Abstract
Lifestyles involving sleep deprivation are common, despite mounting evidence that both acute total sleep deprivation and chronically restricted sleep degrade neurobehavioral functions associated with arousal, attention, memory and state stability. Current research suggests dynamic differences in the way the central nervous system responds to acute versus chronic sleep restriction, which is reflected in new models of sleep-wake regulation. Chronic sleep restriction likely induces long-term neuromodulatory changes in brain physiology that could explain why recovery from it may require more time than from acute sleep loss. High intraclass correlations in neurobehavioral responses to sleep loss suggest that these trait-like differences are phenotypic and may include genetic components. Sleep deprivation induces changes in brain metabolism and neural activation that involve distributed networks and connectivity.
Introduction
Sleep as an adaptive state of dormancy is found widely throughout the animal kingdom [1]. Although its biological and behavioral functions have not been fully understood, there is substantial evidence that human sleep must be of sufficient duration and physiological continuity to ensure coherent levels of waking alertness, attention, cognitive performance and neurobehavioral effectiveness [2-4], and to avoid predisposing humans to adverse health outcomes [5]. Epidemiological evidence has linked habitually short sleep duration to excessive sleepiness, accidents, cognitive deficits, and more recently to increased risk of obesity [6], diabetes [7], hypertension [8], and all-cause mortality. Despite growing awareness of these risks, current surveys indicate that 35%-40% of the adult US population chronically restrict their sleep to less than 7 hours on weekday nights [9], primarily for lifestyle reasons [10]. This makes chronic sleep restriction more common in modern cultures than acute total sleep deprivation, and it highlights the need to understand the dynamics of neurobehavioral changes induced by chronic sleep restriction intermittently followed by extended sleep for recovery [3]. Below we focus on recent scientific evidence on human neurobehavioral differences in response to acute total versus chronic partial sleep deprivation and the implications for the two-process model of sleep-wake regulation; phenotypic and genotypic factors related to responses to sleep deprivation; and neuroimaging evidence for the neural basis of the behavioral effects of sleep deprivation.
Chronic sleep restriction induces cumulative neurobehavioral deficits
Increased scientific focus on dynamic changes in sleep physiology and waking neurobehavioral functions during sleep restriction and recovery has revealed that the results of decades of experiments on acute total sleep deprivation cannot be used to precisely predict the effects of chronic partial sleep restriction. Although the former experiments are more cost-effective to perform than the latter, and hence more common, experiments on chronic sleep restriction have revealed the importance of much longer time constants in the biology of sleep homeostasis and waking functions.
A decade ago, well-controlled sleep-dose-response experiments found that chronic restriction of sleep to between 3 h and 7 h time in bed per 24 h, for a period of 1 to 2 weeks, resulted in near-linear declines across days in behavioral alertness and cognitive performance [11,12]. The rate of these cumulative changes varied systematically with the degree of sleep restriction. The experiments also revealed that no matter what psychometric scales were used, participants subjectively underestimated the growing degradation of their neurobehavioral functions across days of sleep restriction [12]. Since then, the effects of chronic sleep restriction on human biology and behavior have been extensively replicated and expanded [4,13-22]. This has included experiments confirming that the neurobehavioral effects of chronic sleep restriction are modulated by endogenous circadian phase—manifesting most severely at times of circadian “night” [23-25].
Remarkably, the cumulative deficits in vigilant attention performance that developed over 14 nights of sleep restricted to 4 h per night were comparable to those recorded after 3 nights (64-88 h) of total sleep deprivation [12], indicating that chronic partial sleep loss has the potential to induce waking brain deficits equivalent to even the most severe total sleep deprivation. These findings also suggested that the neurobiology underlying the behavioral effects of chronic sleep debt could continue to undergo long-term changes. Further evidence of such long time constants in homeostatic sleep pressure manifesting in waking neurobehavioral functions comes from an experiment by Rupp and colleagues [26] in which the amount of baseline nightly sleep obtained prior to chronic sleep restriction affected both the rate at which behavioral and physiological alertness was degraded and the rate at which these deficits were reversed by repeated nights of recovery sleep.
Neurobehavioral consequences of sleep loss
Both acute total and chronic partial sleep deprivation induce neurobehavioral changes in humans beyond subjective sleepiness, despite motivation to prevent these effects. The most reliable changes include increased lapses of sustained attention (i.e., errors of omission) and compensatory response disinhibition (i.e., errors of commission); psychomotor and cognitive slowing; working memory deficits; slow eyelid closures; and reduced physiological latency to sleep, even when it is being resisted [3,4]. A recent experiment by Lo and colleagues [14], and a meta-analysis [27], have called into question the claim that sleep loss primarily degrades executive functions and reasoning. High-order cognitive functions can be diminished by sleep loss, but when this occurs, it is likely mediated by deficits in the ability to sustain wakefulness, alertness, attention, and to respond accurately in a timely manner. Moreover, sleep deprivation may prevent the now well-documented benefits of sleep for memory consolidation [28].
The most sensitive measures of sleep loss appear to be those that precisely track moment-to-moment changes in neural indicators of state (especially EEG, EOG, and fMRI), or behavioral indicators of the stability of sustained attention, such as the psychomotor vigilance test (PVT). The latter has proven to be among the most sensitive measures of acute and chronic sleep loss [2,29] in part because it prevents compensatory stimulation and lacks the aptitude and learning affects that confound other cognitive measures. It also has the advantages of reflecting performance that has ecological validity (i.e., vigilant attention is required for learning, safe driving, etc.). These characteristics and performance parameter optimizations make the new brief PVT-B a rapid assay for tracking the dynamic interaction of sleep homeostatic drive and circadian phase relative to sleep loss [30]. As importantly, rodent versions of the PVT have recently been developed and validated to be sensitive to both acute total sleep deprivation [31] and chronic partial sleep loss [32], enhancing feasibility of translational studies.
Sleep deprivation and the two-process model
According to the two-process model [33] sleep-wake behavior is regulated by a homeostatic process S (integrating pressure for sleep during wakefulness that dissipates during sleep) and a circadian process C (modulating sleep pressure depending on time of day). The two-process model is a theoretical and mathematical description of sleep-wake dynamics [34]. It predicts that the homeostatic drive for sleep decays during sleep at a much faster exponential rate than its build-up during wakefulness, as putatively reflected in the intensification of sleep EEG slow wave activity (SWA). The accelerated recovery is evident in sleep SWA increasing well above pre-deprivation (baseline) levels after acute total sleep deprivation. A recent study by Banks and colleagues [13] revealed that this SWA response was much less dramatic following chronic partial sleep deprivation, accumulating modestly as sleep duration increased, exceeding pre-deprivation (baseline) levels only when sleep duration was increased to approximately 9-10 h. This finding is supported by recent experiments on recovery responses in chronically sleep-deprived rats [35,36], and humans [21,37-39]. Thus, both recovery sleep duration and elevated SWA are correlated with essential neurobiological elements of sleep homeostatic response and recovery. Critical questions that remain to be answered include: (1) why some neurobehavioral functions (e.g., subjective sleepiness) recover much faster than others (e.g., PVT performance stability); and (2) whether “recovery” actually “resets” the sleep homeostatic drive, or whether it harbors underlying neurobehavioral vulnerability to further sleep loss. Both of these issues are major gaps in our current understanding of the meaning of “recovery.”
While the neurobiology underlying escalating behavioral deficits induced by chronic partial sleep deprivation remains to be discovered, a promising advance recently has been made on the neurobiology of the two-process model prediction of a nonlinear interaction between process S and process C, which produces the dynamic modulation of neurobehavioral functions during acute total and partial sleep deprivation [23,24]. A new report from Paul Franken’s laboratory [40] provides evidence that forebrain expression of the clock gene PER2 responds to both sleep loss and time of day, making it a prime candidate for integrating C and S processes in the expression of neurobehavioral profiles during sleep loss.
Mathematical modeling of neurobehavioral dynamics
Modifications of the mathematical models based on the two-process model have been underway for two decades, in an effort to predict “safe” and “unsafe” work-rest schedules in a wide range of human activities (e.g., military, commercial transport and industrial operations) as part of Fatigue Risk Management Systems [41]. Among the challenges to these applications is that the two-process model predicts sleep SWA and neurobehavioral responses to acute total sleep deprivation, but it fails to adequately predict the dynamic degradation of performance observed during chronic sleep restriction. In an important development, McCauley et al. [42] recently showed that the two-process model belongs to a broader class of models formulated in terms of coupled non-homogeneous first-order ordinary differential equations. They proposed a new model that includes an additional component modulating the homeostatic process across days and weeks to better reflect the neurobehavioral changes observed under both acute total and chronic partial sleep loss (Figure 1).
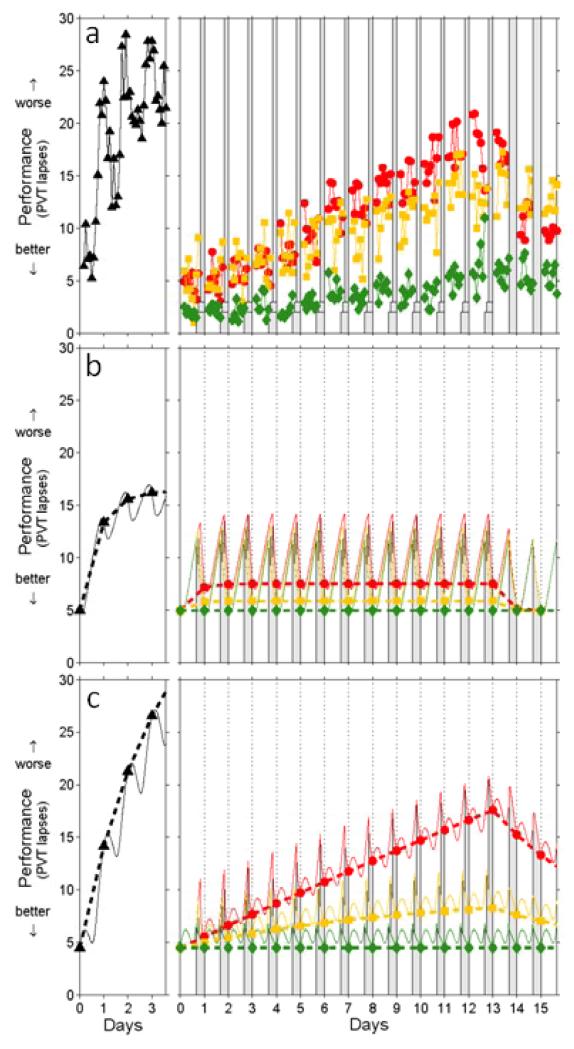
Figure 1.
Neurobehavioral performance observations and predictions by different models. A total of 48 healthy young adults were subjected to one of four laboratory sleep deprivation protocols [12]. Each protocol began with several baseline days involving 16 h scheduled wake time (SWT)/8 h time in bed (TIB); the last of these baseline days is labeled here as day 0. Subsequently, 13 subjects were kept awake (24 h SWT/0 h TIB) for three additional days, for a total of 88 h awake (left panels), after which they received varied amounts of recovery sleep (not shown). The other subjects underwent various doses of sleep restriction for 14 consecutive days, followed by two recovery days with 16 h SWT/8 h TIB (right panels). The sleep restriction schedule involved 20 h SWT/4 h TIB per day for 13 subjects (circles; red); 18 h SWT/6 h TIB per day for another 13 subjects (boxes; yellow); and 16 h SWT/8 h TIB per day for the remaining nine subjects (diamonds; green). Awakening was scheduled at 07:30 each day. Neurobehavioral performance was tested every 2 h during scheduled wakefulness using the PVT, for which the number of lapses (reaction times greater than 500 ms) was recorded. (a) Observed neurobehavioral performance (PVT lapses) for each test bout (dots represent group averages). The first two test bouts of each waking period are omitted in order to avoid confounds from sleep inertia. Gray bars indicate scheduled sleep periods. (b) Corresponding performance predictions according to the two-process model [34], linearly scaled to the data. Data points represent performance predictions at wake onset. Thin curves represent predictions within days, but the focus here is on changes across days (dashed lines). Note the rapid stabilization across days predicted to occur in the chronic sleep restriction conditions (right panel), which does not match the observations shown in (a). (c) Corresponding predictions according to the model introduced by McCauley et al. [42] as defined by their Eqs. (21) and (26). Note the improved fit to the experimental observations across days for total sleep deprivation (left panel), as well as for the 20 h SWT/4 h TIB condition (right panel). Performance impairment in the 18 h SWT/6 h TIB and 16 h SWT/8 h TIB conditions (right panel) is under-predicted. However, the group-average impairment levels observed for these conditions are inflated due to a few outliers [12]. (Figure and caption modified based on J Theor Biol 256 (2009), 227-239, McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HPV, A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance, Copyright 2009, with permission from Elsevier).
Importantly, this revised two-process model predicts a critical amount of daily wake duration of 20.2 h. If daily wake duration is above 15.8 h [12] but below 20.2 h (corresponding to a total sleep time of 3.8-8.2 h), the model converges over a period of weeks to an asymptotically stable equilibrium (i.e., performance impairment will stabilize). If daily wake duration is above 20.2 h, the model diverges from an unstable equilibrium and, similar to acute total sleep deprivation, performance impairment escalates [42]. The model also predicts the recent findings of Banks et al. [13] that a single night of recovery sleep is inadequate to recover from a prolonged period of sleep restriction (Figure 2). McCauley et al. speculate that adenosine receptor up-regulation (wakefulness) and down-regulation (sleep) could constitute the underlying neurobiological mechanism of longer time constants for behavioral changes from chronic partial sleep restriction [42].
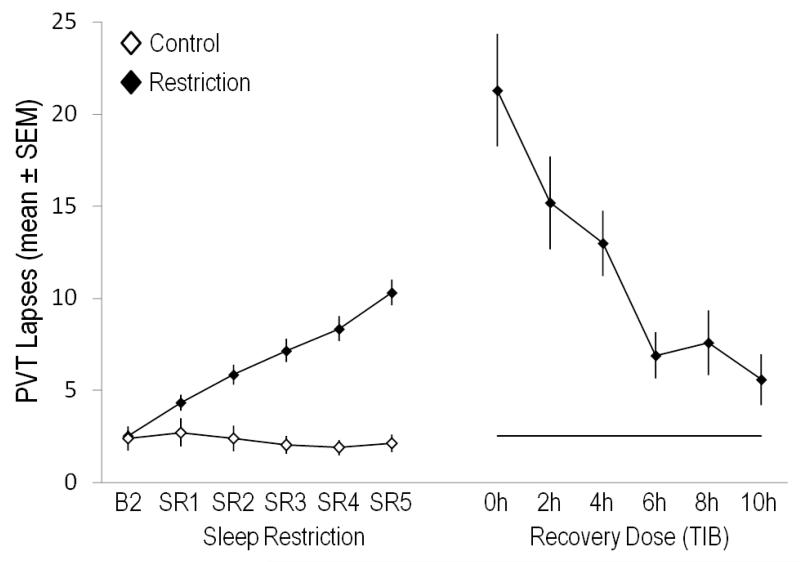
Figure 2.
Lapses of attention (reaction times ≥500 ms) on a 10-minute Psychomotor Vigilance Test (PVT) during a period of sleep restriction (5 nights with 4 hours time in bed [TIB], SR1-SR5, N=142 subjects, left panel) and on the day after one “recovery” night that varied by six different sleep doses (0 h, 2 h, 4 h, 6 h, 8 h, 10 h TIB, right panel). PVT performance lapses on B2 (10 h TIB) are also shown as a black horizontal line in the right panel for the group with restricted sleep. The control group (white diamonds, N=17) received 10 h TIB on all nights throughout the protocol. PVT lapses increased in a near-linear fashion during sleep restriction, further increased in a sleep-dose dependent manner after one night of 0 h (N=13), 2 h (N=27), or 4 h (N=29) TIB, and decreased in a sleep-dose dependent manner after one night of 6 h (N=25), 8 h (N=21), or 10 h (N=27) TIB. However, performance did not return to baseline levels even after a sleep dose of 10h TIB in the recovery night, suggesting that a longer TIB or more than one night are needed to fully recover from this degree of chronic sleep restriction. The figure is based on data from Banks et al. [13].
Phenotypic differential vulnerability to sleep loss
Recent evidence from our laboratory as well as from other groups has indicated large and highly replicable, trait-like individual differences in the magnitude of homeostatic sleep responses and waking measures of fatigue, sleepiness, and cognitive performance to both acute total [43,44] and to chronic partial sleep deprivation [12,45-47]. While some individuals are highly vulnerable to performance deficits when sleep deprived, others show remarkable levels of neurobehavioral resistance to sleep loss, and the remainder display intermediate responses [44,48] (Figure 3). Thus far, our laboratory studies indicate these responses occur as a normal distribution [43], which suggests they may be a polygenetic trait. However, our laboratory distribution may not reflect the distribution of responses in the general population, due to the self-selection bias of studies relying on volunteers (i.e., people are more likely to volunteer for sleep deprivation experiments if they feel they can cope with the sleep loss). Thus far, these differences have not been found to be evident in neurobehavioral functions at baseline when subjects are fully rested. Rather, inter-subject variability in waking measures of sleep loss (e.g., state instability evident in PVT lapse rates [2]) increases systematically as homeostatic pressure for sleep increases during acute and chronic sleep deprivation, exposing inter-subject differential vulnerability.
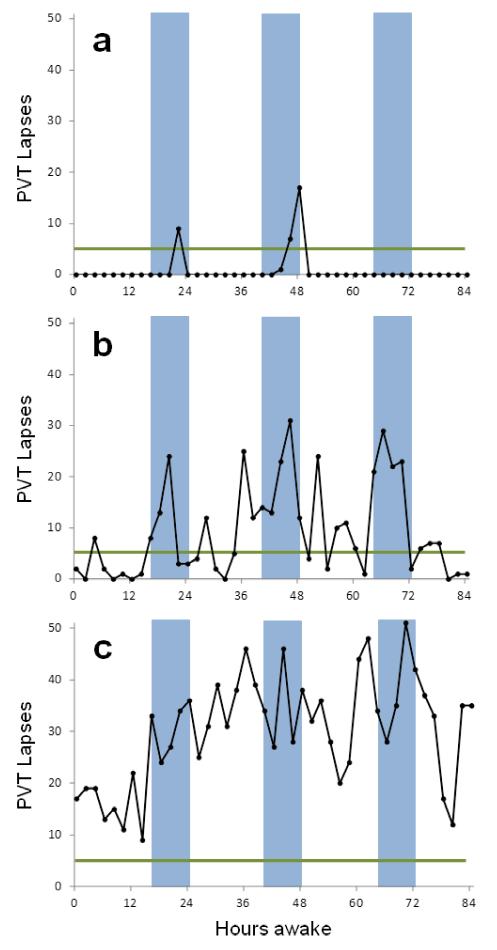
Figure 3.
Inter-individual differences in the vulnerability to sleep loss (unpublished data from David F. Dinges). The three male subjects in panels a-c performed a 10-minute psychomotor vigilance test (PVT) every 2 hours during an 88-h period of acute total sleep deprivation. The green horizontal line reflects 5 lapses (RTs ≥ 500 ms), and the blue bars indicate the period from 0000h to 0800h each day for deprivation. (a) This subject demonstrated a type 1 response, indicative of resilience to the effects of sleep loss. He had PVT lapses above baseline during only three test bouts in the period between 0600h-0800h near 24 h and 48 h awake. (b) This subject was somewhat vulnerable to the effects of total sleep deprivation (type 2 response), with more lapses during the night, but substantial improvement of PVT performance during the daytime (i.e., circadian rescue). (c) This subject was very vulnerable to the effects of sleep loss (type 3 response). PVT lapses were evident early in deprivation (which began for all subjects after a final baseline night of 8 h sleep disrupted by blood draws from an indwelling venous catheter every 1.5 h). As deprivation continued into the first night, his lapse rates escalated to very high levels, never returning to baseline levels. These inter-individual differences in vulnerability to sleep deprivation on a sensitive vigilant attention task were not accounted for by demographic factors, IQ or sleep need. Other studies of large numbers of healthy adults studied during chronic partial sleep deprivation also reveal systematic inter-individual differences in neurobehavioral vulnerability to sleep loss that have thus far have not been found to be predictable with psychometric scales [50].
It is not known whether the same individuals vulnerable to the adverse neurobehavioral effects of chronic partial sleep deprivation are also vulnerable to acute total sleep deprivation. Some studies have reported differences in behavioral, sleep homeostatic and/or physiological responses to chronic partial versus acute total sleep loss [12,15,49]. A few studies have systematically examined the same subjects undergoing both acute total and chronic partial sleep deprivation [14,16-19]. However, they report inconsistent results, likely due to small sample sizes, different populations, varying doses of sleep restriction, and different outcome measures.
The neurobiological bases of phenotypic differential vulnerabilities to sleep loss are unknown. Thus far, they have not been accounted for by demographic factors, IQ, habitual sleep duration, and psychometric scales [50]. However, the stable, trait-like inter-individual differences observed in response to acute total sleep deprivation have yielded intraclass correlation coefficients accounting for 58%-92% of the variance in neurobehavioral measures [43,44,51], which strongly suggests an underlying genetic component. Common genetic polymorphisms involved in sleep-wake, circadian, and cognitive regulation may underlie the large phenotypic differences in neurobehavioral vulnerability to sleep deprivation in healthy adults [3,50,52]. Two examples—one from a genetic variation involved in circadian regulation and one from a genetic variation involved in a cognitive regulation—illustrate this point.
The PERIOD3 VNTR polymorphism (PER3) has been reported by Derk-Jan Dijk’s laboratory to be associated with individual differences in sleep homeostatic and executive performance responses to acute total sleep loss [53,54]. More recently, we found that this polymorphism related to individual differences in sleep homeostatic responses, but not to performance responses to chronic partial sleep loss [45]. By contrast, two very recent studies [14,20] reported that PER3 is related to individual differences in neurobehavioral responses to sleep restriction. It remains uncertain whether differences in important methodological details— including the need for much larger replicate subject samples—underlie the discrepancies relative to PER3 as a marker for neurobehavioral vulnerability to sleep loss.
More work is needed on other potential genotypic markers of phenotypic vulnerability to sleep loss. We recently reported that the Catechol-O-Methyltransferase (COMT) Val158Met polymorphism predicted individual differences in sleep homeostatic responses to chronic sleep restriction [47], but such prediction has not been found for response to acute total sleep deprivation. [55]. A new experiment on Drosophila found that flies with high levels of protein kinase G (PKG) relative to the FORAGING gene (FORR) did not display deficits in short-term memory following 12 h of sleep deprivation, but their memory was more susceptible to disruption from starvation, suggesting that resistance to the effects of sleep deprivation may confer vulnerability to other environmental factors [56].
Brain metabolism and neural activity changes after sleep loss
Early investigations of the effects of sleep deprivation on brain metabolism and neural activation using Positron Emission Tomography (PET) found metabolic rate reductions in thalamic, parietal, and prefrontal regions during prolonged sleep loss [57,58]. More recent studies using blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) demonstrated significant decreases in regional brain activation during cognitive task performance following a night of total sleep deprivation, including reduced fronto-parietal activation during lapses on a visual selective attention task after sleep loss [59,60]. These activation changes were observed mainly in those vulnerable subjects with the larger performance deficits, while resilient individuals showed a trend toward increased parietal activation during performance lapses [59], suggesting a potential neurobiological compensatory mechanism after sleep loss (Figure 4). New PET studies on neurotransmitter receptors have observed down-regulation of striatal dopamine receptors [61] and increased cerebral serotonin receptor binding with sleep loss [62], which may reflect a complex adaptive brain response to sleep deprivation.
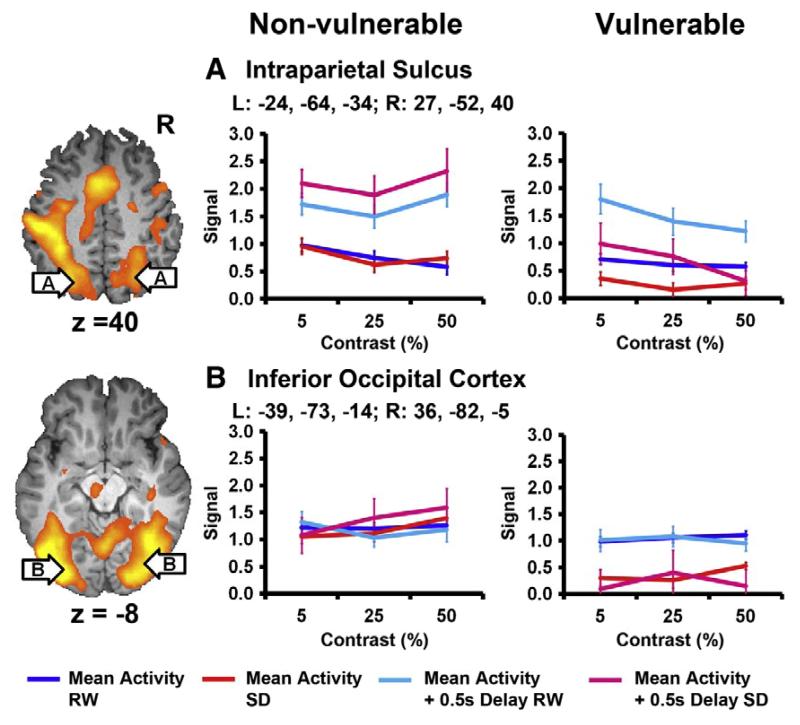
Figure 4.
Inter-individual differences in the brain activation responses to a night of total sleep loss. Twenty healthy young adults were scanned twice in the MR scanner while they performed visual selective attention tasks. One scan was at rested wakefulness (RW) after a normal night’s sleep, the other scan was after one night of acute total sleep deprivation (SD). The 20 subjects were median split into Vulnerable and Non-vulnerable (Resilient) groups, according to their change in PVT performance accuracy after sleep deprivation. Lapses refer to the trials that subjects’ responses were at least 0.5 s longer than the mean reaction time (+ 0.5s Delay). This figure shows the state-specific mean and lapse associated BOLD signal in the intraparietal sulcus (IPS) and inferior occipital cortex for the Vulnerable and Non-vulnerable groups. Lapses were associated with a stronger signal in the bilateral intraparietal sulcus during both RW and SD. Both regions showed a decline in activation following SD for the Vulnerable but not for the Non-vulnerable subjects. Figure and modified caption based on Neuroimage 51 (2010), 835-843, Chee MW, Tan JC, Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals, Copyright 2010, with permission from Elsevier.
Arterial spin labeled (ASL) perfusion fMRI permits non-invasive measures of absolute cerebral blood flow (CBF) that are tightly coupled to regional brain function [63], providing a method to quantify neural activity changes after sleep loss. We used ASL to quantify CBF changes after prolonged cognitive workload without sleep deprivation [64]. A recent study by Poudel and colleagues [65] used ASL to measure resting CBF changes after partial sleep deprivation. Significantly reduced fronto-parietal CBF was observed only in drowsy participants, while non-drowsy participants maintained fronto-parietal CBF and increased CBF in basal forebrain and cingulate regions following sleep deprivation. These results support a compensatory mechanism for drowsiness after sleep loss [65], which may be the difference between those resilient to sleep deprivation, versus those highly vulnerable to it.
Another emerging method for identifying the effects of sleep deprivation on brain activity is resting-state functional connectivity fMRI (FC-fMRI), which examines intrinsic spontaneous neural activity in the absence of external tasks. Recent FC-fMRI studies have consistently indicated an organized mode of resting brain function [66]. Two recent studies using FC-fMRI reported that sleep deprivation reduced functional connectivity within the default mode network (DMN) and between DMN and its anti-correlated network [67,68], suggesting that changes in brain functional connectivity occur as a result of sleep loss.
Currently, nearly all published neuroimaging studies have focused on acute sleep deprivation. There is a critical need to use the newer neuroimaging techniques to identify the dynamic effects of chronic sleep restriction and recovery on brain functions. Findings from the few ASL and resting-state FC-fMRI studies already provide some important new clues to what may be the basis for dynamic changes in neurobehavioral function during and following sleep loss.
Conclusions
This review highlights that there are fundamental differences in the way the central nervous system is affected by and adapts to acute total sleep deprivation and chronic partial sleep restriction. Although logistically challenging, more studies on the neurobehavioral and brain metabolic consequences of chronic sleep restriction (and recovery from it) are needed to improve our understanding of the neuromodulatory changes that recycling through periods of sleep loss induces in the brain, and to find ways to better mitigate the associated neurobehavioral and health consequences.
HIGHLIGHTS.
Acute total and chronic partial sleep loss have common and unique effects on brain and behavior.
Chronic sleep loss and recovery from it induce dynamic changes in physiology and behavior.
Mathematical models of sleep-wake regulation must include chronic effects of sleep duration and circadian modulation.
Vulnerability to sleep loss is substantial, apparently phenotypic, and therefore likely genetic.
Neural bases of the effects of sleep deprivation involve distributed networks and connectivity.
Acknowledgments
Funding provided to MB by the National Space Biomedical Research Institute through NASA NCC 9-58, to HR by NIH HL102119, to NG by ONR N00014-11-1-0361, and to DFD by NIH NR004281.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10:747–753. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 3.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin.Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J.Clin.Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 5.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra143. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath. 2012;16:829–833. doi: 10.1007/s11325-011-0583-0. [DOI] [PubMed] [Google Scholar]
- 7.Chao CY, Wu JS, Yang YC, Shih CC, Wang RH, Lu FH, Chang CJ. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism. 2011;60:799–804. doi: 10.1016/j.metabol.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. 2012 doi: 10.1038/hr.2012.91. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease C, Prevention Effect of short sleep duration on daily activities--United States, 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60:239–242. [PubMed] [Google Scholar]
- 10.Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30:1085–1095. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J.Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 13**.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: Dose-response effects of one night of recovery. Sleep. 2010;33:1013–1026. doi: 10.1093/sleep/33.8.1013. First systematic controlled laboratory evidence of the dose-response relationship between recovery sleep duration following chronic sleep restriction, using cognitive, behavioral, physiological and subjective outcomes. Sleep restriction degraded all neurobehavioral measures across days. Recovery was incomplete even at the longest sleep duration, when EEG slow wave energy was modestly increased above baseline.
- 14*.Lo JC, Groeger JA, Santhi N, Arbon EL, Lazar AS, Hasan S, von Schantz M, Archer SN, Dijk DJ. Effects of Partial and Acute Total Sleep Deprivation on Performance across Cognitive Domains, Individuals and Circadian Phase. PLoS One. 2012;7:e45987. doi: 10.1371/journal.pone.0045987. In this study subjects were exposed to acute total sleep deprivation twice, once after a period of sleep restriction (6 h time in bed per 24 h) and once after a period of sleep extension (10 h time in bed per 24 h), while controlling for circadian phase and stratifying by PER3 genotype. The study found significant differences in the sensitivity to sleep loss between subjective alertness, sustained attention, and a working memory task of varying complexity.
- 15.Drummond SP, Anderson DE, Straus LD, Vogel EK, Perez VB. The effects of two types of sleep deprivation on visual working memory capacity and filtering efficiency. PLoS One. 2012;7:e35653. doi: 10.1371/journal.pone.0035653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tassi P, Schimchowitsch S, Rohmer O, Elbaz M, Bonnefond A, Sagaspe P, Taillard J, Leger D, Philip P. Effects of acute and chronic sleep deprivation on daytime alertness and cognitive performance of healthy snorers and non-snorers. Sleep Med. 2012;13:29–35. doi: 10.1016/j.sleep.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Philip P, Sagaspe P, Prague M, Tassi P, Capelli A, Bioulac B, Commenges D, Taillard J. Acute versus chronic partial sleep deprivation in middle-aged people: differential effect on performance and sleepiness. Sleep. 2012;35:997–1002. doi: 10.5665/sleep.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–987. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- 19*.Rupp TL, Wesensten NJ, Balkin TJ. Trait-like vulnerability to total and partial sleep loss. Sleep. 2012;35:1163–1172. doi: 10.5665/sleep.2010. Important first study to determine responses to acute total sleep deprivation and chronic partial sleep deprivation in the same subjects using intra-class correlation coefficients. The authors found evidence for trait-like vulnerability and resistance to sleep loss across a number of cognitive and mood measures.
- 20.Rupp TL, Wesensten NJ, Newman R, Balkin TJ. PER3 and ADORA2A polymorphisms impact neurobehavioral performance during sleep restriction. J Sleep Res. 2012 Nov 21; doi: 10.1111/j.1365-2869.2012.01062.x. doi: 10.1111/j.1365-2869.2012.01062.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Haavisto ML, Porkka-Heiskanen T, Hublin C, Harma M, Mutanen P, Muller K, Virkkala J, Sallinen M. Sleep restriction for the duration of a work week impairs multitasking performance. J Sleep Res. 2010;19:444–454. doi: 10.1111/j.1365-2869.2010.00823.x. [DOI] [PubMed] [Google Scholar]
- 22.Mollicone DJ, van Dongen HPA, Rogers NL, Dinges DF. Response surface mapping of neurobehavioral performance: Testing the feasibility of split sleep schedules for space operations. Acta Astronautica. 2008;63:833–840. doi: 10.1016/j.actaastro.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen DA, Wang W, Wyatt JK, Kronauer RE, Dijk DJ, Czeisler CA, Klerman EB. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra13. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Zhou X, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, Roach GD. Sleep, Wake and Phase Dependent Changes in Neurobehavioral Function under Forced Desynchrony. Sleep. 2011;34:931–941. doi: 10.5665/SLEEP.1130. This study investigated the influence of circadian phase and time awake on vigilant attention in two forced-desynchrony protocols with 9.33 h and 4.67 h time in bed per 28 h, respectively. Decreases in attention were prominent during the biological night in the sleep restricted group, even when prior wake duration was short, highlighting the importance of the circadian system in modulating neurobehavioral performance during periods of chronic sleep restriction.
- 25.Mollicone DJ, Van Dongen HP, Rogers NL, Banks S, Dinges DF. Time of day effects on neurobehavioral performance during chronic sleep restriction. Aviat Space Environ Med. 2010;81:735–744. doi: 10.3357/asem.2756.2010. [DOI] [PubMed] [Google Scholar]
- 26**.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–321. doi: 10.1093/sleep/32.3.311. Deterioration on a Psychomotor Vigilance Task (PVT) during chronic sleep restriction was greater and recovery after sleep restriction was slower after one week of habitual sleep (7.09 h time in bed) compared to one week of extended sleep (10 h time in bed), suggesting that the physiological mechanisms underlying chronic sleep debt undergo long-term adaptive changes.
- 27**.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–389. doi: 10.1037/a0018883. This meta-analysis provides effect sizes for acute total sleep deprivation effects on various cognitive domains and on both speed and accuracy outcomes reported in 70 experimental studies (147 cognitive tests). Acute total sleep loss had no effect on the accuracy of reasoning, but it had major adverse effects on psychomotor speed and lapses of simple sustained attention. The effects on the speed and accuracy of memory tasks were in between these extremes. These findings suggest that deficits in sustained attention often presage other observable cognitive effects of acute sleep deprivation.
- 28.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 29.Basner M, Dinges DF. Maximizing sensitivity of the Psychomotor Vigilance Test (PVT) to sleep loss. Sleep. 2011;34:581–591. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basner M, Mollicone DJ, Dinges DF. Validity and sensitivity of a brief Psychomotor Vigilance Test (PVT-B) to total and partial sleep deprivation. Acta Astronautica. 2011;69:949–959. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christie MA, McKenna JT, Connolly NP, McCarley RW, Strecker RE. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J Sleep Res. 2008;17:376–384. doi: 10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker JL, Walker BM, Fuentes FM, Rector DM. Rat psychomotor vigilance task with fast response times using a conditioned lick behavior. Behav Brain Res. 2011;216:229–237. doi: 10.1016/j.bbr.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borbely AA. A two process model of sleep regulation. Hum.Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 34.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 35.Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–15944. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–10702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mander BA, Reid KJ, Baron KG, Tjoa T, Parrish TB, Paller KA, Gitelman DR, Zee PC. EEG measures index neural and cognitive recovery from sleep deprivation. J Neurosci. 2010;30:2686–2693. doi: 10.1523/JNEUROSCI.4010-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla Wu N, Darnall LA, Fallon JH, Bunney WE. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 39.Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D. The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res. 2007;16:33–41. doi: 10.1111/j.1365-2869.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 40**.Curie T, Mongrain V, Dorasz S, Mang GM, Emmenegger Y, Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36:311–323. doi: 10.5665/sleep.2440. This insightful experiment used sleep-deprived mice to evaluate whether time of day modulated the effects of increasing sleep pressure on clock-gene expression. The results of this study suggest that Per2 in the brain responds to both sleep loss and time of day, making it a prime candidate for the nexus of the sleep homeostat and circadian clock.
- 41.Dawson D, Ian Noy Y, Härmä M, Åkerstedt T, Belenky G. Modelling fatigue and the use of fatigue models in work settings. Accident Analysis & Prevention. 2011;43:549–564. doi: 10.1016/j.aap.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 42**.McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HPA. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. Journal of Theoretical Biology. 2009;256:227–239. doi: 10.1016/j.jtbi.2008.09.012. The authors extend the 2-process model of sleep-wake regulation to better reflect the neurobehavioral changes observed chronic partial sleep loss. Importantly, the extended model predicts a critical amount of daily wake duration of 20.2 h. If wake duration is less than 20.2 h on a chronic basis, neurobehavioral performance is predicted to converge to an asymptotically stable equilibrium, while it is predicted to diverge from an unstable equilibrium for longer than 20.2 h wake durations.
- 43.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 44.Van Dongen HP, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat.Space Environ.Med. 2004;75:A147–A154. [PubMed] [Google Scholar]
- 45*.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. This study was the first to investigate the role of the variable number tandem repeat (VNTR) polymorphism of the circadian gene PERIOD3 (PER3) in response to chronic sleep restriction in 129 subjects. The PER3 genotypes did not differ at baseline in habitual sleep, physiological sleep structure, circadian phase, physiological sleepiness, cognitive performance, or subjective sleepiness, although during sleep restriction, PER3 5/5 subjects had slightly but reliably elevated sleep homeostatic pressure. PER3 does not contribute to the neurobehavioral effects of chronic sleep loss, in contrast to reported effects following TSD.
- 46**.Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75:1509–1519. doi: 10.1212/WNL.0b013e3181f9615d. This study evaluated whether the DQB1*0602 allele, closely associated with narcolepsy, was a marker of interindividual differences in response to sleep restriction in 129 subjects. During baseline, although DQB1*0602-positive subjects were sleepier and more fatigued, they showed greater sleep fragmentation, and decreased sleep homeostatic pressure and sharper declines during the night. During chronic sleep restriction, DQB1*0602-positive subjects were sleepier and showed more fragmented sleep. DQB1*0602 positivity in a healthy population may be a genetic marker for predicting individual differences to sleep restriction.
- 47*.Goel N, Banks S, Lin L, Mignot E, Dinges DF. Catechol-O-Methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS One. 2011;6:e29283. doi: 10.1371/journal.pone.0029283. This study was the first to investigate the role of the catechol-O-methyltransferase (COMT) valine158methionine (Val158Met) polymorphism, involved in cognitive functions, in response to chronic sleep restriction. Met/Met subjects showed differentially steeper declines in non-REM EEG slow-wave energy (SWE)—the putative homeostatic marker of sleep drive—during sleep restriction, but the genotypes did not differ in cognitive and executing functioning, suggesting these measures may be orthogonal and associated with distinct genetic mechanisms.
- 48.Goel N, Dinges DF. Behavioral and genetic markers of sleepiness. J Clin Sleep Med. 2011;7:S19–S21. doi: 10.5664/JCSM.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abe T, Nonomura T, Komada Y, Asaoka S, Sasai T, Ueno A, Inoue Y. Detecting deteriorated vigilance using percentage of eyelid closure time during behavioral maintenance of wakefulness tests. Int J Psychophysiol. 2011;82:269–274. doi: 10.1016/j.ijpsycho.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Goel N. Genetics of sleep timing, duration, and homeostasis in humans. Sleep Medicine Clinics. 2012;7:443–454. doi: 10.1016/j.jsmc.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Kuna ST, Maislin G, Pack FM, Staley B, Hachadoorian R, Coccaro EF, Pack AI. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35:1223–1233. doi: 10.5665/sleep.2074. This study determined if the large and highly reproducible interindividual differences in rates of PVT performance during 38 hours of total sleep deprivation (TSD) are due to a heritable trait. Using 59 monozygotic and 41 dizygotic same-sex twin pairs, they found heritability (h2) of .836 and that 51.1% of twin variance in PVT response to TSD was attributed to combined additive and dominance genetic effects. Neither the variants in the PERIOD 3 gene or ADA gene explained variance in PVT responses to sleep loss.
- 52.Goel N, Dinges DF. Predicting risk in space: Genetic markers for differential vulnerability to sleep restriction. Acta Astronautica. 2012;77:207–213. doi: 10.1016/j.actaastro.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, von SM, Dijk DJ. PER3 polymorphism predicts sleep structure and waking performance. Curr.Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 54.Groeger JA, Viola AU, Lo JC, von SM, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 55.Bodenmann S, Xu S, Luhmann UF, Arand M, Berger W, Jung HH, Landolt HP. Pharmacogenetics of modafinil after sleep loss: catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 2009;85:296–304. doi: 10.1038/clpt.2008.222. [DOI] [PubMed] [Google Scholar]
- 56.Donlea J, Leahy A, Thimgan MS, Suzuki Y, Hughson BN, Sokolowski MB, Shaw PJ. Foraging alters resilience/vulnerability to sleep disruption and starvation in Drosophila. Proc Natl Acad Sci U S A. 2012;109:2613–2618. doi: 10.1073/pnas.1112623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 58.Thomas ML, Sing HC, Belenky G, Holcomb HH, Mayberg HS, Dannals RF, Wagner HN, Jr, Thorne DR, Popp KA, Rowland LM, et al. Neural basis of alertness and cognitive performance impairments during sleepiness: II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus & Related Systems. 2003;2:199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 59*.Chee MW, Tan JC. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage. 2010;51:835–843. doi: 10.1016/j.neuroimage.2010.02.031. This interesting study used conventional blood oxygenation level dependent (BOLD) fMRI to determine inter-individual differences in brain activation changes after one night of acute sleep deprivation. The authors found robust inter-individual differences in brain activation in the top-down attention network during SD lapses. Sleep-deprived vulnerable individuals showed a reduced fronto-parietal signal, while resilient individuals showed a trend for increased fronto-parietal activation.
- 60.Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J.Neurosci. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Benveniste H, Kim R, Thanos PK, Ferre S. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32:6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. Important study using PET to determine whether reduced dopamine D2/D3 receptor availability after sleep loss reflected dopamine increases or receptor down-regulation by comparing dopamine increases induced by methylphenidate during sleep deprivation versus rested sleep. The authors showed that dopamine increases induced by methylphenidate were associated with increased alertness and reduced sleepiness after sleep deprivation and did not differ between rested sleep and sleep deprivation.
- 62.Elmenhorst D, Kroll T, Matusch A, Bauer A. Sleep deprivation increases cerebral serotonin 2A receptor binding in humans. Sleep. 2012;35:1615–1623. doi: 10.5665/sleep.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Detre JA, Rao H, Wang DJ, Chen YF, Wang Z. Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging. 2012;35:1026–1037. doi: 10.1002/jmri.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–3435. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Poudel GR, Innes CR, Jones RD. Cerebral perfusion differences between drowsy and nondrowsy individuals after acute sleep restriction. Sleep. 2012;35:1085–1096. doi: 10.5665/sleep.1994. Important first study using arterial spin labeled (ASL) perfusion fMRI to determine resting cerebral blood flow (CBF) changes after one night sleep restriction. The authors found an overall reduction in regional CBF in the frontoparietal attentional network, which was largely driven by participants who showed strong signs of drowsiness in the eye-video following sleep restriction. In contrast, participants who remained alert following sleep restriction showed increased CBF in the basal forebrain and cingulate cortex.
- 66.Raichle ME. The restless brain. Brain Connect. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Samann PG, Tully C, Spoormaker VI, Wetter TC, Holsboer F, Wehrle R, Czisch M. Increased sleep pressure reduces resting state functional connectivity. MAGMA. 2010;23:375–389. doi: 10.1007/s10334-010-0213-z. This study was the first to use resting-state fMRI to determine resting brain functional connectivity responses to one night of partial sleep deprivation. The authors found reduced functional connectivity within and between the default mode network and its anti-correlated network after sleep restriction.
- 68*.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. This important study used both resting-state fMRI and conventional task-related fMRI to determine brain functional connectivity responses to one night of total sleep deprivation. The authors found reduced sleep deprivation functional connectivity within and between the default mode network (DMN) and its anti-correlated network both at rest and during task performance.