Abstract
Hypoxia is a prevalent attribute of the solid tumor microenvironment that promotes the expression of genes through posttranslational modifications and stabilization of alpha subunits (HIF1α and HIF2α) of hypoxia-inducible factors (HIFs). Despite significant similarities, HIF1 (HIF1α/ARNT) and HIF2 (HIF2/ARNT) activate common as well as unique target genes and exhibit different functions in cancer biology. More surprisingly, accumulating data indicates that the HIF1- and/or HIF2-mediated hypoxia responses can be oncogenic as well as tumor suppressive. While the role of HIF in the hypoxia response is well established, recent data support the concept that HIF is necessary, but not sufficient for the hypoxic response. Other transcription factors that are activated by hypoxia are also required for the HIF-mediated hypoxia response. HIFs, other transcription factors, co-factors and RNA poll II recruited by HIF and other transcription factors form multifactoral enhanceosome complexes on the promoters of HIF target genes to activate hypoxia inducible genes. Importantly, HIF1 or HIF2 require distinct partners in activating HIF1 or HIF2 target genes. Because HIF enhanceosome formation is required for the gene activation and distinct functions of HIF1 and HIF2 in tumor biology, disruption of the HIF1 or HIF2 specific enhanceosome complex may prove to be a beneficial strategy in tumor treatment in which tumor growth is specifically dependent upon HIF1 or HIF2 activity.
Keywords: hypoxia, HIF, enhanceosome, transcription factors, tumor microenvironment, transcription
1. Introduction
Oxygen is a basic requirement for life. Oxygen is necessary for multiple biochemical reactions including ATP generation in the mitochondria, and its relative abundance or absence fuels the processes of embryogenesis, wound healing and stem cell maintenance [1–4]. As such an important physiological factor, oxygen levels and the molecular pathways regulated by O2 are tightly controlled. When these mechanisms fail to function properly in response to low oxygen (hypoxia), or operate outside of the correct physiological context, they can function to promote an array of pathophysiologic afflictions including diabetic retinopathy, peripheral artery disease, ischemic heart disease and cancer [1, 5].
2. Hypoxia in tumorigenesis
Within the microenvironment of solid tumors, large regions of chronic hypoxia exist [6, 7]. These areas form due to an increase in the oxygen consumption of rapidly proliferating tumor cells and a decrease in the access to vasculature [7]. In response to the low levels of oxygen experienced in hypoxic regions of solid tumors, tumor cells activate specific gene expression programs that promote adaptation to hypoxic stress. Activation of one such program results in an increase of vasculature formation due to increased production of VEGF (vascular endothelial growth factor) in a process called angiogenesis [8, 9]. The vascularization of the tumor alleviates a portion of the demand for oxygen, yet due to the irregular morphology and poor quality of the new vasculature, many regions within the solid tumor experience a chronic state of hypoxia. Importantly, the process of tumor angiogenesis is one of the original “hallmarks of cancer” characterized by Hanahan and Weinberg in 2000 as a process crucial to the formation and survival of tumors [10]. In addition to angiogenesis, hypoxia, through induction of hypoxia responsive genes also serves to promote the other five “hallmarks” including cell survival, proliferation, evasion of growth suppressors, enabling of replicative immortality, and activation of invasion and metastasis [11, 12]. Hypoxic areas of solid tumors are reliable indicators of a poor prognosis because hypoxia increases tumor aggressiveness and protects tumors from conventional therapies [12–18]. Because of its prevalence in the tumor microenvironment, hypoxia is an important selective force in promoting the development of many cancers. The role of hypoxia in promoting tumorigenesis and contributing to a poor clinical prognosis has been extensively reviewed [19–22]. This review will focus on the transcription factors involved in the transcriptional activation of hypoxia inducible genes.
3. Hypoxia-inducible factors (HIFs)
Hypoxia Inducible Factors (HIFs) are necessary for hypoxia inducible gene expression in mammalian physiological and pathophysiological processes [20, 23]. HIFs are heterodimeric transcription factors composed of an alpha subunit (HIF1α, HIF2α, or HIF3α) and a beta subunit (HIF1β, also called Aryl hydrocarbon Receptor Nuclear Translocator or ARNT) [24–28]. ARNT, HIF1α and HIF2α are widely expressed in various tissues, however HIF3α is normally expressed only in highly avascular tissues such as the cornea. In additionally, HIF1α/ARNT (HIF1) or HIF2α/ARNT (HIF2) activates gene transcription while HIF3α/ARNT inhibits the HIF1- or HIF2-mediated hypoxia responses. Thus, our review will focus on HIF1 and HIF2 only. Both HIFα and ARNT proteins are basic-helix-loop-helix Per-ARNT-SIM domain containing (bHLH-PAS) transcription factors. The HLH and PAS domains mediate dimerization between the α and β subunits while the basic regions from HIFα and ARNT contribute to the DNA binding. The functional domains of HIF1α and HIF2α proteins have very similar arrangements (Fig. 1). The N-termini of the proteins contain the DNA-binding basic region, followed by the HLH and PAS domains. The C-terminal halves of HIFα proteins contain an N-terminal activation domain (N-TAD) that overlaps with the oxygen-dependent degradation domain (ODD), followed by an inhibitory domain (IH), and the C-terminal activation domain (C-TAD) [29–33]. Both the N-TAD and C-TAD of HIF1α and HIF2α contribute to transcriptional activity of the proteins by mediating physical interaction with CBP and p300 histone acetyltransferases [34–40]. In addition, the NTADs of HIFαs have also been suggested to play a role in determining HIF target gene specificity through interaction with other transcription factors [41, 42].
Figure 1. Structure of HIFα Proteins.

HIF1α and HIF2α are both bHLH and PAS proteins and exhibit a high degree of similarity in their DNA and ARNT binding domains, and the C-terminal transcriptional activation domains. The N-terminal transactivation domains overlap with the oxygen dependent degradation domains containing two hydroxylation-sensitive proline residues. Numbers refer to percentage of amino acid sequence similarity between HIF1α and HIF2α.
4. Oxygen regulates HIFα protein stability and transcription activity
4.1. Oxygen regulates HIFα protein stability
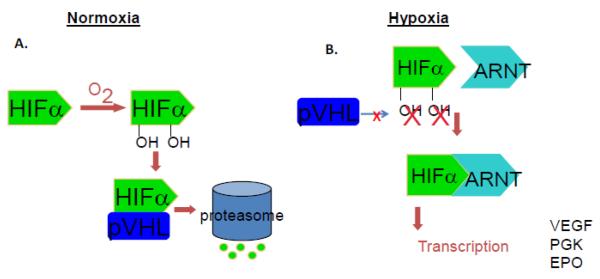
The first and most important function of oxygen in regulation of HIF activity is its effect on HIFα protein stability. In oxygen-rich conditions, although HIFα genes are actively transcribed and HIFα proteins are consistently produced, HIFα proteins are quickly degraded by the proteasome, resulting in a silencing of HIF function [43, 44] (Fig. 2). The presence of oxygen allows for the hydroxylation of two conserved proline residues (P402 and P577 of HIF1α and P405 and P530 of HIF2α in the oxygen dependent degradation domain, ODD) of the HIFα proteins by a family of HIF prolyl hydroxylase enzymes (PHD1, PHD2 and PHD3 in mammals) that require oxygen, iron, ascorbate and 2-oxoglutarate as cofactors [45–50]. The hydroxylation of the HIFα proteins allows for their binding and polyubiquitination by E3 ubiquitin ligases and subsequently for degradation by the 26S proteasome. The tumor suppressor, von Hippel Lindau protein (pVHL) is the recognizing component of the E3 ubiquitin ligase complex, and is necessary for the polyubiquitination and degradation of the HIFα subunits [44, 51–57]. Under hypoxic conditions, hydroxylation does not occur on the HIFα prolines due to a lack of substrate oxygen for PHDs, impairing HIFα recognition by pVHL E3 ubiquitin ligases, resulting in the stabilization of HIFα subunits [58, 59]. The hypoxic stabilization of HIFα allows for nuclear translocation and subsequent heterodimerization with the HIF1β (ARNT) subunit to form the active HIF transcriptional complex. The HIF heterodimer then transactivates the transcription of hypoxia inducible genes by binding to HIF binding sites (HBS, RCGTG) within hypoxia response elements (HREs) on HIF target gene promoters or enhancers [60, 61].
Figure 2. HIFα subunit stability is regulated by oxygen.
A) Hydroxylation of prolyl residues allow for the binding of the von Hippel Lindau protein (pVHL), which targets HIFα for proteasomal degradation. Under hypoxic conditions, HIFα subunits are stabilized due to a lack of hydroxylation. Consequently, transcriptionally active heterodimers can form between HIFα and β (ARNT) subunits and hypoxia inducible genes can be activated.
4.2. Oxygen regulates HIFα protein transcription activity
In addition to oxygen-dependent regulation of HIFα protein stability, HIFα transcriptional activity can also be modulated by molecular oxygen concentration. The aptly named, “Factor Inhibiting HIF” (FIH) is an aspariginyl hydroxlyase which binds to the inhibitory (IH) domain of HIF1α and HIF2α proteins and results in hydroxylation of an asparagine residue located in the HIFα C-TAD (N813 of HIF1α, N851 of HIF2α), which functions to disrupt the physical interaction between HIFα and CBP and/or p300 transcriptional co-activators [62, 63] (Fig. 3). As CBP and p300 proteins mediate the physical interaction between transcription factors and the RNA polymerase II (POLII) basal transcription machinery, FIH activity effectively inhibits HIF transcriptional activity even when HIFα protein is stabilized.
Figure 3. FIH inhibits HIF transactivation.

A) Under hypoxia HIF is transcriptionally active in a complex with ARNT, CBP, p300 and RNA polymerase II (POL II). B) The presence of oxygen allows for FIH-mediated asparaginyl (N) hydroxylation of the HIFα protein interfering with CBP or p300 interaction with the HIFα C-TAD and preventing target gene transcription.
Importantly, like proline hydroxylation, asparagine hydroxylation is dependent upon available substrate oxygen and is therefore not efficient under hypoxic conditions. It has been observed that FIH remains active under lower oxygen concentrations than PHDs, suggesting that HIF stability and activity can be differentially modified under a dynamic range of oxygen concentrations to fine-tune HIF target gene expression [64].
4.3. Hypoxia regulates HIFα transcription activity via posttranslational modifications other than hydroxylation
In addition to PHD or FIH mediated hydroxylation that is directly controlled by oxygen concentration, the transcriptional activity of HIFα can be modulated by a number of HIFα posttranslational modifications under hypoxia including phosphorylation and acetylation (reviewed in [19]).
4.3.1. Phosphorylation
While phosphorylation of both HIF1α and HIF2α proteins has been well documented (see below), recent studies have demonstrated that the two HIFα subunits are subject to different phosphorylation events [65]. Importantly, differences in HIF phosphorylation modulate its activity by determining its physical interaction with other transcription factors. It has been demonstrated that the protein kinase D1 (PKD1)-dependent phosphorylation of Thr324 of HIF2α inhibits HIF2α from binding to the SP1 transcription factor and prevents HIF2 from repressing the expression of the NBS1, a gene crucial in DNA repair [65]. Because this modification is dependent on residues absent in the HIF1α protein, HIF1α is not phosphorylated by PKD1 and so HIF1α interacts with SP1 and inhibits the expression of NBS1. In this way, differential posttranslational modification of the HIFα subunits regulates HIF interaction with co-factors and can contribute to phenotypic differences in tumor progression.
In addition to PKD1, phosphorylation of the HIF1α subunit and consequent modulation of HIF1α localization and activity have also been reported to be dependent upon mitogen-activated protein kinase (MAPK ) [66], casein kinase 1 (CK1) [67], and ataxia telangiectasia mutated (ATM) [68] activity.
MAPK family members including p38α, p38γ, and JNK are activated by hypoxia in some cell lines and have been shown to improve transcriptional activity of HIFα without affecting HIFα gene expression [69–72]. MAPK signaling has been demonstrated to increase HIF target gene expression through phosphorylation of residues in the HIFα C-TAD and IH domains [69–72]. Because of the importance of certain residues within the HIFα IH domain for interaction with the inhibitory factor FIH [62], it has been proposed that IH phosphorylation may antagonize FIH-mediated transcriptional repression of HIF, providing a mechanism by which MAPK signaling can enhance HIF activity [69].
4.3.2. Acetylation
The transcriptional activity of HIFα proteins is also subject to modulation by members of the sirtuin (SIRT) family of deacetylases. SIRT1-mediated deacetylation of lysine residues in the HIF1α or HIF2α N-TAD may achieve opposite results as it reportedly enhances the transcriptional activity of HIF2α but inhibits the activity of HIF1α [73, 74]. Interestingly, it has been proposed that HIF1α activity may inhibit SIRT activity under hypoxic conditions by increasing glycolysis, consequently decreasing the availability of NAD+, a required SIRT co-factor [74]. This may result in an enhancement of HIF1 (and repression of HIF2) activity in cells exposed to prolonged hypoxia. Seemingly contradictory to this hypothesis, results have demonstrated that HIF1α and HIF2α can bind to and activate the SIRT1 promoter under hypoxia, enhancing its expression and likely emphasizing its role in HIF modification [75].
4.3.3. Other modifications
Besides the regulation of HIF by more commonly studied posttranslational mechanisms of phosphorylation and acetylation, HIFα proteins are also reported to be targets of other modifications including sumoylation, S-nitrosylation, and neddylation, all of which modulate HIF protein stability and/or transactivation [19, 76–82].
5. Differential roles of HIF1 and HIF2 in the hypoxia response
Due to the similarity between HIF1α and HIF2α proteins in the oxygen-dependent –degradation domain, both proteins are stabilized by hypoxia. Similarities within the DNA-binding basic region and PAS domains (85% and 70% amino acid sequence identity, respectively), are responsible for HIF1α and HIF2α binding to the same core HIF binding site sequence in target gene promoters/enhancers, and for dimerizing with the same required binding partner protein ARNT/HIF1β [29–33]. It has additionally been shown using ChIP-Seq experiments that in vivo, HIF1 and HIF2 could occupy identical binding sites within HREs of most HIF1 and HIF2 target genes [41, 42, 83, 84].
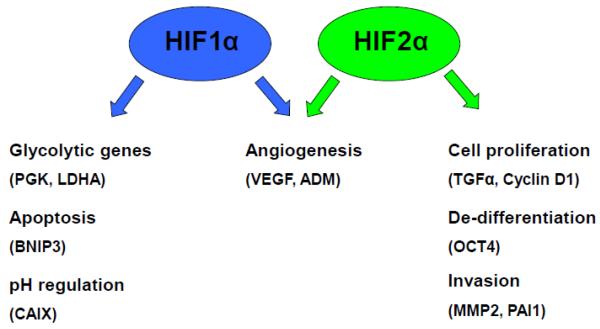
Despite the significant similarity in protein sequence, dimerization partner, and binding sites among HIF1α and HIF2α proteins, it has been well documented that HIF1 and HIF2 activate different subsets of hypoxia inducible genes in cultured cells and animals [41, 85–98]. Though some overlap exists between genes activated by HIF1 and HIF2, multiple laboratories have seen that some target genes are controlled exclusively by one HIF protein [85, 95] (Fig. 4). While specific HIF target genes may vary between cell types, in general HIF1 controls the hypoxic activation of many genes involved in cellular metabolism including all thirteen genes involved in glycolysis, including PGK1 and LDHA, as well as apoptosis such as BCL2/adenovirus E1B 19kDa interacting protein-3 (BNIP3) and pH regulation such as carbonic anhydrase-9 (CAIX) [99–101]. Genes activated exclusively by HIF2 include those involved in several tumorigenic processes including proliferation (transforming growth factor-alpha (TGFα), Cyclin D1 (CCND1)), de-differentiation (OCT4/POU5F1), and invasion (MMP2, plasminogen activator inhibitor-1 (PAI1)) [41, 89, 95].
Figure 4. HIF1 and HIF2 activate overlapping and distinct genes.
HIF1 and HIF2 share target genes involved in angiogenesis. HIF1 uniquely activates genes involved in glycolysis, apoptosis, and pH regulation whereas HIF2 specifically induces expression of genes involved in cell proliferation, de-differentiation, and tumor metastasis.
Because HIF1 and HIF2 can regulate expression of different target genes, HIFs have often been described as possessing differential functions in tumorigenesis. For example, tumors originating from colon or lung tissue have been shown to be dependent upon HIF1 activity for their growth in mouse xenograft experiments. In these tumor types, HIF2 activity represses tumor growth [102, 103]. Alternatively, tumors that develop in the liver and kidneys of VHL−/− mice have been shown to be specifically dependent upon HIF2 activity for their formation [94, 104, 105]. Although most HIF1 or HIF2 target genes are tumoriogenic, HIF1 activates tumor suppressor genes such as cyclin kinase inhibitors p21 and p27 [106] or pro-apoptotic genes such as BNIP3 and BNIP3L [107] while HIF2 promotes tumor suppressor genes such as SCGB3A1 [103]. These findings also indicate that the HIF-mediated hypoxia response could be oncogenic as well as tumor suppressive in a cell-type dependent manner.
6. Other hypoxia-activated transcription factors involved in the hypoxic response
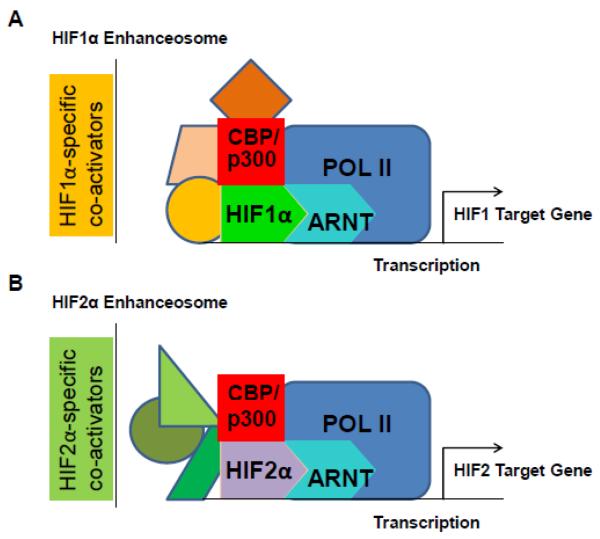
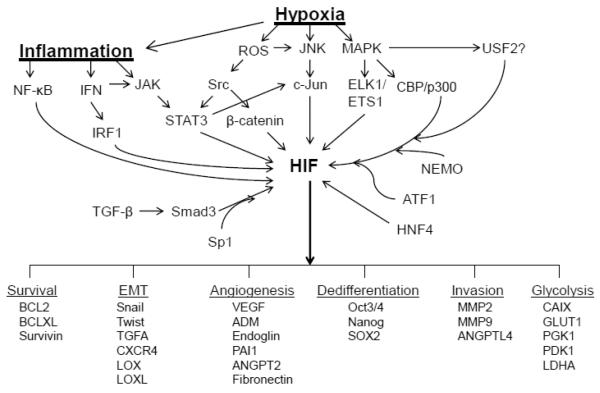
HIF is absolutely required for HIF target gene activation under hypoxia as knockdown of HIFα and/or ARNT significantly reduces or completely blocks hypoxic induction of HIF target genes. However, recent reports demonstrate other transcription factors are also required for HIF target gene expression. These factors have been found to physically interact with HIF1α or HIF2α in transcriptional complexes on HIF target gene promoters, demonstrating the possibility that some of these factors may be components of HIF enhanceosomes. Importantly, the findings that HIFs interact with a diverse variety of co-transcriptional activators suggests a mechanism by which hypoxia activates HIF as well as a number of physiologically and pathophysiologically relevant signaling pathways to induce HIF target gene expression [108, 109] (Fig. 5). Importantly, in several cases, other transcription factors function to selectively enhance expression of HIF1 or HIF2 target genes, demonstrating that distinct HIF1 or HIF2 enhanceosome complexes exist and are composed of different sets of HIF-specific co-transcriptionally activating factors on the promoters of HIF target genes (Fig. 6).
Figure 5. HIFs function in distinct enhanceosome complexes.
HIFs may achieve their target gene specificity through interaction with specific sets of co-transcriptional activating proteins within HIF-specific enhanceosomes on HIF target gene promoters. A) HIF1α interacts with HIF1-specific co-activators on HIF1 target gene promoters. B) HIF2α interacts specifically with HIF2-specific co-activators on HIF2 target gene promoters.
Figure 6. HIF enhanceosomes integrate pro-tumorigenic signaling pathways in response to tumor hypoxic stress.
HIFs interaction in enhanceosome transcriptional complexes with effectors of different signaling pathways (arrows) mediates the activation of a number of genes that promote tumorigenic processes under conditions of intratumoral hypoxia and inflammation. Because of the dependence of gene expression upon HIF/co-factor interaction, inhibition of HIF enhanceosome formation inhibits gene expression mediated by a number of protumorigenic pathways activated in the tumor microenvironment.
The idea that efficient activation of most mammalian genes requires multiple transcription factors within multi-protein enhanceosome complexes is generally accepted [108]. Incorporation of multiple factors in enhanceosome complexes renders regulation of target gene transcription highly sensitive to a variety of stimuli and results in controlled levels of target gene expression under a variety of conditions. Besides the transcriptional versatility conferred by enhanceosomes, these complexes also allow for cooperation between factors acting within the same or overlapping signaling pathways and allow for cooperative or synergistic activation of target gene transcription in the appropriate context [108, 109].
In the following sections, we focus on additional transcription factors that are required to specifically regulate HIF1 or HIF2 target genes and that may be part of HIF1 or HIF2 specific enhancesomes. We divide our review into transcription factors that are specifically required for HIF1 or HIF2 target gene activation.
6.1. Transcription factors interacting with HIF1 to activate HIF1 target genes
6.1.1 ATF-1
Activating transcription factor-1 (ATF-1) is a member of the cAMP response element binding protein (CREB) transcription factor family that binds the consensus sequence `TGACGTCA'. ATF-1's transcriptional activity is enhanced during hypoxia by hypoxia-induced p38 MAP kinase-mediated phosphorylation [110]. Binding of the factor ATF-1/CREB-1 to the HIF target gene promoter of LDHA in a complex with HIF1α has been demonstrated as necessary in order to stabilize binding by the transcriptional co-activator CBP or p300 [36]. Studies have also shown that the HIF binding site alone is inadequate to maintain high levels of CBP and p300 binding, and therefore interaction of HIF with additional co-activating proteins such as ATF-1 in transcriptional complexes promotes HIF target gene transactivation [36].
6.1.2. c-JUN
The activator protein 1 (AP-1) family transcription factor c-Jun has also been demonstrated to interact with and promote transcriptional activity of HIF1α on HIF1 target genes including VEGF [111]. This interaction does not require DNA-binding activity of c-Jun, but is dependent upon its association with HIF1α on the HIF target promoter of the VEGF gene. The physical interaction between HIF1α and c-JUN additionally requires the hypoxia-dependent posttranslational modification of the c-Jun protein by the c-Jun N-terminal kinase (JNK) [111], a modification dependent upon the generation of reactive oxygen species under hypoxia. Interestingly, hypoxia has also been reported to induce expression of, and in some cases promote the DNA-binding activity of several AP-1 proteins including c-Jun, JunB, and c-Fos, suggesting that other members of AP-1 proteins may also be involved in the hypoxia response [112–114]. c-Jun is notably involved in many cellular processes including proliferation, transformation, differentiation, apoptosis, and stress-adaptive responses and so hypoxia-dependent modulation of its activity may be especially important in the context of tumorigenesis by enhancing the HIF-mediated hypoxia response and through its HIF-independent activity [115, 116].
6.1.3. IRF-1
Interferon regulatory factor-1 (IRF-1) is a transcription factor that activates transcription of interferon alpha and beta target genes by binding to the DNA element GAAAGT/CGAAACC. Interestingly, hypoxia has been demonstrated to increase the activity of IRF-1 in hepatocytes by increasing the release of inflammatory cytokines, the upstream activators of IRF-1. Furthermore, HIF1α interaction with the IRF-1 has also been demonstrated to enhance the hypoxic activation of the nitric oxide synthase gene, NOS2, in interferon-primed, hypoxic macrophages [117]. HIF1 and IRF-1 binding sites on the NOS2 regulatory DNA region were identified and found to be required for the synergistic expression of NOS2 under hypoxia, and physical interaction as well as synergistic cooperation between these factors could be demonstrated [117]. The interaction of these factors provides an example of pathway integration occurring between the IFN-mediated inflammatory and hypoxic responses that result in optimal target gene expression only under the appropriate cellular conditions. Importantly, the IRF-1/HIF cooperation within inflammatory and hypoxic tumor microenvironments may function to promote an innate form of anti-tumor immunity.
6.1.4. HNF-4
Hepatocyte nuclear factor-4 (HNF4) and HIF1 have also been shown to cooperate in the induction of the erythropoietin (EPO) gene under hypoxia [118]. HNF4 is a constitutive factor in liver cells and binds to the consensus sequence TGACCT in the EPO 3' enhancer region downstream of the hypoxia response element. HNF4 physically interacts with the HIF1β (ARNT) subunit of the heterodimeric HIF1 transcription complex and is required for efficient transcriptional activation of EPO. Dependence upon both the inducible HIF1α and constitutive HNF4 transcription factors possibly allows for cell type specific hypoxic expression of the EPO gene as both HNF4 and EPO are found highly expressed in hypoxic liver tissue [118].
6.1.5. Smad3/Sp1
Members of the transforming growth factor-β (TGFβ) signaling pathway, a pathway often implicated in cancer and cellular de-differentiation processes, have also been reported to interact functionally with HIF1α on HIF1 target gene promoters. Cytoplasmic Smad proteins mediate TGFβ dependent gene expression by physically interacting with DNA-binding activating factors and have been demonstrated to interact with and enhance the activity of a number of transcription factors including forkhead activin signal transducer (FAST), transcription factor binding to IGHM enhancer 3 (TFE-3), core binding factor (PEP2/CBF), activating transcription factor 2 (ATF-2), ornithine decarboxylase antizyme (OAZ), AP-1, and Sp1 [119–122]. Hypoxia has been reported to increase the expression and activation of TGF-β2 and its downstream effectors, the Smad proteins, effectively upregulating the TGF-β signaling pathway under hypoxia. Additionally it has been shown that Smad3 and HIF1α physically interact and cooperatively activate transcription of the HIF target gene VEGF, and that a complex containing Smad3, Sp1, and HIF1α binds to the promoter and activates expression of the endoglin gene under hypoxia [123, 124]. Importantly, endoglin is a component of the TGFβ receptor complex whose expression is enriched on the surface of endothelial cells and associated with angiogenesis and vascular remodeling, processes which are also driven by hypoxia and HIF target gene activation.
6.1.6. β-catenin
Wnt signaling is often implicated in promoting development, de-differentiation, tissue regeneration, epithelial-mesenchymal transition (EMT), and tumorigenesis [125–132]. Activation of genes by the Wnt pathway is mediated by β-catenin that binds to T cell-specific (TCF) transcription factor and activates TCF target gene expression [129, 133, 134]. Chronic hypoxia has also been demonstrated to upregulate the β-catenin pathway in human macrophages through activation of Akt and deactivation of GSK-3β, a negative regulator of β-catenin activity. Further, hypoxia activation of β-catenin in hepatocellular carcinoma has been shown to promote a metastatic and invasive cancer phenotype. Under hypoxic conditions, it has also been demonstrated that a p-Src dependent phosphorylated form of β-catenin (pY654) physically associates with HIF1α protein and activates HIF target genes, including genes that promote EMT such as Snail and Twist [135, 136]. Importantly, hypoxia itself can increase Src kinase activity through the generation of reactive oxygen species (ROS) [137], increasing the generation of pY654-β-catenin under hypoxia and consequently the expression of HIF and β-catenin target genes.
6.1.7. STAT3
Signal transducer and activator of transcription-3 (STAT3) is a member of the STAT family of transcription factors and is activated in response to cellular stresses including hypoxia, growth factor-mediated signaling, and inflammatory cytokines-mediated signaling [138–141]. STAT3 dimers bind the consensus sequence TTN(4–6)AA and is transcriptionally activated by phosphorylation of residue Y705 and is further modulated by phosphorylation of S727 [141, 142]. It has been demonstrated that hypoxia increases STAT3 Y705 and S727 phosphorylation, promoting its nuclear retention and activation of its target genes [140]. STAT3 has also been shown to function as a component of a transcriptional complex on the vascular endothelial growth factor (VEGF) and haptoglobin gene promoters [143–146]. We have additionally reported that efficient hypoxic expression of HIF1 but not HIF2 target genes require the activity of the transcription factor STAT3 in the breast cancer cell line, MDA-MB-231 and in the renal cell carcinoma cell line, RCC4 [147]. STAT3 functions to increase p300, CBP, and RNA polymerase II recruitment to a transcriptional complex containing HIF1α on HIF1 target genes under hypoxia [147].
6.2. Transcription factors interacting with HIF2 to activate HIF2 target genes
6.2.1. ETS-1
v-ets erythroblastosis virus E26 oncogene homolog (ETS) transcription factors have been implicated in controlling important cellular processes including proliferation, angiogenesis, and metastasis. Interestingly, several members of the ETS family of transcription factors have been demonstrated to specifically interact with HIF2α and promote activation of a number of HIF2-specific target genes [41, 148, 149]. Efficient hypoxic induction of the HIF2 target gene, Flk-1 (vascular endothelial growth factor receptor-2), a receptor tyrosine kinase required for angiogenesis, was shown to be dependent upon expression of Ets-1 [148]. Furthermore, ETS-1 together with HIF2α was demonstrated to cooperatively activate a reporter construct containing a HIF2α/ETS binding element. Additionally, physical interaction was shown between the HIF2α N-TAD and the ETS-1 exon VII protein domain. While HIF1α could also physically interact with ETS-1, the interaction was not functionally cooperative [148]. Importantly, due to the high degree of functional redundancy observed among ETS transcription factors including ETS-1, ETS-2, TEL, NERF2, and ELK-1 [150], the possibility of HIF2α cooperation with other ETS family members is likely and has in fact been demonstrated to occur with ELK-1 (see below).
6.2.2. ELK-1
ELK-1 is a member of the ETS family of transcription factors that, together with HIF2α, has been shown to cooperatively activate HIF2 target genes. Interestingly, ELK-1 is reportedly phosphorylated by MAP kinase in response to hypoxia, activating its transcriptional activity [151, 152]. A subset of specific HIF2α-dependent hypoxia target genes requires the activity of the ELK-1 for optimal hypoxic expression in MCF7 cells [149]. Analysis of the promoters of these genes demonstrated that 10 out of the 11 identified HIF2 target gene promoters possess at least one HRE in proximity to an ETS transcription factor binding site [149]. ELK-1 was further shown in a different study to be required for the hypoxic expression of several other HIF2 target genes in Hep3B cells including CITED-2, EPO, and PAI1, but not the HIF2-specific or HIF2-preferential target genes IGFBP-1, ADM, and NDRG-1, suggesting that ELK-1 is not a general co-activator of HIF2 target genes [41].
6.2.3. NEMO
The NF-κB essential modulator (NEMO) has also been reported to interact with and specifically enhance the activity of HIF2α, but not HIF1α [153]. Interestingly, in comparison to the previously discussed factors, NEMO enhances HIF2α activity specifically under normoxic conditions, making it an especially relevant co-factor in cells exhibiting normoxic activation of HIF2 target genes [154–162]. Endogenous NEMO co-precipitates with endogenous HIF2α C-TAD and enhances HIF2α target gene transactivation by mediating the recruitment of the co-activator, p300 [153].
6.2.4. USF2
Upstream stimulatory factor-2 (USF2) is a ubiquitously expressed transcription factor that binds the E-box consensus sequence CAGCTG or CACGTG as a dimer. We have reported that USF2 is a required component of a transcriptional complex including HIF2, p300, and RNA polymerase II that globally activates HIF2 target genes under hypoxia [163]. We identified that the presence of USF2 is necessary for hypoxic expression of HIF2 target genes due to its function in recruiting the histone acetylases CBP and p300 to HIF2 target promoters under hypoxia. Consequently, inhibition of USF2 expression in RCC4 and PRC3 kidney cancer cell lines (two cell lines dependent upon HIF2 for their tumorigenicity) by shRNA reduces in vitro proliferation, motility and clonogenic survival of these cells. Because USF2 is specifically required for expression of HIF2 target genes and HIF2-dependent tumorigenesis, inhibition of USF2 expression or USF2/HIF2 interaction is expected to reduce tumor growth and associated metastasis of HIF2-dependent tumor types.
7. Perspectives and future directions
The function of CBP and p300 proteins in transcription has been shown to be primarily dependent upon their histone acetyltransferase activity, resulting in gene promoter histone acetylation and nucleosome rearrangement, facilitating DNA binding by additional transcriptional activators and RNA-polymerase II. Additionally, CBP and p300 have been shown to function as physical components of transcriptional complexes, serving as bridges and scaffolds between transcription factors and basal transcription machinery, which due to their size and numerous physical interactions with a multitude of transcription factors, allows the assembly of enhanceosome complexes on target promoters [108]. Several reports indicated that HIF activates its target gene expression by recruiting histone acetylases CBP and/or p300 via their C- and N-TADs [34–40]. One noticeable similarity between many of the identified HIF co-transcriptional activators is their abilities to bind and recruit the general co-activator proteins CBP and p300. Interestingly, we have found that CBP and p300 recruitment to HIF target gene promoters is primarily co-activator (STAT3 or USF2) dependent, with data demonstrating that HIF-dependent CBP and p300 recruitment contributes considerably less to the total CBP and p300 bound to HIF target gene promoters. These findings raised interesting questions that need to be addressed by future studies. What are the functions of HIF in transcription activation of HIF target genes?
Except for STAT3 and USF2, most reported HIF co-transcription factors are involved only in activation of HIF reporter genes or a very limited number of endogenous HIF target genes. Future research is needed to assess the global role of these reported transcription factors in the hypoxia response. In addition, future research in the field of HIF-mediated transcription will undoubtedly uncover additional transcription factors involved in HIF1 or HIF2 target gene activation. One remaining question in the hypoxia field is why HIF1 and HIF2 regulate different target genes despite significant similarities, including their binding to promoters of both HIF1 and HIF2 target genes. It will be interesting to determine if HIF co-transcription factors, particularly those that are required exclusively for HIF1 or HIF2 target gene activation, are important in determining HIF target gene specificity.
Although the HIF-mediated hypoxia response is generally though to be oncogenic, recent data indicates that both the HIF1 and HIF2 mediated hypoxia responses could be oncogenic or tumor suppressive depending on the specific cell or tissue type. While both HIF1 and HIF2 are generally expressed in most cell types, the expression and/or activation of other transcription factors involved in the hypoxia response could be cell-type specific. Thus, the genes and the levels of gene activation by HIF could be significantly different in different cell types, this interplay could determine if the net effect of the HIF-mediated hypoxia response is oncogenic or tumor suppressive.
Because of the opposite roles of HIF1 and HIF2 in a number of cancers, it would be beneficial to develop the means to specifically inhibit HIF1 or 2 protein functions independently of one another. The first step to the development of such therapies would be to identify small molecule inhibitors of the HIF1/co-activator or the HIF2/co-activator interaction through large-scale screening of available pharmacologic compounds. Importantly, because many HIF co-factors possess HIF-independent functions necessary for physiologic processes such as embryogenesis, angiogenesis, cellular proliferation, de-differentiation, anti-inflammatory responses, and cell cycle progression, global inhibition of these factors may be undesirable, necessitating the need to specifically inhibit their physical interaction with HIF. Ideally, the activity of a rationally designed inhibitor could be regulated by oxygen concentration, only becoming functional under hypoxia, in order to further minimize any non-specific effects. While protein/protein interactions are notoriously difficult to drug, success of nutlin and related compounds in the inhibition of the p53/MDM2 interaction have provided an indication that compounds which target specific protein interactions can be effectively developed for tumor therapy.
8. Summary
Hypoxia activates HIF-mediated hypoxia response by stabilizing HIFα protein and increasing the transcription activities of HIFα and other transcription factors.
HIF enhanceosomes include HIF and a variety of co-activating proteins involved in numerous signaling pathways.
HIF1 or HIF2 interacts with specific co-factors to activate HIF1 or HIF2 target genes.
HIF1 and HIF2 activate different sets of genes under hypoxia.
Disrupting the HIF1 or HIF2-specific enhanceosome provides an opportunity for the development of HIF-specific anti-cancer therapies.
Highlights
Hypoxia activates HIF-mediated hypoxia response by stabilizing HIFα protein and increasing the transcription activities of HIFα and other transcription factors.
HIF enhanceosomes include HIF and a variety of co-activating proteins involved in numerous signaling pathways.
HIF1 or HIF2 interacts with specific co-factors to activate HIF1 or HIF2 target genes.
HIF1 and HIF2 activate different sets of genes under hypoxia.
Disrupting the HIF1 or HIF2-specific enhanceosome provides an opportunity for the development of HIF-specific anti-cancer therapies.
Acknowledgments
This work was supported by grant from the National Cancer Institute (RO1CA134687, Hu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
Reference
- [1].Keith B, Simon MC. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Semenza GL. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- [3].Knighton DR, Silver IA, Hunt TK. Surgery. 1981;90:262–270. [PubMed] [Google Scholar]
- [4].Semenza GL. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carmeliet P. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- [6].Kim Y, Lin Q, Glazer PM, Yun Z. Current molecular medicine. 2009;9:425–434. doi: 10.2174/156652409788167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cardenas-Navia LI, Mace D, Richardson RA, Wilson DF, Shan S, Dewhirst MW. Cancer research. 2008;68:5812–5819. doi: 10.1158/0008-5472.CAN-07-6387. [DOI] [PubMed] [Google Scholar]
- [8].Shweiki D, Itin A, Soffer D, Keshet E. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- [9].Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Nature. 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- [10].Hanahan D, Weinberg RA. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [11].Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M. Advanced drug delivery reviews. 2009;61:623–632. doi: 10.1016/j.addr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [12].Ruan K, Song G, Ouyang G. Journal of cellular biochemistry. 2009;107:1053–1062. doi: 10.1002/jcb.22214. [DOI] [PubMed] [Google Scholar]
- [13].Brown JM, Giaccia AJ. Cancer research. 1998;58:1408–1416. [PubMed] [Google Scholar]
- [14].Hockel M, Schlenger K, Hockel S, Vaupel P. Cancer research. 1999;59:4525–4528. [PubMed] [Google Scholar]
- [15].Brown JM. Mol Med Today. 2000;6:157–162. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- [16].Hockel M, Vaupel P. Journal of the National Cancer Institute. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- [17].Vaupel P. Advances in experimental medicine and biology. 2009;645:241–246. doi: 10.1007/978-0-387-85998-9_36. [DOI] [PubMed] [Google Scholar]
- [18].Kalliomaki TM, McCallum G, Wells PG, Hill RP. Cancer letters. 2009;282:98–108. doi: 10.1016/j.canlet.2009.03.009. [DOI] [PubMed] [Google Scholar]
- [19].Keith B, Johnson RS, Simon MC. Nature reviews. Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Semenza GL. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- [21].Kaelin WG., Jr. Nature reviews. Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- [22].Ratcliffe PJ. The Journal of physiology. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wenger RH. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- [24].Wang GL, Jiang BH, Rue EA, Semenza GL. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W. Mechanisms of development. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- [27].Tian H, McKnight SL, Russell DW. Genes & development. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- [28].Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Gene expression. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- [29].Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ. The Journal of biological chemistry. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- [30].Ratcliffe PJ, O'Rourke JF, Maxwell PH, Pugh CW. The Journal of experimental biology. 1998;201:1153–1162. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- [31].O'Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW. The Journal of biological chemistry. 1999;274:2060–2071. doi: 10.1074/jbc.274.4.2060. [DOI] [PubMed] [Google Scholar]
- [32].Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Kidney international. 1997;51:553–555. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- [33].Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. The Journal of biological chemistry. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- [34].Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5271–5276. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ebert BL, Bunn HF. Molecular and cellular biology. 1998;18:4089–4096. doi: 10.1128/mcb.18.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. The EMBO journal. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5367–5372. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kallio PJ, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. The EMBO journal. 1998;17:6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ruas JL, Poellinger L, Pereira T. The Journal of biological chemistry. 2002;277:38723–38730. doi: 10.1074/jbc.M205051200. [DOI] [PubMed] [Google Scholar]
- [41].Hu CJ, Sataur A, Wang L, Chen H, Simon MC. Molecular biology of the cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lau KW, Tian YM, Raval RR, Ratcliffe PJ, Pugh CW. British journal of cancer. 2007;96:1284–1292. doi: 10.1038/sj.bjc.6603675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jiang BH, Semenza GL, Bauer C, Marti HH. The American journal of physiology. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- [44].Huang LE, Gu J, Schau M, Bunn HF. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- [46].Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- [47].Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. The EMBO journal. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- [49].Bruick RK, McKnight SL. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- [50].Yu F, White SB, Zhao Q, Lee FS. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. The Journal of biological chemistry. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- [52].Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. IUBMB life. 2001;52:43–47. doi: 10.1080/15216540252774757. [DOI] [PubMed] [Google Scholar]
- [53].Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- [55].Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Nature cell biology. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- [56].Tanimoto K, Makino Y, Pereira T, Poellinger L. The EMBO journal. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- [58].Kaelin WG., Jr. Biochemical and biophysical research communications. 2005;338:627–638. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- [59].Maxwell PH, Ratcliffe PJ. Seminars in cell & developmental biology. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- [60].Kaluz S, Kaluzova M, Stanbridge EJ. Clinica chimica acta; international journal of clinical chemistry. 2008;395:6–13. doi: 10.1016/j.cca.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wenger RH, Stiehl DP, Camenisch G. Science's STKE : signal transduction knowledge environment. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- [62].Mahon PC, Hirota K, Semenza GL. Genes & development. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- [64].Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J, Mazure NM. Cancer research. 2006;66:3688–3698. doi: 10.1158/0008-5472.CAN-05-4564. [DOI] [PubMed] [Google Scholar]
- [65].To KK, Sedelnikova OA, Samons M, Bonner WM, Huang LE. The EMBO journal. 2006;25:4784–4794. doi: 10.1038/sj.emboj.7601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mylonis I, Chachami G, Samiotaki M, Panayotou G, Paraskeva E, Kalousi A, Georgatsou E, Bonanou S, Simos G. The Journal of biological chemistry. 2006;281:33095–33106. doi: 10.1074/jbc.M605058200. [DOI] [PubMed] [Google Scholar]
- [67].Kalousi A, Mylonis I, Politou AS, Chachami G, Paraskeva E, Simos G. Journal of cell science. 2010;123:2976–2986. doi: 10.1242/jcs.068122. [DOI] [PubMed] [Google Scholar]
- [68].Cam H, Easton JB, High A, Houghton PJ. Molecular cell. 2010;40:509–520. doi: 10.1016/j.molcel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sodhi CP, Batlle D, Sahai A. Kidney international. 2000;58:691–700. doi: 10.1046/j.1523-1755.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- [70].Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. FEBS letters. 2000;468:53–58. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- [71].Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. The Journal of biological chemistry. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- [72].Conrad PW, Rust RT, Han J, Millhorn DE, Beitner-Johnson D. The Journal of biological chemistry. 1999;274:23570–23576. doi: 10.1074/jbc.274.33.23570. [DOI] [PubMed] [Google Scholar]
- [73].Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- [74].Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Molecular cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- [75].Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA. The Journal of biological chemistry. doi: 10.1074/jbc.M110.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cheng J, Kang X, Zhang S, Yeh ET. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].van Hagen M, Overmeer RM, Abolvardi SS, Vertegaal AC. Nucleic acids research. 2010;38:1922–1931. doi: 10.1093/nar/gkp1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, Kim KW. Biochemical and biophysical research communications. 2004;324:394–400. doi: 10.1016/j.bbrc.2004.09.068. [DOI] [PubMed] [Google Scholar]
- [79].Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- [80].Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, Chen Y, Cang H, Li H, Shi G, Cheng J, Tang X, Yi J. The EMBO journal. 2009;28:2748–2762. doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Molecular cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ryu JH, Li SH, Park HS, Park JW, Lee B, Chun YS. The Journal of biological chemistry. 2011;286:6963–6970. doi: 10.1074/jbc.M110.188706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. The Journal of biological chemistry. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. Blood. 2011;117:e207–217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Molecular and cellular biology. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Cancer research. 2005;65:3299–3306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- [87].Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Faseb J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- [88].Warnecke C, Weidemann A, Volke M, Schietke R, Wu X, Knaup KX, Hackenbeck T, Bernhardt W, Willam C, Eckardt KU, Wiesener MS. Experimental cell research. 2008;314:2016–2027. doi: 10.1016/j.yexcr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [89].Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. Genes & development. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. The Journal of clinical investigation. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Molecular and cellular biology. 2005;25:3163–3172. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Molecular and cellular biology. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH. Oncogene. 2008;27:5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Molecular and cellular biology. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mastrogiannaki M, Matak P, Delga S, Deschemin JC, Vaulont S, Peyssonnaux C. Blood. 2012;119:587–590. doi: 10.1182/blood-2011-09-380337. [DOI] [PubMed] [Google Scholar]
- [97].Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. The Journal of clinical investigation. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mastrogiannaki M, Matak P, Mathieu JR, Delga S, Mayeux P, Vaulont S, Peyssonnaux C. Haematologica. 2012;97:827–834. doi: 10.3324/haematol.2011.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Semenza GL, Roth PH, Fang HM, Wang GL. The Journal of biological chemistry. 1994;269:23757–23763. [PubMed] [Google Scholar]
- [100].Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. American journal of respiratory cell and molecular biology. 2005;32:395–403. doi: 10.1165/rcmb.2004-0314OC. [DOI] [PubMed] [Google Scholar]
- [101].Grabmaier K, MC AdW, Verhaegh GW, Schalken JA, Oosterwijk E. Oncogene. 2004;23:5624–5631. doi: 10.1038/sj.onc.1207764. [DOI] [PubMed] [Google Scholar]
- [102].Imamura T, Kikuchi H, Herraiz MT, Park DY, Mizukami Y, Mino-Kenduson M, Lynch MP, Rueda BR, Benita Y, Xavier RJ, Chung DC. International journal of cancer. 2009;124:763–771. doi: 10.1002/ijc.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mazumdar J, Hickey MM, Pant DK, Durham AC, Sweet-Cordero A, Vachani A, Jacks T, Chodosh LA, Kissil JL, Simon MC, Keith B. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14182–14187. doi: 10.1073/pnas.1001296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr. PLoS biology. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WG., Jr. The EMBO journal. 2006;25:4650–4662. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mack FA, Patel JH, Biju MP, Haase VH, Simon MC. Molecular and cellular biology. 2005;25:4565–4578. doi: 10.1128/MCB.25.11.4565-4578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Molecular and cellular biology. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Merika M, Williams AJ, Chen G, Collins T, Thanos D. Molecular cell. 1998;1:277–287. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- [109].Carey M. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- [110].Lu Z, Sack MN. The Journal of biological chemistry. 2008;283:23410–23418. doi: 10.1074/jbc.M801236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Alfranca A, Gutierrez MD, Vara A, Aragones J, Vidal F, Landazuri MO. Molecular and cellular biology. 2002;22:12–22. doi: 10.1128/MCB.22.1.12-22.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ausserer WA, Bourrat-Floeck B, Green CJ, Laderoute KR, Sutherland RM. Molecular and cellular biology. 1994;14:5032–5042. doi: 10.1128/mcb.14.8.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bandyopadhyay RS, Phelan M, Faller DV. Biochimica et biophysica acta. 1995;1264:72–78. doi: 10.1016/0167-4781(95)00116-x. [DOI] [PubMed] [Google Scholar]
- [114].Yao KS, Xanthoudakis S, Curran T, O'Dwyer PJ. Molecular and cellular biology. 1994;14:5997–6003. doi: 10.1128/mcb.14.9.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].van Dam H, Huguier S, Kooistra K, Baguet J, Vial E, van der Eb AJ, Herrlich P, Angel P, Castellazzi M. Genes & development. 1998;12:1227–1239. doi: 10.1101/gad.12.8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wisdom R, Johnson RS, Moore C. The EMBO journal. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tendler DS, Bao C, Wang T, Huang EL, Ratovitski EA, Pardoll DA, Lowenstein CJ. Cancer research. 2001;61:3682–3688. [PubMed] [Google Scholar]
- [118].Huang LE, Ho V, Arany Z, Krainc D, Galson D, Tendler D, Livingston DM, Bunn HF. Kidney international. 1997;51:548–552. doi: 10.1038/ki.1997.76. [DOI] [PubMed] [Google Scholar]
- [119].Attisano L, Wrana JL. Current opinion in cell biology. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- [120].Massague J, Wotton D. The EMBO journal. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Itoh S, Itoh F, Goumans MJ, Ten Dijke P. European journal of biochemistry / FEBS. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- [122].Zhang Y, Derynck R. Trends in cell biology. 1999;9:274–279. doi: 10.1016/s0962-8924(99)01579-2. [DOI] [PubMed] [Google Scholar]
- [123].Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. The Journal of biological chemistry. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- [124].Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. The Journal of biological chemistry. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- [125].Stemmer V, de Craene B, Berx G, Behrens J. Oncogene. 2008;27:5075–5080. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- [126].Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, Cha SY, Ryu JK, Choi YJ, Kim J, Fearon ER, Weiss SJ. Nature cell biology. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- [127].Morin PJ. BioEssays : news and reviews in molecular, cellular and developmental biology. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [128].Polakis P, Hart M, Rubinfeld B. Advances in experimental medicine and biology. 1999;470:23–32. doi: 10.1007/978-1-4615-4149-3_3. [DOI] [PubMed] [Google Scholar]
- [129].Huber O, Bierkamp C, Kemler R. Current opinion in cell biology. 1996;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- [130].Caubit X, Nicolas S, Le Parco Y. Developmental dynamics : an official publication of the American Association of Anatomists. 1997;210:1–10. doi: 10.1002/(SICI)1097-0177(199709)210:1<1::AID-AJA1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [131].Clevers H. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- [132].Thiery JP, Sleeman JP. Nature reviews. Molecular cell biology. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- [133].Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- [134].Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- [135].Xi Y, Wei Y, Sennino B, Ulsamer A, Kwan I, Brumwell AN, Tan K, Aghi MK, McDonald DM, Jablons DM, Chapman HA. Oncogene. 2012 doi: 10.1038/onc.2012.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Nature cell biology. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- [137].Pham NA, Magalhaes JM, Do T, Schwock J, Dhani N, Cao PJ, Hill RP, Hedley DW. International journal of cancer. 2009;124:280–286. doi: 10.1002/ijc.23912. [DOI] [PubMed] [Google Scholar]
- [138].Bowman T, Garcia R, Turkson J, Jove R. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- [139].Horvath CM. Trends in biochemical sciences. 2000;25:496–502. doi: 10.1016/s0968-0004(00)01624-8. [DOI] [PubMed] [Google Scholar]
- [140].Lee MY, Joung YH, Lim EJ, Park JH, Ye SK, Park T, Zhang Z, Park DK, Lee KJ, Yang YM. Breast. 2006;15:187–195. doi: 10.1016/j.breast.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [141].Levy DE, Inghirami G. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10151–10152. doi: 10.1073/pnas.0604042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Schuringa JJ, Schepers H, Vellenga E, Kruijer W. FEBS letters. 2001;495:71–76. doi: 10.1016/s0014-5793(01)02354-7. [DOI] [PubMed] [Google Scholar]
- [143].Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- [144].Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH. Faseb J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- [145].Noman MZ, Buart S, Van Pelt J, Richon C, Hasmim M, Leleu N, Suchorska WM, Jalil A, Lecluse Y, El Hage F, Giuliani M, Pichon C, Azzarone B, Mazure N, Romero P, Mami-Chouaib F, Chouaib S. J Immunol. 2009;182:3510–3521. doi: 10.4049/jimmunol.0800854. [DOI] [PubMed] [Google Scholar]
- [146].Oh MK, Park HJ, Kim NH, Park SJ, Park IY, Kim IS. The Journal of biological chemistry. 2011;286:8857–8865. doi: 10.1074/jbc.M110.150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Pawlus MR, Wang L, Hu CJ. Oncogene. in press. [Google Scholar]
- [148].Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. The Journal of biological chemistry. 2003;278:7520–7530. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- [149].Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Cancer research. 2006;66:5641–5647. doi: 10.1158/0008-5472.CAN-05-3345. [DOI] [PubMed] [Google Scholar]
- [150].Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, Lanier LL, Leiden JM. Immunity. 1998;9:555–563. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- [151].Muller JM, Krauss B, Kaltschmidt C, Baeuerle PA, Rupec RA. The Journal of biological chemistry. 1997;272:23435–23439. doi: 10.1074/jbc.272.37.23435. [DOI] [PubMed] [Google Scholar]
- [152].Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, Stern DM. The Journal of biological chemistry. 1999;274:15030–15040. doi: 10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- [153].Bracken CP, Whitelaw ML, Peet DJ. The Journal of biological chemistry. 2005;280:14240–14251. doi: 10.1074/jbc.M409987200. [DOI] [PubMed] [Google Scholar]
- [154].Brusselmans K, Bono F, Maxwell P, Dor Y, Dewerchin M, Collen D, Herbert JM, Carmeliet P. The Journal of biological chemistry. 2001;276:39192–39196. doi: 10.1074/jbc.C100428200. [DOI] [PubMed] [Google Scholar]
- [155].Mekhail K, Gunaratnam L, Bonicalzi ME, Lee S. Nature cell biology. 2004;6:642–647. doi: 10.1038/ncb1144. [DOI] [PubMed] [Google Scholar]
- [156].Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Cancer research. 2000;60:1541–1545. [PubMed] [Google Scholar]
- [157].Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Cancer research. 1999;59:3915–3918. [PubMed] [Google Scholar]
- [158].Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- [159].Richard DE, Berra E, Pouyssegur J. The Journal of biological chemistry. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- [160].Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Circulation research. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- [161].Tacchini L, Dansi P, Matteucci E, Desiderio MA. Carcinogenesis. 2001;22:1363–1371. doi: 10.1093/carcin/22.9.1363. [DOI] [PubMed] [Google Scholar]
- [162].Thornton RD, Lane P, Borghaei RC, Pease EA, Caro J, Mochan E. The Biochemical journal. 2000;350(Pt 1):307–312. [PMC free article] [PubMed] [Google Scholar]
- [163].Pawlus MR, Wang L, Ware K, Hu CJ. Molecular and cellular biology. 2012;32:4595–4610. doi: 10.1128/MCB.00724-12. [DOI] [PMC free article] [PubMed] [Google Scholar]