Abstract
HIV+ substance dependent individuals (SDIs) make significantly poorer decisions compared with HIV− SDIs, but the neurocognitive mechanisms underlying this impairment have not been identified. We administered the Iowa Gambling Task, a measure of decision making under uncertain risk, and the Cups Task, a measure of decision making under specified risk, to a group of 56 HIV+ and 23 HIV− men who have sex with men (MSMs) with a history of substance dependence enrolled in the Multicenter AIDS Cohort Study. The IGT provides no explicit information regarding the contingencies for each possible choice, and the probability of each outcome remains ambiguous at least for the early trials; in contrast, the Cups Task provides explicit information about the probability of each outcome. The HIV+ group made significantly poorer decisions on the IGT compared with the HIV− group. Cups Task performance did not differ significantly between HIV− and HIV+ groups. Exploratory analyses of the IGT data suggested that HIV+ subjects tended to perform more poorly during the early learning phase when uncertainty about specific outcomes was greatest. Additionally, performance on the final two trial blocks was significantly correlated with Stroop Interference scores, suggesting IGT performance is driven increasingly by executive control during the later portion of the task. Potential cognitive mechanisms to be explored in later studies are discussed, including impairment in implicit learning processing
Keywords: HIV, drug abuse, decision making, executive function, AIDS, neurocognition
Substance dependent individuals (SDIs) regularly make poor decisions by continuing to use drugs despite full knowledge of real life consequences such as incarceration and even death. The neurobiological model of decision making introduced by Bechara and his colleagues (Bechara, 2004, 2005; Bechara & Damasio, 2005; Bechara, Damasio, & Damasio, 2000) has informed a large number of studies of neurocognitive aspects of addiction. Their model broadly conceptualizes adaptive decision making as the executive capacity to select the most advantageous plan of action from a range of available immediate options guided by future goals rather than immediate appetitive or aversive states, (Bechara, 2004, 2005; Bechara & Damasio, 2005; Bechara et al., 2000). Bechara and his colleagues developed the Iowa Gambling Task (IGT), a simulated card game that has been employed frequently to capture drug users’ tendency to persist in making choices with attractive immediate outcomes but negative future outcomes. In a series of studies, our group has demonstrated that SDIs who are seropositive for HIV make significantly more suboptimal choices on the IGT than HIV− SDIs matched on demographic, substance use, and comorbid variables (E. M. Martin et al., 2004) consistent with reports that common prefrontal-striatal brain regions are typically affected by HIV and drug addiction (Martin-Thormeyer & Paul, 2009).
Multiple cognitive mechanisms influence IGT performance (Buelow & Suhr, 2009) including learning and memory (Gupta et al., 2009), future planning (Bechara, Damasio, Damasio, & Anderson, 1994), and responsiveness to reward (Bechara, Damasio, Damasio, & Lee, 1999). Lesion and functional imaging studies demonstrate that the IGT activates a complex network of circuitry including the ventromedial and dorsolateral prefrontal cortices, insula, posterior cingulate cortex, ventral striatum and anterior cingulate/SMA (Li, Lu, D’Argembeau, Ng, & Bechara, 2010). Elements of this network, including ventromedial prefrontal cortex and ventral striatum are also critically involved in addictive processes (Goldstein & Volkow, 2002). Although HIV has demonstrated neuropathological effects in both dorsal and ventral striatum (Wang et al., 2004), and is associated with neuron loss in the prefrontal cortex, specific neurocognitive mechanisms that drive HIV+ SDIs’ impaired decision making have not been identified.
Advances in the neuroeconomics and cognitive neuroscience literature (Fellows, 2004; Sanfey, Loewenstein, McClure, & Cohen, 2006) provide a potential strategy for isolating more specific mechanisms of HIV+ SDIs’ poor decision making performance. Recent models emphasize that decision making represents a dynamic process influenced by multiple environmental and outcome variables. One powerful influence is the degree of uncertainty that a potential outcome bears. During the beginning of the task, the IGT is typically considered to reflect decision making under ambiguous conditions because no information is provided about the likelihood that a given amount of money will be won or lost by each response selection. In contrast, measures of decision-making under specified risk such as the Cups Task provide explicit information about the probability that an outcome will be realized. Notably as more cards are selected during the IGT the subject can deduce the outcome contingencies of each of the choice options and decision-making may take place under conditions of specified risk. This conceptual distinction between ambiguous and specified risk is corroborated by behavioral and fMRI studies as patterns of brain activation during performance on tasks of decision making under specified risk such as the Game of Dice (Brand, Recknor, Grabenhorst, & Bechara, 2007) and the Cups Task (Weller, Levin, & Bechara, 2010; Weller, Levin, Shiv, & Bechara, 2007) are different from those observed with the IGT (Labudda et al., 2010; Xue et al., 2009).
Previous studies have reported that IGT performance is impaired compared to HIV− controls among HIV+ individuals both with (E. M. Martin et al., 2004) and without (Hardy, Hinkin, Levine, Castellon, & Lam, 2006) substance use disorders. The current study sample of self-identified men who have sex with men is unique compared with previous IGT studies of HIV+ SDIs, with distinctly different risk factors and patterns of drug use, Study samples from previous investigations have consisted primarily of male and female polysubstance users infected through injection drug use or heterosexual activity; additionally, use of inhalants (“poppers”) and club drugs such as MDMA, ketamine and GHB is much higher among HIV+ MSMs compared with participants in our previous studies. Performance on the Cups Task has not been studied previously among HIV+ individuals with or without a history of substance dependence, but investigating the pattern of risky choices made by HIV+ individuals under varying degrees of certainty might shed light on their decision making deficit on the IGT. Accordingly, we administered the IGT and the Cups Task to a group of HIV+ and HIV− drug-using men who have sex with men (MSMs) enrolled in the Chicago Multicenter AIDS Cohort Study (MACS) in order to determine if the HIV+ MSM SDIs showed poorer decision making under ambiguity as indexed by the IGT or significantly different patterns of risky decision making (Cups Task) compared with HIV− MSM SDIs.
Methods
Participants
The study was approved by IRBs at the University of Illinois at Chicago and at the three Chicago MACS sites (Northwestern University, Howard Brown Health Center, and the Ruth Rothstein Core Center at Stroger (formerly Cook County) Hospital. Participants consisted of 56 HIV+ and 23 ELISA-verified HIV- self-identified men who have sex with men (MSMs) currently enrolled in MACS. None of these subjects had participated in previous studies by our group. The HIV+ group was 63% African American, 14% Hispanic, and 23% Caucasian. The HIV− group was 61% African American and 39% Caucasian. All HIV+ participants were ambulatory and in generally good health. Their mean CD4 count at testing was 514. HIV RNA levels were currently undetectable at < 40 copies/ml for 59% and approximately 9% had AIDS defining CD4 counts (< 200) at testing. Mean nadir CD4 count was 271. Ninety-three per cent were prescribed potent antiretroviral therapy at testing. Participants were recruited through all three Chicago MACS study sites. MACS subjects reporting a period of at least six months of regular (at least 2–3 times per week) use of cocaine, methamphetamine, opioids or cannabis during at least one of their two core study visits within the previous year were eligible for study, MACS staff at each site contacted eligible participants and described the UIC substudy. Interested participants then received a telephone screening interview by UIC study staff. Exclusion criteria included any AIDS defining or other CNS illness, closed head injury resulting in a period of unconsciousness greater than 30 minutes, open head injury, seizure disorder, schizophrenia, untreated bipolar disorder, or current neuroleptic use. Participants meeting all criteria were scheduled for a testing visit at UIC.
Procedure
Study procedures consisted of one 120–150 minute visit to the Psychiatric Institute at UIC. Testing was conducted by bachelors-level research associates supervised by the PI (EMM). On arrival each participant signed an informed-consent form, provided a urine sample for on-site rapid drug screening) for cocaine, cannabis, opiates and methamphetamine using DrugCheck® NxStep kits, and underwent a breathalyzer test to ensure abstinence from drugs and alcohol at the time of the interview. If a potential participant tested positive, the visit was terminated, the participant received no payment, and the visit was rescheduled1. All participants were informed of these contingencies prior to the testing visit. Participants received $50 cash compensation for their time and $20 cash compensation for transportation costs at study completion.
Measures
Clinical and personality measures
Each subject was administered the Wechsler Test of Adult Reading (Wechsler, 2001) in order to estimate premorbid Verbal IQ, and a series of paper and pencil measures of potentially confounding conditions comorbid with substance use disorders, including the Sensation Seeking Scale - Version V (Zuckerman, 1996), the Beck Depression Inventory-II, (Beck, Steer, Ball, & Ranieri, 1996)and the Self-Report Psychopathy Scale (Levenson, Kiehl, & Fitzpatrick, 1995).
Substance use
All participants were administered the Structured Clinical Interview for DSM-IV- Substance Abuse Module (SCID-SAM) (First, Spitzer, Gibbon, & Williams, 1997) to determine if they met DSM-IV criteria for current or past substance use disorders. Participants also completed the Kreek-McHugh-Schluger-Kellogg Scale (Kellogg et al., 2003) which indexes severity of alcohol, cocaine and opioid use. The KMSK requires the subject to estimate the frequency of use; amount of money spent daily; and time duration of the period of maximum use of each substance. In addition to completing the KMSK, subjects were queried about the total number of years of use and the number of days since last use of alcohol, cannabis, opioids, and cocaine.
Neurocognitive tasks
Each participant was administered two computerized tasks of decision making. The Iowa Gambling Task (IGT: (Bechara, Damasio, Tranel, & Damasio, 1997) is a well-studied measure of decision-making in real time that has been administered to normal and multiple clinical populations, including individuals with focal brain lesions, substance use disorders, mood and personality disorders. The task consists of 100 trials using a simulated display of four card decks labeled A, B, C, and D. The participant is instructed to select cards one at a time from the four decks. Participants are told that they will win some money with each card selection, but occasionally the card also carries a loss. Subjects are told that the object of the game is win as much money as possible but receive no further information about the task. Unbeknownst to the participant, selections from decks A and B (disadvantageous decks) tend to result in large wins but larger and more frequent losses while selections from decks C and D commonly result in small wins but fewer losses. Thus, decks A and B provide attractive immediate outcomes but result in an overall loss while C and D decks are less immediately desirable but will lead to a winning score. No information is provided to the subjects about likelihood of particular wins or losses, so the subject must deduce which decks are advantageous. Over trials, normal participants gradually increase their selections from the advantageous decks.
Cups Task
The original Cups Task (Levin & Hart, 2003) was developed for studies of children. We employed an updated version for adults introduced by Weller and his colleagues. In this task, one can assess overall risk preference as well as sensitivity to the relative expected values (EV; expressed as the product of the magnitude of an outcome and its corresponding probability that it will occur) between choices. The ability to make EV-sensitive judgments can be considered an index of advantageous decision making in the sense that the relative EV signals whether to approach or avoid an uncertain choice option. Briefly, participants receive a computerized block of “gain” trials and a block of “loss” trials, counterbalanced in order across participants. On each gain trial, the participant chooses between one array of cups where each cup contains one quarter and another array of cups where one cup contains multiple quarters (2, 3, or 5 quarters) and the other cups contain no quarters. The number of cups (2, 3, or 5) represented the probability of winning for a risky choice. Thus, the participant can estimate the likelihood of each outcome using the number of cups associated with the risky choice. Thus, on each trial the subject is fully informed about each factor that determines EVs for each available choice. Across trials, the number of cups and the amount to be won are orthogonal, leading to some trials where the expected value (EV) for the risky choice is the same as the sure thing (equivalent, or Equal EV trials) some trials where the EV for the risky choice is greater than the sure thing (“risk advantageous” or RA trials) and some trials where the EV for the risky choice is less than the sure thing (“risk disadvantageous” or RD trials). For the loss trials, the participant starts out with a bank of quarters but can lose some on each trial. Choosing from the “sure thing” array will lead to one quarter being removed, whereas choosing from the “risky” array can lead to no quarters or multiple quarters being removed. Analogous to gain trials, some trials represent equal EV between the two options, some are risk advantageous in that the EV for the risky choice is less negative than the sure thing choice, and some are risk disadvantageous in that the EV for the risky choice is more negative than the “sure thing” choice.
Participants received 27 gain trials and 27 loss trials. Within each domain, participants receive 3 trials, presented in blocks, for each combination of outcome magnitude of the risky option (# of quarters that can be won or lost) and probability level (# of cups), presented in random order. Whether participants win or lose for each risky choice made is dependent on a random process by which p equals 1 divided by the number of cups. Figure 1 includes two representative Cups Task stimulus displays.
Figure 1.
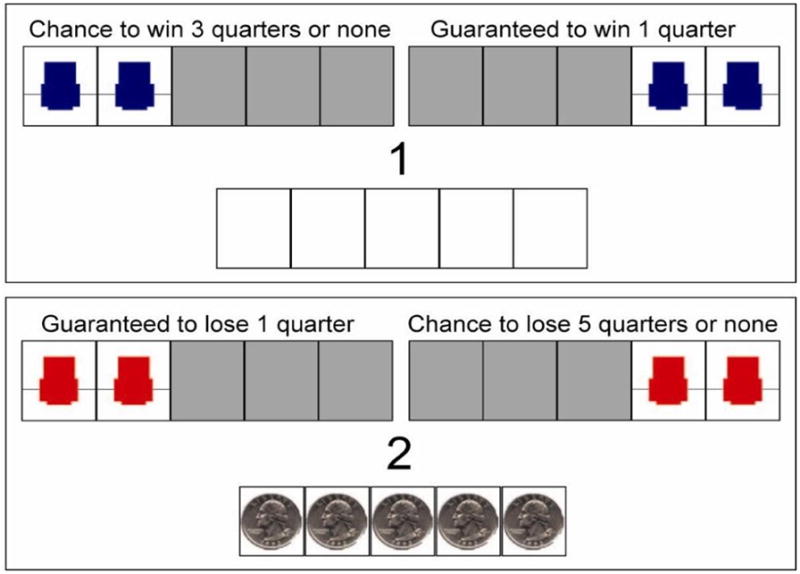
Illustrative Cups Task display.
Results
Group characteristics
Table 1 shows demographic and substance use data for all participants. There were no significant group differences in mean age, years of education or estimated IQ (age: F(1,77) = .05, p = .83; education: F(1,77) = 1.33, p = .25; IQ: F(1,77) = 1.22, p =.27) or race, χ2(1) = 4.78, p = .09. Additionally, groups did not differ significantly in mean SSS-V, BDI-II, or SRPS scores, (SSS-V: F(1,77) = .43, p = .51; BDI-II: F(1,77) = .37, p = .54; SRPS: F (1,78) = .37, p = .54). There were no group differences in severity of peak use of cocaine, heroin, or alcohol (Kreek-McHugh-Schluger-Kellogg (KMSK) Cocaine: F(1,78) = .24, p = .63; Opioids: F(1,77) = 1.98, p = .76: Alcohol, F(1,76) = .07, p = .80). There were no significant group differences in mean number of years of use of alcohol, F(1,78) = .04, p = .84; cannabis, F(1,77) = 1.00, p = .67; cocaine, F(1,58) = .58, p = .45; or opioids, F(1,14) = .24, p =.63, or in median number of days since the last use of alcohol, Mann Whitney Z = −.49, p = .63; cannabis, Z = −.15, p = .88; cocaine, Z = −.99, p = .32; or opioids, Z = −1.11, p = .26.
Table 1.
Demographic and Substance Use Characteristics
| HIV− (n = 23) | HIV+ (n = 56) | F | p | |
|---|---|---|---|---|
| Age* | 47.6 (5.1) | 47.9 (6.4) | .05 | .83 |
| Education | 15.0 (2.2) | 14.3 (2.5) | 1.33 | .25 |
| IQ Estimate | 104.0 (14.0) | 100.0 (12.8) | 1.22 | .27 |
| % African American/Caucasian | 61/39 | 63/23 | χ2 = 4.78 | .09 |
| SSS-V | 19.0 (5.0) | 18.1 (6.0) | .43 | .51 |
| SRPS | 54.7 (12.7) | 56.4 (10.9) | .37 | .54 |
| BDI-II | 5.8 (6.1) | 6.9 (7.8) | .37 | .54 |
| KMSK Cocaine | 8.1 (5.6) | 8.8 (5.9) | .24 | .63 |
| KMSK Opioids | .83 (2.7) | 2.2 (4.2) | 1.98 | .16 |
| KMSK Alcohol | 10.5 (1.7) | 10.7 (2.0) | .07 | .81 |
| Years of Use | ||||
| Alcohol | 26.5 (10.5) | 27.0 (11.0) | .04 | .84 |
| Cannabis | 16.1 (12.1) | 14.8 (11.8) | .18 | .67 |
| Cocaine | 13.7 (6.6) | 16.0 (11.6) | .58 | .45 |
| Opioids | 13.0 (2.8) | 17.9 (13.8) | .24 | .63 |
| Days since Last Use** | Z | |||
| Alcohol | 7 | 9 | −.49 | .63 |
| Cannabis | 240 | 593 | −.15 | .88 |
| Cocaine | 1460 | 300 | −.99 | .32 |
| Opioids | 553 | 1460 | −1.11 | .26 |
Abbreviations: SSS-V – Sensation Seeking Scale, v.5; SRPS – Self-Report Psychopathy Scale; BDI-II – Beck Depression Inventory II; KMSK – Kreek-McHugh-Schluger-Kellogg scale
all values are means unless indicated otherwise
medians
Table 2 provides information about prevalence of current and lifetime DSM-IV substance use disorders. There were no significant group differences in prevalence of current dependence on alcohol, cannabis, methamphetamine, opioids or cocaine (Alcohol: χ2(1) = .49, p = .48; Cannabis: χ2(1) = .42, p = .52; Methamphetamine: χ2(1) = .42, p = .52; Opioids: χ2(1) = .43, p = .51; Cocaine: χ2(1) = .001, p = .98). Approximately 21% of each group met criteria for current cocaine dependence but prevalence of current dependence on other substances was minimal (Cannabis: 1%; Alcohol: 6%; Opioids: 3%; Stimulants: 1%). Similarly, there were no significant group differences in prevalence of DSM-IV lifetime diagnoses of alcohol, cannabis, methamphetamine, or opioid dependence (Alcohol: χ2(1) = .90, p = .34; Cannabis, χ2(1) = .22, p = .64; Methamphetamine: χ2(1) = .23, p = .63; Opioids, χ2(1) = .46, p = .50). There was a nonsignificant trend toward higher prevalence of past cocaine dependence among the HIV+ group, χ2(1) = 3.0, p = .08. Results of these initial analyses indicated that HIV− and HIV+ groups were well matched on demographic, substance use and comorbid variables so that differences in decision making performance could not be attributed to confounding effects of these factors.
Table 2.
Current and Lifetime DSM-IV Substance Dependence Diagnoses
| Lifetime | Current | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HIV− | HIV+ | χ2 | p | HIV− | HIV+ | χ2 | p | |
| Alcohol* | 34.8 | 46.4 | .90 | .34 | 4 | 9 | .49 | .48 |
| Cannabis | 21.7 | 26.8 | .22 | .64 | 0 | 1.8 | .42 | .52 |
| Stimulants | 8.7 | 12.5 | .23 | .63 | 0 | 1.8 | .42 | .52 |
| Opioids | 8.7 | 14.3 | .46 | .50 | 4.3 | 1.8 | .43 | .51 |
| Cocaine | 30.4 | 51.8 | 3.0 | .08 | 21.7 | 21.4 | .001 | .98 |
all values are percentages
Neurocognitive tasks
Iowa Gambling Task
Figure 2 shows performance on the IGT by both groups. A net score is computed for each 20-trial block by subtracting the total number of “disadvantageous” (selections from decks A and B) from the total number of “advantageous” choices (selections from Decks C and D). Performance is plotted using mean net scores across the five trial blocks for each group. These data were analyzed using a (5 × 2) mixed design ANOVA with Serostatus (HIV− or HIV+) as the between factor and Trial Block as the within factor. The results showed a significant main effect for Trial Block, F(4,304) = 3.56, p = .007, and the linear component of the Block effect was statistically significant, F(1,76) = 6.65, p = .012, indicating that mean net scores increased (i.e., performance improved) significantly for each group across trial blocks. There was a significant main effect for Serostatus, F(1,76) = 5.25, p = .02, Cohen’s d = .54, and inspection of the means indicated that overall IGT performance was significantly poorer for the HIV+ compared with the HIV− group. The Serostatus x Trial Block interaction did not reach statistical significance, F(4,304) = .20, p = .94.
Figure 2.
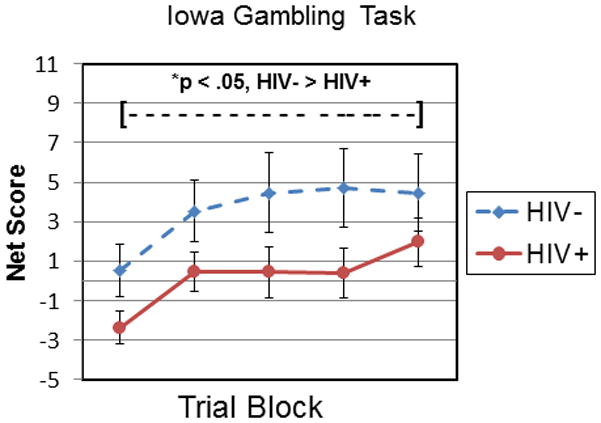
Iowa Gambling Task performance. Net scores at each trial block for HIV+ compared with HIV− subjects. HIV+ < HIV, p < .05
Cups Task
We analyzed Cups data using a (2 × 3 × 2) mixed design ANOVA with Serostatus as the between factor and EV (High, Equivalent, or Low) and Domain (Gain or Loss) as the within group factors. The dependent variable was the number of risky selections made under each EV-Domain condition.
There were no significant differences in number of risky choices for domain, F(1,75) = 2.19, p = .14. Thus, to facilitate ease of viewing, Figure 3 shows performance on the Cups Task collapsed across Domain. There was a significant main effect for EV, F(2,150) = 8.39, p < .001, and the linear component of the EV main effect was statistically significant, F(1,79) = 12.79, p = .001, indicating that the number of risky choices was lower when subjects were less certain about the actual outcome of a risky choice. There was no significant main effect for HIV Serostatus, F(1, 75) = 2.53, p = .12, and no significant interactions involving the Serostatus factor (Serostatus x EV: F(2, 75) = 1.55, p = .22; Serostatus x Domain, F(1,75) = .023, p = .88; Serostatus x EV x Domain, F(2,150) = 1.76, p = .18). Finally, the IGT net score total and the Cups total (sum of all risky choices) were essentially uncorrelated, r = −.09, p = .46.
Figure 3.
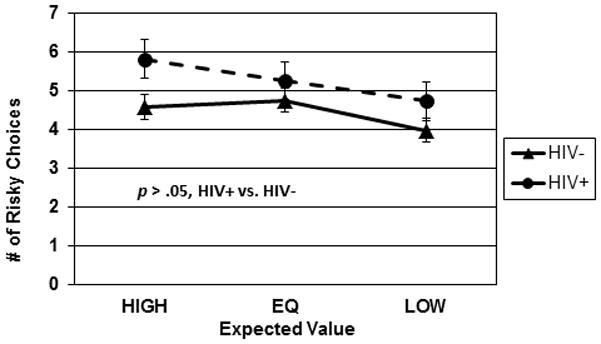
Cups Task performance. Number of risky choices made under Advantageous, Equivocal, and Disadvantageous expected values, collapsed over Doman (gain/loss).
Additional analyses
Ambiguity effects on IGT performance
We then conducted a series of exploratory analyses of potential effects of ambiguity on IGT task performance. Although the Block x Serostatus interaction was not statistically significant, these exploratory analyses seemed justified based on previous reports of the significance of ambiguity as an explanatory construct for differing cognitive mechanisms of early compared with later IGT trial blocks (Brand et al., 2007; (Monterosso, Ehrman, Napier, O’Brien, & Childress, 2001). In other words, if IGT performance was influenced by the degree of uncertainty for each outcome, any detectable effect should be most apparent during the early trial blocks prior to subjects’ deducing the pattern of advantageous and disadvantageous choices; conversely, any shift toward decision making under specified risk should be most apparent on the later trial blocks. To explore this question we compared separate net score totals for IGT Trial Blocks 1–3 and 4–5 for the HIV+ and HIV− groups. Mean net score totals for blocks 1–3 were significantly lower for the HIV+ compared with HIV− groups, F(1,76) = 5.04, p = .03, Cohen’s d = .55, and there was a nonsignificant trend toward lower mean net score totals for blocks 4–5 for the HIV+ group compared to controls, F(1,77) = 2.92, p = .09, Cohen’s d = .42
Expected value effects on Cups Task performance
We then conducted similar exploratory but theoretically justified analyses of potential effects of certainty (e.g., the likelihood that the information provided for each decision is accurate) on Cups Task performance. Compared to HIV− controls, the HIV+ group made significantly more risky choices under conditions of high certainty (high EV), F(1,75) = 4.40, p = .04, Cohen’s d = −.52, but not equivalent, (F(1,75) = .80, p = .37, Cohen’s d = −.21) or low EV, F(1,75) = 1.72, p = .19, Cohen’s d = −.32.
Potential addiction severity effects
We then conducted a series of analyses to investigate potential effects of addiction severity on IGT and Cups Task performance, employing Alcohol, Cocaine, and Opioid subscores from the Kreek-McHugh-Schluger-Kellogg Scale. For the IGT, the main effect for serostatus remained significant when adjusted for severity of cocaine or alcohol use (cocaine: F(1,75) = 5.10, p = .03, d = .55; alcohol, F(1,74) = 4.03, p = .05, d = .48). The main effect for Serostatus showed a marginally significant trend when opioid use was controlled, F(1,75) = 3.80, p = .055; however, the significance of this finding is questionable since more than 80% of the total sample received a score of 0 on the KMSK-Opioid subscale. For the Cups Task, the HIV main effect remained nonsignificant when severity of cocaine, opioid or alcohol use were controlled (Cocaine: F(1,74) = 3.06, p = .09; Opioids: F(1,74) = 2.33, p = .13; Alcohol: F(1,73) = 2.99, p = .09).
Neurocognitive correlates of IGT and Cups performance
We then investigated potential neurocognitive components of IGT and Cups Task performance. All MACS participants complete a brief battery of neuropsychological tests periodically as part of the core longitudinal study, which includes the Trail Making Test - Parts A and B (Reitan, 1958), the Symbol-Digit Modalities Test (Smith, 1982) the Rey Auditory Verbal Learning Test (Rey, 1964), the Rey-Osterreith Complex Figure (Rey, 1941), the Comalli version of the Stroop Task (Comalli, Wapner, & Werner, 1962), and the Grooved Pegboard (Trites, 1989). All core MACS neuropsychological scores are adjusted for age, education, and number of previous test administrations. The adjustment for previous test administrations employed three 0/1 dummy variables for second, third, and later administrations. When baseline score was included as a dependent variable, there were only two dummy variables, for third administration and later administration.
We obtained the scores from each subject’s most recent MACS core visit. Since the literature suggests that executive functions and declarative memory are critical influences on IGT performance, we selected the Rey Auditory Verbal Learning Test and the Interference trial of the Stroop as the most suitable indices of these neurocognitive functions.
We computed a series of exploratory correlations using the entire subject sample between IGT and Cups Task scores with the adjusted RAVLT total scores (for learning trials 1–5), and the adjusted Stroop Interference scores. IGT total, Block 1–3, and Block 4–5 net scores did not correlate significantly with the RAVLT total score, p > .09 for each test. Additionally, IGT total and Block 1–3 net scores did not correlate significantly with Stroop Interference scores (Total: r − .21, p = .09; Blocks 1–3: r = −.10, p = .41); however IGT net scores for Blocks 4–5 were significantly and inversely correlated with Stroop interference scores, r = −.28, p = .02, indicating that those subjects showing relatively greater response inhibition/working memory also showed more advantageous decision making during the final two IGT trial blocks. Total risk taking on the Cups Task and risk taking for RA (high certainty) trials correlated significantly with RAVLT total score (Total: r = .23, p =.05; RA: r =.25, p = .03). Risk taking for EQEV (equivalent certainty) and RD (low certainty) trials did not correlate significantly with RAVLT scores, r =.18, p = .13 and r = .20, p = .10, respectively. These findings indicate that lower verbal memory performance was associated with greater risk aversion, especially for RA trials. That is, individuals with lower verbal memory avoided risks even when the expected value favored the risky choice. There were no significant correlations between Stroop interference and any Cups Task scores, r < .16 for all tests.
Discussion
We administered two measures of decision making to groups of HIV+ and HIV− men who have sex with men (MSMs) with a history of substance dependence recruited from the Multicenter AIDS Cohort Study (MACS) Chicago Cohort. Groups were well matched on demographic, substance use, and comorbid characteristics. Consistent with previous studies (Martin et al., 2004) we found that HIV+ SDIs performed significantly more poorly compared with HIV− SDIs on the Iowa Gambling Task, which requires the subject to learn to avoid selecting “disadvantageous” response options with attractive immediate, but negative future outcomes, in favor of “advantageous” response choices with less appealing immediate but positive future outcomes. In contrast, the HIV+ subjects performed virtually identically to the HIV− controls on the Cups Task, a measure of adaptive decision making that evaluates which key parameters influence the subjects’ willingness to select a risky over a guaranteed outcome and has not been employed previously in studies of neurocognition and HIV. This pattern of findings was generally maintained when indices of severity of drug and alcohol use were covaried, indicating that the results cannot be attributed to nonspecific effects of substance use. The one exception to this pattern was a marginally significant (p = .055) main effect for Serostatus when opioid use was covaried, but the significance of this finding is questionable since more than 80% of the subjects received a score of zero on the KMSK Opioids subscale.
Subjects performing the IGT are initially highly uncertain about the outcome of each choice since no information is provided about the likelihood or magnitude of wins and losses; however most non-clinical subjects gradually increase their advantageous choices over trial blocks presumably as a function of the gain-loss feedback from their decisions. In contrast to the IGT, all Cups Task selections involve deciding between options in which the decision-maker has explicit information about the outcome probabilities. In contrast to the IGT findings, the HIV+ groups’ rates of advantageous and disadvantageous choices on the Cups Task were comparable to those of the HIV− group. In other words, the HIV+ group made significantly more disadvantageous choices when they were largely uncertain of the outcome of their decisions, but appeared to perform similarly to the HIV− group when tested with a task that provides trial by trial explicit information to guide their choices. We note that this interpretation should be considered speculative at present since it is based in part on a negative result; additional data are required in order to address more directly the possibility of a β error and provide stronger conclusion on the validity of the apparent contrast in HIV+ and HIV− groups’ performance.
Investigators have proposed that IGT task demands gradually shift from decision making under ambiguity during the early trial blocks to decision making under risk over later trial blocks as subjects acquire a “sense” of the probabilities associated with various outcomes, and deduce the optimal strategy for increasing wins while minimizing losses. It has been hypothesized that this shift depends primarily on implicit mechanisms (Brand et al., 2007), which are gradually superseded by executive (Brand et al., 2007) or declarative memory processes (Gupta et al., 2009). We have previously reported variable implicit learning performance among HIV+ compared with HIV− SDIs (Gonzalez et al., 2008; E. Martin, Gonzalez, Vassileva, & Maki, 2011), These prompted the question if serostatus effects on IGT performance among SDIs might be more prominent during the first three trial blocks, e.g. that HIV+ SDIs’ decision making might be more susceptible initially to ambiguity but eventually improved to the HIV− drug users’ level of advantageous decision making. This hypothesis is appealing intuitively but results of exploratory analyses did not provide strong support. Although the HIV+ group made significantly more disadvantageous choices during the early IGT trial blocks compared with HIV− controls, they also showed a trend toward significantly poorer performance on the later blocks however, at present it is not possible to determine if this nonsignificant effect is attributable to β error or a true negative result. Thus, these effects demand replication with larger samples to ensure a stronger test of the hypothesis of greater impairment among the HIV+ groups on the earliest blocks prior to more detailed hypothesis of underlying mechanisms. Replication of this effect on early trial blocks is of particular importance because HIV+ non-SDIs’ IGT deficits are typically most apparent over the later trial blocks (for a recent review, see Iudicello, Woods, Cattie, Doyle, & Grant, 2012), and additional data would also permit a more effective comparison of our subjects’ pattern of IGT performance with additional clinical and nonclinical groups (Bolla et al., 2003; Cella, Dymond, Cooper, & Turnbull, 2012; Duarte, Woods, Rooney, Atkinson, & Grant, 2012; Suhr & Tsanadis, 2007).
Larger samples would also shed light on Cups Task performance. We conducted exploratory analyses of the Cups Task results in order to investigate the association between level of certainty and willingness to take risks. We found no evidence of group differences in overall risk taking on the Cups Task, but exploratory comparisons indicated that HIV+ individuals took significantly more risks when highly certain about the outcome of their decisions. This latter finding is not readily explained and requires additional study.
Additionally, neuropsychological test correlates appeared different for the IGT and the Cups Task. Scores on the later IGT trial blocks correlated significantly with Stroop Interference, consistent with speculation in the literature (Monterosso et al., 2001) that performance mechanisms shift to executive processes during the later trial blocks.
In contrast with the IGT, the overall level of risk taking on the Cups Task was associated with higher scores on the RAVLT. The finding that episodic memory performance was higher among subjects willing to take more risks when highly certain of the outcome is not readily explained, but might reflect a greater capacity to actively incorporate ongoing information in order to maximize efficiency of task performance.
Although we found no evidence that IGT performance varied according to severity of immunosuppression (indexed by AIDS-defining CD4 counts < 200), HIV+ subjects with detectable levels of virus performed the IGT significantly more poorly compared with those with undetectable HIV RNA. One might speculate that individuals with poor decision making are less likely to adhere successfully with antiretroviral therapy; conversely, decision making deficits among HIV+ individuals might only be revealed in the context of inadequate viral suppression. The current study builds on previous work by our group in several respects. This is the first study of decision making among drug using HIV+ and HIV− MSMs, whose risk profiles and patterns of drug use are distinct from participants of previous IGT studies in risk factors and patterns of drug use. Our findings demonstrate that decision making deficits can be demonstrated across multiple groups of persons living with HIV/AIDS and suggest that IGT performance might be usefully employed in studies of risk behavior among MSMs as well as drug-using non-MSMs (Gonzalez et al., 2005; Wardle, Gonzalez, Bechara, & Martin-Thormeyer, 2010). In addition, this study is the first to employ the Cups Task with individuals living with HIV/AIDS, regardless of substance use history; the initial results are promising and warrant further investigation.
Although our data provide greater detail regarding potential mechanisms of the underlying IGT deficit among HIV+ SDIs, deconstructing the components of this complex task using behavioral analyses alone is inherently limited. The use of mathematical modeling has revealed distinct cognitive components of IGT performance by SDIs (Stout, Rock, Campbell, Busemeyer, & Finn, 2005) and preliminary data suggest this approach has promise in parsing decision making performance among HIV+ SDIs (Vassileva et al., manuscript under review). However, functional brain imaging studies will be essential for a more detailed analysis of underlying neural mechanisms of the HIV-associated defect in decision making, particularly among HIV+ SDIs, to truly move forward. It is notable that IGT performance activates neural circuitry also central to the pathophysiology of addictive process, and fMRI can potentially differentiate HIV-associated effects on neural activity from “core” processes activated by all drugs of abuse. Additionally, these studies must include a non-drug dependent control group in order to investigate possible additive or interactive effects of HIV and drug dependence on decision making with greater precision.
Current substance abuse treatments employ cognitive remediation strategies targeting executive functions (Bickel, Christensen, & Marsch, 2011; Bickel, Yi, Landes, Hill, & Baxter, 2011). Our findings may be usefully applied in the design of these strategies for HIV+ SDIs; they may derive particular benefits from interventions individually tailored to increase their level of certainty about the future outcomes of their current decisions.
Acknowledgments
Supported by HHS R03 DA025977 to Eileen M. Martin
We thank Kate Lindsay, MA, Carmon Houston RN, MA, Theresa Keeley, RN, Margarita Aguilar, RN, Susheel Reddy, MPH, and John Phair, MD. Supported by HHS R03 DA025977 to Eileen M. Martin. The Chicago MACS is supported by UOI-AI-35039 (PI Steve Wolinsky).
Footnotes
With the exception of participants who tested positive for cannabis. This test result did not necessarily indicate use within 1–2 days prior to testing because of the longer time for elimination of THC metabolites.
References
- Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52(2):336–372. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to the human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GL. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the consequences. Science. 1997;275:1293–1294. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Christensen DR, Marsch LA. A review of computer-based interventions used in the assessment, treatment, and research of drug addiction. Substance Use and Misuse. 2011;46:4–9. doi: 10.3109/10826084.2011.521066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neurology. 2003;57:1001–1007. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. Journal of Clinical & Experimental Neuropsychology. 2007;29:86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Buelow MT, Suhr JA. Construct validity of the Iowa Gambling Task. Neuropsychology Review. 2009;19:102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- Cella Matteo, Dymond Simon, Cooper Andrew, Turnbull Oliver H. Cognitive decision modelling of emotion-based learning impairment in schizophrenia: The role of awareness. Psychiatry Research. 2012;196(1):15–19. doi: 10.1016/j.psychres.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Duarte Nichole A, Woods Steven Paul, Rooney Alexandra, Atkinson J Hampton, Grant Igor. Working memory deficits affect risky decision-making in methamphetamine users with attention-deficit/hyperactivity disorder. Journal of Psychiatric research. 2012;46(4):492–499. doi: 10.1016/j.jpsychires.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK. The cognitive neuroscience of human decision making: a review and conceptual framework. Behavioral and Cognitive Neuroscience Reviews. 2004;3(3):159–172. doi: 10.1177/1534582304273251. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) – Clinician Version. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Jacobus J, Amatya AK, Quartana PJ, Vassileva J, Martin EM. Deficits in complex motor functions, despite no evidence of procedural learning deficits, among HIV+ individuals with history of substance dependence. Neuropsychology. 2008;22(6):776–786. doi: 10.1037/a0013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, Martin EM. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society. 2005;11:121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47(7):1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20(3):355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69(2):137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Labudda K, Brand M, Mertens M, Ollech I, Markowitsch HJ, Woermann FG. Decision making under risk condition in patients with Parkinson’s disease: a behavioural and fMRI study. Behavioral Neurology. 2010;23(3):131–143. doi: 10.3233/BEN-2010-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. Journal of Personality and Social Psychology. 1995;68(1):151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Levin Irwin P, Hart Stephanie S. Risk preferences in young children: early evidence of individual differences in reaction to potential gains and losses. Journal of Behavioral Decision Making. 2003;16(5):397–413. [Google Scholar]
- Li X, Lu ZL, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Human Brain Mapping. 2010;31(3):410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infections as comorbid features of HIV associated neurocognitive disorder: Neurocognitive and neuroimaging features. Neuropsychology Review. 2009;19:215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. Journal of Clinical & Experimental Neuropsychology. 2011;33:112–120. doi: 10.1080/13803395.2010.493150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Loewenstein G, McClure SM, Cohen JD. Neuroeconomics: cross-currents in research on decision-making. Trends in Cognitive Science. 2006;10(3):108–116. doi: 10.1016/j.tics.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Suhr Julie A, Tsanadis John. Affect and personality correlates of the Iowa Gambling Task. Personality and Individual Differences. 2007;43(1):27–36. [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Gonzalez R, Bechara A, Martin-Thormeyer EM. Iowa Gambling Task performance and emotional distress interact to predict risky sexual behavior in individuals with dual substance and HIV diagnoses. Journal of Clinical & Experimental Neuropsychology. 2010;32:1110–1121. doi: 10.1080/13803391003757833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Weller JA, Levin IP, Bechara A. Do individual differences in Iowa Gambling Task performance predict adaptive decision making for risky gains and losses? Journal of Clinical and Experimental Neuropsychology. 2010;32:141–150. doi: 10.1080/13803390902881926. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychological Science. 2007;18(11):958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cerebral Cortex. 2009;19(5):1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. The psychobiological model for impulsive unsocialized sensation seeking: A comparative approach. Neuropsychobiology. 1996;34:125–129. doi: 10.1159/000119303. [DOI] [PubMed] [Google Scholar]


