Introduction
The brain is a common target of HIV infection, and may be damaged through direct and indirect effects of the virus. White matter changes including HIV leukoencephalopathy and vacuolar myelopathy are often seen (Gray and Lescs, 1993). Increased susceptibility to a wide variety of pathogens (CMV, tuberculosis, syphilis, cryptococcus, toxoplasma, JCV, etc.) and neoplastic processes (primary CNS lymphoma) occurs when CD4+ cell counts are greatly diminished.
In rare cases, HIV infection has been associated with tumefactive demyelination (TD), a disorder distinct from either multiple sclerosis (MS) or acute disseminated encephalomyelitis (ADEM). Radiologically, TD consists of an incomplete rim enhancing “tumor-like” lesion (Ball, 2004; Saravanan and Turnbull, 2009; Uriel et al., 2010). We present the neurologic, radiologic, and pathologic findings with one year of follow-up from a patient who presented with neuroimaging findings consistent with TD and who was concomitantly diagnosed with HIV.
Case Report
A 45-year old previously healthy Hispanic male presented to the emergency department complaining of two weeks of imbalance preceded by a few months of personality changes, fatigue, and insomnia. Neurologic exam revealed impaired calculations and concentration, left arm and leg weakness (2/5), and diminished sensation to light touch and pain throughout the left side.
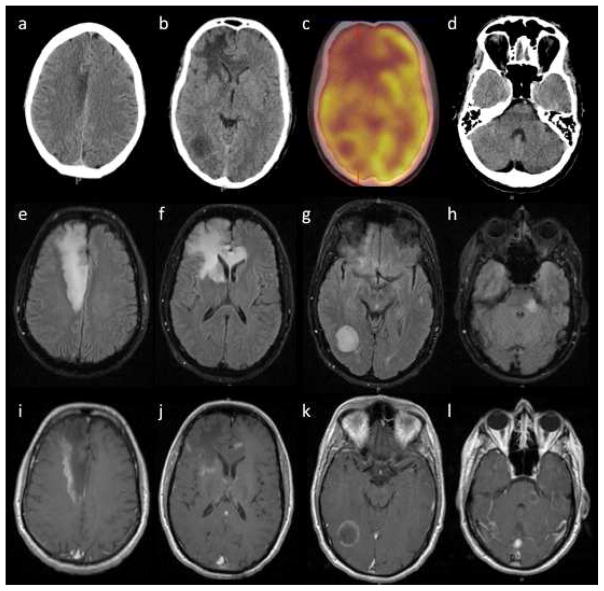
Head CT showed hypodensities in the right frontal lobe, right occipital lobe, and left midbrain and pons (Fig. 1a,b,d). Brain MRI with contrast revealed multiple areas of apparent vasogenic edema involving the right frontal lobe with extension across the corpus callosum, the right occipital lobe, and the left midbrain and pons (Fig. 1e–l). Peripheral gadolinium enhancement was seen for each area (Fig. 1i–l) with corresponding restricted diffusion. MR spectroscopy within the right frontal lesion demonstrated low N-acetyl aspartate, elevated choline, and the presence of lactate, consistent with an inflammatory/infectious etiology. 18F-FDG PET showed focal areas of hypometabolism corresponding to the lesions (Fig. 1c).
Fig. 1.
Radiological findings at initial presentation. a Right frontal lobe, b right occipital lobe, and d left midbrain and pons lesions are demonstrated by hypodense regions on transverse computed tomography (CT) slices, corresponding to c areas of hypometabolism on 18F-FDG position emission tomography (PET), e–h hyperintensity on fluid attenuated inversion recovery (FLAIR) MRI, and i–l incomplete peripheral enhancement on T1-weighted post-contrast MRI
On additional work-up, a rapid HIV test was positive and was subsequently confirmed by Western blotting. Plasma HIV viral load was 49,000 copies/mL with an initial CD4+ cell count of 362 cells/μL. An extensive infectious serological exam for HAV, HBV, HCV, HTLV1/2, CMV, VZV, rubeola, syphilis, toxoplasma, blastomyces, cocciodiodes, and strongyloides was unremarkable. Cerebrospinal fluid (CSF) evaluation for fungal, aerobic and acid fast bacilli, cryptococcus, histoplasmosis, cysticercosis, syphilis, HSV, VZV, CMV, JCV, enterovirus, and toxoplasma were all negative. CSF EBV was positive. CSF flow cytometry was negative for malignancy and CSF oligoclonal bands were not observed.
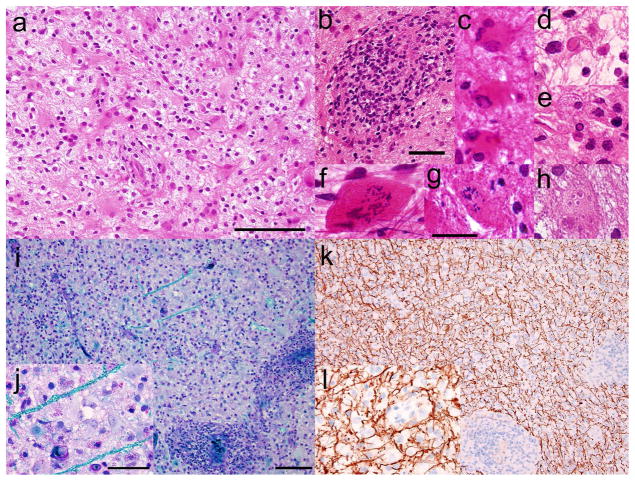
Biopsy of the right frontal lobe lesion demonstrated pale but hypercellular brain tissue with reactive gliosis, foamy macrophages, neuroaxonal spheroids, and perivascular infiltration by benign lymphocytes (Fig. 2a,b). Many astrocytes contained more than one nucleus; some had multiple ‘micronuclei’ or displayed granular mitotic figures (Fig. 2c,f–h). Rare nuclei with glassy eosinophilic centers and marginated chromatin were also observed, suggestive of possible viral inclusions (Fig. 2d,e). Stains for HSV, VZV, CMV, EBV, JCV, HIV, polyoma virus, and toxoplasma and RT-PCR for measles virus were negative. Additional ultrastructural examination identified no viral particles. There was no evidence of neoplasm, necrosis, or infection by parasite, bacteria, or fungus by histology or culture. Sections stained with Luxol fast blue/periodic acid-Schiff stain (Fig. 2i,j) and immunohistochemically for neurofilament protein (Fig. 2k,l) showed severe myelin loss with relative axonal sparing, consistent with a demyelinating process.
Fig. 2.
Stereotactic biopsy of right frontal lobe lesion. a Haematoxylin and eosin staining of paraffin sections shows pale but hypercellular brain tissue with reactive gliosis, foamy macrophages, neuroaxonal spheroids, and b perivascular infiltrates of benign lymphocytes. c Some reactive astrocytes are multinucleated. d,e Rare nuclei show glassy eosinophilic intranuclear bodies and marginated chromatin, suggestive of viral inclusions. Granular mitoses and cells with micronuclei (Creutzfeldt cells) are present in f cytological smear, g frozen section, and h paraffin sections. i Luxol-fast blue/Periodic acid Schiff preparations show severe myelin loss, j highlighting rare remaining myelinated axons in turquoise; k,l in contrast, immunohistochemistry for neurofilament protein, applied to the same tissue and viewed at the same magnifications, highlights abundant relatively preserved axons, indicative of a demyelinating process. Scale bars: a=100 μm; b=50 μm; g=25 μm (applies to c –h); i=100 μm (applies also to k); j=50 μm (applies also to l)
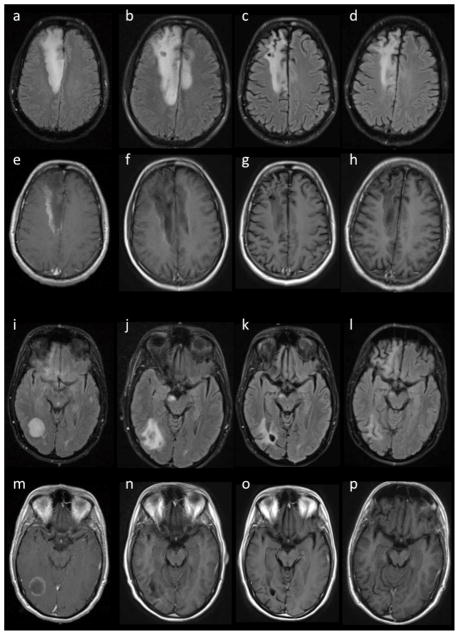
During this admission, the patient was administered highly active anti-retroviral therapy (HAART) and was treated empirically for toxoplasmosis. One month later, he showed mild improvement in cognition and diminished left sided weakness (3/5) despite the continued presence of multiple lesions and increased edema on MRI (Fig. 3b,f,j,n). High dose steroids were initiated and toxoplasmosis therapy discontinued, with significant improvement in strength (4/5) and cognitive function. Steroids were slowly tapered, and at four months the patient had very mild left arm weakness (4+/5) with occasional forgetfulness. Repeat MRI showed a decrease in size of the lesions and degree of edema (Fig. 3c,g,k,o). By eight months the patient had largely stabilized, with left-sided strength of 5−/5, improved concentration and attention, but apathetic mood. Lesions were stable on MRI, with no diffusion restriction or contrast enhancement (Fig. 3d,h,l,p). At one year after initial symptoms, the patient exhibited very mild weakness (5−/5) with some mild dysmetria and continued apathy. The CD4+ cell count was 370 cells/μL and plasma HIV viral load was 42 copies/mL at one year follow-up.
Fig. 3.
Time course of lesions. a–d,i–l FLAIR and e–h,m–p T1-weighted post-contrast MRI slices of a–h right frontal lobe and i–p right occipital lobe lesions at a,e,i,m initial presentation, b,f,j,n with slight worsening at one month, c,g,k,o improvement at four months, and d,h,l,p stable at eight months
Discussion
In contrast to the subtle gross and microscopic white matter changes associated with HIV leukoencephalopathy, tumefactive demyelination typically presents with large areas of selective myelin loss with focal neurological deficits and incomplete ring enhancement on MRI that can mimic intracranial neoplasm or abscess/infection (Kepes, 1993; Nagappa et al., 2012). Our patient had features consistent with TD including subacute time course, characteristic MRI lesions, and demyelination on biopsy. What was unusual was the concomitant diagnosis of HIV infection with a high viral load and relatively spared CD4+ cell count; this pattern raises the possibility that TD arose in the patient in the setting of a longer-standing HIV infection. The clinical presentation and MRI findings were not consistent with HIV associated neurocognitive disorders. TD has been previously described with immunosuppression (high HIV viral load and depressed CD4+ cell count) and improvement after initiation of HAART and steroids (Ball, 2004; Saravanan and Turnbull, 2009; Uriel et al., 2010). Conversely, TD has also been reported in the context of immune reconstitution in an HIV+ patient undergoing HAART treatment, who subsequently improved after discontinuation of HAART and treatment with interferon β-1a (Lindzen et al., 2008).
The differential diagnosis for an HIV-infected patient with focal neurological symptoms and enhancing lesions on MRI includes toxoplasmosis, primary CNS lymphoma, and progressive multifocal leukoencephalopathy (PML) with immune reconstitution. These etiologies were excluded by biopsy, failure of empiric treatment for toxoplasmosis, lack of abnormal lymphocytes on flow cytometry, and negative JCV in CSF. More common demyelinating disorders including MS and ADEM have been occasionally associated with HIV infection (Berger et al., 1989; Narciso et al., 2001). However, the MRI lesions observed in this patient were larger than those typically seen in ADEM and occurred in locations atypical for MS. In addition, no infectious prodrome was identified and oligoclonal bands were not observed. Severe HIV-associated demyelination has also been reported in patients failing HAART, but appears to involve more substantial perivascular invasion by immunoreactive monocytes/macrophages and lymphocytes (Langford et al., 2002; Gray et al., 2013).
Similar to other demyelinating disorders, an as yet unidentified virus has been hypothesized to be responsible for precipitating the anti-myelin immune response in TD (Virtanen and Jacobson, 2012). On histological examination, we observed rare eosinophilic intranuclear bodies, reminiscent of viral inclusions, but failed to identify a specific agent by immunohistochemistry, ancillary PCR-based techniques, or electron microscopy. Inclusions concentrate replicase proteins, virus genomes, and host factors in order to increase replication efficiency; isolation of nucleic acids or peptides from this material could provide the necessary substrate for identification of a causative virus (Netherton et al., 2007).
Acknowledgments
Funding sources include National Institute of Allergy and Infectious Diseases (NIAID) (UM1AI069495) (DBC), National Institute of Mental Health (NIMH) (K23MH081786) (BMA), National Institute of Neurological Disorders and Stroke (NINDS) (U10NS077384) (DBC) and (F30NS063547) (IHS), National Institute of Nursing Research (NINR) (R01NR012907 and R01NR012657) (BMA), NM22005 (DBC), and Alzheimer’s Association (with partial support from Lilly and Roche) (BMA & DBC). DBC has consulted for Pfizer, Genzyme, Millennium, Brinker, Biddle, Reath, Amgen, Quintiles, Biogen Idec, Arnold Todara & Welch.
References
- Ball SC. Rapid neurologic deterioration in a patient with HIV infection. AIDS Read. 2004;14:63–66. 69. [PubMed] [Google Scholar]
- Berger JR, Sheremata WA, Resnick L, Atherton S, Fletcher MA, Norenberg M. Multiple sclerosis-like illness occurring with human immunodeficiency virus infection. Neurology. 1989;39:324–329. doi: 10.1212/wnl.39.3.324. [DOI] [PubMed] [Google Scholar]
- Gray F, Lescs MC. HIV-related demyelinating disease. Eur J Med. 1993;2:89–96. [PubMed] [Google Scholar]
- Gray F, Lescure FX, Adle-Biassette H, Polivka FM, Gallien S, Pialoux G, Moulignier A. Encephalitis with infiltration by CD8+ Lymphocytes in HIV Patients receiving Combination Antiretroviral Treatment. Brain Pathol. 2013 doi: 10.1111/bpa.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepes JJ. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. Aids. 2002;16:1019–1029. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindzen E, Jewells V, Bouldin T, Speer D, Royal W, 3rd, Markovic-Plese S. Progressive tumefactive inflammatory central nervous system demyelinating disease in an acquired immunodeficiency syndrome patient treated with highly active antiretroviral therapy. J Neurovirol. 2008;14:569–573. doi: 10.1080/13550280802304753. [DOI] [PubMed] [Google Scholar]
- Nagappa M, Taly AB, Sinha S, Bharath RD, Mahadevan A, Bindu PS, Saini JS, Prasad C, Shankar SK. Tumefactive demyelination: clinical, imaging and follow-up observations in thirty-nine patients. Acta Neurol Scand. 2012 doi: 10.1111/ane.12071. [DOI] [PubMed] [Google Scholar]
- Narciso P, Galgani S, Del Grosso B, De Marco M, De Santis A, Balestra P, Ciapparoni V, Tozzi V. Acute disseminated encephalomyelitis as manifestation of primary HIV infection. Neurology. 2001;57:1493–1496. doi: 10.1212/wnl.57.8.1493. [DOI] [PubMed] [Google Scholar]
- Netherton C, Moffat K, Brooks E, Wileman T. A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication. Adv Virus Res. 2007;70:101–182. doi: 10.1016/S0065-3527(07)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan M, Turnbull IW. Brain: non-infective and non-neoplastic manifestations of HIV. Br J Radiol. 2009;82:956–965. doi: 10.1259/bjr/14027261. [DOI] [PubMed] [Google Scholar]
- Uriel A, Stow R, Johnson L, Varma A, du Plessis D, Gray F, Herwadkar A, Holland J, Lewthwaite P, Wilkins E. Tumefactive demyelination-an unusual neurological presentation of HIV. Clin Infect Dis. 2010;51:1217–1220. doi: 10.1086/656812. [DOI] [PubMed] [Google Scholar]
- Virtanen JO, Jacobson S. Viruses and multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11:528–544. doi: 10.2174/187152712801661220. [DOI] [PMC free article] [PubMed] [Google Scholar]