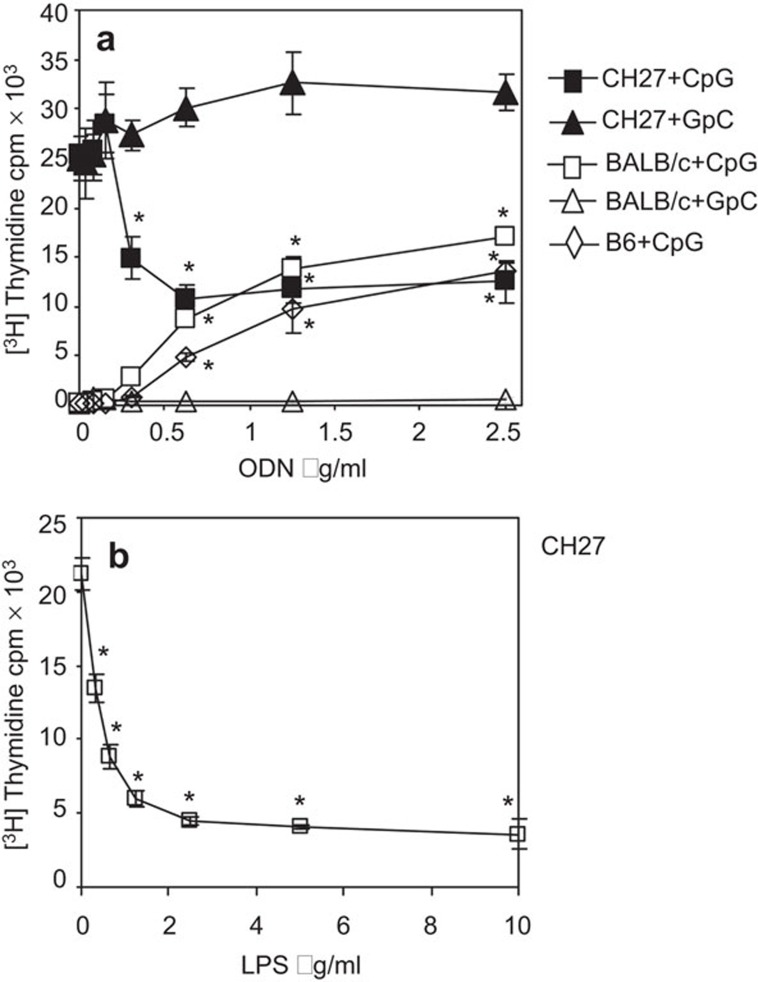
Previous studies have shown that synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODNs) inhibit spontaneous apoptosis of splenic B cells,14 while sensitizing human B-cell chronic lymphocytic leukemia cells to undergo apoptosis.24,25 We sought to compare the effects of CpG ODN on the proliferation of a mouse B-cell lymphoma line, CH27, and murine splenic B cells. The CH27 line was established from lymphatic tumors of 2a4b mice that were repeatedly immunized with sheep red blood cells.30 CH27 cells and splenic B cells from BALB/c and C57BL/6 mice were cultured with varying concentrations of CpG ODN for 66 h, and the rate of cell proliferation was determined using [3H]-thymidine incorporation. An ODN with a GpC motif in place of the CpG motif (GpC ODN) was used as a control. As previously reported,14 there was a dose-dependent increase in the proliferation of splenic B cells from both BALB/c and C57BL/6 upon treatment with CpG ODN, while the control GpC ODN failed to induce splenic B-cell proliferation (Figure 1a). In contrast, CH27 cells, which constitutively proliferate in culture, significantly reduced their proliferation rate as the CpG ODN concentration increased (Figure 1a). When cultured in 0.5 µg/ml CpG ODN, the proliferation rate of CH27 cells was reduced by 60% (Figure 1a). The proliferation rate of CH27 cells did not decrease further at CpG ODN concentrations greater than 0.5 µg/ml. The control GpC ODN did not affect the proliferation of CH27 cells (Figure 1a). Similarly, when treated with LPS, a TLR4 agonist, the proliferation of CH27 cells was significantly inhibited in a concentration-dependent manner, and at 1 µg/ml LPS, proliferation decreased to approximately 30% of the proliferation level of untreated CH27 cells (Figure 1b). These results indicate that CpG ODN and LPS inhibit and stimulate the proliferation of CH27 lymphoma and splenic B cells, respectively.
Figure 1.
Treatment with TLR9 and TLR4 agonists inhibit and stimulate the proliferation of mouse lymphoma and primary B cells, respectively. CH27 cells and splenic B cells from BALB/c and C57BL/6 mice were treated with varying concentrations of CpG ODN or GpC ODN (a), and CH27 cells were treated with varying concentrations of LPS (b) for 66 h. [3H]-thymidine was added to each well for the last 18 h of incubation. Cells were harvested and cell-associated radioactivity was measured using a scintillation counter. Data represent the mean (±s.d.) of triplicate samples. Representative results from three independent experiments are shown. *P<0.01 compared to GpC ODN-treated cells (a) or untreated CD27 cells (b). CpG, cytosyl guanosyl; LPS, lipopolysaccharides; ODN, oligodeoxynucleotide; TLR, Toll-like receptor.
CpG ODN induces apoptosis of CH27 lymphoma B cells
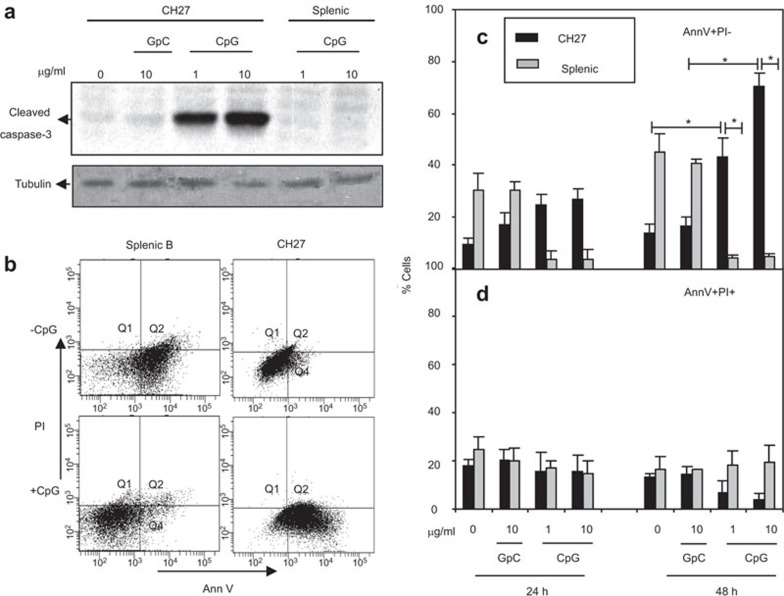
One possible explanation for the growth inhibition of the CH27 B-cell lymphoma line is the induction of apoptosis. To test this hypothesis, we evaluated the levels of activated caspase-3, one of the key mediators of apoptosis. Caspase-3 is activated by the cleavage of its inactive zymogen form (35 kDa) into activated p17 and p12 subunits, which is one of the hallmarks of caspase-mediated apoptosis.31 After treating CH27 and BALB/c splenic B cells with 1 or 10 µg/ml of CpG or control GpC ODN for 24 h, the levels of cleaved caspase-3 were assessed by western blot. CpG ODN but not GpC ODN treatment resulted in increased levels of cleaved caspase-3 in CH27 cells (Figure 2a). In contrast, cleaved caspase-3 was undetectable in splenic B cells under similar treatments. This indicates that CH27 cells undergo caspase-dependent apoptosis in response to CpG ODN.
Figure 2.
CpG ODN induces apoptosis of mouse B-cell lymphoma cells. (a) CH27 cells (2×106 cells/ml) and splenic B cells from BALB/c mice (5×106 cells/ml) were incubated with or without 1 or 10 µg/ml CpG for 24 h. The cells were lysed, and the lysates were analyzed by SDS–PAGE and western blotting and were probed with antibodies specific for cleaved caspase-3. Equal amounts (20 µg proteins/lane) of cell lysate were loaded. Blots were stripped and probed for tubulin as loading controls. Shown are the representative results from four independent experiments. (b–d) CH27 B-cell lymphoma cells and splenic B cells were incubated with 1 or 10 µg/ml GpC and CpG ODNs for 24 and 48 h and stained with PI and AnnV and analyzed by flow cytometry. The representative dot plots of splenic B cells and CH27 cells are shown (b). The percentages of AnnV+ PI− cells (c) and Ann V+ PI+ plus AnnV− PI+ cells (d) are plotted. The mean (±s.d.) from three independent experiments are shown. *P<0.02 compared to GpC ODN-treated CH27 cells and splenic B cells. AnnV, Annexin V; CpG, cytosyl guanosyl; ODN, oligodeoxynucleotide; PAGE, polyacrylamide gel electrophoresis; PI, propidium iodide.
We further determined the timing and prevalence of apoptosis in these cell populations by flow cytometry. CH27 and splenic B cells from BALB/c mice were treated with 1 or 10 µg/ml CpG ODN or control GpC ODN for 24 and 48 h. At the end of the incubation, cells were stained with Alexa Fluor 488-conjugated Annexin V (AnnV) and propidium iodide (PI) and analyzed by flow cytometry. Cells stained with AnnV only (AnnV+ PI−) were considered apoptotic cells, and cells stained with both AnnV and PI (AnnV+ PI+) were considered necrotic cells.32,33 CpG ODN treatment increased the percentage of AnnV+ PI− apoptotic CH27 cells. While less than 20% of GpC ODN-treated cells were apoptotic, CpG ODN increased the percentage of apoptotic CH27 cells to more than 40% at 1 µg/ml and up to 70% at 10 µg/ml after 48 h incubation (Figure 2b and c). In contrast, CpG ODN but not GpC ODN treatment reduced the percentage of apoptotic splenic B cells (Figure 2b and c). The percentages of AnnV+ PI+ necrotic cells were not significantly different between CH27 cells and splenic B cells after 24 or 48 h of treatment with CpG ODN when compared to untreated and GpC ODN-treated cells (Figure 2d). Taken together, these results show that CpG ODN induces apoptosis of CH27 cells but rescues splenic B cells from apoptosis.
The antiproliferative and pro-apoptotic effects of CpG ODN on the CH27 B-cell lymphoma line are TLR9-dependent
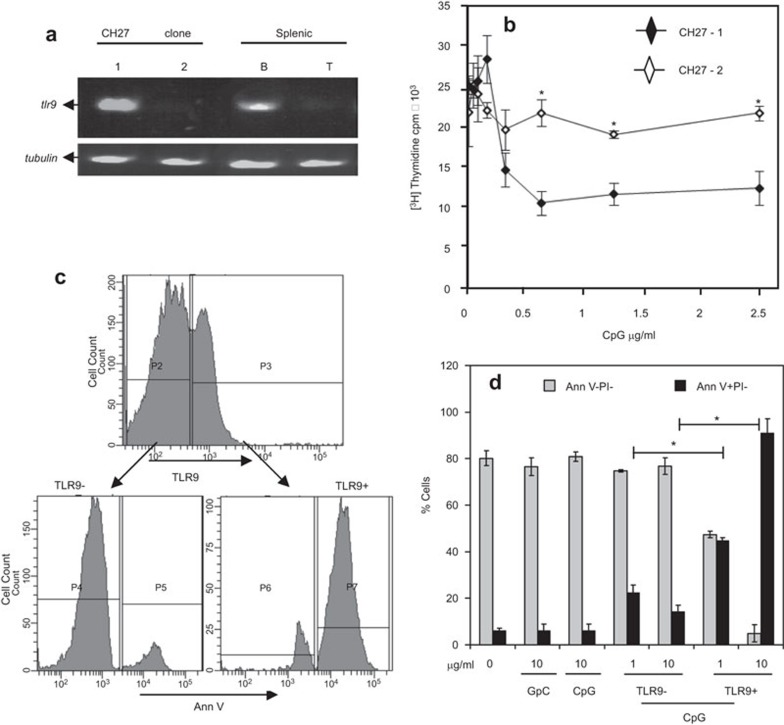
To determine whether the inhibition of cell proliferation and induction of apoptosis of CH27 cells by CpG ODN is dependent on TLR9, we compared the effects of CpG ODN on the proliferation and apoptosis of two different CH27 clones: one that expresses TLR9 and one that does not. TLR9 negative clones were isolated by cloning CH27 cells by limiting dilution and screening for TLR9 expression using RT-PCR. As shown in Figure 3a, CH27 clone 1 and mouse splenic B cells expressed significant levels of tlr9 mRNA, but tlr9 mRNA was undetectable in CH27 clone 2 and splenic T cells. In contrast to the antiproliferative and pro-apoptotic effects of CpG ODN on the tlr9-expressing CH27 clone 1, CpG ODN neither altered the proliferation rate (Figure 3b) nor induced apoptosis (Figure 3d) in CH27 clone 2, which lacks tlr9 mRNA expression. The introduction of mouse TLR9 into CH27 clone 2 by transient transfection caused apoptosis in response to CpG ODN in CH27 cells that expressed TLR9 but not those that underwent transfection and did not express TLR9 (Figure 3c and d). This indicates that CpG ODN inhibits proliferation and induces apoptosis of CH27 cells through TLR9.
Figure 3.
The antiproliferative and pro-apoptotic effects of CpG ODN on CH27 lymphoma B cells are mediated through TLR9. (a) RNA was isolated from two CH27 clones (1 and 2) and splenic B (B) and T (T) cells from BALB/c mice using TRIzol reagent. TLR9 mRNA was detected by RT-PCR. Tubulin mRNA was amplified as a control. (b) CH27 clones 1 and 2 were treated with 1 or 10 µg/ml CpG ODN for 66 h. Cell proliferation was analyzed by [3H]-thymidine incorporation as described in Figure 1. Data represent the mean (±s.d.) of triplicate samples. Representative results from three independent experiments are shown. The P<0.01 is compared to the TLR9-expressing CH27 clone 1. (c and d) CH27 clone 2 cells were transiently transfected with TLR9 cDNA and treated with 10 µg/ml GpC (10 GpC) or CpG (10 CpG) ODN for 48 h. Transfected cells that express TLR9 (TLR9+) or did not express TLR9 (TLR9−) were gated based on staining with TLR9-specific antibody (c). The percentages of Ann V− PI− live cells and Ann V+ PI− apoptotic cells in TLR9+ and TLR9− CH27 clone 2 cells were determined, as described in Figure 2. Shown are the mean (±s.d.) of three independent experiments. *P<0.01 compared to TLR9− CH27 cells. AnnV, Annexin V; CpG, cytosyl guanosyl; ODN, oligodeoxynucleotide; PI, propidium iodide; RT-PCR, reverse transcription polymerase chain reaction; TLR, Toll-like receptor.
Differential effects of CpG ODN on the expression of apoptosis regulatory proteins in CH27 and splenic B cells
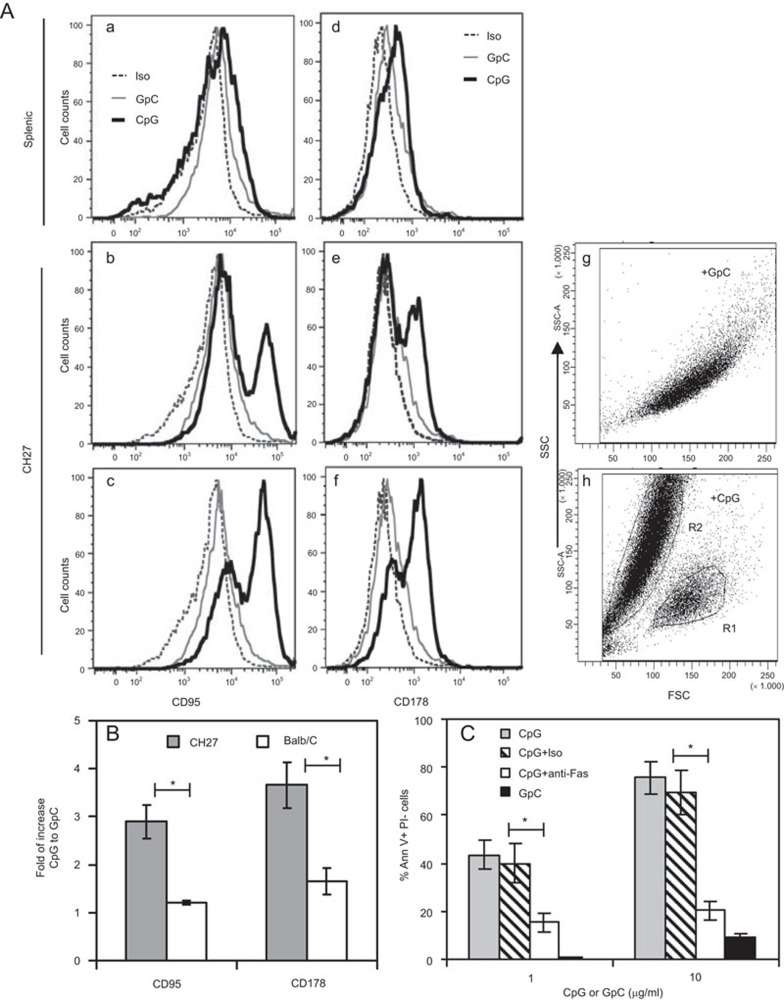
To determine whether CpG ODN induces apoptosis of CH27 cells by regulating the expression of the death receptor, Fas, and its ligand, CH27 cells were treated for 48 h with CpG or control GpC ODN, stained with an antibody specific for Fas (CD95) or Fas ligand (CD178), and analyzed by flow cytometry. CpG ODN treatment resulted in a significant increase in surface expression of both Fas and Fas ligand on CH27 cells (Figure 4A (b), 4A (e) and 4B), but only a slight increase in splenic B cells (Figure 4A (a), 4A (d) and 4B). Cell size analysis using forward scatter plots showed that a population (R2) of CpG ODN-treated CH27 cells decreased in size compared to GpC ODN-treated cells (Figure 4A (g–h)), indicating that these cells were dead or dying. Significantly, these smaller cells exhibited higher Fas and Fas ligand expression than normal size cells (Figure 4A (c) and 4A (f)). To determine if the increases in Fas and Fas ligand expression are involved in CpG ODN-induced apoptosis, CH27 cells were treated with CpG ODN in the presence of the Fas ligand-neutralizing antibody. The Fas ligand-neutralizing antibody but not the control antibody significantly reduced the percentage of apoptotic CH27 cells. These results suggest that CpG ODN-induced apoptosis of CH27 cells depends on the interaction of Fas ligand with Fas.
Figure 4.
CpG ODN-induced apoptosis of CH27 lymphoma B cells depends on Fas and Fas ligand. (A) splenic B cells (a and d) and TLR9 expressing CH27 cells (b–c and e–h) were treated with 10 µg/ml GpC or CpG ODNs for 48 h and stained for CD95 (Fas) or CD178 (Fas ligand). The surface levels of CD95 (a–c) and CD178 (d–f), as well as cell sizes (g–h) were analyzed by flow cytometry. A population of CpG-treated CH27 cells with reduced sizes (R2) were gated, and their CD95 and CD178 expression levels were measured (c and f). Representative flow cytometry plots are shown. (B) The mean (±s.d.) fold increases of the mean fluorescence intensity of CD95 and CD178 in CpG-treated cells compared to GpC-treated cells from three independent experiments are shown. (C) CH27 clone 1 cells were incubated with 1 or 10 µg/ml GpC or CpG ODN in the presence of an isotype control antibody (Iso) or Fas ligand-neutralizing antibody (anti-Fas, 10 µg/ml) for 48 h. The percentages of Ann V+ PI− cells were determined. The mean (±s.d.) of three independent experiments are shown. *P<0.01. AnnV, Annexin V; CpG, cytosyl guanosyl; ODN, oligodeoxynucleotide; PI, propidium iodide; TLR, Toll-like receptor.
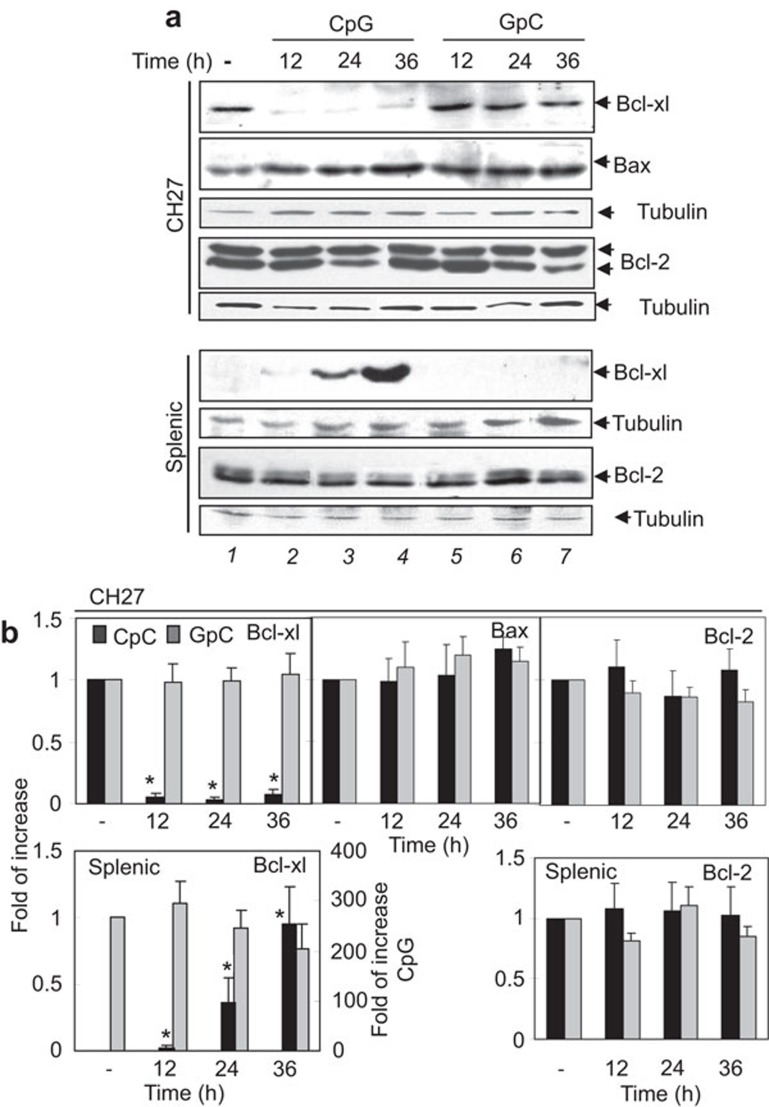
We further examined the effect of CpG ODN on Bcl-2 family protein expression levels in CH27 and splenic B cells by western blot. CH27 cells constitutively expressed high levels of Bcl-xl (Figure 5a, top panel, lane 1; and Figure 5b), which is similar to other B-cell lymphomas that express high levels of Myc.34 CpG ODN treatment significantly decreased Bcl-xl expression in CH27 cells, and this decrease was observed as early as 12 h after CpG ODN treatment (Figure 5a, top panel, lane 2–4; and Figure 5b). In contrast, freshly isolated naive splenic B cells had no detectable Bcl-xl and significantly increased their Bcl-xl expression levels after 24 and 36 h of CpG ODN treatment (Figure 5a, 6th panel, lane 1–4; and Figure 5b). CpG ODN treatment did not result in any significant change in Bcl-2 and Bax protein levels in both CH27 (Figure 5a, 2nd and 4th panel; and Figure 5b) and splenic B cells (Figure 5a, 8th panel; Figure 5b; and data not shown). This indicates that CpG ODN differentially regulates the expression levels of the anti-apoptotic protein Bcl-xl in CH27 and splenic B cells, contributing to the different fates of these two types of B cells.
Figure 5.
Effects of CpG ODN treatment on Bcl-xl, Bcl-2 and Bax expression levels in B-cell lymphoma cells and primary B cells. CH27 clone 1 and splenic B cells from BALB/c mice were cultured in the absence or presence of 10 µg/ml CpG or GpC ODN for 12, 24 and 36 h. The cells were lysed, and the lysates were analyzed by SDS–PAGE and western blotting. Blots were probed with anti-Bcl-xl, Bcl-2, or anti-Bax antibody. Equal amounts (30 µg proteins/lane) of the cell lysates were loaded. Blots were stripped and probed for tubulin as loading controls. The relative amount of Bcl-xl, Bax and Bcl-2 protein was quantified by densitometry and normalized to the relative amount of tubulin in the same sample. The fold increases of Bcl-xl, Bax and Bcl-2 compared to untreated cells were determined. Representative blots (a) and mean values (±s.d.) from quantification (b) from three independent experiments are shown. *P<0.01 compared to untreated cells. CpG, cytosyl guanosyl; ODN, oligodeoxynucleotide; PAGE, polyacrylamide gel electrophoresis; TLR, Toll-like receptor.
Differential effects of CpG ODN on NF-κB activation in CH27 B and splenic B cells
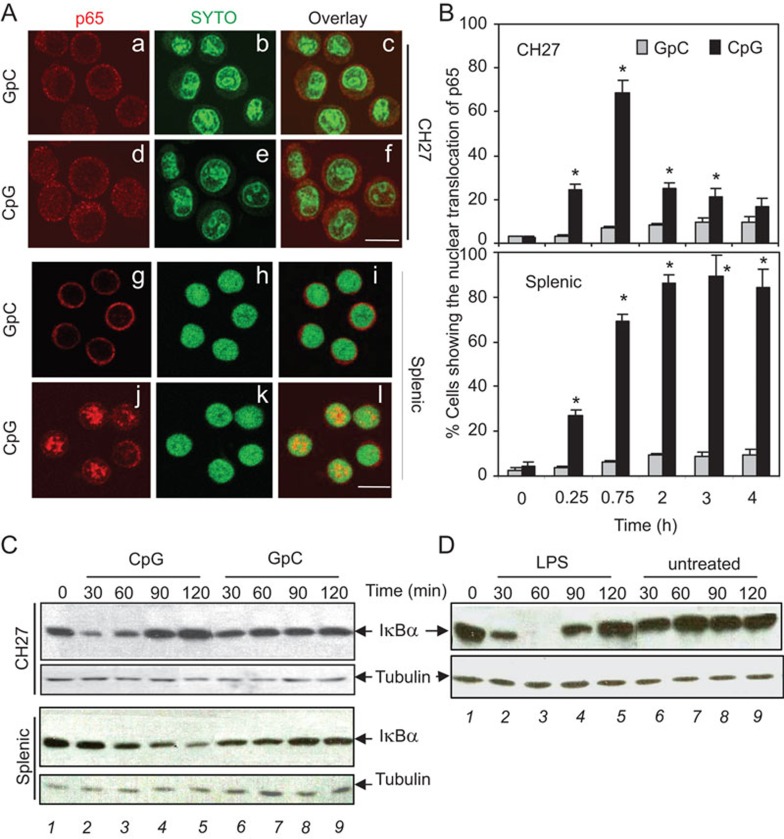
NF-κB is the primary downstream target of TLR9 signaling pathway11,16 and is a key regulator of B cell fate.35 Next, we tested whether CpG ODN treatment affects NF-κB activation in CH27 and splenic B cells by assessing the nuclear translocation of the NF-κB p65 subunit and the degradation of the inhibitory component of NF-κB, IκBα. Nuclear translocation of NF-κB p65 was visualized by immunofluorescence microscopy. CH27 and splenic B cells were treated with CpG ODN (7 µg/ml) or control GpC ODN for varying lengths of time. At each time point, the cells were fixed, permeabilized, and stained with a mAb specific for the p65 subunit of NF-κB and a DNA dye. The basal level of nuclear NF-κB p65 was minimal in both CH27 and splenic B cells (Figure 6A(a–c), 6A (g–i), and Figure 6B). The percentage of cells with nuclear p65 staining increased in both B-cell types after 15 and 45 min of CpG ODN treatment (Figure 6B). The percentage of splenic B cells with nuclear NF-κB increased throughout the time course, reaching approximately 80% after 120 min of CpG ODN treatment and remaining at approximately 80% until 4 h post-treatment (Figures 6A (j–l) and 6B). However, the percentage of CH27 cells with nuclear NF-κB decreased from approximately 70% at 45 min to approximately 20% at 120 min post-CpG ODN-treatment and dropped below 20% by 4 h post-treatment (Figure 6A (d–f) and 6B).
Figure 6.
Effects of CpG ODN on NF-κB activation in a B-cell lymphoma line and primary B cells. (A) CH27 clone 1 (a–f) and splenic B cells from BALB/c mice (g–l) were treated with CpG or GpC ODN (7 µg/ml) for various amounts of time at 37 °C. The cells were then fixed, permeabilized and incubated with a mAb specific for the p65 subunit of NF-κB and SYTO-green for the nucleus. Images were acquired using a confocal fluorescence microscope. Representative images of cells after 120 min of exposure to ODNs are shown. Bar, 10 µm. (B) Quantitative analysis of the immunofluorescence images is shown. One hundred cells were counted from five random fields of each time point from three independent experiments. The percentages of cells showing nuclear translocation of the p65 subunit of NF-κB upon exposure to CpG or GpC ODN were plotted. The mean values (±s.d.) from three independent experiments are shown. *P<0.01 compared to GpC ODN-treated cells. (C–D) CH27 clone 1 and splenic B cells were treated with 10 µg/ml CpG and GpC ODN (C), or CH27 clone 1 cells were treated with or without 1 µg/ml LPS (D) for various times. Cell lysates were analyzed using SDS–PAGE and western blot that was probed for IκBα. Equal amounts (30 µg proteins/lane) of cell lysates were loaded. The experiment was repeated more than three times with similar results. CpG, cytosyl guanosyl; LPS, lipopolysaccharide; mAb, monoclonal antibody; NF, nuclear factor; ODN, oligodeoxynucleotide; PAGE, polyacrylamide gel electrophoresis.
The degradation of the NF-κB inhibitor, IκBα, was measured by western blot. CpG ODN treatment induced a continuous decrease of IκBα in splenic B cells up to 120 min, the final time point tested (Figure 6C, 3rd panel, lane 1–5). However, similarly treated CH27 cells had a transient decrease in IκBα levels at 30 min (Figure 6C, top panel, lane 2) that returned to basal levels by 90 min (Figure 6C, top panel, lane 4). The control GpC ODN did not alter the IκBα protein levels in either cell type (Figure 6C, lane 6–9). Similarly, transient decreases in the IκBα level occurred at 30–60 min in LPS-treated CH27 cells (Figure 6D, lane 2–3), but IκBα returned to basal levels by 120 min (Figure 6D, lane 5). These results show that the TLR agonists CpG ODN and LPS induce a transient activation of NF-κB in CH27 cells, which is in contrast to a relatively sustained increase of NF-κB activation in splenic B cells.
Effects of NF-κB activators and inhibitors on the proliferation of the CH27 B-cell lymphoma line
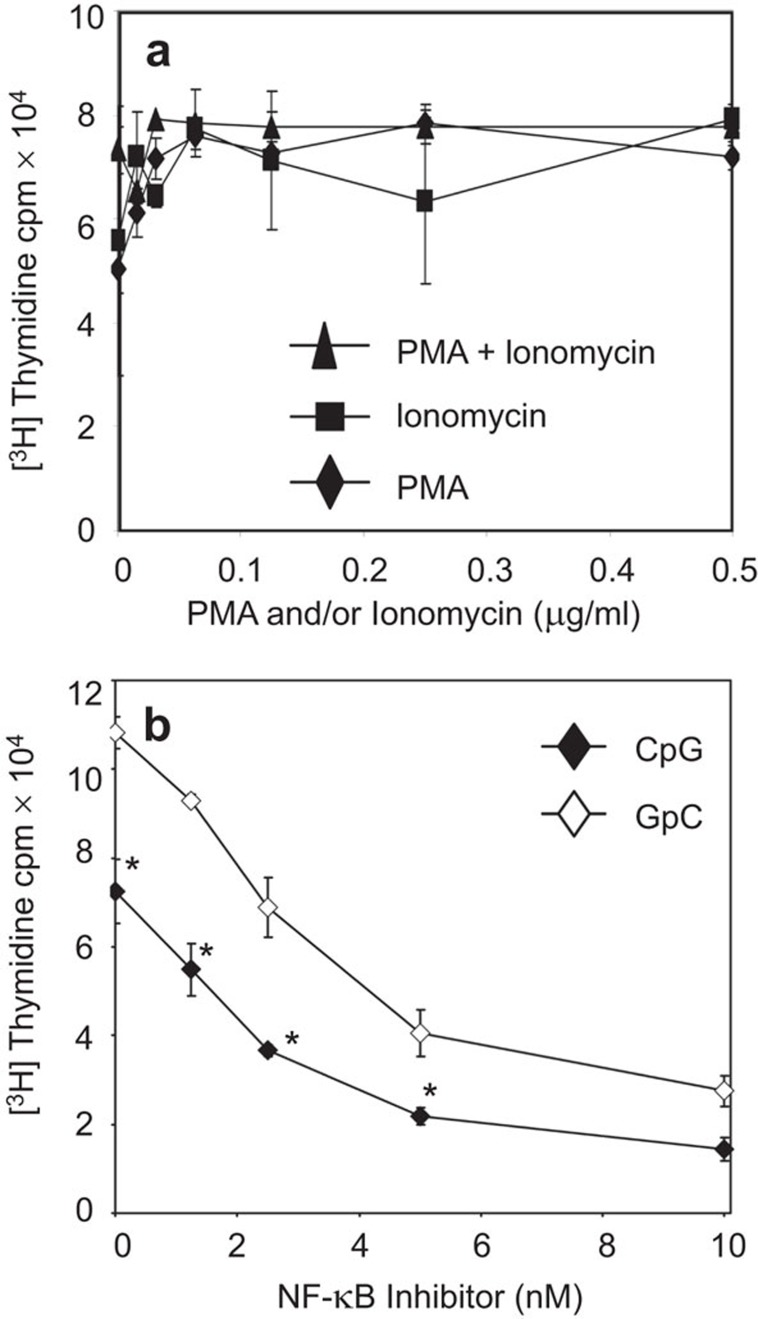
CpG ODN treatment of CH27 cells induced a transient activation of NF-κB, which was followed by a decrease in cell proliferation and an increase in apoptosis. To examine the role of NF-κB in the CpG ODN-induced apoptosis of CH27 cells, we assessed the effect of NF-κB activators and an activation inhibitor on CH27 cell proliferation. In B cells, protein kinase C is the primary upstream activator of NF-κB.36 To activate NF-κB, we treated CH27 cells with varying concentrations of the protein kinase C activators phorbol myristate acetate (PMA), ionomycin, and a combination of the two. To inhibit CpG ODN-induced transient NF-κB activation, CH27 cells were treated with various concentrations of the NF-κB activation inhibitor 6-amino-4-(4-phenoxyphenylethylamino) quinazoline in the presence of CpG or GpC ODN. The protein kinase C activators slightly increased the proliferation rate of CH27 cells (Figure 7a). The combination treatment of CpG ODN and the NF-κB inhibitor induced a stronger inhibition of CH27 proliferation than CpG ODN alone or NF-κB inhibitor plus GpC ODN (Figure 7b). This suggests that CpG ODN-induced CH27 cell apoptosis is associated with the inhibition but not the transient activation of NF-κB.
Figure 7.
Effects of NF-κB activators and inhibitor on proliferation of lymphoma B cells. (A) CH27 clone 1 cells were treated with varying concentrations of PMA, ionomycin, or a combination of PMA and ionomycin in the absence of CpG or GpC ODN. (B) CH27 clone 1 cells were treated with an NF-κB inhibitor in the presence of CpG or GpC ODN (7 µg/ml). The rate of cell proliferation was determined as described in Figure 1. Data represent the mean (±s.d.) of triplicate experiments. Representative results from three independent experiments are shown. *P<0.01 compared to GpC ODN-treated cells. CpG, cytosyl guanosyl; NF, nuclear factor; ODN, oligodeoxynucleotide; PMA, phorbol-12-myristate-13-acetate.
Differential effects of CpG ODN on c-Myc expression in the CH27 B-cell lymphoma line and splenic B cells
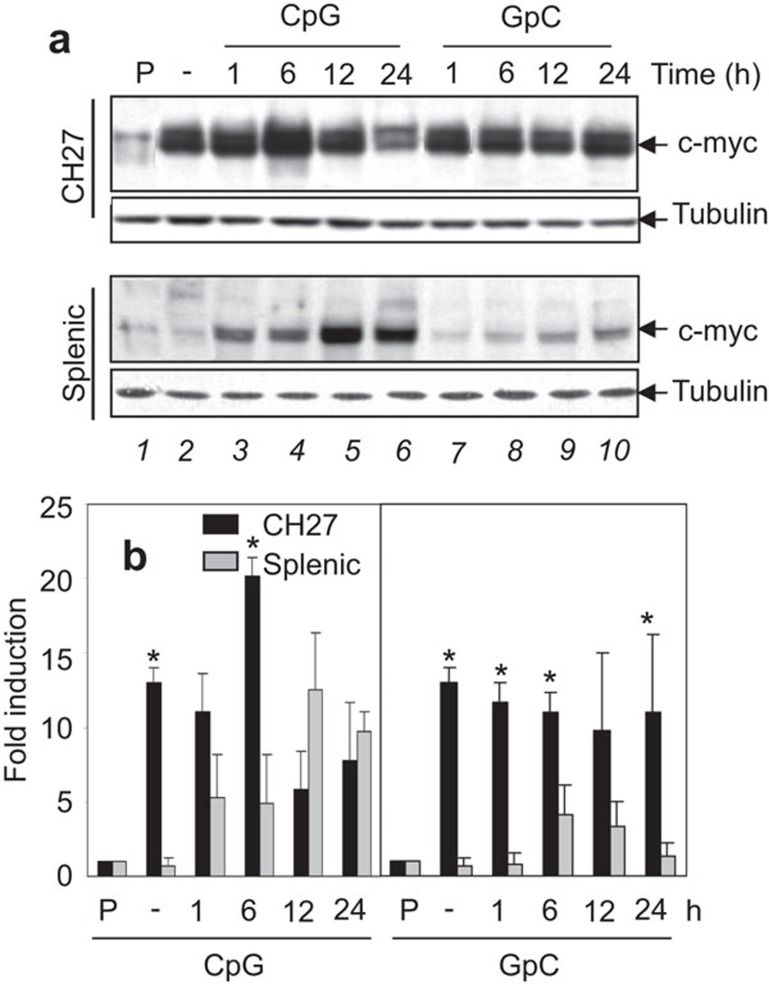
c-Myc is a key regulator in cell proliferation and survival. CpG DNA has been reported to induce an increase in c-Myc protein levels, conferring a proliferating effect on splenic B cells.15 To compare the effect of CpG DNA treatment on c-Myc expression in CH27 and splenic B cells, cells were treated with 10 µg/ml CpG ODN or control GpC ODN for 1, 6, 12 and 24 h, and the lysates were analyzed by Western blot to determine c-Myc protein levels. As observed in some B lymphomas,37 a significantly higher level of constitutive c-Myc protein expression was observed in unstimulated CH27 cells (Figure 8a, top panel, lane 2; and Figure 8b) compared to the resting splenic B cells (Figure 8a, 3rd panel, lane 2; and Figure 8b). CpG ODN-treated CH27 cells exhibited increased c-Myc protein levels at 6 h, which decreased to the constitutive expression level at 12 h (Figure 8a, top panel, lane 2–6; and Figure 8b). CpG ODN-treated splenic B cells showed an increase in c-Myc expression at 1 h that continued to increase over time and reached a level similar to the constitutive level of CH27 cells at 12 h after exposure to CpG ODN (Figure 8a, 3rd panel, lane 2–6; and Figure 8b). Control GpC ODN had no major effect on the expression of c-Myc in either cell type (Figure 8a, lane 7–10; and Figure 8b). Thus, the CH27 B cell lymphoma line constitutively expressed a high level of c-Myc, and CpG ODN treatment induced a transient increase at 6 h; in contrast, a sustained increase of c-Myc expression occurred in primary splenic B cells.
Figure 8.
Effects of CpG DNA on c-Myc expression in lymphoma and primary B cells. (a) CH27 lymphoma B cell clone 1 and splenic B cells from BALB/c mice were treated with or without (−) 10 µg/ml of GpC or CpG ODNs for specified times. Cells were then lysed, and the lysates were analyzed by SDS–PAGE and Western blotting, probing for c-Myc. A representative blot of three independent experiments is shown. P, a control of HL-60 cell lysates. (b) The relative amount of c-Myc protein expressed in CH27 clone 1 and splenic B cells was quantified by densitometry and normalized to the relative amount of tubulin in the same sample. The results were plotted as the fold of densitometric value of the c-Myc-positive control sample. Average results from three independent experiments are shown. *P<0.01 compared to splenic B cells. CpG, cytosyl guanosyl; ODN, oligodeoxynucleotide; PAGE, polyacrylamide gel electrophoresis.
Discussion
Recent studies have shown that the binding of ligands to TLR2, 3, 4, 6 and 9 can lead to apoptosis of cancer cells and bacterially infected macrophages.19,24,25,38,39 However, the cellular mechanisms underlying the induction of apoptosis remain elusive. By comparing the effects of the TLR9 agonist CpG ODN on a mouse B-cell lymphoma line with those on naive mouse splenic B cells, we found that CpG ODN induced apoptosis in the B-cell lymphoma line CH27, which constitutively expresses Bcl-xl and c-Myc, but promotes the proliferation of splenic B cells. CpG ODN-induced apoptosis of CH27 cells is dependent on the expression of TLR9 and the interaction of Fas and Fas ligand and is associated with aborted NF-κB activation of and a decrease in Bcl-xl expression.
The finding that CpG ODN induces apoptosis of a mouse B-cell lymphoma line, CH27, corroborates recent reports that TLR9 ligands induce apoptosis in B cells from patients with B-CLL.24,25 Our study further supports a pro-apoptotic effect of TLR9 ligands on TLR9-expressing tumor cells. The CH27 murine B-cell lymphoma line was generated in mice immunized with sheep red blood cells30 and expresses CD5 and mIgM specific for phosphocholine, which is similar to the phenotype of B-CLL cells.26,40,41 Surface immunoglobulins (Igs) of most B-CLL cells react to self-antigens, such as DNA, phosphatidylcholine and Igs.42,43 Here, we show that CH27 cells constitutively express high levels of Bcl-xl and c-Myc, two genes that are commonly deregulated and translocated to the IgH locus in B-cell cancers, such as human Burkitt's lymphoma.34,44,45 Even though it is rare, c-Myc has also been shown to be constitutively expressed in B-CLL cells.46 Because CH27 cells have properties similar to human B-cell lymphomas, this cell line has the potential to become an in vitro model in understanding the mechanisms underlying the pro-apoptotic function of TLR9 on B-cell cancers.
This study provides strong evidence that CpG ODN-induced apoptosis is mediated by TLRs. Only ODN that contain the CpG motif and is known to bind to TLR9, but not ODN that has an inverted CpG motif (GpC), exhibit pro-apoptotic effects. Furthermore, CpG ODN induces apoptosis of TLR9-expressing CH27 cells but had no effect on the proliferation and apoptosis of CH27 cells that do not express TLR9. Introducing TLR9 to TLR9-negative CH27 cells restores their apoptotic response to CpG ODN. In addition, our results show that the TLR4 agonist LPS also can induce apoptosis of CH27 cells, indicating that the pro-apoptosis effect is not limited to TLR9. This is similar to the finding by Gruppi et al.47 that LPS increases the sensitivity of B cells to Fas/Fas ligand mediated apoptosis. These data suggest that common signaling cascades downstream of TLRs are responsible for the induction of apoptosis of CH27 cells, and the TLR signaling pathways in CH27 cells are likely to be different from those in splenic B cells. Indeed, recent studies have found mutations in MyD88, the key signaling adaptor protein of TLRs, in human cancer B cells.48,49 While it remains to be determined whether or not TLR signaling components in CH27 cells are mutated, the induction of CH27 apoptosis by TLR agonists implicates their therapeutic potential in treating TLR-expressing B-cell lymphomas.
To further understand how CpG ODN induces apoptosis of CH27 cells, we assessed the effects of CpG ODN treatment on the levels of cleaved executioner caspase 3, Fas and Fas ligand, and the Bcl-2 family proteins Bcl-2, Bcl-xl and Bax that regulate mitochondrial stability. This study shows that CpG ODN induces caspase-3 cleavage in CH27 cells but not in splenic B cells, suggesting that CpG ODN-induced apoptosis is mediated by a caspase-dependent pathway in CH27 cells. Furthermore, CH27 cells treated with CpG ODN show increased levels of both Fas and Fas ligand on the surface of dying cell populations, and Fas ligand-neutralizing antibody reduces the percentage of apoptotic CH27 cells to a level close to that of cells treated with GpC ODN. These results indicate that the Fas/Fas ligand pathway is the primary mechanism that mediates CpG ODN-induced CH27 cell apoptosis. In addition, CpG ODN treatment drastically decreased Bcl-xl protein levels in CH27 cells, while increasing Bcl-xl protein levels in naive primary B cells. This result suggests that mitochondrial destabilization induced by a decrease in Bcl-xl expression may also contribute to CH27 apoptosis induced by CpG ODN. We did not detect any significant effect of CpG ODN treatment on Bcl-2 or Bax protein levels in CH27 cells. The functional redundancy between Bcl-xl and Bcl-2 might account for the lack of change in Bcl-2 protein levels in CH27 cells undergoing CpG ODN-induced apoptosis. Unaltered Bax levels do not exclude it from playing a role in CpG ODN-induced apoptosis, because the activation of Bax during apoptosis generally does not require an increase in its expression level.50 In addition to Bcl-xl, other members of Bcl-2 family,51 could also play roles in CpG ODN-induced apoptosis of CH27 cells.
NF-κB is a key TLR-activated transcription factor8 and a primary regulator of c-Myc expression.52,53 Other than activating the transcription of c-Myc, NF-κB can induce the transcription of anti-apoptotic genes, such as Bcl-xl,54,55 which promote cell survival. The deficiency of Rel-A/p65, a member of the NF-κB family expressed in B cells, increases cell sensitivity to TNF-mediated apoptosis,56 indicating a role for NF-κB in suppressing apoptosis in B cells. The c-Myc protein is expressed from immature stages of B-cell development,57 and its expression level increases upon stimulation.58 c-Myc has long been known as an inducer of apoptosis.59,60 Overexpression of c-Myc induces apoptosis in fibroblasts and myeloid cell lines in the absence of stimuli.60,61 c-Myc sensitizes cells to TNF-mediated apoptosis by inhibiting NF-κB activation.62 The expression of c-Myc is also important for cell proliferation. c-Myc has been found to be constitutively activated or expressed in a number of human B-cell lymphomas via gene translocation, which has been implicated in tumorigenesis.44,57 The relative activation and expression levels of NF-κB and c-Myc have been shown to be critical for controlling the balance between cell proliferation and apoptosis.63,64 c-Myc-induced cell proliferation accompanied by impaired activation of NF-κB and insufficient levels of anti-apoptotic signals leads to apoptosis,65,66,67 while overexpression of the p65 subunit of NF-κB rescues cells from c-Myc-dependent cell death.62 Therefore, cooperation between NF-κB and c-Myc is crucial to cell fate.
Our study shows that CH27 cells constitutively express a high level of c-Myc independent of NF-κB activation and that CpG ODN and LPS induce a transient activation of NF-κB, which contrasts sharply with the steady increase of NF-κB activity in naive splenic B cells. While the mechanism of this transient NF-κB activation is not defined, c-Myc has been shown to induce apoptosis by inhibiting NF-κB activation.62 Based on the discussion above, impaired NF-κB activation plus a constitutively high level of c-Myc could cause the downregulation of Bcl-xl and the upregulation of Fas and Fas ligand expression in CpG ODN-treated CH27 cells. Our findings that the activation NF-κB using protein kinase C activators increases CH27 cell proliferation and the inhibition NF-κB activation further enhances the inhibitory effect of CpG ODN on CH27 cell proliferation, further supporting the idea that the CH27 cell proliferation is partially dependent on NF-κB activation and that CpG ODN-induced apoptosis of CH27 cells is, at least in part, the result of hypoactivation of NF-κB.
Our data clearly show that CpG ODN-induced CH27 cell apoptosis is dependent on Fas and the Fas ligand and is associated with the increase of Fas and Fas ligand surface expression. Both c-Myc and NF-κB are involved in the regulation of Fas and Fas ligand expression. The expression of c-Myc is associated with an increase in Fas and Fas ligand expression. B cell-specific c-myc gene deletion reduces stimulus-induced expression of Fas and the Fas ligand and increases resistance to Fas ligand-induced apoptosis.68 In contrast, NF-κB appears to suppress Fas-mediated apoptosis. While the regulation of Fas/Fas ligand expression by NF-κB has not been well studied in B cells, it has been shown that NF-κB activation suppresses Fas-mediated apoptosis in T cells and macrophages.69,70 These data support our hypothesis that the transient activation of NF-κB and the persistently high c-Myc levels create a situation where c-Myc-induced expression of Fas and the Fas ligand in the absence of sufficient anti-apoptotic signals from NF-κB culminates in apoptosis.
Cellular alterations in apoptosing CH27 cells are very similar to what was previously observed in apoptosing WEHI 231 B cells, a widely used B cell model for apoptosis research.71 WEHI 231 is a B-cell line that behaves like immature B cells and undergoes apoptosis in response to anti-IgM antibody.72,73 The cross-linking of surface IgM causes transient activation and expression of NF-κB and c-Myc, respectively, followed by decreases of both in WEHI cells.74,75 However, CpG ODN rescues WEHI cells from anti-IgM antibody-induced apoptosis.74,76 This rescue has been linked to the sustained activation of NF-κB and expression of c-Myc induced by CpG ODN,74 similar to what we observed in CpG ODN-treated splenic B cells. This suggests that apoptosis of both CH27 and WEHI B cells may be mediated by similar downstream signaling cascades, even though apoptosis is induced by different receptors.
Our results suggest that TLR-induced apoptosis of CH27 cells is associated with the imbalanced activation of NF-κB and c-Myc, but our results do not exclude the involvement of other mechanisms. CpG ODN has been shown to cause apoptosis of human B-CLL cells by inducing autocrine IL-10 and IL-10-activated JAK/STAT pathway.24 CH27 cells are B-1 B cells. Normal B-1 cells increase IL-10 and Fas ligand expression in response to TLR activation.77 However, the induction of IL-10 autocrine signaling in both normal B cells and B-CLL cells has been shown to be NF-κB-dependent.24,78 It is unclear whether the transient activation of NF-κB in CpG ODN-treated CH27 cells is sufficient to induce a significant amount of IL-10. TLRs are known to induce the activation of the MAP kinase JNK. JNK and its downstream transcription factors have been shown to be required for the survival of lymphoma B cells.79,80,81 However, CpG ODN does not appear to have any effect on JNK phosphorylation (data not shown) in CH27 cells. Therefore, JNK may not be a major contributor of CH27 cell apoptosis. Previous studies have shown that anti-IgM antibody-induced apoptosis of WEHI B cells is dependent on an increased production of ceramide.82,83 Ceramide, a lipid secondary messenger that induces apoptosis,84 has been suggested to act upstream of Bcl-xl with protein kinase C in WEHI B cells.82 Because ceramide has been shown to be involved in apoptosis induced by many stimuli including Fas and Fas ligand interaction,85 it is expected to contribute to CpG ODN-induced apoptosis as well, potentially through Fas and/or TLR-induced or lymphoma B-cell intrinsic alterations in ceramide metabolism.
While the molecular mechanism by which CpG ODN induces NF-κB activation via TLRs has been well studied, why TLRs in CH27 cells fail to induce sustained NF-κB activation remains unclear. Such a failure is likely to be associated with high levels of basal signaling activities in cancer B cells, such as the constitutively high expression levels of c-Myc and Bcl-xl in CH27 cells. Because different cancer B cells have different genetic mutations and translocations, they will respond to TLRs differently, and apoptosis will be triggered through different pathways in different cancer cells. Further studies on the responses of different cancer B cells to TLR signaling will increase our understanding of their oncogenic mechanisms and allow us to match treatments with the cancer genotype.
Acknowledgments
This work was funded by the National Institute of Health, USA (AI059617). The funding agency had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. K-H Kim, Dr. S Janz and Dr. J Zheng and the imaging core and flow cytometry core of the Maryland Pathogenesis Research Institute for technical assistance.
References
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2 8:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Burge C, Clayton DA, Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proc Natl Acad Sci U S A. 1994;91 9:3799–3803. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374 6522:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168 9:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5 10:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32 7:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4 7:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Hacker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, Wagner H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J Exp Med. 2000;192 4:595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412 6844:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Yi AK, Yoon JG, Krieg AM. Convergence of CpG DNA- and BCR-mediated signals at the c-Jun N-terminal kinase and NF-kappaB activation pathways: regulation by mitogen-activated protein kinases. Int Immunol. 2003;15 5:577–591. doi: 10.1093/intimm/dxg058. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164 2:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996;93 7:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998;160 12:5898–5906. [PubMed] [Google Scholar]
- Yi AK, Hornbeck P, Lafrenz DE, Krieg AM. CpG DNA rescue of murine B lymphoma cells from anti-IgM-induced growth arrest and programmed cell death is associated with increased expression of c-myc and bcl-xL. J Immunol. 1996;157 11:4918–4925. [PubMed] [Google Scholar]
- Yi AK, Peckham DW, Ashman RF, Krieg AM. CpG DNA rescues B cells from apoptosis by activating NFkappaB and preventing mitochondrial membrane potential disruption via a chloroquine-sensitive pathway. Int Immunol. 1999;11 12:2015–2024. doi: 10.1093/intimm/11.12.2015. [DOI] [PubMed] [Google Scholar]
- Haase R, Kirschning CJ, Sing A, Schrottner P, Fukase K, Kusumoto S, et al. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J Immunol. 2003;171 8:4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, et al. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285 5428:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176 8:4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5 6:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Antitumor applications of stimulating Toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6 2:88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, et al. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 2001;167 9:4878–4886. doi: 10.4049/jimmunol.167.9.4878. [DOI] [PubMed] [Google Scholar]
- Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clin Cancer Res. 2003;9 7:2693–2700. [PubMed] [Google Scholar]
- Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, et al. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115 24:5041–5052. doi: 10.1182/blood-2009-03-213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrsdorfer B, Wooldridge JE, Blackwell SE, Taylor CM, Griffith TS, Link BK, et al. Immunostimulatory oligodeoxynucleotides induce apoptosis of B cell chronic lymphocytic leukemia cells. J Leukoc Biol. 2005;77 3:378–387. doi: 10.1189/jlb.0604373. [DOI] [PubMed] [Google Scholar]
- Haughton G, Arnold LW, Bishop GA, Mercolino TJ. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Jelachich ML, Grusby MJ, Clark D, Tasch D, Margoliash E, Pierce SK. Synergistic effects of antigen and soluble T-cell factors in B-lymphocyte activation. Proc Natl Acad Sci U S A. 1984;81 17:5537–5541. doi: 10.1073/pnas.81.17.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Fukazawa T, et al. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg Med Chem. 2003;11 3:383–391. doi: 10.1016/s0968-0896(02)00440-6. [DOI] [PubMed] [Google Scholar]
- Baader E, Toloczko A, Fuchs U, Schmid I, Beltinger C, Ehrhardt H, et al. Tumor necrosis factor-related apoptosis-inducing ligand-mediated proliferation of tumor cells with receptor-proximal apoptosis defects. Cancer Res. 2005;65 17:7888–7895. doi: 10.1158/0008-5472.CAN-04-4278. [DOI] [PubMed] [Google Scholar]
- Pennell CA, Arnold LW, Lutz PM, LoCascio NJ, Willoughby PB, Haughton G. Cross-reactive idiotypes and common antigen binding specificities expressed by a series of murine B-cell lymphomas: etiological implications. Proc Natl Acad Sci U S A. 1985;82 11:3799–3803. doi: 10.1073/pnas.82.11.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996;93:2239. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31 1:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Laszlo DJ, Noble PW, Weinstein L, Riches DW, Henson PM. Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. J Immunol. 1993;151 8:4274–4285. [PubMed] [Google Scholar]
- Kelly PN, Grabow S, Delbridge AR, Strasser A, Adams JM. Endogenous Bcl-xL is essential for Myc-driven lymphomagenesis in mice. Blood. 2011;118 24:6380–6386. doi: 10.1182/blood-2011-07-367672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5 6:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41 6–7:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6 8:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- Into T, Kiura K, Yasuda M, Kataoka H, Inoue N, Hasebe A, et al. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kappa B activation. Cell Microbiol. 2004;6 2:187–199. doi: 10.1046/j.1462-5822.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- Ruckdeschel K, Pfaffinger G, Haase R, Sing A, Weighardt H, Hacker G, et al. Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J Immunol. 2004;173 5:3320–3328. doi: 10.4049/jimmunol.173.5.3320. [DOI] [PubMed] [Google Scholar]
- Mercolino TJ, Arnold LW, Haughton G. Phosphatidyl choline is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J Exp Med. 1986;163 1:155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligaris-Cappio F. B-chronic lymphocytic leukemia: a malignancy of anti-self B cells. Blood. 1996;87 7:2615–2620. [PubMed] [Google Scholar]
- Sthoeger ZM. Production of autoantibodies by CD5-expressing B lymphocytes from patients with chronic lymphocytic leukemia. J Exp Med. 1989;169:255. doi: 10.1084/jem.169.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MR, Cohen HJ, Huntley CC, Waite BM, Spees L, Spurr CL. A monoclonal IgM with antibodylike specificity for phospholipids in a patient with lymphoma. Blood. 1974;43 4:493–504. [PubMed] [Google Scholar]
- Gelmann EP, Psallidopoulos MC, Papas TS, Dalla-Favera R. Identification of reciprocal translocation sites within the c-myc oncogene and immunoglobulin mu locus in a Burkitt lymphoma. Nature. 1983;306 5945:799–803. doi: 10.1038/306799a0. [DOI] [PubMed] [Google Scholar]
- Schlaifer D, March M, Krajewski S, Laurent G, Pris J, Delsol G, et al. High expression of the bcl-x gene in Reed-Sternberg cells of Hodgkin's disease. Blood. 1995;85 10:2671–2674. [PubMed] [Google Scholar]
- Put N, Van Roosbroeck K, Konings P, Meeus P, Brusselmans C, Rack K, et al. Chronic lymphocytic leukemia and prolymphocytic leukemia with MYC translocations: a subgroup with an aggressive disease course. Ann Hematol. 2012;91 6:863–873. doi: 10.1007/s00277-011-1393-y. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Craxton A, Hendricks DW, Merino MC, Montes CL, Clark EA, et al. BAFF and LPS cooperate to induce B cells to become susceptible to CD95/Fas-mediated cell death. Eur J Immunol. 2007;37 4:990–1000. doi: 10.1002/eji.200636698. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470 7332:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Kim Y, Lee JH, Kim YS.MYD88 expression and L265P mutation in diffuse large B-cell lymphoma. Hum Pathol 2013. in press [DOI] [PubMed]
- Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM. Mitochondria as the target of the pro-apoptotic protein Bax. Biochim Biophys Acta. 2006;1757 9–10:1301–1311. doi: 10.1016/j.bbabio.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Duyao MP, Kessler DJ, Spicer DB, Bartholomew C, Cleveland JL, Siekevitz M, et al. Transactivation of the c-myc promoter by human T cell leukemia virus type 1 tax is mediated by NF kappa B. J Biol Chem. 1992;267 23:16288–16291. [PubMed] [Google Scholar]
- Grumont RJ, Strasser A, Gerondakis S. B cell growth is controlled by phosphatidylinosotol 3-kinase-dependent induction of Rel/NF-kappaB regulated c-myc transcription. Mol Cell. 2002;10 6:1283–1294. doi: 10.1016/s1097-2765(02)00779-7. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Kamalakaran S. Pro-apoptotic role of NF-kappaB: implications for cancer therapy. Biochim Biophys Acta. 2006;1766 1:53–62. doi: 10.1016/j.bbcan.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441 7092:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Prendes M, Zheng Y, Beg AA. Regulation of developing B cell survival by RelA-containing NF-kappa B complexes. J Immunol. 2003;171 8:3963–3969. doi: 10.4049/jimmunol.171.8.3963. [DOI] [PubMed] [Google Scholar]
- Rui L, Goodnow CC. Lymphoma and the control of B cell growth and differentiation. Curr Mol Med. 2006;6 3:291–308. doi: 10.2174/156652406776894563. [DOI] [PubMed] [Google Scholar]
- Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983;35 3 Pt 2:603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Askew DS, Ashmun RA, Simmons BC, Cleveland JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6 10:1915–1922. [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69 1:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Thompson EB. The many roles of c-Myc in apoptosis. Annu Rev Physiol. 1998;60:575–600. doi: 10.1146/annurev.physiol.60.1.575. [DOI] [PubMed] [Google Scholar]
- Klefstrom J, Arighi E, Littlewood T, Jaattela M, Saksela E, Evan GI, et al. Induction of TNF-sensitive cellular phenotype by c-Myc involves p53 and impaired NF-kappaB activation. Embo J. 1997;16 24:7382–7392. doi: 10.1093/emboj/16.24.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc's broad reach. Genes Dev. 2008;22 20:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21 2:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, Kim SS, Penn LZ. The Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol. 2006;16 4:275–287. doi: 10.1016/j.semcancer.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22 56:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- You Z, Madrid LV, Saims D, Sedivy J, Wang CY. c-Myc sensitizes cells to tumor necrosis factor-mediated apoptosis by inhibiting nuclear factor kappa B transactivation. J Biol Chem. 2002;277 39:36671–36677. doi: 10.1074/jbc.M203213200. [DOI] [PubMed] [Google Scholar]
- de Alboran IM, Baena E, Martinez AC. c-Myc-deficient B lymphocytes are resistant to spontaneous and induced cell death. Cell Death Differ. 2004;11 1:61–68. doi: 10.1038/sj.cdd.4401319. [DOI] [PubMed] [Google Scholar]
- Lu B, Wang L, Medan D, Toledo D, Huang C, Chen F, et al. Regulation of Fas (CD95)-induced apoptosis by nuclear factor-kappaB and tumor necrosis factor-alpha in macrophages. Am J Physiol Cell Physiol. 2002;283 3:C831–C838. doi: 10.1152/ajpcell.00045.2002. [DOI] [PubMed] [Google Scholar]
- Dudley E, Hornung F, Zheng L, Scherer D, Ballard D, Lenardo M. NF-kappaB regulates Fas/APO-1/CD95- and TCR-mediated apoptosis of T lymphocytes. Eur J Immunol. 1999;29 3:878–886. doi: 10.1002/(SICI)1521-4141(199903)29:03<878::AID-IMMU878>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Carey GB, Donjerkovic D, Mueller CM, Liu S, Hinshaw JA, Tonnetti L, et al. B-cell receptor and Fas-mediated signals for life and death. Immunol Rev. 2000;176:105–115. doi: 10.1034/j.1600-065x.2000.00502.x. [DOI] [PubMed] [Google Scholar]
- Boyd AW, Schrader JW. The regulation of growth and differentiation of a murine B cell lymphoma. II. The inhibition of WEHI 231 by anti-immunoglobulin antibodies. J Immunol. 1981;126 6:2466–2469. [PubMed] [Google Scholar]
- Ralph P. Functional subsets of murine and human B lymphocyte cell lines. Immunol Rev. 1979;48:107–121. doi: 10.1111/j.1600-065x.1979.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Yi AK, Krieg AM. CpG DNA rescue from anti-IgM-induced WEHI-231 B lymphoma apoptosis via modulation of I kappa B alpha and I kappa B beta and sustained activation of nuclear factor-kappa B/c-Rel. J Immunol. 1998;160 3:1240–1245. [PubMed] [Google Scholar]
- Levine RA, McCormack JE, Buckler A, Sonenshein GE. Transcriptional and posttranscriptional control of c-myc gene expression in WEHI 231 cells. Mol Cell Biol. 1986;6 11:4112–4116. doi: 10.1128/mcb.6.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane DE, Manzel L, Krieg AM. Unmethylated CpG-containing oligodeoxynucleotides inhibit apoptosis in WEHI 231 B lymphocytes induced by several agents: evidence for blockade of apoptosis at a distal signalling step. Immunology. 1997;91 4:586–593. doi: 10.1046/j.1365-2567.1997.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy SK. Killer B lymphocytes: the evidence and the potential. Inflamm Res. 2009;58 7:345–357. doi: 10.1007/s00011-009-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton's tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B Cells. J Biol Chem. 2008;283 17:11189–11198. doi: 10.1074/jbc.M708516200. [DOI] [PubMed] [Google Scholar]
- Walczynski J, Lyons S, Jones N, Breitwieser W.Sensitisation of c-MYC-induced B-lymphoma cells to apoptosis by ATF2. Oncogene 2013. in press [DOI] [PubMed]
- Gururajan M, Chui R, Karuppannan AK, Ke J, Jennings CD, Bondada S. c-Jun N-terminal kinase (JNK) is required for survival and proliferation of B-lymphoma cells. Blood. 2005;106 4:1382–1391. doi: 10.1182/blood-2004-10-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizundia R, Chaussepied M, Huerre M, Werling D, Di Santo JP, Langsley G. c-Jun NH2-terminal kinase/c-Jun signaling promotes survival and metastasis of B lymphocytes transformed by Theileria. Cancer Res. 2006;66 12:6105–6110. doi: 10.1158/0008-5472.CAN-05-3861. [DOI] [PubMed] [Google Scholar]
- Wiesner DA, Kilkus JP, Gottschalk AR, Quintans J, Dawson G. Anti-immunoglobulin-induced apoptosis in WEHI 231 cells involves the slow formation of ceramide from sphingomyelin and is blocked by bcl-XL. J Biol Chem. 1997;272 15:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- Chmura SJ, Nodzenski E, Crane MA, Virudachalam S, Hallahan DE, Weichselbaum RR, et al. Cross-talk between ceramide and PKC activity in the control of apoptosis in WEHI-231. Adv Exp Med Biol. 1996;406:39–55. doi: 10.1007/978-1-4899-0274-0_5. [DOI] [PubMed] [Google Scholar]
- Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758 12:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A, Dumitru C, Schenck M, Gulbins E. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008;264 1:1–10. doi: 10.1016/j.canlet.2008.02.020. [DOI] [PubMed] [Google Scholar]