Abstract
Background
An NIA-sponsored workgroup on preclinical Alzheimer’s disease (AD) articulated the need to characterize cognitive differences between normal aging and preclinical AD.
Methods
71 apolipoprotein E (APOE) e4 homozygotes (HMZ), 194 e3/4 heterozygotes (HTZ), and 356 e4 noncarriers (NC) aged 21–87 years who were cognitively healthy underwent neuropsychological testing every two years. Longitudinal trajectories of test scores were compared between APOE subgroups.
Results
There was a significant effect of age on all cognitive domains in both APOE e4 carriers and NC. A significant effect of APOE e4 gene dose was confined to the memory domain and the Dementia Rating Scale. Cross sectional comparisons did not discriminate the groups.
Conclusions
While cognitive aging patterns are similar in APOE e4 carriers and NC, preclinical AD is characterized by a significant e4 gene dose effect that impacts memory and is detectable longitudinally. Preclinical neuropsychological testing strategies should emphasize memory sensitive measures and longitudinal design.
Keywords: preclinical Alzheimer’s disease, cognitive aging, age-related memory loss, mild cognitive impairment, apolipoprotein E, longitudinal testing
1. Introduction
Interest in preclinical Alzheimer’s disease (AD) is driven by the need for earlier therapeutic intervention. The National Institute on Aging-Alzheimer’s Association (NAI-AA) workgroup on diagnostic guidelines for the preclinical stages of Alzheimer’s disease (AD) drew a distinction between the pathophysiological disease process (AD-P) that begins before symptoms are evident, from the clinical manifestations (AD-C) of mild cognitive impairment (MCI) and dementia1 that ultimately ensue. In previous work, we showed that apolipoprotein E (APOE) e4 carriers, a powerful genetic risk factor for AD2, experienced accelerating memory decline that correlated with e4 gene dose, and preceded MCI by over a decade3, identical to the preclinical stage envisioned by the NIA-AA workgroup. Howieson et al showed that three to four years before the diagnosis of MCI, decline further accelerates in memory as well as in some executive and spatial measures4. The preclinical stage of AD is characterized by the progressive accumulation of cerebral amyloid5,6 that amyloid-PET studies have shown is maximal, not in medial temporal regions, but in prefrontal and posterior cingulate regions7–12. In a subsequent study we compared performances on neuropsychological measures of executive function (that are known to depend on the normal function of the prefrontal cortices) in APOE e4 carriers who are expected to have an elevated prefrontal amyloid burden with noncarriers (NC), but despite the wealth of evidence that executive measures are sensitive to aging13–15 we found the differences between APOE e4 carriers and NC surprisingly limited, in contrast to the more robust memory differences16 presumably mediated by medial temporal tau-based pathology in e4 carriers17.
At what point should AD-C be defined? The workgroup felt that MCI was the appropriate starting point, a stage that would follow their proposed preclinical AD stage 3, but the cognitive profile distinguishing this stage from normal aging was felt to require further clarification. More specifically the workgroup hypothesized that patients may have objective “decline from their own baseline” especially on challenging episodic memory measures, and possibly subjective impairment or some combination of objective and subjective changes1. Building upon our previous work, we therefore sought to more comprehensively characterize the longitudinal changes in neuropsychological performance that may distinguish normal from pathological (AD-P) cognitive aging in APOE e4 carriers (who are at higher risk for both AD-P and AD-C) and e4 NC.
2. Methods
2.1 Study participants and enrollment
Since January 1, 1994, cognitively normal residents of Maricopa County age 21 years and older were recruited through local media advertisements into a longitudinal study of cognitive aging (the Arizona APOE Cohort) requiring APOE genotyping16. Demographic, family, and medical history data were obtained, and identity was coded by a study assistant. All individuals gave their written, informed consent to participate in the study, which was approved by the Institutional Review Boards of all participating institutions. The participants agreed to have the results of the APOE test withheld from them as a precondition to their participation in this study. Genetic determination of APOE allelic status was performed using a polymerase chain reaction (PCR) based assay.
The recruitment strategy for the Arizona APOE Cohort involved recruiting all identified e4 homozygotes (HMZ), matching them by age, gender, and education to one heterozygote (HTZ; all with the e3/4 genotype) and two NC. We identified many more HTZ and NC than HMZ, (especially those persons over age 70 reflecting the greater number of HMZ developing MCI and AD by this age) who were also eligible for enrollment so that the final match paradigm involved matching two e4 carriers to two noncarriers with priority given to HMZ.
Each potential participant had screening tests to confirm their neuropsychiatrically normal state that included a neurological examination, the Folstein Mini-Mental Status Exam18 (MMSE), the Hamilton Depression (Ham-D) Rating Scale19, the Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for DSM-IIIR20. There were no potentially confounding medical (for example, end organ failure), neurologic (for example, stroke), or psychiatric problems (for example, psychotic disorder). None met the published criteria for MCI21, AD22, other forms of dementia or major depressive disorder20 at entry or during subsequent followup (to insure ours was a truly preclinical cohort and that the data would not be skewed by a few potentially impaired individuals; individuals developing MCI during followup were identified either because they had sought medical attention for cognitive impairment that was then evaluated by the patient’s physician with results reviewed by R.J.C., or else were identified on the basis of their study results). Entry criteria for all participants included a score of at least 27 on the MMSE (and scoring at least 1 out of 3 on the recall subtest), a score of 10 or less on the Ham-D rating scale at the time of their first visit, and no indication of loss of function according to the FAQ and IADL. The resulting study population was identical to that previously reported16. Those fulfilling these requirements were administered an extensive standardized battery of neuropsychological tests repeated every one to two years.
2.2 Neuropsychological testing
The neuropsychological tests within our battery are detailed in reference 18, and encompass four broadly defined cognitive domains19. The scores used were as follows:
Memory: Auditory Verbal Learning Test (AVLT) Total Learning (TL) and Long Term Memory Score (LTM); Buschke Free and Cued Selective Reminding Test Total Free (SRT-free) and Cued (SRT-cued) Recall, Rey-Osterrieth Complex Figure Test Absolute Recall (CFT-recall) and Percent Recall (CFT%) scores; and the Benton Visual Retention Test total number correct (VRT).
Executive: Wisconsin Card Sorting Test Categories Completed (WCST-Cat), Total Errors (WCST-TERR), and Perseverative Errors (WCST-PERR), Paced Auditory Serial Attention Task 3 and 2 second versions total correct (PASAT-3, PASAT-2), and Age Scaled Scores of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) subtests including Digit Span (DigSp), Mental Arithmetic (MArith), and Digit Symbol Substitution Age Scaled Score (DSS).
Language: Boston Naming Test (BNT; 60 item), Controlled Oral Word Association Test total words (COWAT), Token Test total correct, and Age Scaled Scores on the WAIS-R Vocabulary (Voc) and Similarities (Sim) subtests
Visuospatial: Judgment of Line Orientation total correct (JLO), Facial Recognition Test Short Form corrected long form score (FRT), WAIS-R Block Design Age Scaled Score (BD), and the CFT copy score.
Additionally each subject received the Mattis Dementia Rating Scale (DRS).
2.3 Subjective cognition
Participants and their informants completed the paired Multidimensional Assessment of Neurodegenerative Symptoms Questionnaire including both self (MANS-Self) and Observer (MANS-Observer) versions23. The MANS are paired self and informant based questionnaires comprised of 87 questions that assess changes over the preceding year in daily habits, personality, and motor functioning. It employs a quantitative scale for rating the frequency of a symptom from 0 (never) to 4 (routinely), with intermediate values of 1 (once), 2 (occasionally), and 3 (more than monthly); scores can range from 0–344 with higher scores indicating more frequent and severe symptoms.
2.4 Statistical methods
To compare the relative effects of age by APOE subgroups, the acceleration of the rate of decline for each measure for carriers (collectively and separately for HMZ and HTZ subgroups) was compared to NC by using a mixed model approach for modeling cross-sectional and longitudinal data24,25. See supplementary material for detailed description. Modeling was carried out using SAS PROC MIXED (SAS Version 9, SAS Institute, Cary, NC). To examine the relative rate of change in test performance, scores for each measure were standardized using the sample mean and standard deviation (resulting in each measure having a mean of 0 and standard deviation of 1), and the predicted annual change was computed based on the previously described models24,25 at ages 50, 60, and 70 for each measure for each APOE subgroup. Baseline characteristics and followup were compared among groups by using the two-sample t-test / analysis of variance (ANOVA) F-test or Pearson chi-square test.
2.5 Testing a preclinical AD battery
Based upon evidence that between ages 50 and 70 years asymptomatic APOE e4/4 HMZs are manifesting neuropathological26 and biomarker7,27 evidence of AD in the absence of symptoms we defined them as a “preclinical AD subset”. Using unpaired t-tests (two tailed) we compared this preclinical AD subset to the NCs in our cohort between ages 50 and 70 on the tests our analyses identified as most sensitive to preclinical decline, as well as on the other tests in our battery at entry and at the time of last followup excluding the MCI epoch in any who developed MCI (n=27).
Results
265 APOE e4 carriers, including 71 e4 HMZ and 194 HTZ, and 356 NC were included. Demographic data are summarized in table one, and the breakdown by age decile is summarized in table two. The number and proportion of HMZ, HTZ, and NC were uneven between age deciles with more participants overall, and a higher proportion of e4 carriers (especially HMZ) in the 50–59 and 60–69 year old deciles. Overall, carriers had a higher reported rate of a first-degree relative with dementia, but adjusting for family history did not significantly alter the results for any of the measures. Gender (70% women), mean years of education (15.6 +/− 2.4), and number of participants with more than one epoch of testing (77.1%) did not differ between the carrier groups. Mean duration of followup among those with more than one epoch of testing was 6.3 +/− 3.2 years overall.
Table 1.
Demographics
| APOE e4 Noncarriers |
APOE e3/4 Heterozygotes |
APOE e4/4 Homozygotes |
p | |
|---|---|---|---|---|
| N | 356 | 194 | 71 | |
| Mean Age (SD) | 57.2 (10.9) | 56.0 (12.7) | 55.6 (9.0) | .37 |
| Mean Yrs Education (SD) | 15.5 (2.3) | 15.7 (2.5) | 15.5 (2.6) | .57 |
| % Female | 69.4% | 70.6% | 70.4% | .95 |
| % Non-Caucasian | 3.9% | 4.1% | 4.2% | .84 |
| % with first degree relative | 56.1% | 72% | 85.9% | <.001 |
| % with > 1 epoch | 74.7% | 77.8% | 87.3% | .07 |
| Mean (SD) duration followup (yrs) | 6.2 (3.2) | 6.3 (3.0) | 6.6 (3.5) | .72 |
Table 2.
Age Deciles at Entry
| Age | APOE e4 Noncarriers |
APOE e3/4 Heterozygotes |
APOE e4/4 Homozygotes |
Total |
|---|---|---|---|---|
| 20–29 | 9 (2.5%) | 5 (2.6%) | 0 | 14 (2.3%) |
| 30–39 | 20 (5.6%) | 17 (8.8%) | 6 (8.5%) | 43 (6.9%) |
| 40–49 | 27 (7.6%) | 32 (16.5%) | 4 (5.6%) | 63 (10.1%) |
| 50–59 | 154 (43.3%) | 66 (34%) | 41 (57.7%) | 261 (42%) |
| 60–69 | 110 (30.9%) | 48 (24.7%) | 15 (21.1%) | 173 (27.9%) |
| 70–79 | 33 (9.3%) | 20 (10.3%) | 5 (7%) | 58 (9.3%) |
| ≥ 80 | 3 (0.8%) | 6 (3.1%) | 0 | 9 (1.4%) |
We first examined the main effect of age on neuropsychological measures (table three). Executive and memory measures generally showed the greatest changes in both carriers and noncarriers: the WCST showed the greatest decline (supplementary figure one) and the PASAT-2 the greatest test-retest improvement (supplementary figure two) at three age points (50, 60, and 70 years).
Table 3.
Main Effects of Age on Neuropsychological Scores in APOE e4 Noncarriers and Carriers
|
Non-carriers (n=356) |
Carriers (n=265) |
|||||||
|---|---|---|---|---|---|---|---|---|
| annual change (%)** | annual change (%)** | |||||||
| P* | 50 | 60 | 70 | P* | 50 | 60 | 70 | |
| Memory | ||||||||
| AVLT-TL | .001 | +3.6% | +1.4% | −0.9% | <.001 | +1.2% | −2.1% | −5.3% |
| AVLT-LT | .04 | +3.2% | +1.8% | +0.3% | <.001 | +2.5% | −1.1% | −4.6% |
| SRT-free | <.001 | +7.9% | +5.6% | +3.2% | <.001 | +7.6% | +1.4% | −4.8% |
| SRT-cued | .003 | −7.5% | −5.5% | −3.5% | <.001 | −8% | −2.3% | +3.4% |
| CFT-R | <.001 | +5.3% | +2.6% | −.02% | <.001 | +5.0% | +1.6% | −1.9% |
| CFT-% | .001 | +5.3% | +2.9% | +0.5% | <.001 | +5.6% | +1.8% | −2.2% |
| VRT | .17 | +0.2% | −0.8% | −1.9% | <.001 | +1.4% | −2.8% | −7.0% |
| Executive | ||||||||
| WCST-Cat | .02 | −1.5% | −3.5% | −5.5% | <.001 | +0.1% | −3.7% | −7.5% |
| WCST-TErr | .03 | +1.3% | +3.0% | +4.8% | <.001 | −0.4% | +2.9% | +6.3% |
| WCST-Perr | .06 | +0.5% | +2.1% | +3.8% | <.001 | −0.8% | +2.5% | +5.8% |
| PASAT-3 | .007 | +5.6% | +3.7% | +1.9% | <.001 | +4.6% | +1.4% | −1.9% |
| PASAT-2 | .02 | +10.6% | +9.0% | +7.4% | <.001 | +10.3% | +7.1% | +3.9% |
| COWAT | .26 | +4.0% | +3.2% | +2.5% | <.001 | +5.3% | +3.0% | +0.7% |
| DigSp | .78 | +1.2% | +1.0% | +0.8% | .6 | +0.1% | +0.5% | +0.8% |
| MArith | .19 | +1.3% | +0.5% | −0.3% | .08 | +0.6% | −0.5% | −1.7% |
| DSS | .36 | +6.2% | +5.6% | +5.0% | .27 | +4.0% | +3.3% | +2.5% |
| Language | ||||||||
| BNT | <.001 | +3.6% | +0.7% | −2.2% | .04 | +0.1% | −1.5% | −3.1% |
| Token | .02 | −0.6% | −2.7% | −4.9% | .17 | −3.3% | −4.7% | −6.1% |
| Vocab | .007 | +6.3% | +4.7% | +3.0% | .08 | +3.4% | +2.3% | +1.1% |
| Sim | .76 | +4.3% | +4.0% | +3.8% | .02 | +6.1% | +4.1% | +2.1% |
| Spatial | ||||||||
| JLO | .46 | +0.9% | +0.3% | −0.2% | .07 | −.05% | −1.5% | −3.0% |
| FRT | .23 | −1.2% | −2.3% | −3.3% | .004 | +0.1% | −2.6% | −5.4% |
| CFT-copy | .05 | +1.2% | −0.5% | −2.2% | .99 | −1.4% | −1.4% | −1.3% |
| BD | <.001 | +5.5% | +3.3% | +1.2% | <.001 | +4.5% | +1.7% | −1.2% |
| General | ||||||||
| MMSE | .51 | +0.5% | −0.2% | −.08% | .02 | −0.7% | −3.2% | −5.6% |
| DRS | .12 | +1.5% | +.02% | −1.4% | <.001 | +1.9% | −3.7% | −9.3% |
| Observer | .003 | −3.9% | −0.7% | +2.5% | <.001 | −3.9% | +0.9% | +5.7% |
| Self | .61 | −1.5% | −1.0% | −0.5% | .01 | −0.5% | +2.3% | +4.7% |
Test of significance for the longitudinal quadratic effect of aging for the stated group based on a mixed model.
Scores for each measure were standardized to have mean 0 and standard deviation 1 with annual changes reported as percentages of a standard deviation.
Auditory Verbal Learning Test (AVLT) Total Learning (TL) and Long Term Memory Score (LTM); Buschke Free and Cued Selective Reminding Test Immediate Free (SRT-IF) and Delayed Free (SRT-DF) Recall, Rey-Osterrieth Complex Figure Test Absolute Recall (CFT-recall) and Percent Recall (CFT%) scores; and the Benton Visual Retention Test total number correct (VRT); Wisconsin Card Sorting Test Categories Completed (WCST-Cat), Total Errors (WCST-TERR), and Perseverative Errors (WCST-PERR), Paced Auditory Serial Attention Task 3 and 2 second versions total correct (PASAT-3, PASAT-2), Age Scaled Scores of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) subtests including Digit Span (DigSp), Mental Arithmetic (MArith), and Digit Symbol Substitution Age Scaled Score (DSS); Boston Naming Test 60 item (total spontaneous correct), Controlled Oral Word Association Test total words (COWAT), Token Test total correct, and Age Scaled Scores on the WAIS-R Vocabulary (Vocab) and Similarities (Sim) subtests; Judgment of Line Orientation total correct (JLO), Facial Recognition Test Short Form corrected long form score (FRT), WAIS-R Block Design Age Scaled Score (BD), and the CFT copy score.
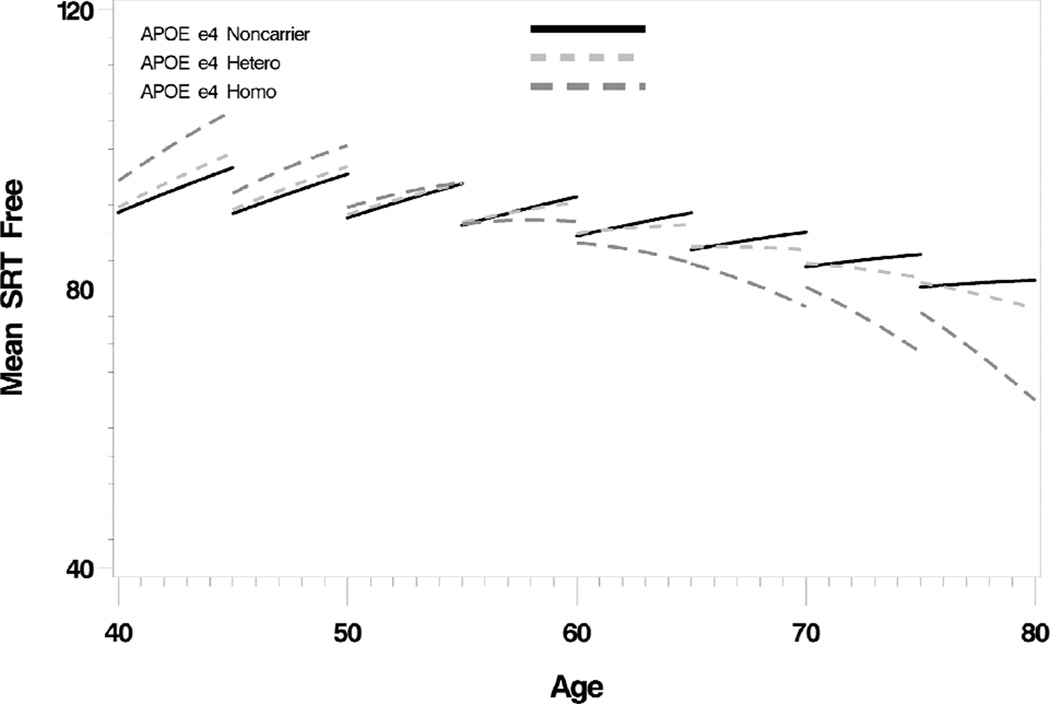
The relative age effect differences between APOE subgroups are summarized in table four. A significant e4 gene dose effect was observed for three of the four memory tests (figure one) but not for any other domain-specific tests. The only other measure to show a gene dose effect was the DRS. All four memory tests had at least one significant difference between HMZs and NCs in either linear or quadratic effects (and usually both) while in HTZs the main difference was confined to verbal learning scores (AVLT-total learning, linear effect p=.02; SRT-free, linear effect = .02, quadratic effect =.01; SRT-cued, quadratic effect = .01). Significant differences between HTZs (but not HMZs) and NCs included DSS (linear effect, p=.03), BNT (linear effect, p=.01), WAIS-R Voc (linear effect, p=.01), CFT copy (quadratic effect, p=.03), WAIS-R BD (linear effect, p=.046), and DRS (linear effect, p=.007, quadratic effect, p=.005), but only the DRS showed a significant gene dose effect. Applying Bonferroni correction, only memory effects remain significant (table four).
Table 4.
Comparison of Age Effects In APOE Subgroups (P values)
| Test | C v NC Linear |
C v NC Quadratic |
E44 v NC Linear |
E44 v NC Quadratic |
E34 v NC Linear |
E34 v NC Quadratic |
Gene Dose |
|---|---|---|---|---|---|---|---|
| Memory | |||||||
| AVLT-TL | <.001* | .35 | <.001* | .03 | .02 | .97 | .09 |
| AVLT-LTM | .002 | .04 | <.001 | .01 | .19 | .23 | .01 |
| SRT-free | <.001* | <.001* | <.001* | <.001* | .02 | .01 | <.001* |
| SRT-cued | <.001* | <.001* | <.001* | <.001* | .25 | .01 | <.001* |
| CFT-R | .25 | .42 | .05 | .59 | .8 | .51 | .44 |
| CFT-% | .24 | .16 | .03 | .8 | .87 | .13 | .33 |
| VRT | .045 | .004 | .049 | <.001* | .2 | .08 | <.001* |
| Executive | |||||||
| WCST-Cat | .88 | .16 | 1.0 | .1 | .91 | .38 | .09 |
| WCST-TErr | .91 | .19 | .56 | .09 | .56 | .46 | .1 |
| WCST-Perr | .81 | .19 | .62 | .09 | .62 | .47 | .1 |
| PASAT-3 | .02 | .16 | .01 | .11 | .11 | .35 | .1 |
| PASAT-2 | .06 | .12 | .004 | .06 | .51 | .32 | .06 |
| COWAT | .78 | .1 | .6 | .13 | .99 | .19 | .08 |
| DigSp | .49 | .57 | .51 | .24 | .58 | .86 | .35 |
| MArith | .2 | .71 | .048 | .1 | .71 | .76 | .29 |
| DSS | .01 | .86 | .12 | .23 | .03 | .74 | .49 |
| Language | |||||||
| BNT | .03 | .25 | .62 | .35 | .01 | .07 | .88 |
| Token | .15 | .58 | .13 | .9 | .35 | .48 | .76 |
| Vocab | .008 | .57 | .14 | .95 | .01 | .51 | .72 |
| Sim | .96 | .13 | .78 | .29 | .93 | .19 | .14 |
| Spatial | |||||||
| JLO | .09 | .41 | .15 | .006 | .25 | .81 | .06 |
| FRT | .78 | .19 | .31 | .45 | .8 | .23 | .25 |
| CFT-copy | .44 | .18 | .62 | .19 | .22 | .03 | .96 |
| BD | .04 | .45 | .26 | .23 | .046 | .72 | .28 |
| General | |||||||
| MMSE | .02 | .18 | .08 | .04 | .06 | .6 | .06 |
| DRS | .007 | .003 | .2 | .12 | .007 | .005 | .01 |
| Observer | .27 | .29 | .41 | .03 | .36 | .71 | .08 |
| Self | .02 | .16 | .008 | .89 | .09 | .13 | .33 |
Remain significant after Bonferroni correction
Auditory Verbal Learning Test (AVLT) Total Learning (TL) and Long Term Memory Score (LTM); Buschke Free and Cued Selective Reminding Test Immediate Free (SRT-IF) and Delayed Free (SRT-DF) Recall, Rey-Osterrieth Complex Figure Test Absolute Recall (CFT-recall) and Percent Recall (CFT%) scores; and the Benton Visual Retention Test total number correct (VRT); Wisconsin Card Sorting Test Categories Completed (WCST-Cat), Total Errors (WCST-TERR), and Perseverative Errors (WCST-PERR), Paced Auditory Serial Attention Task 3 and 2 second versions total correct (PASAT-3, PASAT-2), Age Scaled Scores of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) subtests including Digit Span (DigSp), Mental Arithmetic (MArith), and Digit Symbol Substitution Age Scaled Score (DSS); Boston Naming Test 60 item (total spontaneous correct), Controlled Oral Word Association Test total words (COWAT), Token Test total correct, and Age Scaled Scores on the WAIS-R Vocabulary (Vocab) and Similarities (Sim) subtests; Judgment of Line Orientation total correct (JLO), Facial Recognition Test Short Form corrected long form score (FRT), WAIS-R Block Design Age Scaled Score (BD), and the CFT copy score.
Figure one. Selective Reminding Test, Free Recall (Total).
There is accelerating decline with increasing age that exhibits a significant APOE e4 gene dose effect.
Regarding subjective cognition, significant differences between HMZs and NCs included MANS-self (linear effect, p=.008) and observer (quadratic effect, p=.03; and a nonsignificant trend for gene dose effect, p=.08).
Comparing 47 HMZs with 254 NCs between ages 50 and 70 (our preclinical AD subset and controls) who have not to date manifested symptomatic memory loss and who have been followed for 75.8 +/− 57.4 and 68.0 +/− 58.2 months respectively, there were no differences at entry or at the time of their most recent followup on any neuropsychological measure (and this result did not change if we altered the age range to 60 and older, either included or excluded e3/4 heterozygotes, or summed the scores into a single composite value).
Discussion
This is the first study to report the pattern and magnitude of longitudinal changes on such a large battery of objective neuropsychological as well as subjective cognitive measures based upon APOE e4 gene dose. Cognitive aging patterns were very similar, with executive skills showing the greatest relative changes in both APOE e4 carriers and noncarriers. Comparing these longitudinal changes in cognitive domains across APOE genetic subgroups, however, showed that only memory decline exhibits an e4 gene dose effect, and despite these longitudinal changes, we were unable to show that cross sectional comparisons at either entry or last followup distinguished preclinical AD from controls. Our results support the use and continued development of more sensitive memory measures as well as a longitudinal design (as posited by the NIA-AA workgroup) for preclinical AD assessment.
Previous studies of preclinical AD based upon the retrospective analysis of longitudinal data in older patients who developed AD have generally supported the early emergence of memory decline as a defining feature28–35, with some showing accelerated psychometric decline as much as 12 years in advance of dementia32 and seven years in advance of MCI35. In the Baltimore Longitudinal Study of Aging, for example, memory decline accelerated seven years and executive skills two to three years before dementia diagnosis in a cohort of 92 patients whose mean age at entry was 79 years33. We previously reported that some executive skills (primarily mental arithmetic/working memory and possibly problem solving) decline in the two to four years or so preceding the diagnosis of MCI, but that memory declines well before that16.
Another strategy for studying preclinical AD has been to evaluate presymptomatic autosomal dominant mutation carriers. Asymptomatic presenilin 1 (PSEN1)mutation carriers from multiple Mexican kindreds who were within 5.6 years of the expected age of dementia diagnosis performed significantly less well on multiple tests, but younger carriers did not differ from controls36. Asymptomatic PSEN1 mutation carriers from a single large Colombian kindred were selectively impaired on a novel color-shape “binding” task that conventional measures of memory failed to detect37 (although in a subsequent study mutation carriers also performed less well on standard verbal memory measures38). More recently, the Dominantly Inherited Alzheimer’s Network (DIAN) study reported that memory declined 10 years and a general mental status measure 5 years in advance of the expected age of symptomatic onset39.
One of the earliest proposed indicators of preclinical AD was the presence of subjective cognitive concerns in the absence of objective decline40. While subjective cognitive complaints in unimpaired individuals often reflect emotional factors41,42, our data showed that both self and observer based subjective cognition changed more in APOE e4 HMZs than HTZ or NC (though we could not demonstrate an e4 gene dose effect) congruent with previous findings in a French32, German43 and another American44 cohort. Further, Perrotin and colleagues showed that cognitively normal individuals with higher cerebral amyloid levels had more subjective cognitive complaints than those with lower levels45 supporting the potential biological validity of subjective cognition. Nonetheless the subjective measures in our study showed less change longitudinally than objective memory measures.
Our working definition for preclinical AD, supported by biomarker studies, was based upon APOE genotype and age, an approach that would be easy to replicate. However, cross sectional comparisons did not distinguish the two groups. The Mayo Clinic Study of Aging instead used the arbitrary cut point of lowest 10th percentile on objective composite psychometric measures in a much older cohort, and showed a correlation with biomarker indicators of preclinical AD and APOE genotype46. While this remarkable study provides strong support for a proposed model of preclinical AD1, that is not the same as showing that individuals with biomarker evidence of preclinical disease differ on a predefined cognitive battery (as the authors themselves explained). The further along in their disease course are patients, the more readily will they be distinguished on cognitive measures, and while where one draws the line between preclinical and clinical can be debated, it must be early enough for disease modifying therapy to be effective.
A limitation of our study is that due to its prospective nature and focus on a younger population, we lack final clinical outcomes in most of our subjects so that AD risk is based exclusively upon APOE genotype. Nonetheless, APOE genotype is a major genetic risk factor for AD2 that even correlates with incidental AD pathology in nondemented decedents27,47,48, and given that memory declines a decade in advance of expected diagnosis in patients with autosomal dominantly inherited AD39, the earliest cognitive changes in APOE e4 carriers (whose age of onset is generally older and rate of progression slower) likely begin even more than a decade in advance of clinical symptoms. Another limitation is that despite the extensive breadth and depth of our battery, some tests may be more sensitive to decline than others. We addressed this by normalizing all results to a common mean and calculating percent change over time from that mean. It is also possible that the sensitivity of the WAIS-R subtests to decline may have been blunted by our use of age-scaled scores. However, abnormal decline should exceed the age scaling, and since the age scaling was applied to all groups, decline should still be evident if one group is more affected than another. Although our cohort was enriched for APOE e4, we were unable to balance APOE genotypes across each age decile. This was accommodated, however, by our statistical model. Finally, separate discovery and test cohorts would have been preferable for assessing the “discovered” preclinical battery, but the fact that even within the same discovery cohort cross sectional comparisons failed to yield a difference makes it even less likely that a different cohort would. Nonetheless we would welcome such a test in an appropriate separate cohort.
Preclinical AD represents the stage of progressive cerebral amyloidosis that is already advanced at the onset of MCI5,6. Well designed therapeutic trials have failed to staunch the progression of dementia when initiated later than this, and so new efforts are aimed at preclinical intervention49,50. Longitudinal (but probably not cross sectional) memory assessment may be a biologically valid and relatively sensitive approach to the identification of treatment eligible presymptomatic patients as well as a means for monitoring treatment effects. It is not yet known how early intervention must begin to be effective, but it is known that once symptoms emerge, nothing has worked.
Supplementary Material
Supplementary figure one: Wisconsin Card Sorting Test, Categories Completed. In both APOE e4 noncarriers and carriers there is steady decline with age.
Supplementary figure two. Paced Auditory Serial Attention Task, 2-second version. In both APOE e4 noncarriers and carriers there is prominent test-retest improvement that diminishes with age.
Acknowledgments
The authors gratefully acknowledge funding support from NIA R01AG031581, NIA P30AG19610, and the Arizona Alzheimer's Consortium. The authors wish to thank Bruce Henslin, Sandra Yee-Benedetto, Travis Johnson, Marci Zomok, Jessie Jacobsen, Jeanne Young, Jennifer Pichon, Lynn Autry, Allyson Jensen, and Andrea Fowler for expert technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions and declarations
Richard J. Caselli. Co-principal investigator. Designed study, analyzed neuropsychological data, wrote main draft of manuscript. No conflicts.
Dona E.C. Locke. Collected neuropsychology data, provided groundwork for utilizing the paired self and informant questionnaires, contributed critical revisions to the manuscript. No conflicts.
Amylou C. Dueck. Biostatistician, performed all statistical analyses, contributed statistical sections to manuscript, and provided critical revisions. No conflicts.
David Knopman. Provided critical revisions to the manuscript and content expertise.
Associate Editor, Neurology.
Bryan K. Woodruff. Helped collect and analyze data, provided critical revision to the manuscript. Receives research funding support from Genetech.
Charlene Hoffman-Snyder. Helped collect and analyze data, provided critical revision to the manuscript. No conflicts.
Rosa Rademakers. Performed genetic testing for apolipoprotein E, provided genetic data, and critical revision to the manuscript. No conflicts.
Adam S. Fleisher. Contributed critical revision to the manuscript. No conflicts.
Eric M. Reiman. Principal investigator, contributed critical revisions to the manuscript. No conflicts.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alz Dem. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 3.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howieson DB, Carlson NE, Moore MM, Wasserman D, Abendroth CD, Payne-Murphy J, Kaye JA. Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc. 2008;14:192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- 5.Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994;4:138–150. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 7.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci (USA) 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mormino EC, Kluth JT, Madison CM, Rabinovici VD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated B-amyloid deposition in elderly subjects. Brain. 2008;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villemagne VL, Pike KE, Darby D, Maruff B, Savage G, Ng S, et al. AB deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia. 2008;46:1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinno JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–1511. doi: 10.1212/WNL.0b013e3181a2e896. [DOI] [PubMed] [Google Scholar]
- 12.Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 14.Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neurosci Behav Rev. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 15.Treitz FH, Heyder K, Daum I. Differential course of executive control changes during normal aging. Aging, Neuropsychology, and Cognition. 2007;14:370–393. doi: 10.1080/13825580600678442. [DOI] [PubMed] [Google Scholar]
- 16.Caselli RJ, Dueck AC, Locke DE, Hoffman-Snyder CR, Woodruff BK, Rapcsak SZ, Reiman EM. Longitudinal modeling of frontal cognition in APOE epsilon4 homozygotess, heterozygotes, and noncarriers. Neurology. 2011;76:1383–1388. doi: 10.1212/WNL.0b013e3182167147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 18.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental satae examination by age and education level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 19.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th edition. New York: Oxford University Press; 2004. [Google Scholar]
- 20.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- 21.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurol. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Locke DEC, Dassel KB, Hall G, Baxter LC, Woodruff BK, Hoffman Synder C, Miller BL, Caselli RJ. Assessment of patient and caregiver experiences of dementia-related symptoms: development of the Multidimensional Assessment of Neurodegenerative Symptoms questionnaire. Dem Geriatr Cogn Dis. 2009;27:260–272. doi: 10.1159/000203890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware JH, Dockery DW, Louis TA, XU X, Ferris BG, Speizer FE. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiol. 1990;132:685–700. doi: 10.1093/oxfordjournals.aje.a115710. [DOI] [PubMed] [Google Scholar]
- 25.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken: John Wiley & Sons, Inc.; 2004. (Section 15.4) [Google Scholar]
- 26.Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- 29.Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 30.Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to diagnosis. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 31.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 32.Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, et al. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 33.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringman JM, Diaz-Olavarrieta C, Rodriguez Y, Chavez M, Fairbanks L, Paz F, et al. Neuropsychological function in nondemented carriers of presenilin-1 mutations. Neurol. 2005;65:552–558. doi: 10.1212/01.wnl.0000172919.50001.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Salla S. Visual short term memory binding deficits in familial Alzheimer’s disease. Brain. 2010;133:2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- 38.MacPherson SE, Parra MA, Moreno S, Lopera F, Della Salla S. Dual task abilities as a possible marker of Alzheimer’s disease in carriers of the E280A presenilin-1 mutation. J Int Neuropsych Soc. 2012;18:1–8. doi: 10.1017/S1355617711001561. [DOI] [PubMed] [Google Scholar]
- 39.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reisberg B, Ferris SH, De Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 41.Comijs HC, Deeg DJH, Dik MG, Twisk JWR, Jonker C. Memory complaints; the association with psych-affective and health problems and the role of personality characteristics. A 6 year follow-up study. J Affect Disord. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 42.Jorm AF, Butterworth P, Anstey KJ, Christensen H, Easteal S, Maller J, et al. Memory complaints in a community sample aged 60–64 years: associations with cognitive functioning, psychiatric symptoms, APOE genotype, hippocampus and amygdale volumes, and white matter hyperintensities. Psychol Med. 2004;34:1495–1506. doi: 10.1017/s0033291704003162. [DOI] [PubMed] [Google Scholar]
- 43.Jessen F, Wiese B, Bachman C, Eifflaender-Gorfer S, Haller F, Kolsch H, et al. for the German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiat. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 44.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alz Dem. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imagine. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Venuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer’s disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett DA, De Jager PL, Leurgans SE, Schneider JA. Neuropathologic intermediate phenotypes enhance association to Alzheimer susceptibility alleles. Neurol. 2009;72:1495–1503. doi: 10.1212/WNL.0b013e3181a2e87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci Lett. 2010;473:168–171. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alz Res Therapy. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiman EM, Langbaum JB, Tariot PN. Alzheimer's prevention initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomarkers in medicine. 2010;4:3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure one: Wisconsin Card Sorting Test, Categories Completed. In both APOE e4 noncarriers and carriers there is steady decline with age.
Supplementary figure two. Paced Auditory Serial Attention Task, 2-second version. In both APOE e4 noncarriers and carriers there is prominent test-retest improvement that diminishes with age.