Abstract
Involvement of the maternal and fetal immune systems in the events of pregnancy was generally overlooked by reproductive biologists until the mid-twentieth century when many landmark explorations were reported. Now, more than half a century later, it is well understood that with the initiation of pregnancy, immune cells in mammalian uteri are reprogrammed, losing their cytotoxic potential and providing an immunosuppressive environment suitable for harboring the genetically different fetus. We propose that it is the placenta that is mainly responsible for this conversion and maintenance throughout pregnancy. Studies in our laboratory indicate that trophoblast-derived soluble HLA-G has a subtle but well defined role in programming uterine placental macrophages, a potentially destructive immune cell population. Thus, placental HLA-G plays a critical role in assuring that the developing fetus emerges unscathed at parturition.
Keywords: Placenta, Trophoblast, Macrophage, HLA-G
1. Introduction
Immunological events at the maternal-fetal interface and their consequences for pregnancy have now been studied for more than half a century. Most experiments have been based on principles developed earlier in other areas of immunology, notably infectious disease and transplantation. For a precise and informative listing of the immunological studies conducted during the 19th and 20th centuries that underlie today’s experiments, see Ref. [1]. The citations within include both summaries and original publications.
The human uterus contains readily detectable subpopulations of leukocytes, as is true to a greater or lesser degree of all other tissues and organs. The work of Bulmer [2] and others describes these subpopulations under various conditions that include cycling, pregnant and infected uteri. Notably, proportions of specific subpopulations of immune competent cells in pregnant uteri shift dramatically 1) during stages of the menstrual cycle, 2) when implantation has been initiated, and 3) during infections. Yet uterine leukocytes are appropriately programmed at all times to aid and abet implantation, maintain pregnancy and sustain and protect the fetus.
Here, we discuss our proposition that the placenta is the main origin of substances that drive uterine leukocytes into profiles consistent with successful pregnancy, focusing primarily on macrophages, the primitive defenders of tissue integrity that are abundant in the uterus and are characterized by their ready responses to environmental signals [3–6].
2. Uterine leukocytes and immune modulation
Two subpopulations of uterine leukocytes have received considerable attention. The first subpopulation is uterine macrophages [2–7]. This cell type is dense and stable throughout pregnancy. It is best known for its ability to phagocytose cell debris as well as foreign cells and particles, particularly when coated with antibody, as well as present antigens to T cells and synthesize multiple active proteins. From the functional viewpoint it is important to note that these cells are peculiarly sensitive to microbial products such as lipopolysaccharides (LPS) from gram negative bacteria, exhibiting high levels of receptors for LPS and other endogenous mediators. Phagocytosis or receptor activation is normally followed quickly by production of various substances; many of these fall into the category of inflammation-related cytokines and include interleukin-1 (IL-1) and tumor necrosis factor (TNF) as well as their inhibitors such as corticosteroids and IL-10.
Unexpectedly, uterine macrophages are extremely subdued, thus giving evidence of environmental programming conducive to pregnancy success where toxicity to the newly established semiallogeneic fetus must be bypassed or eliminated. Production of IL-1 and TNF is reduced whereas levels of prostaglandin E as well as IL-10, both down-regulators of macrophage production of cytotoxic cytokines, are high. The immunological circumstances of pregnancy negatively impact these responses as the expected vigorous proinflammatory responses are displayed by macrophages in nonpregnant uteri.
Active immunity in the pregnant uterus is clearly reversed, dominated by immunosuppressive molecules accessible to the immune cell inhabitants. This odd situation is believed to have been designed through evolution to permit the comfortable residence of genetically different [fetal] cells side-by-side with the potentially destructive maternal macrophages that crowd the maternal-fetal interface (Fig. 1). Because the progress of pregnancy toward uneventful parturition is known to be interrupted by infections activating local uterine immune cells with subsequent production of cytotoxic molecules [3, 7], the ways in which these cells are normally programmed for quietude during pregnancy is of the utmost interest.
Fig. 1. Infiltrating cytotrophoblast cells and maternal uterine macrophages reside side by side in early (8 week) human decidua.

Macrophages were stained with anti-CD14 (green, arrows) and cytotrophoblasts (red, arrowheads) were stained with anti-cytokeratin-8. The figure also reveals that glandular epithelial cells stain orange in this double stain. Confocal microscopy; original magnifications (A) x200 (bar, 50 μm) and (B) x400 (bar, 20μm).
The second of the leukocyte populations of major interest is the uterine natural killer (uNK) cell lineage. Although the functions of these cells are not as yet entirely clear, in mice, the cells assist in placental growth and development, as first illustrated in an early study by Croy et al [8]. Moreover, the cells are poorly cytotoxic, thus denying one of their most outstanding qualities. Collectively, the data to date indicate that uNK cells are environmentally programmed in a manner that is, as with macrophages, specific for pregnancy. Substances responsible for this overall programming remain as yet poorly explored although it is clear that at least one method, encounter with fetal class I Human Leukocyte Antigens (HLA), has been implicated [9]. Uterine NK cells have not been investigated in our laboratory and will not be further discussed. For reviews see numerous publications by Ashley Moffett King.
The results to date lead us to believe that during most of pregnancy, it is the placenta that shoulders the responsibility for creating an immunologically safe harbor for the developing fetus. In the paragraphs below we discuss documented influences, particularly those that have concerned our laboratories.
3. Early programming for uterine immunosuppression
Prior to establishment of the defined placenta several major conditions set the stage for early regulation of local maternal immunity. First, the ovarian corpus luteum produces progesterone, which targets cells in the uterus and drives decidualization. Progesterone also has a calming effect on macrophages much like that of corticosteroids but significantly milder [10–12]. Second, powerful but transient immunosuppressive cytokines are delivered in the ejaculate. This novel mode of delivery of programming substances, first documented in mice by Robertson [13], and has very recently been confirmed in human studies in her laboratory [14]. Seminal fluid also induces a second reaction in both mice and women: an inflammation wherein macrophages and dendritic cells arrive for clearing debris and possibly presenting fetal antigens to T cells. T cells themselves [CD3+CD8+CD45RO memory cells] also arrive in large numbers. Their functions remain to be fully dissected.
It should be noted that these early events may be highly susceptible to breakdown; nearly 50% of expected pregnancies, as predicted by hormone levels, do not take place. Collectively, these preliminary events can be seen as part of a carefully scheduled program that fosters decidualization, sets the stage for protection of semiallogeneic fetal cells driving into the uterus, permits clearing of the cellular debris that accompanies implantation and, interestingly, may also arrange extended maternal immunological programming via attracted lymphocytes.
4. Placentas govern localized maternal immune responses
As these initial regulatory events expire, the placenta takes over. A novel concept of pregnancy is that controlling the activities of the highly responsive uterine immune cells is achieved primarily, if not exclusively, by the unusual fetal cells that make up the placenta, i.e., the trophoblastic lineage. This is not unique in the catalogue of immune cell regulation; tumors, which are oft compared with the fetal implant, also regulate immune cells in a localized fashion, preventing immune cell toxicity and encouraging blood circulation to facilitate adequate nourishment.
Clearly, the placenta operates as a gateway between the mother and the developing fetus, as explored in many publications by visionary placentologists that include P. Kaufmann, A. Enders, two generations of Boyds and K. Thornburg. Considerable emphasis has been placed on how the placenta receives maternal nutrients and disposes of the products of metabolism through the maternal circulation. The syncytiotrophoblast layer that covers the placental face of the maternal-fetal interface is highly involved in this bidirectional transport. The layer is also instrumental in the production of polypeptide and steroid hormones that include progesterone. To this group, publications from our laboratory have added secretion of an unusual form of HLA-G that is soluble and lacks its usual associate, β2-microglobulin (β2m) [15, 16].
5. Placental HLA-G – a major modulator of uterine immunosuppression
Geraghty et al. reported in the late 1980s on genes encoding non-classical HLA class IB proteins, HLA-E, -F, and –G [17]. Of these, HLA-G has been of the greatest interest. Generous donation of his probes resulted in the identification of HLA-G on cytotrophoblasts invading the decidua [18]. Since that time, many investigators have studied the expression, structures and functions of these novel HLA class proteins. Much structural work on HLA-G has revealed that as many as seven different isoforms are generated from the single gene. Some are membrane-bound and others are soluble due to interruptions in intron 4; these include HLA-G5 and HLA-G6, both of which have been studied in our laboratory.
The rationale for studying soluble HLA-G is strong. In a number of experiments, ELISA procedures have assisted in identifying HLA-G: LeBouteiller’s group showed that term placental explants secrete HLA-G [19]; we learned that one isoform of HLA-G, HLA-G5, circulates in maternal blood [20] whereas a second, HLA-G6, does not (Fig. 2). Further evidence from our laboratory and others suggests that chorionic villus trophoblast-derived HLA-G5 enters blood via small membrane vesicles that bud from the plasma membrane and/or intralumenal vesicles, termed exosomes [21, 22]. Exosomes, which are produced by many cell types, may have either immune stimulatory or inhibitory properties; cargo carried by placental exosomes includes not only HLA-G5 but also members of the B7 family and Fas Ligand (CD95L; Tumor Necrosis Factor superfamily member 6). Additionally, they carry placenta-specific microRNAs encoded by the chromosome 19 miRNA cluster (C19MC) [23]. Presently little is known about the function of placental exosomes, but given their cargo, they likely have potent impacts on maternal and/or fetal target cells. Key questions include how/whether cargos are targeted into these vesicles, and how/whether they traffic to specific cellular targets.
Fig. 2. HLA-G5 in pregnancy sera.
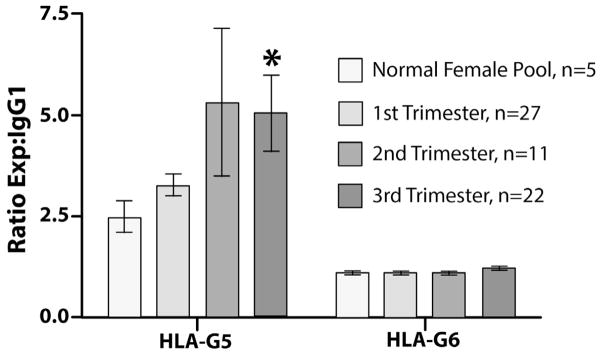
Throughout the three trimesters of pregnancy, maternal blood contains HLA-G5 but little HLA-G6. Values were obtained in capture ELISA assays against monoclonal antibody 1-2C3 (IgG1, anti-HLA-G5) and monoclonal antibody 26-2H11 (IgG1, anti-HLA-G6) [15]. Levels of HLA-G5 increased through pregnancy. * = P<0.05 when levels in pregnancy sera were compared with levels in nonpregnant female sera.
Not surprisingly, given a well-known lack of ability of paternally-derived HLA class IA antigens (HLA-A, -B) to harm pregnancy by stimulating maternal cytotoxic antibodies, we found that mothers generate anti-HLA-G but that this is not allele-specific and has no observable negative effect on the progress of pregnancy [20, 24]. Reviews of this work include several from our group [25, 26]. Further, many clinical investigators have benefited from sharing information during the several HLA-G symposia led by E. Carosella.
In our laboratory we have investigated the less differentiated cytotrophoblast cells lying below the syncytium as primary producers of the unique form of HLA-G that is soluble and lacks the normally associated light chain, β2m. This is not a trivial observation. The critical nature of non-association of β2m in receptor binding has been studied thoroughly by Maenaka [27] and others.
Work in our laboratory has broadened our understanding of this unusual form of HLA-G. We first reported placental expression and the generation of recombinant, soluble β2m-free HLA-G-5 and HLA-G6 [15]. Functional data in this study demonstrated that CD8, a molecule required for T cell activation, is down-regulated by recombinant, soluble β2m-free HLA-G5 and –G6. Subsequently, the lack of β2m production by cytotrophoblasts from term placentas was more thoroughly investigated [16] and we have since generated preliminary data for lack of β2m in quiescent chorionic plate cytotrophoblast cells (J.S. Platt and J.S. Hunt, unreported data).
How the recombinant proteins might function to program monocytes and macrophages has been the subject of more recent experiments. In resting monocytes, this modulator minimally decreases production of inflammatory cytokines such as TNF and IL-1 yet it cannot entirely overcome stimulation of higher levels when the cells are activated [28]. Concurrently, soluble β2m-free HLA-G increases IL-10, which has the ability to reverse production of inflammatory cytokines from activated monocytes. Thus, activation stimulates its own negative feedback pathways. Unreported experiments from our laboratory (B. Ding and J. S. Hunt, unreported data) show clearly that both HLA-G5 and HLA-G6 operate by interfering with the JAK-STAT pathway via LILRB2 inhibitory receptor interactions, ultimately diminishing STAT-1 mRNA while having little effect on other pathways that lead to macrophage inactivation.
Perhaps drama in the introduction of immunosuppression in pregnancy is to be avoided so that the reversal in case of infection remains possible. This idea is supported by conclusions from our experiments as, unlike many immunoregulatory substances, soluble β2m-free HLA-G exerts very subtle effects. This is also true of a second critical modulatory substance produced in the placenta, progesterone.
6. Summary
At this time we may conclude that much has been learned about regulation of immunity in mammalian pregnancy over the past several decades. However, progress is ongoing, and understanding of this complex and tightly controlled biological imperative continues to improve as new information appears. For example, macrophages remain a highly studied cell population in the uterus. We reported on factors that drive monocytes into the alternative activation profile found in pregnancy [29], a finding supported by Svensson et al [30]. In a related study, Houser and coworkers [31] defined two functionally distinct macrophage populations in the uteri of pregnant women. The role of the paternal major histocompatibility antigens expressed by trophoblast in mice in promoting fetal viability has recently been explored by Madeja et al [32]. Importantly, the importance of programming of the uterine environment is now reviving with a new understanding of decidual cell resistance to T cell infiltration [33] and explanation of how placental mammals develop regulatory T cells [34, 35].
The emerging area of epigenetic programming is providing exciting new ideas and insights into just how conditions of pregnancy may impact this delicate process. Among those may eventually be some that relate to the localized immunosuppression of the mammalian uterus during pregnancy. It is wise to stay conversant with this area as the original work of Barker [36] and subsequent studies by others demonstrate significant relationships with adult health and disease. Finally, as a consequence of compelling information from placentologists such as K. Thornburg, L. Myatt and several others, funding agencies are showing interest.
Acknowledgments
The authors of this manuscript are deeply in debt to the late Harold Fox, founder with Page Faulk of the journal Placenta as well as to R. K. Miller, the founder of Trophoblast Research. In these two journals many of the exciting new findings in reproductive immunology have been and continue to be featured. This work was supported by NIH grant PO1 049480 and its predecessors to J.S.H. as well as NIH grant R01 HD 045611 to M.G.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Bibel DJ. Milestones in immunology. New York: Springer-Verlag; 1988. [Google Scholar]
- 2.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54(2–3):281–94. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JS. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989;16:1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JS. Current Topic: The role of macrophages in the uterine response to pregnancy. Placenta. 1990;11:467–75. doi: 10.1016/s0143-4004(05)80192-4. [DOI] [PubMed] [Google Scholar]
- 5.Hunt JS, Robertson SA. Uterine macrophages and environmental programming for pregnancy success. J Reprod Immunol. 1996;32:1–5. doi: 10.1016/s0165-0378(96)88352-5. [DOI] [PubMed] [Google Scholar]
- 6.Petroff MG, Sedlmayr P, Azzola D, Hunt JS. Decidual macrophages are potentially susceptible to inhibition by class Ia and class Ib HLA molecules. J Reprod Immunol. 2002;56:3–17. doi: 10.1016/s0165-0378(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 7.Hunt JS, McIntire RH. Inflammatory cells and cytokine production. In: Peebles DM, Myatt L, editors. Inflammation and Pregnancy. Abingdon, United Kingdom: Informa Health Care; 2006. pp. 1–12. [Google Scholar]
- 8.Croy BA, Luross JA, Guimond MJ, Hunt JS. Uterine natural killer cells: insights into lineage relationships and functions from studies of pregnancies in mutant and transgenic mice. Nat Immun. 1997;15(1):22–33. [PubMed] [Google Scholar]
- 9.Chazara O, Xiong S, Moffett A. Maternal KIR and fetal HLA-C: a fine balance. J Leukoc Biol. 2011;90(4):703–16. doi: 10.1189/jlb.0511227. [DOI] [PubMed] [Google Scholar]
- 10.Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JSL. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukocyte Biol. 1996;59:442–50. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- 11.Miller L, Hunt JS. Regulation of TNF-α production in activated mouse macrophages by progesterone. J Immunol. 1998;160:5098–5104. [PubMed] [Google Scholar]
- 12.Hunt JS, Petroff MG. Placental Immunology. In: Henry HL, Norman AW, editors. Encyclopedia of hormones. Vol. 3. New York: Academic Press, NY; 2003. pp. 224–31. [Google Scholar]
- 13.Robertson SA, Mau VJ, Hudson SN, Tremellen KP. Cytokine-leukocyte networks and the establishment of pregnancy. Am J Reprod Immunol. 1997;37(6):438–42. doi: 10.1111/j.1600-0897.1997.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–54. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 15.Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, Hunt JS. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol. 2003;171:6215–24. doi: 10.4049/jimmunol.171.11.6215. [DOI] [PubMed] [Google Scholar]
- 16.Morales PJ, Pace JL, Platt JS, Langat DL, Hunt JS. Synthesis of β2-microglobulin-free, disulfide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunol. 2007;122:179–188. doi: 10.1111/j.1365-2567.2007.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geraghty DE, Koller BH, Orr HT. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci USA. 1987;84(24):9145–9. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248(4952):220–3. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 19.Solier C, Aquerre-Girr M, Lenfant F, Campan A, Berrebi A, Rebmann V, et al. Secretion of pro-apoptotic intron4-retaining soluble HLA-G1 by human villous trophoblast. Eur J Immunol. 2002;32(12):3576–86. doi: 10.1002/1521-4141(200212)32:12<3576::AID-IMMU3576>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Hunt JS, Jedhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in mothers during pregnancy. Amer J Obstet Gynecol. 2000;183:682–8. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 21.Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M, Billstrand C, Hunt JS, Petroff MG. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012 doi: 10.1016/j.placenta.2012.10.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 23.Donker RB, Mouillet JF, Chu T, Hubel CA, Stolz DB, Morelli AE, Sadovsky Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod. 2012;18:417–424. doi: 10.1093/molehr/gas013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt JS, Pace JL, Morales PJ, Ober C. Immunogenicity of the soluble isoforms of HLA-G. Mol Human Reprod. 2003;9:729–35. doi: 10.1093/molehr/gag087. [DOI] [PubMed] [Google Scholar]
- 25.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 26.Hunt JS. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiroishi M, Kuroki K, Rasubala L, Tsumoto K, Kumagai I, Kurimoto E, et al. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d) Proc Natl Acad Sci USA. 2006;103(44):16412–7. doi: 10.1073/pnas.0605228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt JS, Langat DL. HLA-G: a human pregnancy-related immunomodulator. Curr Opin Pharmacol. 2009;9:462–69. doi: 10.1016/j.coph.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIntire RM, Ganacias K, Hunt JS. Programming of human monocytes by the uteroplacental environment. Reprod Sci. 2008;15:437–47. doi: 10.1177/1933719107314065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Emerudh J. Macrophages at the maternal-fetal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187(7):3671–82. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 31.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. Two unique human decidual macrophage populations. J Immunol. 2011;186:2633–42. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natnl Acad Sci. 2011;108(10):4012–17. doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nancy P, Tagliana E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–21. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker DJ. Human growth and chronic disease: a memorial to Jim Tanner. Ann Hum Biol. 2012 Oct 4; doi: 10.3109/03014460.2012.712717. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]


