Abstract
Selectins are carbohydrate-binding adhesion molecules critically involved in leukocyte recognition of endothelium. The endothelial selectins have been implicated in homing of hematopoietic stem/progenitor cell(s) (HSPC) to the bone marrow (BM) during bone marrow transplant (BMT), but the precise role(s) of individual selectins in this process have never been defined. BMT of lethally irradiated mice lacking both endothelial selectins (E/P KO) with limiting numbers of wild-type BM cells rescued significantly fewer E/P KO than WT recipients, but higher numbers of transplanted WT cells rescued E/P KOs in a dose-dependent fashion. Short term homing assays confirmed a substantial defect in HSPC homing to BM in E/P KO mice. In contrast, BMT of E-selectin null (E KO) or P-selectin null (P KO) mice at limiting cell number uniformly rescued >95% of the transplanted animals. Consistent with these functional results, flow cytometric analysis revealed both E-selectin ligands and P-selectin ligands on distinct subsets of HSPC. Taken together, these results demonstrate overlapping functions for the endothelial selectins in HSPC homing to BM in the setting of BMT, and define a novel aspect of HSPC heterogeneity linked to selectin ligand expression.
Keywords: hematopoietic stem cell, selectin, bone marrow transplantation, homing
INTRODUCTION
Leukocyte-endothelial recognition is controlled by several different families of molecules that govern distinct steps in the overall process of leukocyte recruitment [1]. The initial steps of interaction between blood borne leukocytes and the vessel wall are mediated by selectins, a family of carbohydrate-binding adhesion molecules whose attachment and rolling activity is crucial for the subsequent steps of leukocyte activation, firm adhesion and transmigration [2, 3]. L-selectin is expressed exclusively on leukocytes, whereas both E- and P-selectin are expressed on activated endothelium, and P-selectin is also expressed on activated platelets. Numerous studies firmly establish the critical importance of L-selectin in normal homeostatic lymphocyte recirculation, and all three selectins function in the tissue-specific recruitment of all classes of leukocytes to sites of inflammation in specific tissues. Thus, inhibition of selectin activity by monoclonal antibodies (mAb) or by targeted gene disruption inhibits leukocyte recruitment in a variety of settings of acute and chronic inflammation [4-9].
Hematopoietic reconstitution via transplantation of bone marrow or mobilized peripheral blood is a widely used clinical intervention for hematological disorders that depends upon the ability of intravenously infused hematopoietic stem and progenitor cells (HSPC) to home from the blood to the marrow cavity to re-establish productive hematopoiesis. Despite its clinical value, molecular mechanisms governing HSPC homing in the context of bone marrow transplantation (BMT), or even during steady-state hematopoiesis [10], remain incompletely defined. Both E- and P-selectin are constitutively expressed on the endothelium of murine bone marrow sinusoids [11, 12], although in distinct patterns [13], and one or both are required for efficient homing of HSPC to BM [14]. A critical role for VLA-4/VCAM-1 interactions in murine HSPC homing to BM is also well documented [12, 14, 15]. However, existing studies do not address the specific, possibly unique, roles of individual selectins in HSPC function, and their distinct patterns of expression [13] raise the possibility of unique functions. In the present study, we analyzed the role of individual endothelial selectins in the homing of HSPCs to BM during BMT, and detailed the expression of E- and P-selectin ligands on highly enriched hematopoietic stem cells and progenitor populations.
MATERIALS & METHODS
Mice
C57BL6/J mice expressing the CD45.1 allotypic marker (congenic C57BL6/J mice are normally CD45.2) were purchased from Jackson Labs and were bred and maintained in our colony. Mice with homozygous null mutations in either E-selectin, P-selectin, or both E- and P-selectin (E KO, P KO or E/P KO, respectively) [8] backcrossed to C57BL6/J were kindly supplied by Dr. Dan Bullard, UAB, Birmingham AL, and were bred and maintained in our colony. Mice were 4-8 weeks old when used. Both sexes were used for these experiments, but were never mixed in BMT experiments (i.e. male mice received BM from male mice only, and female mice received BM from female mice only).
Bone marrow transplantation (BMT)
Total BM cells were obtained from CD45.1 mice by flushing femurs and tibia with ice cold HBSS/2% FCS followed by hypotonic lysis of erythrocytes. Mice (n = 10-12) of the indicated genotypes were irradiated with 1100 Rads in split doses 3-4 hours apart using a Cs137 source and transplanted within 2 hours with the indicated numbers of BM cells by intravenous injection into the tail vein under sterile conditions. Irradiation control mice were irradiated as above and were either transplanted with PBS or were not transplanted. All BMT mice were maintained on a combination of neomycin, tetracycline and trimethoprim for at least 4 weeks following BMT, by which time all deaths following BMT had occurred. No evidence of graft vs host disease, infection, or other complication was observed upon necropsy for any animals in the results reported here. Surviving mice were followed for up to 6 months, and were then sacrificed for FACS analysis of donor contribution to the hematopoietic compartment.
CFC assays
BM was isolated at various timepoints from mice that had undergone BMT, and was plated at 5 × 104, 105 and 2 × 105 cells/dish in duplicate in 0.9% methylcellulose containing either PWM-conditioned media (myeloid colonies) or IL-7-containing media (pre-B colonies) (Stem Cell Technologies, Vancouver, BC, Canada), according to the manufacturer’s instructions. Colonies were identified by standard morphologic criteria and enumerated single blind after 7-8 days (pre-B), 8-10 days (CFU-GM) or 14 days (BFU-E) by individuals unfamiliar with the identity of experimental groups.
Flow cytometry
To ascertain the contribution of donor cells to hematopoietic reconstitution in mice following BMT, two color FACS analysis was performed, for CD19 and separately CD11b vs CD45.1 (donor) and CD45.2 (host). BM was isolated as above from mice 4-6 months after BMT, and 5 × 105 cells were stained on ice with empirically determined optimal concentrations of PE-conjugated mAb to mouse CD19 or CD11b, or FITC-conjugated mAb to CD45.1 or CD45.2 (BD Biosciences). The percent of CD19+ or CD11b+ cells that were CD45.1+ was then determined, and considered to represent donor contribution.
For analysis of selectin ligand expression on HSPCs, bone marrow mononuclear cells were enriched by magnetic selection for HSPCs by either c-Kit positive selection or lineage depletion (using biotinylated antibodies to the lineage markers CD3, CD4, CD5, CD8, CD19, B220, Mac-1 (CD11b/CD18), Gr-1, and Ter119). C-kit selection was performed using a Miltenyi Biotech AutoMACS and anti-c-Kit microbeads; lineage depletion was performed using a Dynal magnetic particle concentrator (MPC-L) and sheep anti-rat IgG Dynabeads (Invitrogen). HSPC-enriched BM cells were stained with either E-selectin receptor-IgG (as tissue culture supernatant from CHO cells stably transfected with the E-RIgG construct; kindly supplied by Dietmar Vestweber) or P-selectin receptor-IgG [16] (BD Biosciences) and appropriate AF647- or AF488-conjugated anti-human IgG (Jackson Immunoresearch), followed by rat IgG to block unbound sites on the 2nd step. Cells were then stained with PE-TR-conjugated streptavidin (to detect lineage antibodies) and antibodies to c-Kit (APC-Cy7), Sca-1 (PE-Cy7), CD150 (PE or APC), and CD48 (FITC or Pacific Blue) or CD44 (PerCP-Cy5.5) and CD162 (PE) (eBioscience).
Statistical analysis
Differences between groups in Kaplan-Meier plots were analyzed in a pairwise fashion with the log rank test, using the Curve Comparison function within the Prism4 program. All other statistical comparisons were by pairwise 2 way Student’s t-test. Coefficients of correlation (Pearson’s product-moment correlation coefficients) were calculated in Microsoft Excel using .fcs files exported from FlowJo.
RESULTS
Function of endothelial selectins in rescue and reconstitution of mice undergoing BMT following lethal irradiation
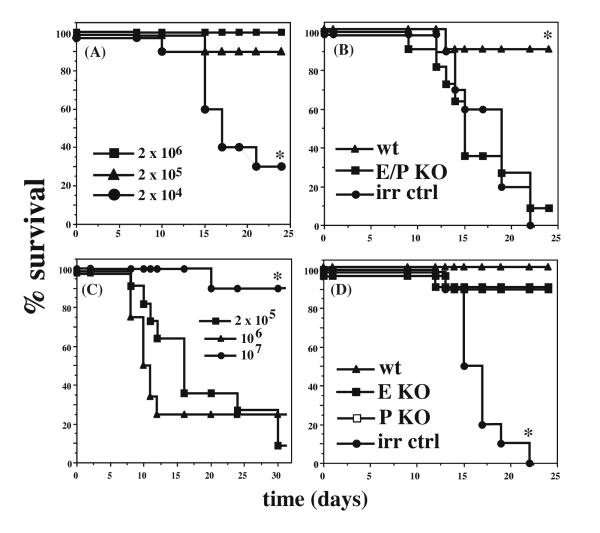
To identify the most sensitive system for detection of altered homing or reconstitution by HSPCs, we first sought to define the lowest dose of BM cells required for complete or nearly complete rescue of lethally irradiated C57BL6/J mice. Groups of 10-12 mice were lethally irradiated and injected with graded numbers of BM cells from CD45.1 mice of the same sex. All mice receiving 2 × 106 BM cells, and 90% of mice receiving 2 × 105 cells, survived for >4 weeks. However, at cell doses below 2 × 105 total BM cells/mouse, survival of lethally irradiated mice past 4 weeks fell precipitously, with <30% of mice surviving after transplant with 2 × 104 BM cells (fig 1A, p<0.01). These results are consistent with those previously reported by others [14, 17]. With the exception of a single mouse, all irradiation control mice, which were lethally irradiated but not transplanted, died within 25 days, with most dead by day 17. These kinetics of mortality are consistent with death due to hematopoietic failure, and examination of mice at necropsy revealed no evidence of graft-vs.-host disease or disseminated bacterial infection.
Fig 1. Role of endothelial selectins in rescue of lethally irradiated mice.
Kaplan-Meier plots of survival following BMT of WT and selectin-deficient mice. (A) WT mice were lethally irradiated and transplanted with the indicated numbers of WT (CD45.1+) BM cells. (B) WT or E/P KO mice were lethally irradiated and transplanted with 2 × 105 BM cells or WT mice were irradiated but not transplanted (irr ctrl). (C) E/P KO mice were irradiated and transplanted with increasing numbers of WT BM cells, as indicated. (D) WT, E KO or P KO mice were transplanted with 2 × 105 WT BM cells or WT mice were irradiated but not transplanted (irr ctrl). Each plot represents 1 representative experiment out of at least 4, except for (C), which was performed three times. * indicates statistically different from all other groups (p < 0.01 by log rank test).
We next examined the survival of mice deficient in both E- and P-selectin [8] (E/P KO) after lethal irradiation followed by BMT with wild-type (WT) BM cells. Consistent with previous studies [14], E/P KO mice transplanted with 2 × 105 BM cells survived poorly after BMT, with only ~20% of mice surviving >4 weeks (fig 1B, p<0.01). The kinetics of death in the E/P KO group was indistinguishable from that of the irradiation controls, suggesting death from hematopoietic failure, and examination of these mice at necropsy also failed to show any evidence of graft-vs.-host disease or disseminated bacterial infection. To determine if the engraftment defect we observed in E/P KO recipients could be overcome by transplantation with higher cell numbers, we examined survival post-transplant of lethally irradiated E/P KO mice receiving 106 or 107 WT BM cells. E/P KO mice transplanted with 106 cells showed equivalently high rates of mortality as E/P KO mice transplanted with 2 × 105 BM cells, whereas transplant of E/P KO mice with 107 BM cells/mouse rescued all or nearly all recipient animals (fig 1C). The requirement for higher doses of transplanted cells to rescue lethally irradiated E/P KO mice likely reflects inefficient homing and/or engraftment of HSPCs in the absence of both endothelial selectins. These results confirm previous findings [14] indicating that lethally irradiated E/P KO mice engraft poorly when transplanted with limiting numbers of WT BM cells following lethal irradiation.
These results strongly support the hypothesis that the endothelial selectins are important for homing of HSPCs to BM in the context of lethal irradiation and BMT, but do not determine whether it is the absence of E-selectin, P-selectin, or both, which is responsible for the defective HSPC homing and hematopoietic reconstitution in E/P KO animals. Results of hematopoietic reconstitution following BMT in mice deficient in individual endothelial selectins have not been previously reported. We therefore carried out BMT experiments using mice deficient in either E-selectin or P-selectin as recipients. In contrast to the results with E/P KO mice, E KO and P KO mice survived as well as WT mice after BMT using limiting numbers (2 × 105 WT BM cells/mouse) of transplanted cells, with >90% survival in all three groups (fig 1D). Cumulative results of all experiments performed with 2 × 105 BM cells/mouse for all recipient genotypes are summarized in Table 1.
Table 1. Cumulative survival of WT and selectin-deficient recipients following lethal irradiation and BMT with 2 × 105 BM cells.
Summary of all BMT experiments performed in this study using a dose of 2 × 105 BM cells/mouse. Experiments using lower or higher doses of cells are excluded from this analysis. *Different (p<0.05) from all other groups by pairwise Student’s t test.
| genotype: | |||||
|---|---|---|---|---|---|
|
| |||||
| WT | E KO | P KO | E/P KO | WT (Irr ctrl) | |
| Number of experiments | 9 | 5 | 6 | 4 | 5 |
|
| |||||
| Number surviving/total | 84/92 | 47/52 | 52/65 | 9/43* | 1/50* |
| (%) | (91.3) | (90.3) | (80) | (20.9) | (2) |
Equivalent donor cell contributions to long term reconstitution in surviving selectin-deficient mice transplanted with WT BM cells
To address the possibility that surviving mice, particularly in the E/P KO recipient cohort, were rescued in part by contribution from residual host HSCs [17, 18], we analyzed the relative contribution of donor vs. host cells to reconstitution of the hematopoietic compartment in surviving mice 3-6 months after BMT. BM cells were stained in two colors for either CD19, a pan-B cell marker expressed early in B cell development, or CD11b, a pan-myeloid marker expressed early after myeloid commitment, and for either CD45.1 (donor) or CD45.2 (host) (Table 2). For nearly all mice across all recipient genotypes, the vast majority of both B lymphoid and myeloid cells were of donor origin, with a small but reproducible host component present in virtually all mice examined. These data indicate that in both WT and selectin-deficient recipients, mice were reconstituted by donor LT-HSCs, which homed to BM, leading to engraftment and long-term reconstitution of the hematopoietic compartment, independent of recipient genotype or surviving fraction.
Table 2. Donor contribution to long term surviving mice following BMT.
Mice surviving for at least 3 months following BMT, as indicated in Table 1, were sacrificed, BM was harvested and stained for donor (CD45.1) or host (CD45.2) cells within the B cell (CD19+) or total myeloid cell (CD11b+) compartment. Data are presented as mean +/− SD. There are no statistically significant differences between any groups (p>0.05 by Student’s t test for all pairwise comparisons).
| genotype: | ||||
|---|---|---|---|---|
| WT | E KO | P KO | E/P KO | |
| Number analyzed | 28 | 21 | 25 | 7 |
|
| ||||
| B cell (CD19+, %) | 88.9 +/− 14.5 | 91.2 +/− 15.7 | 89.6 +/− 17.3 | 80.3 +/− 17.3 |
| Myeloid (CD11b+, %) | 72.9 +/−30 | 90.2 +/− 21 | 86 +/− 26 | 76.4 +/− 30 |
Impaired homing of HSPC to BM of E/P KO mice
Our data demonstrate that expression of either E-selectin or P-selectin is sufficient for full rescue and radioprotection of lethally irradiated mice following BMT at limiting cell number, thereby suggesting an overlapping function for endothelial selectins in HSPC homing to murine bone marrow. To examine more directly the issue of HSPC homing in the absence of endothelial selectins, we next quantified the number of hematopoietic colony forming cells (CFU-C) detectable in the BM of WT recipients following ablative irradiation and BMT at early time points, prior to death from hematopoietic failure. WT mice were irradiated at 1100rad and transplanted with either 2 × 105 or 2 × 106 unfractionated BM cells. As expected, control experiments showed zero CFU-C in irradiated, untransplanted animals (data not shown). Very few CFU-C were detected one day after BMT (fig 2A). When analyzed at later timepoints, however, the total number of CFU-C increased logarithmically until at least day 7, and was proportional to the input dose of BM cells for all timepoints (fig 2A), reaching the level of normal unmanipulated WT bone marrow within two weeks (data not shown). These data establish a time course for the normal homing of CFC precursors, which include HSC, to the BM following intravenous injection into irradiated recipients, and confirm that the homed cells subsequently give rise to more differentiated cells, which can then be detected in conventional CFU-C assays. Based on these data, we next determined the number of CFU-C detectable in the BM of WT or E/P KO mice 7 days following BMT with 2 × 106 WT cells, to evaluate possible differences in the efficiency of HSPC homing in transplanted E/P KO mice. For all CFU-C types examined, including CFU-GM, BFU-E and CFU-pre-B, the number of CFUs in the E/P KO recipients was diminished by 70-80% (fig 2B, p< 0.05). These data are closely concordant with previous work [14], and clearly show that homing to bone marrow of HSPC (which subsequently give rise to CFU-C) is substantially impaired in E/P KO mice.
Fig 2. Short term homing of HSC/HPC to BM requires endothelial selectins.

(A) Mice were irradiated and transplanted with either 2 × 105 or 2 × 106 WT BM cells, as indicated, sacrificed on the indicated days (either 1, 4 or 7), and total CFU/femur were enumerated as described in Materials & Methods. (B) Mice were irradiated and transplanted with 2 × 106 WT BM cells, and sacrificed on day 7. CFU/femur were enumerated for each class (myeloid, lymphoid, erythroid) of hematopoietic lineage. Filled columns, WT; hatched columns, E/P KO.
Expression of selectin ligands on HSCs and HPCs
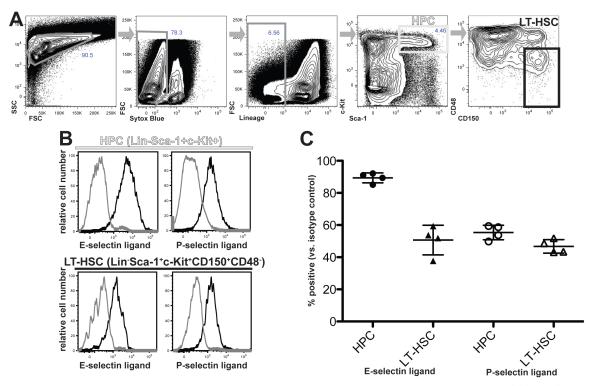
The data above implicate E- and/or P-selectin as critical homing receptors in the trafficking of HSPC to BM. To begin to dissect the relative importance of these two adhesion molecules in the migration of various subsets of HSPCs, we used 7-color flow cytometry with selectin-IgG chimera proteins to quantitatively assess the expression and distribution of E- and P-selectin ligands on primitive hematopoietic cells. HPC were defined as lineage marker-negative, Sca-1+, c-kit+ (LSK) BM cells, and within this subset, the most primitive, long term-reconstituting HSC (LT-HSC) were defined as LSK, CD48-negative, CD150-positive [17, 19] (fig 3A). E-selectin ligands were detected on a majority of LSK cells (89.4+/−3.1%, n=4) and on a significant fraction of LT-HSC (50.7+/−9.2%, n=4) isolated from the BM of WT mice (fig 3B and 3C). Similarly, P-selectin ligands were easily detected on LSK cells (55.4+/−4.5% n=4) and LT-HSC (46.7+/−4.2% n=4) (fig 3B and 3C). These data are consistent with the BMT data presented above, yet indicate that only a subset of HSPC, including a subset of LT-HSC, expresses ligands for both endothelial selectins. Thus, HSPC exhibit heterogeneity with respect to selectin ligand expression that likely influences their migratory capacity and contributions to hematopoietic reconstitution following BMT.
Fig 3. Expression of selectin ligands on HSPCs.
Hematopoietic cells from WT mice were harvested from long bones and pelvic bones and enriched for HSPCs by magnetic bead selection, as described in Materials and Methods. (A) Strategy for discrimination of mouse HPC and LT-HSC. HSPC-enriched cells were stained in 7 colors for viability (Sytox Blue dye), lineage markers (see Methods), c-Kit, Sca-1, CD48, and CD150. These markers were used in sequential gating, left-to-right as indicated by grey arrows, to differentiate Lin−Sca1+c-kit+ HPC (light grey gate) and Lin−Sca1+c-kit+CD150+CD48− LT-HSC (black gate). (B) Expression of E-selectin ligands or P-selectin ligands on HPCs or LT-HSCs, gated as in (A) and detected using IgG chimera reagents. Data are depicted as histograms of chimera staining intensity for HPCs or LT-HSCs stained with the indicated selectin chimera (black trace) or with secondary alone as a negative control (grey trace). (C) Summary of flow cytometry data, depicting individual data points (circles or triangles) and mean (+/− SD) percentage of HPC or LT-HSC showing positive staining for the indicated selectin ligand.
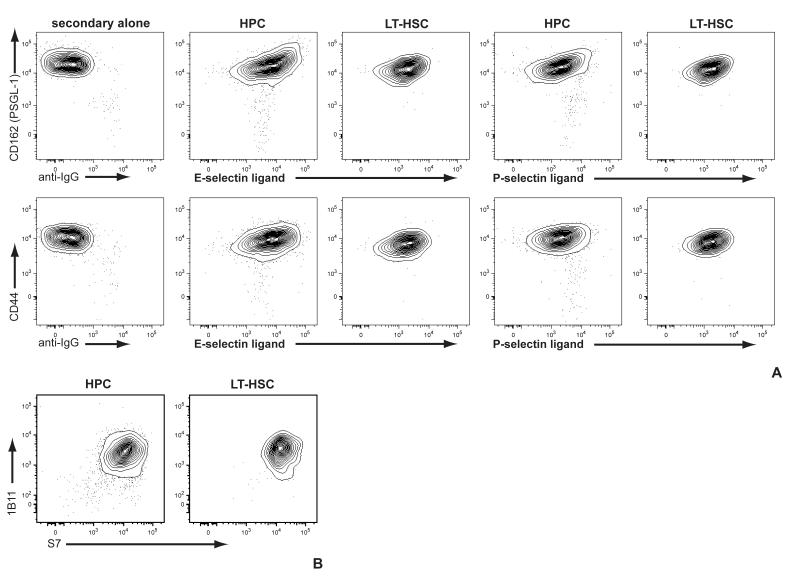
To determine whether expression of selectin ligands on HSPC was associated with upregulation of specific glycoprotein scaffolds, we examined the correllated expression of E- and P-selectin ligands with CD44 and PSGL-1. We found minimal correlation (r < 0.4) between either CD44 or PSGL-1 and ligands for either E- or P-selectin (fig 4A). Similarly, levels of CD43, which has also been implicated as an E-selectin ligand scaffold on HSPC [20], were uniformly high (fig 4B), and uniformly exhibited high levels of core 2 O-glycosylation (fig 4B). These data show that the heterogeneity of expression of selectin ligands on HSPCs is not a function of selective expression of these known or candidate glycoprotein scaffolds.
Fig 4. Expression of selectin ligand glycoprotein scaffolds does not determine selectin ligand expression on HSPC.
HPC and LT-HSC were identified as in fig 3 and analyzed for (A) E- or P-selectin ligands vs either PSGL-1 or CD44; or (B) O-glycosylated (1B11) vs non-O-glycosylated (S7) CD43.
DISCUSSION
Molecular mechanisms governing HSC traffic, including the homing of HSC to specific microenvironments in the BM, remain incompletely understood. For mature leukocytes, it is well established that a cascade of overlapping molecular interactions underlies the distinct steps of leukocyte attachment, rolling, progressive slowing, arrest, and transmigration. Selectins mediate the initial steps of attachment and rolling, following which progressive activation of leukocyte integrins by lumenal chemokines leads to their slowing and eventual arrest [1]. To a significant extent, this model has been thought to apply to HSPC homing to bone marrow as well. Specifically, attachment and rolling of HPC/HSC is thought to involve one or both endothelial selectins, with VLA-4 (α4β1) integrin mediating firm adhesion via interaction with VCAM-1 [12, 14, 15]. The chemokine SDF-1 (CXCL12) and its receptor CXCR4 play important roles in both induction of firm adhesion as well as maintenance of progenitors within appropriate marrow microenvironments [21]. Thus, homing of HSC/HPC to BM appears to follow similar rules as recruitment of mature leukocytes to sites of inflammation and normal lymphocyte recirculation [1, 22].
Despite this, the particular roles of the endothelial selectins have not previously been individually evaluated, and the expression of selectin ligands on HSC has not been studied. We present the first genetic evidence showing that expression of either E-selectin or P-selectin alone is sufficient to support rescue and hematopoietic reconstitution of lethally irradiated recipients following BMT. Single selectin deficient mice survived as well as WT mice under conditions in which limiting numbers of BM cells were transplanted. In contrast, doubly-deficient E/P KO mice showed significantly reduced survival under the same experimental conditions. Importantly, this impaired survival could be overcome by increasing the number of BM cells transplanted, suggesting a defect in the efficiency of HSPC homing to BM in E/P KO recipients.
Consistent with this hypothesis, direct homing studies of HSPC in E/P KO mice demonstrated a substantial impairment in the rapid homing to BM of HSPC which give rise to CFU-C. In WT mice, donor-derived reconstitution of BM CFU-C was dose-dependent and led to restoration of normal levels of BM CFU-C within two weeks. In E/P KO recipients at day 7 after transplant, the levels of CFU-C of all lineages examined were 70-80% lower than WT. These data are consistent with a defect in HSPC homing to BM in the absence of functional endothelial selectins in the recipient animals. Our data thus reinforce the idea [14] that endothelial selectins play an important role in efficient homing of HSPCs during BMT.
Papayannopoulou and colleagues have argued [23] that the death of E/P KO mice following lethal irradiation and BMT seen in previous work [14] was attributable solely or primarily to infectious complications and not to defective HSPC homing, and hence that endothelial selectins are not critical for HSPC homing to BM. Several lines of evidence argue against this conclusion, and support the idea that E/P KO mice that died following BMT died of hematopoietic failure rather than from bacterial infection, at least in the present study. First, all of the BMT mice in this study were maintained on multiple broad-spectrum antibiotics for at least four weeks after transplant, by which time all BMT-associated deaths had occurred. Second, the kinetics of death in transplanted animals were indistinguishable from the kinetics of death in the irradiation control group (which consisted of WT animals), and this was independent of the genotype of the recipient or the number of transplanted BM cells. Infectious disease would be expected to act both more quickly and less synchronously. Third, although E/P KO mice sometimes experience chronic subclinical bacterial infections, these infections are limited to the skin; the blood and internal organs of these mice are routinely sterile [8]. Papayannopoulou and colleagues argued that the high neutrophil counts of the post-BMT animals was indicative of ongoing infection. However, this conclusion preceded the understanding that high neutrophil counts are a characteristic and stable feature of E/P KO animals, as well as other mice with genetically engineered disruptions of neutrophil recruitment [5, 8, 24-26], suggesting that the high neutrophil counts in E/P KO recipients following BMT simply represent restoration of the “normal”, unperturbed E/P KO hematopoietic phenotype. Indeed, more recent work has clearly shown that high neutrophil counts in adhesion molecule-deficient mice occur due to interruption of a feedback loop that maintains blood neutrophil counts within a narrow window, and thus are unrelated to infection [27]. Finally, and most compellingly, E/P KO mice transplanted with higher numbers of BM cells survived, which is inconsistent with the idea that infectious complications are a primary factor in the deaths of mice undergoing BMT. Taken together, these considerations make it unlikely that fatal bacterial infections played a significant role in either the present study or that of Frennette and colleagues [14], and reinforce the idea that endothelial selectins play a critical role in HSPC homing to BM.
The survival of both E-selectin KO and P-selectin KO mice following lethal irradiation and BMT implies the expression and function of both E-selectin ligands and P-selectin ligands on at least a subset of HSPC. This inference was directly confirmed by flow cytometric detection of E-selectin and P-selectin ligands on HPCs and LT-HSCs. These results are consistent with a recent report that also found P-selectin ligands expressed at a low level on HSC [28], and with the BMT data presented above showing that survival of single selectin KO mice is equivalent to that of WT following lethal irradiation and BMT. That report also showed an increase in P-selectin ligands with differentiation, consistent with our finding that LSK cells express higher levels of both E- and P-selectin ligands. Since expression levels of known selectin ligand scaffold glycoproteins are largely constant during early hematopoiesis and do not strongly correlate with levels of selectin ligands, it is likely that this enhanced expression of the selectin ligands on HPC vs LT-HSC is primarily a consequence of rising levels of relevant glycosyltransferases which participate in selectin ligand biosynthesis [25, 29-31].
Available evidence strongly indicates that PSGL-1 is the sole P-selectin ligand on both mature leukocytes and HSPC [32-37], but the nature and identity of E-selectin ligands on HSPC remains poorly understood. A number of glycoproteins have been suggested as E-selectin ligands on one or more hematopoietic cell types, including CD44, PSGL-1, CD43, and ESL-1 [20, 38-43], and glycosphingolipids may also function as E-selectin ligands [44, 45]. Which of these are relevant for HSPC is still unclear, and may vary with cell type, species, and/or state of differentiation. One recent report [20] identified CD43 and PSGL-1, but not CD44, as E-selectin ligands on mouse HPC, although no functional data were provided. These investigators also failed to detect ESL-1 on these cells [20]. In contrast, another report [45] detected ESL-1 on HPC, but whether ESL-1 functions as an E-selectin ligand on these cells remains unclear.
More interestingly, this report [45] indicated that neither PSGL-1 nor CD44 were responsible for a novel function of E-selectin as a regulator of HSC quiescence, and suggested that glycosphingolipids may subserve this function. These findings add complexity to the identification of E-selectin ligands by raising the possibility that the ligands that act during homing of HSPC to the marrow niche are distinct from those that enable the non-homing functions of E-selectin within the niche. These functions of selectins may similarly be important for the migration and function of long-lived plasma cells [46] and subsets of effector/memory T cells [47], as the bone marrow serves as an important reservoir for these more differentiated hematopoietc cells as well [48, 49]. Thus, future studies to further dissect selectin ligand heterogeneity will help to clarify the shared and distinct mechanisms that maintain and support homeostatic migration of multiple classes of primitive and mature leukocytes to BM.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Joslin’s HSCI/DERC Flow Cytometry Core (NIH Award Number P30DK036836) for excellent flow cytometry support and H. Ziltener for helpful advice during preparation of the manuscript. AJW is an Early Career Scientist of the Howard Hughes Medical Institute. LDW is a Damon Runyon-Sohn Foundation Fellow supported by the Damon Runyon Cancer Research Foundation (DRSG 2-12) and the St. Baldrick’s Foundation.
SUPPORT: Supported by NIH HL058710 to GSK, 1RO1 HL088582 and DP2 OD004345 to AJW and 5T32 HL007574 to LDW.
Footnotes
CONFLICTS OF INTEREST: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- [2].Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3286. [PubMed] [Google Scholar]
- [3].Ley K, Kansas GS. Selectins in T cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- [4].Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P-selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- [5].Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- [6].Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin (CD62L) deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- [7].Ley K, Bullard D, Arbones ML, et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bullard DC, Kunkel EJ, Kubo H, et al. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;183:2329–2337. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Labow MA, Norton CR, Rumberger JM, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- [10].Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- [11].Schweitzer KM, Drager AM, van der Valk P, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Path. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- [12].Mazo IB, Gutierrez-Ramos JC, Frennette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci (USA) 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci (USA) 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lenter M, Levinovitz A, Isenmann S, Vestweber D. Monospecific and common glycoprotein ligands for E- and P-selectin on myeloid cells. J Cell Biol. 1994;125:471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin-Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Na Nakorn T, Traver D, Weissman IL, Akashi K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J Clin Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- [20].Merzaban JS, Burdick MM, Gadhoum SZ, et al. Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells. Blood. 2011;118:1774–1783. doi: 10.1182/blood-2010-11-320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- [22].Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- [23].Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- [24].Scharfetter-Kochanek K, Lu H, Norman K, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maly P, Thall AD, Petryniak B, et al. The α(1,3) fucosyltransferase FucT-VII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- [26].Cacalono G, Lee J, Kikly K, et al. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- [27].Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [28].Sultana DA, Zhang SL, Todd SP, Bhandoola A. Expression of functional P-selectin glycoprotein ligand 1 on hematopoietic progenitors is developmentally regulated. J Immunol. 2012;188:4385–4393. doi: 10.4049/jimmunol.1101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- [30].Ellies LG, Sperandio M, Underhill GH, et al. Sialyltransferase specificity in selectin ligand formation. Blood. 2002;100:3618–3625. doi: 10.1182/blood-2002-04-1007. [DOI] [PubMed] [Google Scholar]
- [31].Yang WH, Nussbaum C, Grewal PK, Marth JD, Sperandio M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood. 2012;120:1015–1026. doi: 10.1182/blood-2012-04-424366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4423. [PubMed] [Google Scholar]
- [33].Yang J, Hirata T, Croce K, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand-1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Snapp KR, Ding H, Atkins K, Warnke R, Luscinskas FW, Kansas GS. A novel P-selectin glycoprotein ligand-1 (PSGL-1) monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 amd blocks recognition of both P- and L-selectin. Blood. 1998;91:154–164. [PubMed] [Google Scholar]
- [35].Snapp KR, Wagers AJ, Craig R, Stoolman LM, Kansas GS. P-selectin glycoprotein ligand-1 (PSGL-1) is essential for adhesion to P-selectin but not E-selectin in stably transfected hematopoietic cell lines. Blood. 1996;89:896–901. [PubMed] [Google Scholar]
- [36].Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- [37].Hidalgo A, Weiss LA, Frenette PS. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110:559–569. doi: 10.1172/JCI14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xia L, Sperandio M, Yago T, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nacher M, Blazquez AB, Shao B, et al. Physiological contribution of CD44 as a ligand for E-Selectin during inflammatory T-cell recruitment. Am J Pathol. 2011;178:2437–2446. doi: 10.1016/j.ajpath.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Steegmaier M, Levinovitz A, Isenmann I, et al. The E-selectin ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373:615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- [44].Nimrichter L, Burdick MM, Aoki K, et al. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Winkler IG, Barbier V, Nowlan B, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- [46].Underhill GH, Minges Wols HA, Fornek JL, Witte PL, Kansas GS. IgG plasma cells display a unique spectrum of leukocyte adhesion and homing molecules. Blood. 2002;99:2905–2912. doi: 10.1182/blood.v99.8.2905. [DOI] [PubMed] [Google Scholar]
- [47].Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- [49].Mazo IB, Honczarenko M, Leung H, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]