Abstract
Background
Our aim was to determine if age, sex, positive family history (FH+) for colorectal cancer, and body mass index (BMI) are important predictors of advanced neoplasia in the setting of screening CT colonography (CTC).
Methods
Consecutive patients referred for first-time screening CTC from 2004 to 2011 at a single medical center were enrolled. Results at pathology were recorded for all patients undergoing polypectomy. Logistic regression was used to identify significant predictor variables for advanced neoplasia (any adenoma ≥10mm or with villous component, high-grade dysplasia, or adenocarcinoma). Odds ratio (OR) was used to express the association between study variables (age, sex, BMI and FH+) and advanced neoplasia.
Results
7,620 patients underwent CTC screening. Of these, 276 (3.6%; 95% CI: 3.2–4.1%) were ultimately diagnosed with advanced neoplasia. At multivariate analysis, age (mean OR per 10-year increase: 1.8; 95% CI: 1.6–2) and male sex (OR: 1.7; 95% CI: 1.3–2.2) were independent predictors of advanced neoplasia, whereas BMI and FH+ were not. Number needed to screen (NNS) to detect one case of advanced neoplasia varied from 51 in women ≤55 years to 10 in men >65 years. The number of post-CTC colonoscopies needed to detect one case of advanced neoplasia varied from two to four.
Conclusions
Age and sex were important independent predictors of advanced neoplasia risk in subjects undergoing screening CTC, whereas BMI and positive family history were not. These results have implications for appropriate patient selection.
Keywords: Advanced adenomas, Advanced neoplasia, CT colonography, Colorectal cancer prevention, Colorectal cancer screening
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in developed countries. 1 Evidence suggests that screening asymptomatic populations can reduce CRC mortality2 and that removal of precursor adenomas may reduce the incidence of colorectal cancer.3 Computed tomographic colonography (CTC) has been well-studied and found to be a valid screening test for colorectal cancer and other advanced neoplasia,4–9 as well as demonstrating both cost-effectiveness11, 12 and a high degree of acceptance among patients.13, 14
Advanced neoplasia has been considered a valid target for CRC screening tests, including CTC.4, 9, 10 Identification of important risk factors for advanced colonic neoplasia could impact both risk stratification and development of risk reduction strategies.15 Age and sex have been shown to be strictly associated with the detection of advanced neoplasia at colonoscopy screening,16, 17 while the role of body mass index (BMI) is still controversial.17–21 Positive family history (FH+) for CRC has also been shown to predict the risk of advanced neoplasia at screening colonoscopy.16, 17 The role of these risk factors in stratification of screening CTC patients is currently unclear.
The primary aim of this study was to derive and validate a model for the detection of advanced colorectal neoplasia during screening CTC using age, sex, FH+, and BMI as predictive variables. Our secondary aims were to determine the number needed to screen (NNS) at CTC and the number of post-CTC colonoscopies needed in order to detect one case of advanced neoplasia for those variables shown to be predictive of advanced neoplasia risk. This information may be useful to refine future screening recommendations.
Methods
Study Design
The study protocol was HIPAA-compliant and approved by our institutional review board. The need for signed informed consent was waived. We prospectively enrolled 8,321 consecutive asymptomatic individuals for first-time CTC examination for the purpose of colorectal cancer screening. We excluded patients with a prior history of colorectal cancer, inflammatory bowel disease, polyposis syndromes, or prior history of colorectal surgery, as well as those referred from incomplete OC. After appropriate exclusions, a total of 7,620 asymptomatic patients comprise the screening CTC study population (Table 1). Patient age, sex, FH+ for CRC, and body mass index (BMI), were recorded. FH+ for CRC was strictly defined according to ACS criteria as having one first-degree relative diagnosed by age 60, or two first-degree relatives diagnosed at any age.
Table 1.
Characteristics of the study population.
| Characteristic | All (N=7,620) | Women (N=4,093) | Men (N=3,527) | |
|---|---|---|---|---|
| Age – yr | ||||
| Mean ± SD | 56.7±7.3 | 56.5±7.3 | 57.0±7.5 | |
| Age group – N (%) | ||||
| <50 | 294 (3.9) | 162 (4.0) | 132 (3.7) | |
| 50–54 | 3,123 (41.0) | 1,720 (42.0) | 1,403 (39.8) | |
| 55–59 | 2,024 (26.5) | 1,103 (26.9) | 921 (26.1) | |
| 60–64 | 1,266 (16.6) | 660 (16.2) | 606 (17.2) | |
| ≥65 | 913 (12.0) | 448 (10.9) | 465 (13.2) | |
| Positive family history | ||||
| N (%) | 200 (2.6%) | 119 (2.9) | 81 (2.3) | |
| BMI | ||||
| Mean ± SD | 28.1±7.1 | 27.8±8.5 | 28.4±5.1 | |
| ≥25, N (%) | 5,125 (67.3) | 2,411 (58.9) | 2,714 (76.9) | |
| Positive CTC, N (%) | 1,087 (14.3) | 480 (11.7) | 607 (17.2) | |
| Patients to polypectomy, N (%) | 735 (9.6) | 341 (8.3) | 394 (11.2) | |
A positive CTC examination was defined as any examination in which any polyp ≥6 mm in size was found. In accordance with C-RADS, positive patients with any large polyp (≥10mm in size) or more than two small polyps (6–9mm in size) found at CTC were referred for colonoscopy with polypectomy.23 Patients with one or two small polyps were given the option of either colonoscopy with polypectomy or CTC polyp surveillance. Patients with a negative exam result at CTC were recommended to undergo routine screening in five years.
The results of colonoscopy and polypectomy, as well as the final histologic result at pathology, were recorded for all patients undergoing colonoscopy. Concordance was recorded for the CTC findings of patients undergoing colonoscopy with polypectomy using colonoscopy findings as the reference standard. In discordant cases where the CTC findings were not confirmed, the finding was considered a CTC false positive unless additional imaging or repeat colonoscopy demonstrated that the lesion was missed at initial colonoscopy.
The findings on colonoscopy were categorized on the basis of the most advanced lesion identified.16 Advanced neoplasia was defined as any adenoma ≥10mm in diameter, any adenoma with a villous component or high grade dysplasia at histology, or any adenocarcinoma.16, 17
CT Colonography Technique
The CTC technique used in our screening program has been described elsewhere.24 Briefly, patients underwent a bowel preparation protocol beginning one day prior to CTC consisting of a cathartic cleansing agent. Residual fluid and fecal material was tagged using 2.1% w/v barium and diatrizoate. Colonic insufflation was achieved and maintained throughout image acquisition using automated continuous carbon dioxide delivered through a rectal catheter. Patients were routinely scanned in both supine and prone positions with decubitus positioning used as needed.25 Images were acquired with 8-to-64-section multidetector CT scanners using 1.25 mm collimation, 1-mm reconstruction interval, 120 kVp, and either a fixed tube current-time product (50–75 mAs) or tube-current modulation (range, 30–300 mA). Interpretation of CT examinations by the radiologists was performed using both three-dimensional reconstructions for initial polyp detection and two-dimensional cross-sectional images for secondary detection and polyp confirmation.6, 7, 26
Statistical Analysis
Stepwise multiple logistic regression analysis was used to identify significant predictor variables for advanced neoplasia on a per-patient basis. The prediction model was built using JMP 98.0 version 8.0 (SAS; Cary, North Carolina; USA) stepwise logistic regression analysis with an entry criteria of p<0.05. Odds ratio (OR) was used to express the association between study variables (age, sex and BMI) and the selected outcome. To test the accuracy of the multivariate model in predicting the selected outcome, the study population was randomly divided into derivation and validation groups of equal size. Once the model was established using the exploratory group, the parameter estimates were tested in the validation group in order to check goodness-of-fit (Hosmer–Lemeshow test). The procedure was repeated 20 times. Point estimates for NNS were derived from the inverse of the point estimates for prevalence. We derived the confidence intervals (CI) for the NNS by inverting the values for the 95% CIs for risk proportions. As noted previously, patients with one or two small polyps (6–9mm) found at CTC were given the option of either colonoscopy with polypectomy or CTC polyp surveillance, presumably resulting in an underestimation of the advanced neoplasia rate. Consequently, in a secondary analysis, we adopted the same regression model to predict the rate of advanced neoplasia for small polyps undergoing CTC surveillance and generate an adjusted advanced neoplasia detection rate.
Results
A total of 7,620 persons had first-time CTC screening in the study period (Table 1). The mean age [±SD] in years for the entire population was 56.7±7.3. Men accounted for 46.3% of this cohort. A positive family history for CRC was reported by 200 (3%) subjects. Mean BMI was 28.1±7.1, with 5,062 (66.4%) presenting with a BMI ≥25. CTC examination was positive (at least one polyp ≥6 mm) in 1,087 (14.3%; 95% CI: 13.5–15.1%) patients. Of these, 735 (67.6%) underwent colonoscopy, while the remaining 352 (32.4%) entered CTC surveillance for small polyps. Overall, 480/7,620 patients (6.3%) had at least one adenoma. Of these, 276/7,620 (3.6%; 95% CI: 3.2–4.1%) had at least one advanced neoplastic lesion. Table 2 reports the distribution of colorectal lesions according to histology and risk factors, as well as the association between the study variables and the risk of advanced neoplasia at univariate analysis.
Table 2.
Findings at post-CTC colonoscopy according to histology and risk factors. Association between study variables and (advanced) neoplasia at univariate analysis is also reported.
| Characteristic | All (N=7,620) |
Women <55 years of age (N=1,882) |
Women ≥55 years of age (N=2,211) |
Men <55 years of age (N=1,535) |
Men ≥55 years of age (N=1,992) |
BMI<25 (N=2,495) |
BMI≥25 (N=5,125) |
±FH (N=200) |
−FH (N=7,420) |
|---|---|---|---|---|---|---|---|---|---|
| Advanced Neoplasia, N (%) | 276 (3.6) | 37 (2.0) | 75 (3.4)* | 48 (3.1) | 116 (5.8)* | 69 (2.8) | 207 (4,0)* | 9 (4.5) | 267 (3.6) |
| Any Adenoma ≥6 mm, N (%) | 480 (6.3) | 75 (4) | 126 (5.7)* | 96 (6.3) | 183 (9.2)* | 119 (4.8) | 361 (7.0)* | 18 (9.0) | 462 (6.2) |
| Non-neoplastic Polyp ≥6 mm, N (%) | 152 (2) | 24 (1.3) | 44 (2.0) | 38 (2.5) | 46 (2.3) | 50 (2.0) | 102 (2.0)* | 7 (3.5) | 145 (2.0) |
p<0.05 (Chi-square test); BMI: body mass index; FH: family history of CRC
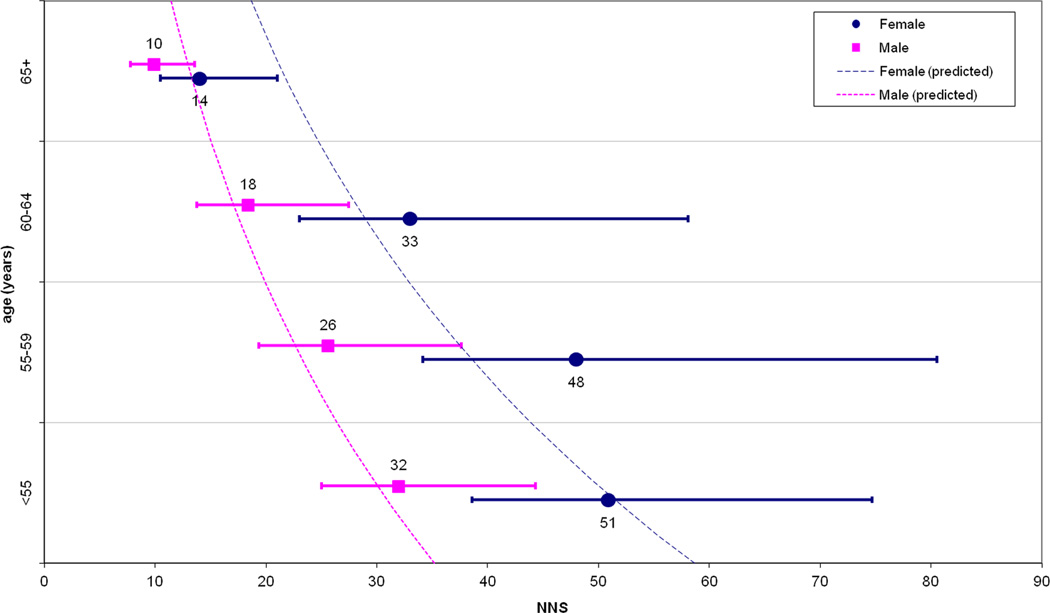
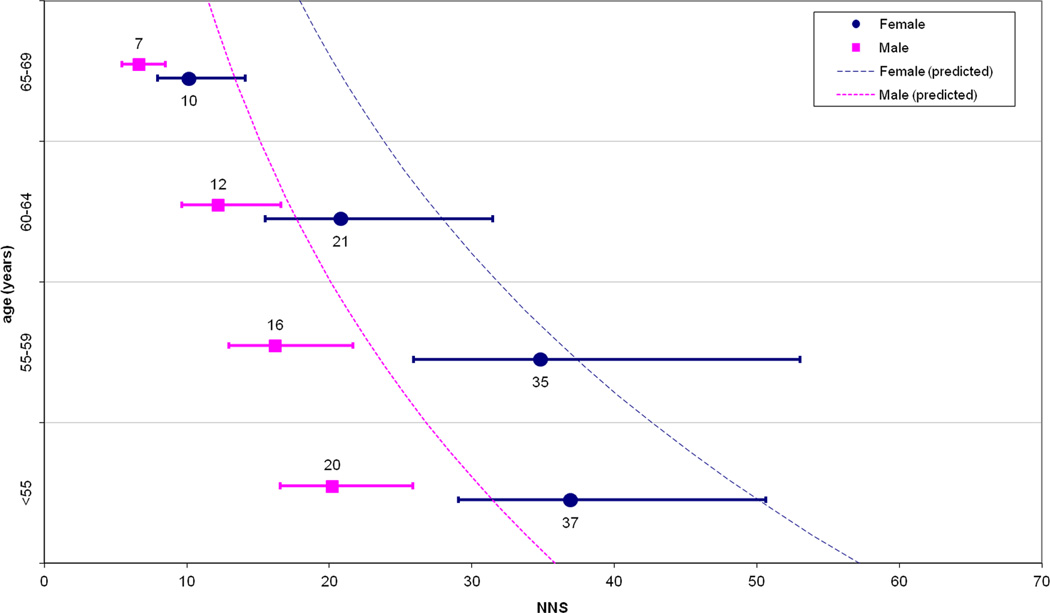
Multivariate modeling indicated age (mean OR per 10-year increase: 1.8; 95% CI: 1.6–2.0) and male sex (OR: 1.7; 95% CI: 1.3–2.2) as independent predictors of advanced neoplasia risk, while FH+ (OR: 1.2; 95% CI: 0.6–2.4) and BMI (OR: 1.0; 95% CI: 0.9–1.1) were not. When dividing the study population into derivation and validation samples, the Hosmer–Lemeshow test confirmed the goodness-of-fit of the model with a mean p-value (among 20 iterations) of 0.3 for the validation data set (where p <0.05 would indicate a lack of fit). Figure 1(a) and Table 3 show the NNS to detect advanced neoplasia according to age and sex, with figure 1(b) depicting the same analysis after adjustment with the multivariate model predicting advanced neoplasia rate for the positive patients with small detected lesions (6–9 mm) entering CTC polyp surveillance. Table 3 shows the number of post-CTC colonoscopies needed to detect one case of advanced neoplasia according to age and sex.
Figure 1.
(a) Numbers needed to screen (NNS) in order to detect advanced neoplasia, according to age and sex. (b) The same data are reported after simulation to predict advanced neoplasia in those subjects undergoing CTC surveillance for small polyps. Dotted lines represent the yearly values of NNS as estimated by the multivariate model.
Table 3.
Number needed to screen (NNS) with CTC to detect one case of advance neoplasia and number of post-CTC colonoscopies to detect one case of advanced neoplasia, according to age and sex.
| Age/Sex | No. Needed to Screen to Detect Advanced Neoplasia (95% CI) | No. of post-CTC Colonoscopies Needed to Detect Advanced Neoplasia (95% CI) | |
|---|---|---|---|
| <55 years | |||
| Male | 32 (25–44) | 3.1 (2.5–4) | |
| Female | 51 (39–75) | 3.4 (2.6–4.6) | |
| 55–59 years | |||
| Male | 26 (19–38) | 2.4 (2–3.3) | |
| Female | 48 (34–81) | 4.0 (2.9–6.1) | |
| 60–64 years | |||
| Male | 18 (13–25) | 2.2 (1.8–3) | |
| Female | 33 (23–58) | 3.1 (2.2–4.8) | |
| ≥65 years | |||
| Male | 10 (8–14) | 1.8 (1.5–2.2) | |
| Female | 14 (10–21) | 2 (1.6–2.7) | |
Discussion
According to our study, age and sex are significantly associated with the detection of advanced neoplasia in a large cohort of first-time CTC screening patients, allowing for clinically relevant risk stratification of the study population. The wide interval in the estimates of the NNS with CTC to detect one case of advanced neoplasia, ranging from 10 to 51 (7–37 when adjusting for 6–9 mm polyps still in surveillance) closely approximates the 10–36 NNS interval shown by Regula, et al. in a large cohort of patients undergoing primary screening colonoscopy.16 This suggests equivalence in the role of age and sex in stratifying average-risk subjects between CTC and colonoscopy. The more than four-fold variation in NNS to detect advanced neoplasia according to age and sex may be informative when planning for general population screening. In cases of limited resources, which is likely to occur when considering all adults over 50 are recommended for CRC screening, it makes sense for health systems to target patients with a higher expected benefit in order to best utilize available economic and medical resources. This would not be new in the field of CRC screening, as shown by the adoption of a selective cut-off for the exploitation of colonoscopy resources following sigmoidoscopy or immunochemical fecal testing.2, 3, 10 Our observation of an exponential reduction of the NNS according to increasing age would also be consistent with the adoption of a higher age cut-off for initial population screening as with the recently recommended 55–65 age interval for sigmoidoscopy screening.3 Although gender has not been explicitly included in any guidelines, our analysis would confirm a substantial difference in prevalence of advanced neoplasia between men and women, as already outlined by Regula et al.16 For example, as shown by the prediction model in Figure 1, women appeared to reach the same NNS as men with an approximate 10-year delay. It would appear reasonable to propose different age recommendations for screening by CTC according to sex, while perhaps a less costly strategy, such as fecal occult blood testing, could be employed earlier. Specifically, women <65 years and men <55 years have an NNS below the study-wide mean NNS of 28, suggesting that screening these patients represents a somewhat less efficient use of CTC.
The adoption of non-invasive CRC screening approaches, including CTC, necessitates post-test referral to colonoscopy following positive examinations. The ultimate efficiency of any non-invasive approach would greatly depend upon the number of post-test colonoscopies needed to detect one case of advanced neoplasia as a matter of avoiding unnecessarily redundant procedures. When stratifying our study population according to age and sex, there is a two-fold difference in the efficiency of post-CTC colonoscopy exploitation, ranging from 2 to 4 procedures to detect one case of advanced neoplasia. In the case of limited endoscopic capacity, this information could trigger more conservative non-endoscopic surveillance policies for those CTC-positive subjects at lower risk of advanced neoplasia or the adoption of more selective post-CTC dimensional cut-off for referring subjects to polypectomy.
Despite an apparent association at univariate analysis, our study failed to confirm any association between BMI and the detection of advanced neoplasia at multivariate analysis, casting doubt on the relevance of this factor in stratifying the average-risk population for CRC screening with CTC. Of note, two-thirds of the study population reported a ≥25 BMI, so any eventual impact of this variable would have been substantial. Our result supports a previous predictive model for advanced neoplasia at colonoscopy.17 The lack of association between BMI and advanced neoplasia was also confirmed by a recent meta-analysis – including 168,201 subjects – that failed to show any statistically significant association between these two variables.18, 19 Of note, the same meta-analysis showed a slight – but statistically significant – increase in colorectal adenomas prevalence (i.e. also including non-advanced adenomas), when comparing subjects with a BMI of ≥25 with those with BMI<25.18, 19 This is also in line with a previous report from our group in a separate CTC screening series, also showing an association between higher visceral adiposity measurements on CTC and the presence of adenomas (including non-advanced).20 Therefore, as suggested by the same Authors of the meta-analysis, obesity seems to be involved in the tumor initiation (i.e. adenoma formation) rather than in tumor progression (i.e. advanced neoplasia).18
We could not confirm the role of a positive family history for CRC in stratifying for advanced neoplasia risk. When considering the relatively low prevalence of FH+ – 3% in our cohort as compared with 13.3% in a previous endoscopic series –16, we cannot exclude that such lack of association may be simply due to a sample size bias. In a recent well-design endoscopic study, a moderate association between a positive family history for CRC and the risk of advanced neoplasia has been shown in a Chinese population. Thus, further studies specifically looking at siblings of CRC-patients are needed to clarify the role of CTC in these high-risk subjects.21
We acknowledge limitations to our study. One-third of our positive patients entered non-invasive CTC surveillance of their 6–9 mm polyps, potentially underestimating the prevalence of advanced neoplasia in our population. We attempted to address this bias by calculating the adjusted advanced neoplasia rate at multivariate analysis. In addition, although our CTC screening cohort is among the largest ever reported, the confidence intervals for our five-year age groups are quite large. However, the four-fold difference among point-estimates for NNS, as well as the linear model prediction according to age and sex, are likely to be informative for policy makers.
In conclusion, age and sex appear to be useful variables in predicting risk of advanced neoplasia at screening CT colonography, whereas BMI and FH+ do not appear to have a major impact. These findings could be useful in stratifying patients undergoing CTC screening.
Acknowledgements
Funding: This work was supported in part by NIH (NCI) grant 1R01CA144835-01
Footnotes
Competing interests: None
Contributors: All authors were involved in conception and design, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published; CH and BDP had full access to all the data in the study; PJP had final responsibility for the decision to submit for publication.
References
- 1.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 3.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 4.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CD, Chen M-H, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. New England Journal of Medicine. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357:1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 8.Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal Cancer: CT Colonography and Colonoscopy for Detection-Systematic Review and Meta-Analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regge D, Laudi C, Galatola G, et al. Diagnostic Accuracy of Computed Tomographic Colonography for the Detection of Advanced Neoplasia in Individuals at Increased Risk of Colorectal Cancer. Jama-Journal of the American Medical Association. 2009;301:2453–2461. doi: 10.1001/jama.2009.832. [DOI] [PubMed] [Google Scholar]
- 10.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Hassan C, Zullo A, Laghi A, et al. Colon cancer prevention in Italy: Cost-effectiveness analysis with CT colonography and endoscopy. Digestive and Liver Disease. 2007;39:242–250. doi: 10.1016/j.dld.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ, Hassan C, Laghi A, et al. Cost-effectiveness of colorectal cancer screening with computed tomography colonography - The impact of not reporting diminutive lesions. Cancer. 2007;109:2213–2221. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 13.Moawad FJ, Maydonovitch CL, Cullen PA, et al. CT Colonography May Improve Colorectal Cancer Screening Compliance. American Journal of Roentgenology. 2010;195:1118–1123. doi: 10.2214/AJR.10.4921. [DOI] [PubMed] [Google Scholar]
- 14.Pooler BD, Baumel MJ, Cash BD, et al. Screening CT colonography: multicenter study of patient experience, preference, and potential impact on adherence. American Journal of Roentgenology. 2012;198:1361–1366. doi: 10.2214/AJR.11.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JMG, Jungner G. Principles and practice of screening for disease. Who Chronicle. 1968;22:473. [Google Scholar]
- 16.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA, Prindiville S, Weiss DG, et al. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959–2967. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 18.Okabayashi K, Ashrafian H, Hasegawa H, et al. Body mass index category as a risk factor for colorectal adenomas: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1175–1185. doi: 10.1038/ajg.2012.180. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JC. Editorial: Body mass index and colorectal adenomas. Am J Gastroenterol. 2012;107:1187–1188. doi: 10.1038/ajg.2012.182. [DOI] [PubMed] [Google Scholar]
- 20.Hassan C, Pickhardt PJ, Marmo R, et al. Impact of lifestyle factors on colorectal polyp detection in the screening setting. Dis Colon Rectum. 2010;53:1328–1333. doi: 10.1007/DCR.0b013e3181e10daa. [DOI] [PubMed] [Google Scholar]
- 21.Summers RM, Liu J, Sussman DL, et al. Association between visceral adiposity and colorectal polyps on CT colonography. AJR Am J Roentgenol. 2012 Jul;199(1):48–57. doi: 10.2214/AJR.11.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng SC, Lau JY, Chan FK, et al. Increased Risk of Advanced Neoplasms Among Asymptomatic Siblings of Patients With Colorectal Cancer. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.11.011. PMID: 23159367. [DOI] [PubMed] [Google Scholar]
- 23.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: A consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 24.Pickhardt PJ. Screening CT colonography: How I do it. American Journal of Roentgenology. 2007;189:290–298. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 25.Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdominal Imaging. 2011;36:538–544. doi: 10.1007/s00261-010-9666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT Colonography. American Journal of Roentgenology. 2007;189:1451–1456. doi: 10.2214/AJR.07.2291. [DOI] [PubMed] [Google Scholar]