Abstract
Adolescence is a unique developmental period characterized by major physiological, psychological, social, and brain changes, as well as an increased incidence of maladaptive, addictive behaviors. With the use of magnetic resonance imaging techniques, researchers have been able to provide a better understanding of adolescent brain maturation and how neurodevelopment affects cognition and behavior. This review discusses adolescent brain development and its potential influence on psychotherapeutic change. We focus on cognitive-behavioral and mindfulness-based approaches for treating substance use and highlight potential brain mechanisms underlying response to psychotherapy. Finally, we discuss integrative neuroimaging and treatment studies and potential opportunities for advancing the treatment of adolescent addictive behaviors.
Keywords: Adolescence, Substance use, Neuroimaging, Psychotherapy
INTRODUCTION
Adolescence is a critical developmental period that is often associated with the emergence of substance use and behavioral risk-taking. The prevalence of adolescent substance use and associated negative outcomes emphasize the need for evidence-based treatments and interventions in this population. Over the past decade, evidence-based treatments have been developed specifically for adolescents (Deas, 2008; Waldron & Turner, 2008), and advances in adolescent addiction psychotherapy are likely to stem from improved understanding of developmental processes that may affect psychotherapeutic change. This review will present the current research on adolescent brain development, potential mechanisms underlying adolescent response to psychotherapy, and how neuromaturation might influence psychotherapeutic change. Research on evidence-based treatments and neuroimaging studies will be discussed, emphasizing therapeutic mechanisms and measurement of change using neuroimaging. We conclude with suggestions for future research.
ADOLESCENT SUBSTANCE USE
National survey data indicate that the prevalence of substance use increases markedly from early to late adolescence, peaking during the transition into young adulthood (SAMHSA, 2011). Past year alcohol use rates increase from 29% to 65% between 8th and 12th grade, with similar increases in illicit drug use from 16% to 38% (Johnston, O'Malley, Bachman, & Schulenberg, 2010). Over a third (36%) of 8th graders and 71% of high school seniors have consumed alcohol, and 21% and 48% of 8th and 12th graders, respectively, have at least tried illicit drugs (Johnston et al., 2010). Together, these findings highlight that substance use is normative during adolescence, yet this is a period of vulnerability for the emergence of substance use disorders (SUD) (Rutherford, Mayes, & Potenza, 2010), as nearly 8% of adolescents ages 12 to 17 and 21% of 18 to 25 year-olds meet diagnostic criteria for a SUD (SAMHSA, 2011).
Although substance use is common during adolescence, it is concerning, as early initiation increases the likelihood of developing a SUD (Grant & Dawson, 1997). Adolescent substance use is associated with negative health, social, and behavioral outcomes, including alterations in neurodevelopment (Newcomb & Locke, 2005; Squeglia, Jacobus, & Tapert, 2009a). Therefore, understanding adolescent brain development and the influence of substance use on neuromaturation are important factors to consider when examining brain mechanisms underlying adolescents’ response to psychotherapeutic intervention.
ADOLESCENT BRAIN DEVELOPMENT
Developmental advances during adolescence occur in the context of complex physiological, psychological, and social transitions that interact and influence the dynamic brain changes that lead to more efficient, advanced cognitive processing (Bava & Tapert, 2010; Gogtay et al., 2004; Squeglia et al., 2009a). Specifically, synaptic refinement eliminates superfluous neural connections, resulting in cortical volume and thickness reductions (Giedd et al., 1999; Gogtay et al., 2004). Although this pruning process takes place at varying times across brain regions (Wilke, Krageloh-Mann, & Holland, 2007), cortical reductions are most evident in prefrontal and temporal cortices, as well as subcortical structures (Bava & Tapert, 2010; Sowell et al., 2004). While cortical volume decreases across adolescence, white matter volume increases, particularly in the prefrontal cortex, and is reflected in enhanced cortical connections (Bava et al., 2010; Giedd, 2008). Both gray matter decreases and white matter organization increases during adolescence are associated with increasingly efficient neural activity (Huttenlocher & Dabholkar, 1997), allowing for improved cognitive processing (Luna, Garver, Urban, Lazar, & Sweeney, 2004).
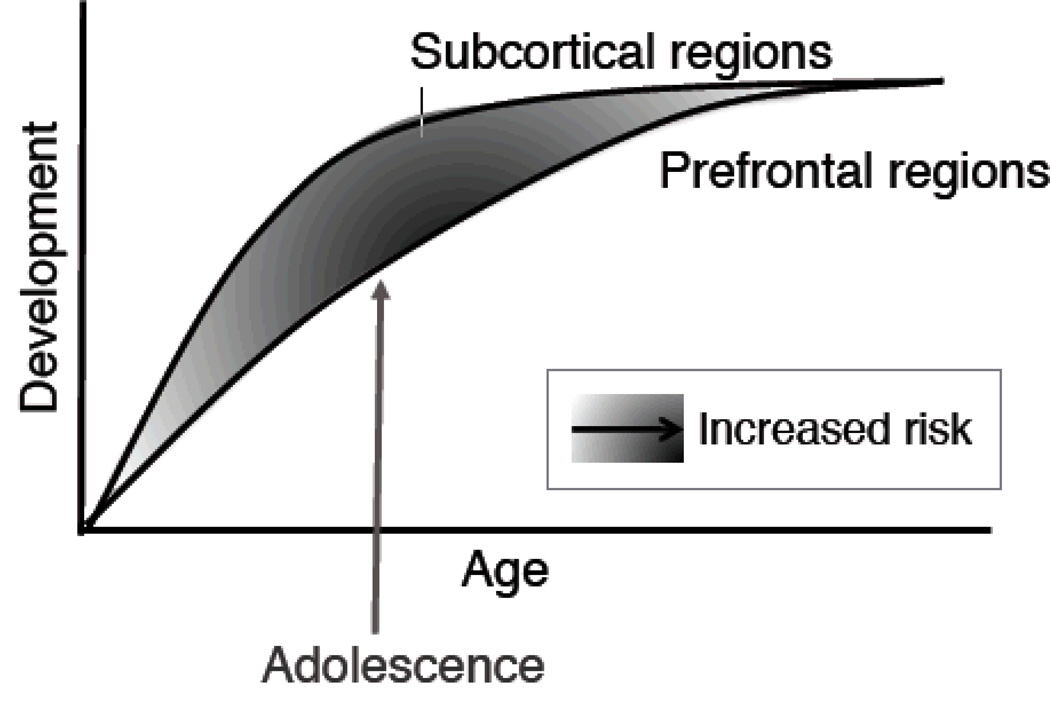
Although neuromaturation is linked to cognitive and behavioral advancements, brain development and improvements in cognitive control do not occur in a linear manner, as evidenced by adolescents’ propensity to engage in impulsive, risk-taking behaviors. Instead, these behaviors appear to be related to asynchronous, nonlinear developmental changes in brain structure and function (see Figure 1) (Casey, Jones, & Hare, 2008; Clark, Thatcher, & Tapert, 2008; Somerville, Jones, & Casey, 2010). Based on both animal (Laviola, Adriani, Terranova, & Gerra, 1999; Spear, 2000) and recent adolescent imaging studies (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Hare et al., 2008; Somerville et al., 2010), subcortical circuitry and projections (e.g., ventral striatum, amygdala, orbitofrontal cortex) involved in motivation and reward develop early in adolescence relative to cortical (i.e., frontal) circuitry involved in top-down cognitive control. This imbalance in adolescent frontolimbic circuitry has been associated with a heightened sensitivity to motivational cues and immediate rewards, as well as an increased likelihood of engaging in behavioral risks, including substance use (Casey & Jones, 2010; Galvan, Hare, Voss, Glover, & Casey, 2007; Somerville & Casey, 2010). Thus, temporal variations in adolescent brain development likely contribute to the behavioral disinhibition and affective dysregulation that is characteristic of adolescence, substance use, and psychopathology (Rutherford et al., 2010; Tessner & Hill, 2010).
Figure 1.
Neurobiological model depicting asynchronous, nonlinear development of prefrontal and subcortical regions. Reprinted with permission from Somerville et al., 2010, Brain & Cognition, 72, 124–133.
EFFECTS OF SUBSTANCE USE ON ADOLESCENT BRAIN AND COGNITIVE FUNCTION
Given the brain changes occurring during adolescence, exposure to neurotoxins, such as alcohol and illicit drugs, may interrupt neurodevelopment and associated cognitive and behavioral functioning. The potentially adverse effects of substance use on the developing adolescent brain have been seen in neuroimaging studies of white matter microstructure (Bava et al., 2009; Jacobus et al., 2009; McQueeny et al., 2009); brain morphometry (Medina et al., 2008; Medina et al., 2009; Nagel, Schweinsburg, Phan, & Tapert, 2005); brain functioning (Schweinsburg, Schweinsburg, Nagel, Eyler, & Tapert, 2011; Squeglia, Schweinsburg, Pulido, & Tapert, 2011; Tapert et al., 2007) and neuropsychological functioning among adolescent substance users (Medina et al., 2007; Squeglia, Spadoni, Infante, Myers, & Tapert, 2009b; Tapert, Granholm, Leedy, & Brown, 2002).
Advances in neuroimaging techniques have provided considerable information on the neural consequences of substance use among adolescents. For example, magnetic resonance imaging (MRI) has examined structural differences in hippocampal and prefrontal cortex volumes (brain areas associated with memory, and executive functions of planning, inhibition, self-regulation, respectively) and indicate that heavy drinking adolescents show smaller hippocampal (De Bellis et al., 2000; Nagel et al., 2005) and prefrontal (Medina et al., 2008) volumes than non-drinking controls. Similarly, diffusion tensor imaging (DTI), a neuroimaging technique that characterizes white matter integrity by examining water molecule diffusion in white matter tissue, indicates that heavy drinking adolescents show abnormal white matter tissue integrity in the corpus callosum (De Bellis et al., 2008) and in frontal, cerebellar, temporal, and parietal regions (McQueeny et al., 2009).
Neural activity implications of adolescent substance use have been measured using functional magnetic resonance imaging (fMRI), which measures changes in blood oxygen level-dependent (BOLD) signal. Overall, adolescent heavy drinkers show aberrant frontal and parietal neural responses during spatial working memory (Squeglia et al., 2009a; Tapert, Pulido, Paulus, Schuckit, & Burke, 2004), verbal encoding (Schweinsburg, McQueeny, Nagel, Eyler, & Tapert, 2010), and inhibition (Tapert et al., 2007) tasks. Similarly, adolescent marijuana users show increased activation in right frontal and parietal areas during tasks that require attentional control compared to non-using controls (Abdullaev, Posner, Nunnally, & Dishion, 2010), suggesting that marijuana users exert more effort when trying to self-regulate (Dishion, Felver-Grant, Abdullaev, & Posner, 2011). Adolescent substance use also has been linked to disadvantaged attention (McQueeny et al., 2009; Tapert, Baratta, Abrantes, & Brown, 2002), memory (Brown, Tapert, Granholm, & Delis, 2000; Squeglia et al., 2009b), and executive functioning (Giancola & Moss, 1998; Hanson, Cummins, Tapert, & Brown, 2011; Thoma et al., 2011). Together, these findings suggest that the deficits observed among substance using adolescents may reflect pre-existing cognitive and behavioral deficits, pre-existing deficits that may have been exacerbated by substance use, or consequences of substance use on brain maturation.
EVIDENCE-BASED TREATMENT FOR ADOLESCENT SUBSTANCE USE
Evidence-based treatments generally refer to treatment approaches that are validated by scientific evidence (SAMHSA, 2011). Historically, evidence-based treatments for adults with substance use disorders have been applied to adolescents without considering the complex, developmental differences between adolescents and adults. Adolescents in substance use treatment differ from adults in important factors that affect treatment outcome. Specifically, adolescents often show lower intentions and readiness to change substance use behaviors (Melnick, De Leon, Hawke, Jainchill, & Kressel, 1997; Ramo, Prochaska, & Myers, 2010), which may be related to adolescents being referred to treatment by external pressure (e.g., criminal justice system, school, family) (SAMHSA, 2010). Furthermore, adolescents typically have shorter duration of use and lower prevalence of dependence (Deas, Riggs, Langenbucher, Goldman, & Brown, 2000) and often present to treatment with co-occurring psychopathology (Waldron & Turner, 2008). Adding to the complexity of providing treatment to adolescent substance users is the interaction between adolescent brain development and substance use. Given these differences, clinical researchers have modified evidence-based treatments for adults with substance use disorders to address the needs of adolescents and have developed substance abuse treatments tailored to adolescent substance users (Deas, 2008; Winters, 1999).
Adolescent psychotherapies differ in approach and therapeutic mechanisms, but typically involve motivational interviewing (MI), behavioral therapy, cognitive-behavior therapy (CBT), mindfulness, or family-based approaches. A comprehensive description of each treatment is beyond the scope of this review; however, a brief description of each approach is warranted. MI enhances motivation to make changes in substance-related behaviors through the use of empathy, developing discrepancy, minimizing resistance to change, and supporting self-efficacy for change (Colby et al., 1998; Miller & Rollnick, 2002). CBT approaches address substance use by providing training in identifying patterns of use (e.g., triggers and consequences) and ways to cope with the desire to use drugs (Carroll, 1998; Kaminer, Burleson, Blitz, Sussman, & Rounsaville, 1998). Mindfulness-based approaches help individuals recognize triggers and craving while also focusing on one’s present experiences with acceptance (Bowen et al., 2009). Family-based interventions foster change in the adolescent’s family and social environment through skills training and interpersonal relationships (Barton & Alexander, 1981; Liddle, Dakof, Turner, Henderson, & Greenbaum, 2008; Szapocznik, Kurtines, Foote, Perez-Vidal, & Hervis, 1986; Szapocznik, Kurtines, Foote, Perez-Vidal, & Hervis, 1983). Empirical evidence supports the efficacy of these psychotherapies for adolescent substance use, but a recent meta-analysis indicates that no treatment is clearly superior to another (Waldron & Turner, 2008).
Whereas many treatments have now proven to be effective, the exact brain-based mechanisms underlying successful therapeutic change remain poorly understood. In adolescent substance use treatment, neural mechanisms of change are likely related to the intervention approach employed and the neurobiological circuitry associated with substance use, specifically the incentive motivation and cognitive control networks (Bechara, 2005; Hutchison, 2010; Kalivas & Volkow, 2005). As previously mentioned, these neural networks develop at different rates during adolescence and are associated with motivation, reward, and regulatory control. Therefore, we focus on CBT and mindfulness, which provide training in self-regulation (Bowen, Chawla, & Marlatt, 2011; Brewer, Bowen, Smith, Marlatt, & Potenza, 2010; Carroll, 1998) to consider brain mechanisms underlying adolescents’ response to substance use treatments.
CBT for substance use (for treatment manual see Carroll, 1998) is a short-term, psychotherapeutic approach that relies on 1) functional analysis to identify the substance use patterns, specifically antecedents and consequences of use, and 2) skills training to recognize thoughts, feelings, and situations that place individuals at risk for substance use, to avoid these situations, and to cope with these situations by using cognitive and behavioral strategies (Carroll, 1998; Carroll & Onken, 2005). Treatment modules focus on psychoeducation, coping with craving, increasing motivation and commitment to abstain from use, drug refusal/social skills, and problem solving (Carroll, 1998). During CBT for substance abuse, individuals learn to 1) identify triggers of craving (i.e., strong desire to use), 2) avoid triggers (e.g., avoiding persons and places where drugs were used; getting rid of drug paraphernalia), and 3) cope with craving by using distraction, talking about craving with supportive family and friends, going with the craving, recalling the negative consequences of drug use, and using self talk. Thus, CBT can be viewed as a method that helps improve cognitive control over automatic urges and emotions by helping individuals attend to their environment, self-monitor their thoughts and feelings, and regulate emotions and urges in order to resist the desire to use.
Mindfulness, or “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally” (Kabat-Zinn, 1990), has become a popular intervention for the treatment of SUDs. Mindfulness-based approaches, including mindfulness-based stress reduction (MBSR) (Kabat-Zinn, 1982), mindfulness-based cognitive therapy (MBCT) (Teasdale et al., 2000), and mindfulness-based relapse prevention (MBRP) (Bowen et al., 2011), emphasize the importance of maintaining attention on one’s immediate experience and adopting an attitude of acceptance toward the experience (Brewer et al., 2011). Like CBT, mindfulness training for substance use helps individuals recognize emotional, cognitive, and situational cues associated with substance use, develop coping skills, and enhance self-efficacy (Bowen et al., 2009). Mindfulness-based treatments for substance use focus on psychoeducation, bringing awareness to triggers and craving, practicing mindfulness in life and high-risk situations, acceptance and skillful action, seeing thoughts as thoughts, and self-care and balance (Bowen et al, 2011). As such, mindfulness-based approaches have been hypothesized to increase awareness, self-regulation, and tolerance of craving and relapse (Bowen et al., 2009; Brewer et al., 2011).
Although there are many similarities between CBT and mindfulness for substance use, the two therapeutic approaches differ in attentional focus. CBT encourages avoidance of cues/triggers (Carroll & Onken, 2005; Marlatt & Donovan, 2005); whereas, mindfulness encourages tolerance of cues and craving (Brewer et al., 2011). While differences may exist, both treatments have been associated with reductions in substance use among adolescents (Cavallo et al., 2007; de Dios et al., 2012; Dennis et al., 2004). In one of the first studies to examine CBT for substance use with adolescents, Kaminer and colleagues (1998) randomly assigned 32 adolescents (ages 13–18) to 12-weeks of group CBT or to 12-weeks of interactional group therapy. Adolescents in the CBT intervention showed significantly greater reductions in substance use from baseline to 3-month follow-up compared to youth in the interactional group therapy condition. Similarly, Britton and colleagues (2010) examined the effects of mindfulness among adolescents (ages 13–19) who completed a 6-week intervention followed by 8-, 20-, and 60-week post-entry follow-up assessments. For youth who completed the 60-week follow-up (n = 18), mindfulness was associated with reductions in substance use and emotional distress.
These findings, which are consistent with the adolescent and adult substance use treatment literature, indicate that CBT and mindfulness likely address developmental and psychosocial needs of adolescent substance users. To date, the brain-based mechanisms of adolescent psychotherapeutic change during substance use treatment remain unknown; however, recent studies of CBT and mindfulness among adults in substance use treatment may provide insight into potential brain-based mechanisms among adolescents and may help expand on the most beneficial therapeutic elements.
POTENTIAL BRAIN-BASED MECHANISMS OF CBT AND MINDFULNESS FOR SUBSTANCE USE
Owing to advances in cognitive neuroscience and treatment outcome research, new information on the neurobiological basis of substance abuse treatment response is coming to light (for reviews see Ewing, Filbey, Sabbineni, Chandler, & Hutchison, 2011; Potenza, Sofuoglu, Carroll, & Rounsaville, 2011; Richards, Lee, & Daughters, 2011). Examining and understanding how changes in brain activity are associated with treatment response and outcomes may provide mechanistic explanations for psychotherapeutic change. As described below, recent neuroimaging studies provide great promise in understanding how CBT and mindfulness for substance abuse affect neural circuitry (i.e., incentive motivation and cognitive control networks) and cognitive processes associated with substance use.
CBT and mindfulness approaches train individuals to increase inhibitory control and regulate automatic emotional responses, suggesting that these strategies might enhance prefrontal cortex (cognitive control network) activity while attenuating limbic reactions (incentive motivation network) associated with emotion and reward. Recent findings from adult neuroimaging studies examining the effects of cognitive control over drug craving are consistent with this formulation (Kober et al., 2010; Volkow et al., 2010). Using PET and 2-deoxy-2[18F]fluoro-D-glucose, Volkow and colleagues (2010) measured brain glucose metabolism among 24 cocaine abusers who watched a cocaine-cue video with and without instructions to cognitively inhibit craving, as well as at baseline (without video). Brain activation comparisons revealed lower metabolism in limbic brain regions (i.e., right orbitofrontal cortex and right nucleus accumbens) when instructed to inhibit cue-induced craving. Further, Kober and colleagues (2010) examined brain activity in 21 cigarette smokers during a regulation of craving task and found increased activity in prefrontal regions and decreased activity in several subcortical, reward-related regions (i.e., ventral striatum, cingulate, amygdala, ventral tegmental area). Subjective craving was correlated with brain activity, with associations between decreased craving and decreased activity in the ventral striatum, and increases in craving and increased prefrontal activity. Association between increased prefrontal activity and increases in craving was fully mediated by ventral striatum activity. Thus, cognitive inhibition and control over cue-induced craving appears to decrease brain activity in regions of the incentive motivation network associated with emotional salience and reward.
In a recent examination of neural effects of CBT for substance use (Devito et al., 2012), 12 treatment-seeking, substance-dependent adults (SUD group) completed fMRI scanning while completing a cognitive control task before and after 8-weeks of computer-assisted CBT. Twelve healthy, non-using controls completed the same tasks under test-retest conditions. Findings revealed that the SUD group demonstrated improved cognitive control performance and attenuated BOLD response change in brain regions of the cognitive control and incentive motivation networks, including the anterior cingulate cortex, right inferior frontal gyrus, and the midbrain. These findings suggest that CBT cognitive strategies improve cognitive control over automatic cue-related responses, and that this improvement may be driven by enhanced recruitment of cognitive control networks that regulate limbic reward-related functioning.
Whereas CBT strategies appear to enhance top-down cognitive control over limbic, reward-related response to emotional and situational cues, a recent study suggests that mindfulness-related psychotherapeutic change may operate through a different mechanism. Specifically, 47 meditation-naïve, treatment-seeking adult smokers viewed smoking and neutral images during fMRI scanning, and were trained and instructed to view the images passively or with mindful attention (Westbrook et al., 2011). During the mindful attention condition, participants were instructed to actively focus on their responses, while maintaining a nonjudgmental attitude toward those responses, as opposed to a strategy to reduce craving. Findings indicated that mindful attention reduced craving to smoking images, reduced neural activity in the reward-related subgenual anterior cingulate cortex, and reduced functional connectivity between the subgenual anterior cingulate cortex and other craving-related regions compared to passively viewing smoking images. Thus, mindfulness may operate through “bottom-up” processes; whereby, attention to present experience alters incentive motivation network activity and decreases subjective drug craving. Although neuroimaging has provided insight into potential brain mechanisms underlying adult psychotherapeutic change, future research is needed to better understand psychotherapeutic change among adolescents and to examine how brain development may affect psychotherapeutic change processes.
ADOLESCENT BRAIN DEVELOPMENT AND PSYCHOTHERAPEUTIC CHANGE
Based on the findings described above, both CBT for substance use and mindfulness-based approaches appear to alter brain activity in prefrontal and limbic systems involved in inhibitory control and reward. CBT strategies appear to help substance users overcome biases toward substance-related cues by increasing prefrontal activity and inhibitory control and by decreasing limbic activity (Devito et al., 2012; Kober et al., 2010; Volkow et al., 2010). Although this hypothesis has not been confirmed among adolescent substance users, we speculate that CBT for substance use may operate in a similar fashion among adolescents; however, there are important neurodevelopmental issues to consider. Depending on the stage of neurodevelopment, adolescents’ prefrontal circuitry is not fully developed, which may affect psychotherapeutic change. For example, adolescents may have a difficult time with increasing inhibitory control given that the prefrontal brain regions and circuitry needed for adequate inhibitory control might not be fully developed or process efficiently. With that in mind, adolescents may require additional sessions of CBT or an adjunct treatment to enhance cognitive functioning in order to overcome developmental lags in prefrontal brain development and associated cognitive abilities. Further, substance use during adolescence interferes with prefrontal brain structure, function, and associated executive cognitive functions, and as such, it may be helpful to use neuroimaging and neuropsychological assessments when planning treatment for adolescent substance users. These assessments would provide information about inhibitory, attentional, and emotional reactivity that could prove useful in personalizing treatment for adolescents with varying cognitive abilities and coping skills, which in turn could improve treatment outcomes.
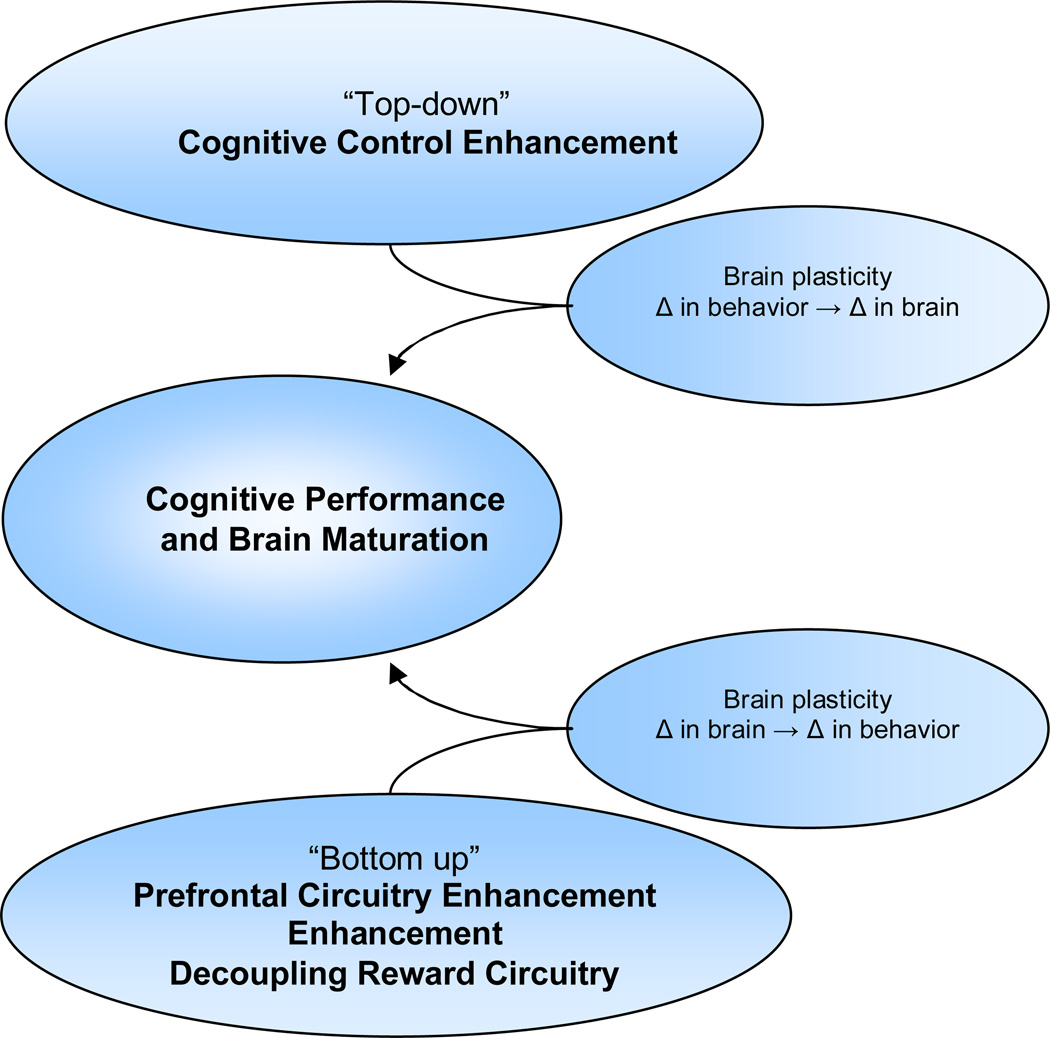
Whereas CBT for substance use appears to work through prefrontal-limbic circuitry changes that improve inhibitory control and self-regulation, mindfulness-based approaches appear to improve attention and change beliefs about use through “bottom-up” subcortically-based changes (for a proposed conceptual model, see Figure 2). Similar to the research with CBT, the neural mechanisms of mindfulness for substance use have yet to be confirmed among adolescent substance users. Given the early development of limbic circuitry and projections, mindfulness-based approaches may be ideal for targeting intermediate phenotypes of affective dysregulation and stress that are characteristic of adolescence. According to a recent review of mindfulness (Brewer et al., 2010), mindfulness training to shift attention towards the present moment and acceptance of thoughts and sensations could help adolescents gain a non-attached viewpoint and increase tolerance of unpleasant situations, feelings, and stress that could lead to substance use. The recent finding that mindful attention reduces craving among adult substance users by decoupling subcortical circuitry (Westbrook et al., 2011) emphasizes the potential utility of mindfulness among adolescents with highly responsive limbic systems.
Figure 2.
Conceptual model of psychotherapeutic change during adolescence
Overall, adolescents appear to have the cognitive capacity to engage in and benefit from treatments and interventions for substance use. It is important to note, however, that some adolescents may benefit from certain therapeutic approaches more than others and may require additional adjunctive therapies to maintain abstinence. Adolescents with extensive substance use histories may require a period of abstinence before engaging in treatment due to the effects of substance use on cognition (Hanson et al., 2010; Medina et al., 2007). Adolescent marijuana users may show deficits in verbal learning, working memory, and attention tasks that can persist up to three weeks post last marijuana use (Hanson et al., 2010). Given that these functions are required to successfully respond to various types of treatments and are associated with prefrontal functioning, some adolescents may benefit from a period of abstinence before initiating intensive psychotherapy. Adolescents with co-occurring psychopathologies show worse outcomes and may require individualized treatments targeting brain mechanisms underlying the comorbid disorders (Elkins, McGue, & Iacono, 2007; Grella, Hser, Joshi, & Rounds-Bryant, 2001; Hawkins, 2009).
Gender differences during adolescent brain development are yet another factor to consider when discussing neural mechanisms of therapeutic change and substance use (Hines, 2011). In general, males have a higher rate of substance use and SUDs (Greenfield et al., 2007; Grella, 2008); however, females are more likely to experience negative consequences from substance use (Broome, Hurley, & Taber, 2010). Interestingly, females show earlier peaks in cortical gray matter volume (Giedd et al., 1999; Lenroot & Giedd, 2010), as well as earlier improvements in cognitive functioning (Vuontela et al., 2003), yet heavy drinking during adolescence alters females’ cognitive functioning and brain activity more than males (Squeglia et al., 2011). Together, these findings emphasize the need to personalize treatments for adolescent substance users given the various factors that contribute to use and the varying effects of brain development on cognition and emotion.
RESEARCH USING NEUROIMAGING AND TREATMENT TAILORED TO THE NEEDS OF DEVELOPING ADOLESCENTS
In the following section we present a potential neurobehavioral treatment for adolescent substance use as an example of how we can translate neuroimaging data to interventions and treatments that target mechanisms underlying substance use while also addressing adolescent neurodevelopment. Based on the neuropsychological and neuroimaging data showing adolescent prefrontal deficits (e.g., attention, inhibitory control) contribute to substance use, enhancing prefrontal functioning could remediate adolescents’ heightened sensitivity to emotional, social, and reward-related cues, and thus, help treat substance use. Although CBT for substance use appears to invoke such changes, a targeted approach, such as cognitive training, may enhance changes and lead to sustained abstinence.
Cognitive training has been implemented among several populations (i.e., schizophrenia: Eack et al., 2010; Hogarty, Greenwald, & Eack, 2006; Twamley, Savla, Zurhellen, Heaton, & Jeste, 2008; acquired brain injury Powell, Letson, Davidoff, Valentine, & Greenwood, 2008; and aging-related deficits Barnes et al., 2009; Jean et al., 2010) and are typically used to address prefrontal cognitive functioning abnormalities. Cognitive training approaches aim to improve and correct cognitive and neurobiological weaknesses through practice and use of new cognitive strategies (Bellack, Gold, & Buchanan, 1999; Twamley, Jeste, & Bellack, 2003; Twamley et al., 2008). In addition to improving prefrontal functioning and behavioral outcomes, cognitive training has also been shown to produce neurobiological changes (Eack et al., 2010; McNab et al., 2009), resulting in measurable changes in the brain. For example, adults who participated in 35 minutes of daily working memory training over the course of 5 weeks showed significant increases in prefrontal and parietal dopamine binding potential, a key neurotransmitter involved in cognitive functioning and reward (McNab et al., 2009). It should be noted, however, that the effects of restorative training approaches (e.g., memory strategy training) show limited generalizability to other cognitive functions (Gates, Sachdev, Fiatarone Singh, & Valenzuela, 2011). Thus, researchers have begun to integrate targeted cognitive training with behavioral and environmental modifications to encourage global behavioral change (Bryck & Fisher, 2012; Twamley et al., 2008). Thus, cognitive training approaches may be ideal for addressing neural risk factors associated with addictive behavior onset and escalation. Among adolescents, cognitive training could be used to address cognitive control network weaknesses (e.g., frontoparietal circuitry) by having youth complete computerized working memory and attentional training while also teaching youth how to modify their behavior and environment to compensate for such weaknesses. With repeated practice and modifications in environment, youth may show improved cognitive functioning, self-regulation, and inhibitory control, which may reduce the likelihood and intensity of substance use.
Neuroimaging can be used to measure treatment efficacy in producing neurobiological and associated cognitive change. In fact, similar studies are being conducted among adult populations and show working memory training improved delayed discounting among stimulant users (Bickel, Yi, Landes, Hill, & Baxter, 2011), which may be correlated with reduced substance use or decreased likelihood for relapse. Further, these findings are particularly relevant given the recent study showing that high rates of delay discounting predicted marijuana treatment outcomes in youth (Stanger et al., 2011). Future studies will assess whether improved delayed discounting is associated with neurobiological changes and improved treatment response to contingency management interventions. While there may be other potential approaches to addressing the neurodevelopmental aspects of adolescence, cognitive training approaches show great promise in producing cognitive, neurobiological, and functional change.
SUMMARY AND FUTURE DIRECTIONS
Adolescent substance use continues to be a prevalent concern with long-term negative consequences. Research has led to new, effective treatments for substance use; however, given the developmental changes that occur during adolescence, treatments must be tailored to meet adolescents’ needs. Cognitive neuroscience and treatment research has provided important insight into adolescent brain development and brain-based mechanisms of psychotherapeutic change during CBT and mindfulness treatments for adult substance use disorders. This information can help researchers and practitioners select appropriate cognitive and behavioral strategies for each adolescent. For example, by knowing that both prefrontal circuitry and cognitive control continue to develop during adolescence, researchers and clinicians may choose to employ therapeutic techniques known to increase cognitive control and enhance prefrontal activity, such as CBT for substance use. Similarly, mindfulness may be especially helpful for adolescents who have highly reactive limbic systems and show significant deficits in self-regulation of emotion and behavior because mindfulness attention appears to decouple limbic, reward-related circuitry, potentially allowing for decreased emotional reactivity. Given the current neurobiological models of SUDs and adolescent brain development and advances in understanding neural mechanisms of adult psychotherapeutic change, future research may be able to personalize and fine tune treatments to address the needs of the developing adolescent. Furthermore, given that adolescents are undergoing significant biopsychosocial changes during adolescence and lack of highly effective interventions for addictive behaviors among youth, it is time to focus attention on developing and assessing developmentally-sensitive and neurobiologically-focused treatments and interventions for adolescent substance users.
Acknowledgements
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R01 AA13419, PI: Tapert; T32 AA013525, PI: Riley) and the National Institute on Drug Abuse (R01 DA021182, P20 DA024194, P20 DA027834).
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behavioural Brain Research. 2010;215:45–57. doi: 10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, Kramer JH. Computer-based cognitive training for mild cognitive impairment: Results from a pilot randomized, controlled trial. Alzheimer Disease & Associated Disorders. 2009;23:205–210. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C, Alexander JF. Functional family therapy. In: Gurman AS, Kniskern DP, editors. Handbook of Family Therapy. New York: Brunner/Mazel; 1981. pp. 403–443. [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Gold JM, Buchanan RW. Cognitive rehabilitation for schizophrenia: Problems, prospects, and strategies. Schizophrenia Bulletin. 1999;25:257–274. doi: 10.1093/oxfordjournals.schbul.a033377. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Marlatt A. Mindfulness-based relapse prevention for substance use disorders: A pilot efficacy trial. Substance Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Marlatt GA. Mindfulness-Based Relapse Prevention for Addictive Behaviors: A Clinician's Guide. New York: The Guilford Press; 2011. [Google Scholar]
- Brewer JA, Bowen S, Smith JT, Marlatt GA, Potenza MN. Mindfulness-based treatments for co-occurring depression and substance use disorders: What can we learn from the brain? Addiction. 2010;105:1698–1706. doi: 10.1111/j.1360-0443.2009.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, Rounsaville BJ. Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug and Alcohol Dependence. 2011;119:72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Bootzin RR, Cousins JC, Hasler BP, Peck T, Shapiro SL. The contribution of mindfulness practice to a multicomponent behavioral sleep intervention following substance abuse treatment in adolescents: A treatment-development study. Substance Abuse. 2010;31:86–97. doi: 10.1080/08897071003641297. [DOI] [PubMed] [Google Scholar]
- Broome MV, Hurley RA, Taber KH. Substance use disorders: Do males and females have differential vulnerability? The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22:v. doi: 10.1176/jnp.2010.22.3.iv. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Bryck RL, Fisher PA. Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. The American Psychologist. 2012;67:87–100. doi: 10.1037/a0024657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction (NIH publication 98-4308) Rockville, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. The American Journal of Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo DA, Cooney JL, Duhig AM, Smith AE, Liss TB, McFetridge AK, Krishnan-Sarin S. Combining cognitive behavioral therapy with contingency management for smoking cessation in adolescent smokers: A preliminary comparison of two different cbt formats. The American Journal on Addictions. 2007;16:468–474. doi: 10.1080/10550490701641173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcoholism: Clinical and Experimental Research. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Colby SM, Monti PM, Barnett NP, Rohsenow DJ, Weissman K, Spirito A, Lewander WJ. Brief motivational interviewing in a hospital setting for adolescent smoking: A preliminary study. Journal of Consulting and Clinical Psychology. 1998;66:574–578. doi: 10.1037//0022-006x.66.3.574. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. The American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dios MA, Herman DS, Britton WB, Hagerty CE, Anderson BJ, Stein MD. Motivational and mindfulness intervention for young adult female marijuana users. Journal of Substance Abuse Treatment. 2012;42:56–64. doi: 10.1016/j.jsat.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas D. Evidence-based treatments for alcohol use disorders in adolescents. Pediatrics. 2008;121(Suppl 4):S348–S354. doi: 10.1542/peds.2007-2243G. [DOI] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M, Brown S. Adolescents are not adults: Developmental considerations in alcohol users. Alcoholism: Clinical and Experimental Research. 2000;24:232–237. [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Funk R. The Cannabis Youth Treatment (CYT) study: Main findings from two randomized trials. Journal of Substance Abuse Treatment. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Devito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence. 2012;122:228–235. doi: 10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Felver-Grant JC, Abdullaev Y, Posner MI. Self-regulation and adolescent drug use: Translating developmental science and neuroscience into prevention practice. In: Bardo MT, Fishbein DH, Milch R, editors. Inhibitory Control and Drug Abuse Prevention: From Research to Translation. New York: Springer; 2011. pp. 281–301. [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: Results from a 2-year randomized controlled trial. Archives of General Psychiatry. 2010;67:674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Ewing SWF, Filbey FM, Sabbineni A, Chandler LD, Hutchison KE. How psychosocial alcohol interventions work: A preliminary look at what fmri can tell us. Alcoholism: Clinical and Experimental Research. 2011;35:643–651. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: A systematic review. BMC Geriatrics. 2011;11:55. doi: 10.1186/1471-2318-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Developments in Alcoholism: An Official Publication of the American Medical Society on Alcoholism, the Research Society on Alcoholism, and the National Council on Alcoholism. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: Insights from neuroimaging. The Journal of Adolescent Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal mri study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with dsm-iv alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Miele GM. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug and Alcohol Dependence. 2007;86:1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE. From generic to gender-responsive treatment: Changes in social policies, treatment services, and outcomes of women in substance abuse treatment. Journal of Psychoactive Drugs. 2008;(Suppl 5):327–343. doi: 10.1080/02791072.2008.10400661. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Joshi V, Rounds-Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. The Journal of Nervous and Mental Disease. 2001;189:384–392. doi: 10.1097/00005053-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychology of Addictive Behaviors. 2011;25:127–142. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins EH. A tale of two systems: Co-occurring mental health and substance abuse disorders treatment for adolescents. Annual Review of Psychology. 2009;60:197–227. doi: 10.1146/annurev.psych.60.110707.163456. [DOI] [PubMed] [Google Scholar]
- Hines M. Gender development and the human brain. Annual Review of Neuroscience. 2011;34:69–88. doi: 10.1146/annurev-neuro-061010-113654. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald DP, Eack SM. Durability and mechanism of effects of cognitive enhancement therapy. Psychiatric Services. 2006;57:1751–1757. doi: 10.1176/ps.2006.57.12.1751. [DOI] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology, Vol 6. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean L, Simard M, Wiederkehr S, Bergeron ME, Turgeon Y, Hudon C, van Reekum R. Efficacy of a cognitive training programme for mild cognitive impairment: Results of a randomised controlled study. Neuropsychological Rehabilitation. 2010;20:377–405. doi: 10.1080/09602010903343012. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2009: Volume I, Secondary school students. (NIH publication no. 10-7584) Bethesda, MD: National Institute on Drug Abuse; 2010. [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Dell Publishing; 1990. [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. The American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaminer Y, Burleson JA, Blitz C, Sussman J, Rounsaville BJ. Psychotherapies for adolescent substance abusers: A pilot study. The Journal of Nervous and Mental Disease. 1998;186:684–690. doi: 10.1097/00005053-199811000-00004. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience and Biobehavioral Reviews. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle HA, Dakof GA, Turner RM, Henderson CE, Greenbaum PE. Treating adolescent drug abuse: A randomized trial comparing multidimensional family therapy and cognitive behavior therapy. Addiction. 2008;103:1660–1670. doi: 10.1111/j.1360-0443.2008.02274.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DME, editors. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. 2nd ed. New York, NY: Guilford Press; 2005. [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine d1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcoholism: Clinical and Experimental Research. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addiction Biology. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick G, De Leon G, Hawke J, Jainchill N, Kressel D. Motivation and readiness for therapeutic community treatment among adolescents and adult substance abusers. The American Journal of Drug and Alcohol Abuse. 1997;23:485–506. doi: 10.3109/00952999709016891. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New york: Guilford; 2002. [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb MD, Locke TF. Childhood adversity and poor mothering: Consequences of polydrug abuse use as a moderator. Addictive Behaviors. 2005;30:1061–1064. doi: 10.1016/j.addbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Letson S, Davidoff J, Valentine T, Greenwood R. Enhancement of face recognition learning in patients with brain injury using three cognitive training procedures. Neuropsychological Rehabilitation. 2008;18:182–203. doi: 10.1080/09602010701419485. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Prochaska JJ, Myers MG. Intentions to quit smoking among youth in substance abuse treatment. Drug and Alcohol Dependence. 2010;106:48–51. doi: 10.1016/j.drugalcdep.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JF, Lee M, Daughters SB. Why should clinical researchers care about cognitive affective neuroscience? The Behavior Therapist. 2011;34:121–132. [Google Scholar]
- Rutherford HJ, Mayes LC, Potenza MN. Neurobiology of adolescent substance use disorders: Implications for prevention and treatment. Child and Adolescent Psychiatric Clinics of North America. 2010;19:479–492. doi: 10.1016/j.chc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clinical EEG and Neuroscience. 2009a;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcoholism: Clinical and Experimental Research. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009b;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and Clinical Psychopharmacology. 2011 doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Service Administration, Office of Applied Studies. The TEDS Report: Substance Abuse Treatment Admissions Involving Abuse of Pain Relievers: 1998 and 2008. Rockville, MD: 2010. Jul 15, [Google Scholar]
- Substance Abuse and Mental Health Services Administration. State Estimates of Substance Use and Mental Disorders from the 2008–2009 National Surveys on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. NSDUH Series H-40, HHS Publication No. (SMA) 11-4641. [Google Scholar]
- Szapocznik J, Kurtines WM, Foote F, Perez-Vidal A, Hervis O. Conjoint versus one-person family therapy: Further evidence for the effectiveness of conducting family therapy through one person with drug-abusing adolescents. Journal of Consulting and Clinical Psychology. 1986;54:395–397. doi: 10.1037//0022-006x.54.3.395. [DOI] [PubMed] [Google Scholar]
- Szapocznik J, Kurtines WM, Foote FH, Perez-Vidal A, Hervis O. Conjoint versus one-person family therapy: Some evidence for the effectiveness of conducting family therapy through one person. Journal of Consulting and Clinical Psychology. 1983;51:889–899. doi: 10.1037//0022-006x.51.6.889. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. Journal of Studies on Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional mri of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychology Review. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, Yeo RA. Adolescent substance abuse: The effects of alcohol and marijuana on neuropsychological performance. Alcoholism: Clinical and Experimental Research. 2011;35:39–46. doi: 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophrenia Bulletin. 2003;29:359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Savla GN, Zurhellen CH, Heaton RK, Jeste DV. Development and pilot testing of a novel compensatory cognitive training intervention for people with psychosis. American Journal of Psychiatric Rehabilitation. 2008;11:144–163. doi: 10.1080/15487760801963678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuontela V, Steenari MR, Carlson S, Koivisto J, Fjallberg M, Aronen ET. Audiospatial and visuospatial working memory in 6–13 year old school children. Learning & Memory. 2003;10:74–81. doi: 10.1101/lm.53503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. Journal of Clinical Child and Adolescent Psychology. 2008;37:238–261. doi: 10.1080/15374410701820133. [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Krageloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Experimental Brain Research. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC. Treating adolescents with substance use disorders: An overview of practice issues and treatment outcome. Substance Abuse. 1999;20:203–225. doi: 10.1080/08897079909511407. [DOI] [PubMed] [Google Scholar]