Abstract
Objectives
Cortical auditory evoked potentials (CAEPs) to tones and speech sounds were obtained in infants to: 1) further knowledge of auditory development above the level of the brainstem during the first year of life; 2) establish CAEP input-output functions for tonal and speech stimuli as a function of stimulus level and to 3) elaborate the data-base that establishes CAEP in infants tested while awake using clinically relevant stimuli, thus providing methodology that would have translation to pediatric audiological assessment. Hypotheses concerning CAEP development were that the latency and amplitude input-output functions would reflect immaturity in encoding stimulus level.
In a second experiment, infants were tested with the same stimuli used to evoke the CAEPs. Thresholds for these stimuli were determined using observer-based psychophysical techniques. The hypothesis was that the behavioral thresholds would be correlated with CAEP input-output functions because of shared cortical response areas known to be active in sound detection.
Design
36 infants, between the ages of 4-12 months (mean= 8 months, s.d.=1.8 months) and 9 young adults (mean age 21 years) with normal hearing were tested. First, CAEPs amplitude and latency input-output functions were obtained for 4 tone bursts and 7 speech tokens. The tone bursts stimuli were 50 ms tokens of pure tones at 0.5, 1.0, 2.0 and 4.0 kHz. The speech sound tokens, /a/, /i/, /o/, /u/, /m/, /s/, and /∫/, were created from natural speech samples and were also 50 ms in duration. CAEPs were obtained for tone burst and speech token stimuli at 10 dB level decrements in descending order from 70 dB SPL. All CAEP tests were completed while the infants were awake and engaged in quiet play.
For the second experiment, observer-based psychophysical methods were used to establish perceptual threshold for the same speech sound and tone tokens.
Results
Infant CAEP component latencies were prolonged by 100-150 ms in comparison to adults. CAEP latency-intensity input output functions were steeper in infants compared to adults. CAEP amplitude growth functions with respect to stimulus SPL are adult-like at this age, particularly for the earliest component, P1-N1.
Infant perceptual thresholds were elevated with respect to those found in adults. Furthermore, perceptual thresholds were higher, on average, than levels at which CAEPs could be obtained. When CAEP amplitudes were plotted with respect to perceptual threshold (dB SL), the infant CAEP amplitude growth slopes were steeper than in adults.
Conclusions
Although CAEP latencies indicate immaturity in neural transmission at the level of the cortex, amplitude growth with respect to stimulus SPL is adult-like at this age, particularly for the earliest component, P1-N1. The latency and amplitude input-output functions may provide additional information as to how infants perceive stimulus level. The reasons for the discrepancy between electrophysiologic and perceptual threshold may be due to immaturity in perceptual temporal resolution abilities and the broad-band listening strategy employed by infants.
The findings from the current study can be translated to the clinical setting. It is possible to use tonal or speech sound tokens to evoke CAEPs in an awake, passively alert infant, and thus determine whether these sounds activate the auditory cortex. This could be beneficial in the verification of hearing aid or cochlear implant benefit.
Keywords: infant, evoked potential, perception
Introduction
During the past 35 years, much knowledge of infant auditory system development and capacity has been obtained from the auditory brainstem responses (ABR). ABR absolute and interpeak latencies of components Wave I- V reflect increased capacity for neural synchrony and temporal processing that follows the time-course for brainstem myelination over the first 18 months of life (Hecox & Galambos, 1974). ABR thresholds for clicks and tone bursts suggest that adult-like sensitivity is obtained during the first year of life, at least in the mid-high frequencies, well before perceptual thresholds approximate adult levels (Werner et al, 1993).
Much less is known about the how cortical auditory evoked potentials (CAEPs) may reflect aspects of perceptual development in infants. One hypothesis is that perceptual thresholds are dependent upon maturation at more rostral levels of the auditory system, particularly the auditory cortex. By testing CAEPs in awake, alert infants, we aimed to show that these evoked potentials could be used as a metric of physiological development in the same way that ABRs are used at the level of the brainstem. That is, the motivation was to use CAEP to understand underlying physiological processes and the neural substrates of perception.
Wunderlich & Cone-Wesson (2006) provided a comprehensive review of the development of CAEP in early infancy summarizing the immaturities of CAEP recorded in infants and children compared to responses obtained from adults. At term, and in the early months of life, the typical CAEP waveform recorded in the midline has as its most prominent features a broad positive peak, P2, followed by a broad negative trough, N2. Earlier peaks, P1 and N1 may sometimes be seen but are much less frequently evoked (Little et al, 1999; Molfese, 2000; Ohlrich and Barnet, 1972; Rapin and Graziani, 1967; Shucard et al, 1987; Weitzman & Graziani, 1968) and, when present, are small relative to P2 and N2. In the newborn period P2 has a peak latency around 200–250 ms and N2 around 300–550 ms (Barnet et al, 1975; Kurtzberg et al, 1984; Rotteveel et al, 1987a,b). The source of the P2 CAEP component in newborn infants has been localized to the temporal lobe in or near the auditory areas (Huotilainen et al, 2003). Developmental changes during the first year 3 years of life include decreasing response latencies and greater prominence of later components (Ohlrich et al, 1978; Barnet et al, 1975). The peak latencies of P2 and N2 recorded at the vertex in sleeping infants have generally been found to decrease with age, most markedly over the first months of life. When tested in awake infants, CAEP peak latencies do not necessarily show the same developmental trend although the waveforms have similar morphology to those in sleeping infants. The latency of P2 becomes less variable with age over the first 4 months of life (Thomas et al, 1997).
Clicks and tones have been used to evoke CAEP in infants but there are few systematic studies of stimulus effects in this age group. One exception is the research by Wunderlich et al (2006) who investigated the effect of frequency on the CAEP in infants and young children. They found that 400 Hz tones evoked larger amplitude CAEP than did tones at 3000 Hz. To date, there has been no systematic study of infant CAEP latency and amplitude as a function of stimulus level, nor are there studies that have sought to relate the CAEP latencies and amplitudes to how infants perceive the same stimulus.
Speech sounds have also been used to evoke CAEPs in young infants (Kurtzberg et al, 1986). Kurtzberg et al (1986) found topographical differences in the CAEP of newborns that reflected frequency based differences in the place of articulation of consonants (/da/ vs. /ba/) and morphological differences that reflected voice onset time (/ta/ vs. /da/ and /ba/). Novak et al. (1989) recorded CAEPs to formants extracted from synthesized CV syllables but found no systematic effect of formant centre frequency on the responses recorded during the first 6 months of life. Wunderlich et al (2006) also used speech tokens to evoke CAEP in infants and young children. In newborns, the speech tokens evoked a much larger amplitude response than did tones, but this finding was not consistent in older infants or children. In these previous studies, the speech tokens were presented at one level, known to be above threshold and to approximate a conversational level, that is, 50-60 dB HL. There were no attempts to determine how stimulus level would affect CAEP latencies and amplitudes.
Despite the paucity of research on stimulus effects on infant CAEPs, these evoked responses have been used in clinical research to evaluate neurological integrity and to estimate hearing loss in infants since the 1960's (Barnet & Lodge, 1967; Rapin and Graziani, 1967). Although the use of CAEP use for audiometric purposes was largely eclipsed by the ABR during the past 30 years, some recent clinical research results have re-invigorated their relevance. Sharma and colleagues (2002; 2005) have demonstrated that CAEPs indicate cortical plasticity and development brought on by the use of cochlear implants. Their studies indicate that CAEP latency change in the first months of implant use can be used as a “biomarker” of expected auditory maturation or plasticity following electrical stimulation of the auditory nerve. They have also shown, furthermore, that those implanted after 7 years of age do not demonstrate the CAEP latency shifts to age-appropriate values, despite long periods of electrical stimulation with cochlear implant use. These findings are correlated with attenuated speech perception benefits from implantation in comparison to those who are implanted before 3.5 years of age.
Another clinically-relevant study was completed by Rance et al (2002). They tested for CAEPs in a group of infants and young children diagnosed with auditory neuropathy spectrum disorder (ANSD) and an age-matched group of children with sensorineural hearing loss (SNHL). Rance et al found that CAEPs for tones and speech tokens were present in over 85% of those with SNHL, but for only 60% of those with ANSD. Whereas absence of CAEPs in the SNHL group could be accounted for on the basis of severity of loss and stimulus output limits, this was not the case for those with ANSD. That is, CAEP presence/absence in response to a suprathreshold stimulus was not related to the severity of the pure tone hearing loss, nor was it attributable to age. There was a strong positive correlation between the presence of CAEP and the child's speech perception abilities. These findings suggested that CAEP could be used clinically as a prognostic measure in infants and young children with ANSD.
More recently, the use of CAEPs in the verification of hearing aid fittings in infants has also been suggested as a clinical application (Carter et al, 2010). To this end, Golding et al (2007) obtained CAEPs in response to brief speech sound tokens in infants with hearing loss. The infants were tested in sound field with and without their hearing aids. The presence of a CAEP indicated that the hearing aids provided enough gain to evoke a response to speech sound tokens presented at a conversational level. The CAEP findings were positively correlated with responses to a parent questionnaire regarding functional hearing abilities demonstrated by infants when using their amplification.
Although Rance et al (2002); Sharma et al (2002; 2005) and Golding et al (2007) correlated their CAEP results with behavioral indicators of auditory function, the routine use of CAEP in pediatric audiology is limited by lack of information about CAEP stimulus dependencies in this age group. The effect of stimulus level on CAEP has not been systematically investigated in infants. Whereas otoacoustic emissions reflect mature cochlear function in the newborn period (Abdala, 2007) and ABR thresholds and latency-intensity functions are adult-like by 6 months of age, in contrast, CAEP latencies for stimuli presented at normal speech levels (~ 50-60 dB HL) indicate immaturity into early childhood (Wunderlich & Cone-Wesson, 2006). Immaturity in CAEP latency and scalp topography suggested that there could be developmental-dependencies in the encoding of stimulus level at the level of the cortex that would ultimately affect perceptual detection thresholds.
These issues motivated a study to further knowledge of auditory development above the level of the brainstem during the first year of life by establishing CAEP latency and amplitude input-output functions for tonal and speech stimuli as a function of stimulus level. Hypotheses concerning CAEP development were that the latency and amplitude input-output functions would reflect immaturity in encoding stimulus level. An additional objective was to significantly extend the data-base that establishes CAEP in infants tested while awake, using clinically relevant stimuli so that these methods could be used in pediatric audiological assessment.
In a second experiment, infants were infant's behavioral response was evaluated using the same stimuli used to evoke the CAEPs. The hypothesis was that the behavioral thresholds would be correlated with CAEP input-output functions because of shared cortical response areas known to be active in sound detection.
Methods
Subjects
Thirty-six (36) full-term infants (20 girls), with no risk factors for hearing loss participated in the study. All had passed their newborn hearing screening test (as reported by the parent) and all passed tympanometry and distortion product otoacoustic emission (DPOAE) screening tests upon admission to the study. Tympanometry and DPOAE tests were repeated during the course of the infant's enrollment if the parent reported a recent history of upper respiratory or ear infection. The data included in this report are only those obtained when tympanometry and DPOAE screening tests were normal.
The infant age at the time of testing was between 4-12 months (mean= 8 months, s.d.=1.8 months) and infants were allowed to participate in up to 5 test sessions to obtain responses to as many stimuli as possible. Infants were enrolled sequentially over a period of 13 months. There were 5 additional infants who were enrolled from whom no data could be obtained owing to intolerance of the CAEP procedures during the first visit.
Nine adult young adult subjects (three men) were also tested. The young adults ranged in age from 19-24 years (mean age 21 years). All adults passed a pure tone hearing threshold test on the day of enrollment. None had a history of otologic or neurologic disease. All were students at the University of Arizona.
This research was reviewed and approved by the Human Subjects Protection Program at the University of Arizona.
Stimuli
There were 11 different stimuli used in the tests, 4 tone bursts and 7 speech tokens. The tone-burst stimuli were 50 ms tokens of pure tones at 0.5, 1.0, 2.0 and 4.0 kHz. The tone bursts had a 10 ms linear rise-fall time and had a 10% frequency modulation of the base frequency at 100 Hz. The tone bursts were created using the stimulus generation software module of the Intelligent Hearing Systems Smart EP evoked potential system.
The speech sound tokens were created from natural speech samples. The speaker (B.C.) recorded sustained (>3 s) productions of each of the following tokens /a/, /i/, /o/, /u/, /m/, /s/, and /∫/ using Praat 5.1.05 software (Boersma, 2001). The time-domain waveform of the sample was visualized, and a 100 ms portion of the sample showing steady amplitude and frequency, typically mid-way through the production, was selected for further editing, also using Praat software. This sample was further truncated to 50 ms with a 10 ms linear rise-fall time. The digitized samples in *.wav format were converted to the format used by Intelligent Hearing Systems for stimulus presentation using their software.
The frequency range of the tone tokens were those conventionally used to estimate a speech awareness threshold. The speech tokens, except for /o/, are the “Ling Speech Sounds”. These speech sounds are representative of the speech spectrum. The token /m/, along with the fundamental frequency and first formant of the vowels are comprised of the low frequency range in the long-term speech spectrum. The /s/ and /∫/ tokens are comprised of high frequency energy, and the second and third vowel formants are comprised of mid- and high-frequency energy. These “Ling Sounds” are commonly used in the daily listening checks for young children who use hearing aids or cochlear implants and also in research contexts (Baudhuin et al, 2012; Davidson et al, 2009) . The reason for using them to evoke CAEPs was to develop an electrophysiologic method for testing pre-verbal infants/toddlers who cannot participate in a (speech sound) imitative task. The duration of the tokens, 50 ms, was chosen for two reasons. First, the brevity of the speech sound tokens is representative of the duration of consonants and vowels during running speech. Second, brief tokens limit the duration of the large electrical artifact produced by a cochlear implant on CAEP recordings, so these would be beneficial for translation to clinical methods.
Tone bursts and speech samples were presented in the sound field, via a JBL Control 1x model speaker with a Crown D-75A amplifier. The stimuli were calibrated in the sound field using a Larsen Davis Model 824 sound pressure level meter using with a half-inch microphone suspended from the ceiling in the position approximating the position of the infant's head during testing. An A-weighting scale was used. During calibration, the samples were presented in a steady-state fashion with an inter-stimulus interval of < 1.0 ms.
For CAEP tests, individual speech sound or tone burst tokens were presented at a rate of 0.5 Hz. For behavioral tests, the stimuli were presented in trains of 10 tokens with a 100 ms offset-to-onset interval; the total stimulus duration was 1500 ms.
Procedure for CAEP Tests
CAEPs were recorded using silver-silver chloride disposal electrodes placed at Cz (vertex, non-inverting), A2 (right mastoid, inverting), and A1 (left mastoid, ground) using electrode paste and paper tape, after cleansing each site with Nu-Prep. Electrode impedances were maintained at <10 kΩ and with < 3 kΩ inter-electrode impedance. If an electrode became displaced during testing it was re-placed and electrode impedances checked prior to resuming recording. The Cz-A1 bi-polar montage has been shown to yield the largest amplitudes for CAEP components P1-N1-P2 compared to other bi-polar channels (Wunderlich et al, 2006) and so was used for this investigation of stimulus level effects on CAEP amplitude.
All recordings were made using Intelligent Hearing Systems Smart EP system. The EEG was amplified by a factor of 94 dB and filtered at 1-30 Hz and digitized at 0.5 kHz. Amplitude-based artifact rejection level was set at between 50-90 μV for infant tests.
CAEPs were obtained for 10 dB level decrements from 60 or 70 dBA SPL. At least fifty (50) artifact-free samples were obtained at each level tested. Replications of responses were usually completed at these higher levels and for some lower levels. In addition, a sub-threshold, 0 dBA SPL “control” trial recording, consisting of 50 artifact-free averaged samples was obtained from each infant for each stimulus used. Responses were obtained over a stimulus range of at least 20 dB (3 levels) for most test sessions. The design of this experiment emphasized obtaining the CAEP input-output function over at least a 20 dB range, as opposed to defining the threshold of the CAEP response. That is, the protocol emphasized obtaining responses over a range of stimulus levels, rather than using time-consuming threshold seeking paradigms that may have unreasonably time-consuming for infants.
The stimulus presentation rate was 0.5 Hz. This rate is nearly half that used in many other studies of infant CAEP, for which stimulus rates of 1.0 or 1.2 Hz are typical. When faster rates are used, more samples are required to be averaged, usually 75-100, to obtain CAEPs with an adequate response-to-noise ratio. However, Wunderlich et al (2006) showed that infant CAEPs obtained at stimulus rates slower than 1.0 Hz exhibited response components not present at 1.0 Hz or faster rates. Faster rates allow a greater number of averages to be completed, but at the expense of CAEP adaptation or habituation. The response-to-noise ratio of the CAEP is improved by the use of stimulus rates of less than 1.0 Hz.
CAEP Data Analyses
CAEP waveforms were analyzed using rule-based visual detection methods, by the principal investigator (BC) and research assistant (RW) who had been trained by the principal investigator. The rules for visual detection of CAEP components included latency and amplitude criteria for each component, based upon values derived from the published literature on infant CAEP (Wunderlich & Cone-Wesson, 2006). For an individual subject, template waveforms created from responses at higher stimulus levels were also used to guide peak latency measures at lower levels. CAEP component peaks P1, N1, P2, and N2 were marked with a cursor that also calculated the peak to succeeding trough amplitude, such as P1-N1. In addition, the response-to-noise ratios (RNR) of the averaged responses were estimated off-line using method derived from Schimmel's (1967) “plus-minus average”. For each trial, alternate sweeps were held in one of two buffers, so that the averaged response consisted of the averaged sweeps held in the A and B buffers. The peak-to-peak amplitude of the averaged response: (A+B)/2, was used as the estimate of the response amplitude. The peak-to-peak amplitude of the difference between the buffers (A-B) was used as an estimate of the noise. The RNR was calculated as: [(A+B/2)-(A-B)]÷(A-B).
Descriptive and inferential statistical analyses (analyses of variance, t-tests) were completed using Statview (v5.0.2) software. The value of p< 0.05 was used as the criterion for statistical significance.
The amount of CAEP and behavioral data obtained from each infant varied. Twelve infants had CAEP tests with one stimulus type. Fourteen infants had CAEP tests with three or more stimulus types. Eight infants had tests with five or more stimuli. An emphasis was put upon obtaining data for stimulus tokens /a/, /s/ and /m/ and so the results are weighted for those stimuli.
Results for Experiment I
Response presence as a function of level
Figure 1 presents examples of CAEP waveforms from individual infants, plotted as a function of decreasing stimulus level. The components have been labeled P1, N1, P2 and N2 according to the convention established by Barnet & Lodge (1967). At least one component of the CAEP was detected in all infants for stimulus levels of > 50 dB SPL. P1 was present in 96% of trials, and N1 in 99%, whereas P2 was present in only 87% of trials. A summary of component presence as a function of level is shown in Table 1. Whereas infants are less likely that adults to exhibit a P2 at any stimulus level, P1 or P2 are present in infants at least 50% of the time at stimulus levels of 30 dBA SPL.
Figure 1.
A – C: Individual subject waveforms in response to speech sound tokens. Each waveform is the average of at least 50 artifact-free sweeps.
Example A: Individual infant responses to speech token /u/. The waveforms are replicated at 55, 35 and 25 dB SPL. There is also a sub-threshold control (0 dB SPL). Responses are present and replicable for stimulus levels of 35 dB SPL and above.
Example B: Individual infant responses to speech token /a/. Waveforms at 60 dB SPL indicate excellent waveform replicability. Two control trials with the stimulus level well below threshold (0 dB SPL) are also shown. Responses are clearly present at levels of 20 dB SPL and above.
Example C: Individual infant responses to speech sound token /m/. Stimulus trials were repeated at 10 dB decrements from 65 to 25 dB SPL. Responses are present and replicable at 45 dB SPL and above, and there are questionable responses at 35 dB, with a small amplitude P2 apparent in both trials at a latency nearly 50 ms delayed in comparison to responses at higher levels.
Table 1.
CAEP component percent present as a function of stimulus level (collapsed across stimulus types) for infants and adults
| Component | P1 | N1 | P2 | |||
|---|---|---|---|---|---|---|
| Level, dB SPL | Infant % | Adult % | Infant % | Adult % | Infant % | Adult % |
| 20 | 50 | 70 | 60 | 96 | 73 | 90 |
| 30 | 77 | 73 | 85 | 94 | 77 | 97 |
| 40 | 83 | 86 | 97 | 100 | 78 | 98 |
| 50 | 96 | 88 | 98 | 97 | 81 | 100 |
| 60 | 96 | 86 | 100 | 100 | 91 | 100 |
The use of a response-to-noise (RNR) criterion for judging the presence of a CAEP was evaluated. The RNR as a function of stimulus level is plotted for infants and adults in Figure 2. Clearly, adults achieve higher RNRs at suprathreshold levels than do infants; not surprisingly, the noise levels were much lower in adults than in infants. Further post-hoc inspection of the RNR noise estimates revealed that noise levels increased with stimulus level in infants, whereas they did not in adults.
Figure 2.
Response-to-noise ratio values as a function of stimulus level (pooled over stimulus type) for infants and adults.
CAEP Latency Input-Output Functions
The infant CAEP component latencies are much longer than those seen in adults. At stimulus levels of 50 dB SPL or greater, the P1 latency is 132-138 ms, N1 at 215- 226 ms and P2 at 333-339 ms. These latencies are 100-150 ms longer than in responses obtained from adults at the same levels. The latency data were pooled over stimulus type and are summarized in Table 2 for levels of 30-60 dB SPL for infants and adults.
Table 2.
CAEP component latencies as a function of stimulus level for infant and adult groups. Latencies for different stimulus types have been collapsed across level.
| Component | dBA SPL | Infant Latency, ms (s.d.) | Adult Latency, ms (s.d.) |
|---|---|---|---|
| P1 | |||
| 30 | 149.78 (23.5) | 75.93 (22.9) | |
| 40 | 141.76 (23.8) | 67.33 (11.1) | |
| 50 | 137.89 (24.4) | 68.83 (10.3) | |
| 60 | 132.00 (21.3) | 65.46 (7.7) | |
| N1 | |||
| 30 | 238.27 (31.3) | 128.69 (13.9) | |
| 40 | 221.10 (31.3) | 119.49 (15.5) | |
| 50 | 226.09 (32.5) | 119.44 (13.8) | |
| 60 | 215.70 (24.7) | 116.13 (11.8) | |
| P2 | |||
| 30 | 356.07 (55.7) | 215.06 (24.5) | |
| 40 | 343.19 (54.4) | 205.95 (21.2) | |
| 50 | 338.57 (54.5) | 207.15 (19.3) | |
| 60 | 333.08 (56.5) | 199.29 (19.4) | |
| N2 | |||
| 30 | 451.78 (81.2) | 311.29 (30.1 | |
| 40 | 451.10 (88.0) | 301.89 (28.0) | |
| 50 | 463.28 (80.2) | 304.5 (25.5) | |
| 60 | 461.67 (85.0) | 305.35 (22.5) |
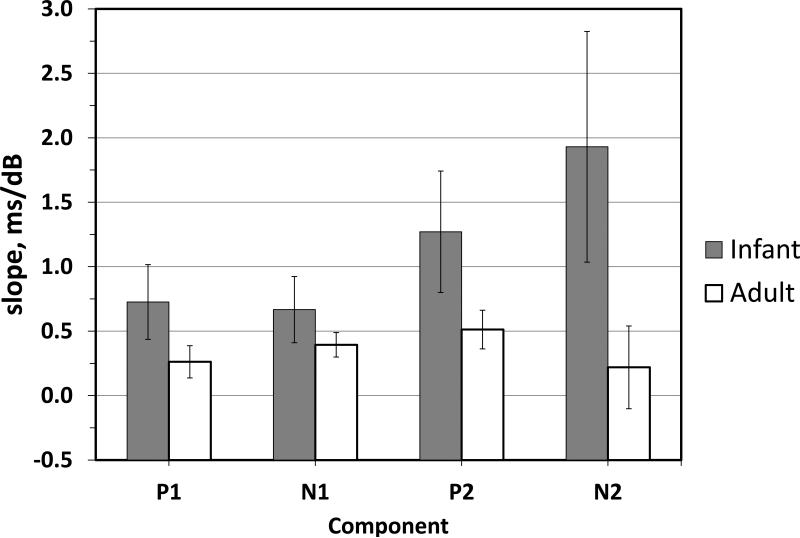
The mean latencies for P1, N1, P2 and N2 latencies decrease inversely with stimulus level (Table 2). The slopes of the latency input-output functions for infants and adults, calculated using mean data at 30 and 60 dB SPL, and pooled across stimulus type are shown in Figure 3. The infants appear to have more rapid change in CAEP component latencies than adults as levels are increased. The slopes are at least twice as steep for infants. These infant-adult differences in the slopes of the latency-level functions are statistically significant for components P2 (T37=1.70, p= 0.049, one-tailed) and N2 (T1, 37 2.23, p = 0.02, one-tailed) but not for N1; the trend for P1 (T33 = 1.38, p = 0.09) is in the same direction as for the later components.
Figure 3.
Latency change slope (ms/dB) calculated for each CAEP component between 30 and 60 dB SPL. Data are collapsed across stimulus type for infant and adult groups.
Latency differences for different stimulus types were also evaluated. The stimuli were divided into 3 groups: vowel, consonant, and tone burst. Analyses of variance were used to determine if latency differences as a function of stimulus type were significant. Latency data were pooled across level, only using suprathreshold levels (at 50 dB SPL or greater) for which there is no significant change in latency as level is varied. The group mean component latencies as a function of stimulus type for adults and infants are shown in Table 3. The N1 latency change with stimulus type was statistically significant for adults (F 2, 85 = 3.47, p=0.036), based upon an analyses of variance for 3 different stimulus types. Specifically, N1 latencies for tone bursts were significantly longer than those for vowels or consonants. The P1 latency change with stimulus type was also significant for infants (F2,180 = 3.17, p = 0.044), and there was a trend (F 2, 193 = 2.94, p = 0.055) for N1. In both cases, latencies for vowels appeared to be shorter than those for consonants or tone bursts.
Table 3.
CAEP component latencies as a function of stimulus type for infant and adult groups. Latencies for each stimulus type are collapsed across the 50-70 dB SPL range for adults, and 60-80 dB SPL range for infants.
| Component | Adult | Infant |
|---|---|---|
| P1 | ||
| vowel | 66.37 (7.17) | 131.81 (24.90) |
| consonant | 65.71 (10.02) | 140.00 (19.07) |
| tone burst | 71.00 (11.93) | 141.28 (24.99) |
| N1 | ||
| vowel | 117.32 (11.03) | 212.51 (34.29) |
| consonant | 117.88 (12.11) | 223.558 (27.68) |
| tone burst | 126.12 (12.74) | 221.625 (22.94) |
| P2 | ||
| vowel | 202.11 (21.18) | 332.68 (63.27) |
| consonant | 196.88 (17.18) | 341.11 (52.90) |
| tone burst | 209.25 (19.19) | 337.94 (33.73) |
| N2 | ||
| vowel | 310.53 (25.49) | 449.88 (92.24) |
| consonant | 304.59 (31.19) | 459.45 (64.39) |
| tone burst | 309.00 (28.23) | 476.64 (61.72) |
CAEP Amplitude Input-Output Functions
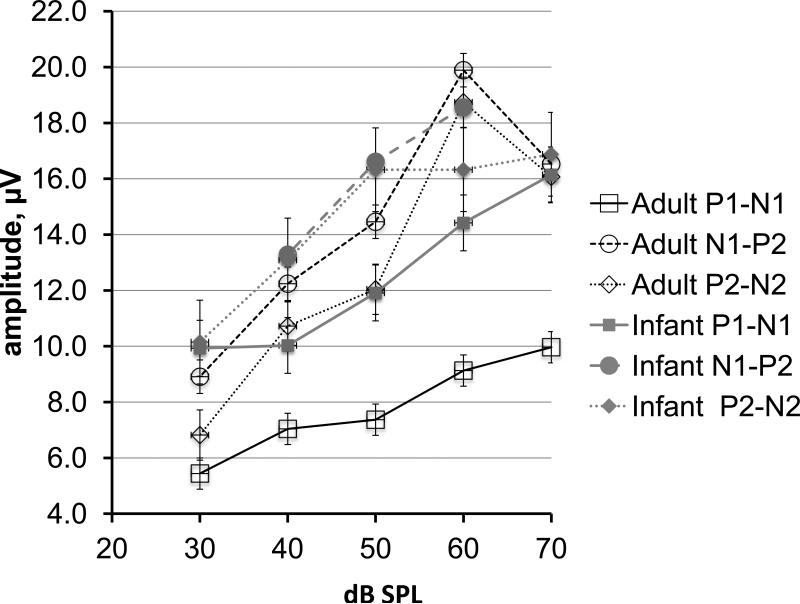
CAEP component peak-to-trough amplitudes as a function of stimulus level are shown in Figure 4 for infant and adult subjects. Although component amplitudes are larger in infants compared to adults, the response-to-noise ratios of infant CAEPs were lower in infants owing to higher noise levels, as previously discussed (see also, Figure 2). There are similarities between the adult and infant data. First, the P1-N1 amplitude functions are less steep than those for N1-P2 and P2-N2. Second, the N1-P2 and P2-N2 functions are very similar to one another, an expected finding, owing to the shared value for P2 amplitude contributing to both means. Third, the N1-P2 and P2-N2 amplitudes appear to asymptote at 60 dB SPL. The slopes of the amplitude input-output functions are summarized in Table 4. There were no statistically significant differences in the slope values calculated with respect to level for infants compared to adults.
Figure 4.
CAEP component mean amplitudes plotted as a function of stimulus level for infant and adults groups. Amplitudes are collapsed across stimulus type.
Table 4.
CAEP amplitude input-output function slopes, and mean amplitudes at 0 dB and 10 dB SL for infant and adult groups.
| Infant | Adult | |
|---|---|---|
| Slope 30-60 dBA SPL | μV/dB | μV/ dB |
| P1-N1 | 0.21 (0.20) | 0.13 (0.22) |
| N1-P2 | 0.28 (0.30) | 0.39 (0.30) |
| P2-N2 | 0.24 (0.36) | 0.39 (0.27) |
| Slope 0-10 dB SL | ||
| P1-N1 | 0.39 | 0.09 |
| N1-P2 | 0.78 | 0.22 |
| P2-N2 | 0.88 | 0.02 |
| Amplitude at 0 dB SL | μV | μV |
| P1-N1 | 9.45(6.56) | 4.25 (1.25) |
| N1-P2 | 9.41(5.51) | 4.87(1.83) |
| P2-N2 | 7.08 (4.25) | 5.98(3.79) |
| Amplitude at 10 dB SL | ||
| P1-N1 | 13.39(4.66) | 5.17(1.67) |
| N1-P2 | 17.22 (10.51) | 7.08(4.25) |
| P2-N2 | 15.85 (10.45) | 6.18 (3.62) |
There were also amplitude differences that were apparent as a function of stimulus type. As was the case for the latency analyses (above), only supra-threshold data were used to avoid the confounding effects of stimulus on amplitude. Vowel sounds evoked larger N1-P2 amplitudes than consonants or tone bursts in both infants (F 2,178 = 4.93 p = 0.008), and adults (F 2, 85 = 4.68, p =0 .012). This was also the case for component P1-N1 in infants (F2,174 = 6.42, p = .0020) and P2-N2 (F2, 85 = 5.35, p = 0.006) in adults.
CAEP threshold estimates
Although the experimental design was focused on the latency and amplitude dynamics as a function of level, estimates of CAEP thresholds can be inferred from these data. One method is to use CAEP component presence as a criterion, that is, to determine the stimulus level at which CAEPs are present 50% of the time. From Table 1, a summary of CAEP component presence as a function of stimulus level for vowel stimuli, the infant CAEP threshold appears to be at or below 20 dBA SPL for components P1 and N1.
The RNR offers another method by which CAEP threshold could be inferred. The RNR for the waveforms obtained at 0 dB SPL, the control condition, can be used as a baseline value. A doubling of the RNR over this control condition could be used as a criterion for threshold. For adults, the RNR stays well above twice the baseline value for levels of 40 dBA SPL and above. For infants, a doubling of the baseline value is not achieved until a stimulus level of 50 dB SPL. This level is approximately 20 dB higher than what was achieved with rule-bound visual detection methods.
A third method for estimating threshold is from the amplitude input-output function. It is a common practice in auditory electrophysiology to extrapolate auditory nerve action potential amplitude or firing rate input-output functions to estimate threshold (May, 2003; Moore; 1981; Rose et al, 1971; Sachs & Abbas, 1974). Using the amplitude input-output slope values for component N1-P2 (Table 4), and a noise floor criterion of 7 μV, CAEP threshold in infants is 24 dBA SPL.
Discussion of Experiment I
One aim of the project was to establish a comprehensive and systematic data set for clinical use of CAEPs in pediatric audiology. The CAEP data set reported is the largest of its kind for infants under the age of 1 year, tested while awake. This is the first report of dynamic change in CAEPs recorded from awake infants as stimulus level was systematically varied.
Infant CAEP components
CAEPs were reliably obtained in awake infants for tonal and speech tokens as stimulus level was varied (Table 1). At a moderate stimulus level (60 dBA SPL) CAEP component N1 was present in all infants. CAEP component P1 was more reliably obtained in infants than in adults, and was present in infants over 90% of the time at stimulus levels above 40 dB SPL. CAEP component P2 was less reliably obtained in infants compared to adults but was present in over 80% of the infants for stimulus levels above 40 dB SPL.
A notable aspect of the present results is that CAEP component N1 was obtained in >90% of the infants. The N1 component has a complex set of neural generators (Naatanen and Picton, 1987) in primary auditory cortex. Stimulus rate has a profound effect on N1 as does maturational level, and so consistent N1 responses may not be observed until later childhood when using stimulus rates at or greater than 1/s. In the current work, a stimulus rate of 0.5 Hz was used and the N1 component was readily observed. This result is similar to those of Wunderlich et al (2006) who used stimulus rates of 0.1-0.2 Hz. If CAEP are to be used in clinical evaluations to document auditory pathway integrity and maturation up to the level of the cortex, it is important that the rate-dependency of the components be considered. With the 0.5 Hz rate used in this study, N1 was just as readily recorded as was P1, even at low stimulus levels.
Detection of CAEPs
An automatic detection algorithm for CAEPs was proposed by Schimmel in 1967, and received some discussion for clinical application (Schimmel et al 1974; 1975) but this approach did not achieve widespread use. A modified version of Schimmel's “plus-minus averaging” algorithm was applied in a post hoc fashion to the current infant CAEP data set. As with any statistical technique applied to electrophysiologic data, the test performance of a metric for response detection must be verified with empirical experimentation. When using a criterion that is double the RNR value found at 0 dB SPL, there were many “misses”, that is, CAEPs were visually detected at levels that did not meet this criterion. Using a more lax criterion resulted in too many false positives: waveforms that met criterion for which a response could not be visually detected. This simply means that the test performance of the algorithm that we used was not high, particularly given the baseline noise levels in the infant responses. The performance of the algorithm may be improved by increasing the number of samples to decrease the noise level, if, in fact, the structure of the infant noise is moderately Gaussian distributed, as assumed by this approach.
Recently, a statistical approach to CAEP detection utilizing Hotelling's t-test has been implemented in a commercially available instrument dedicated for CAEP recordings (Carter et al, 2010; Golding et al, 2009; Munro et al, 2011). The refinement of this and other algorithms will useful for translation of CAEP methods to the clinic. It is still the case, however, that much of “evoked response audiometry”, even for ABRs, is accomplished clinically using expert visual detection methods.
CAEP latency and amplitude dynamics
The hypotheses of the current study were that the latency and amplitude input-output functions would reflect immaturity in encoding stimulus level. There are substantial maturational changes in the CAEP that take place through the first 20 years of life (Eggermont & Ponton, 2003; Wunderlich & Cone-Wesson, 2006) and these can be tied to neuroanatomical findings (Moore & Guang, 2001). CAEPs for infants aged 4.5-12 months appear to be generated in cortical layer I as this is the only layer that shows consistent neurofilament staining. This layer receives direct thalamic input. The lack of neurofilament staining at deeper layers until later in childhood suggests that the CAEP components in infancy could have different stimulus-response properties owing to a lack of intra-cortical connectivity that would be needed for efferent and inhibitory networks. Thus, the neural representations of stimulus level, and spectral and temporal complexity reflected in the evoked potentials would be immature.
The immaturities of infant cortical generators result in CAEP components that have much longer latencies than do CAEPs in adults. This was apparent in the current findings. Latency can be interpreted as the speed of processing, and as stimulus level is increased more neural resources are synchronized to contribute to the response and latencies become shorter. The latency change with level may also be interpreted as a change in place and extent of activation of the cochlear elements along the basilar membrane. As level increases there are greater areas of the cochlea that are activated by the traveling wave and the place of maximum stimulation may shift more basalward (von Bekesy, 1960; Kiang, 1965) and this results in decreasing response latency. It is difficult to explain why infants had a steeper CAEP latency-change function with increased stimulus level compared to adults as was found in the current study. This is not seen in the latency change functions recorded at lower levels of the auditory system, such as for the ABR. One possibility is to consider how latency-level functions vary with stimulus spectrum. At the level of the ABR, the latency vs. level functions are steeper for low frequency tone bursts as compared to high frequency tone bursts (Klein & Teas, 1978). The finding of steeper functions for CAEP may be related to the spectral content of the stimuli. The infant data set was heavily weighted with responses to the vowel sound /a/, which has substantial low frequency spectral content .
The amplitude of an evoked potential is conventionally considered to be an estimate of the amount of neural responses devoted to a response. Infant and adult CAEP amplitude input-output functions are similar when plotted as a function of stimulus level in dB SPL. This is particularly evident for the earliest component, P1-N1, and so suggests that the cortical encoding of stimulus level is similar in adults and infants over 6 months of age. The amplitude input-output (I/O) functions obtained in adults tested in this study are similar to those described by Davis et al (1968) and Davis & Zerlin (1966) and Muller (1973). These I/O functions have an asymptote at 40-60 dB above threshold and generally follow the compressive characteristics of the cochlear active process. They found differences in the slope of the I/O function relating to stimulus frequency, with slopes for low frequency (250 Hz) tone bursts being nearly twice those for high frequency (4000 Hz) tone bursts. Post-hoc analysis of the I/O slopes as a function of stimulus type reveals the same trends for adults tested in this study. The I/O slope for the responses to speech token /a/, a primarily low frequency vowel, was more than twice as steep as those for /s/, a high frequency consonant. The slope for the 500 Hz tone-burst was 60% steeper than for 2000 Hz. This was not the case for infants, however, whose amplitude I/O slopes were not differentiated by stimulus spectrum. It should be noted, however, that given the sample size, and considerable variability in the infant data, there were substantial statistical power limitations in being able to detect these differences in CAEP amplitude I/O slopes as a function of stimulus type.
Conclusions from Experiment I
Reliable and robust CAEPs can be obtained from awake infants. The latency input-output functions as a function of level reflect immaturity in neural transmission time that can be tied to immaturity of cortical generators and also to stimulus spectral content. The amplitude input-output functions indicate that neural resources recruited into the response increase with stimulus level in a similar fashion for infants and adults.
Experiment II
Behavioral Thresholds in Infants
The first experiment evaluated the effect of stimulus level and complexity on CAEP latency and amplitude growth functions obtained from infants. Another goal of this investigation was to determine the relationship between CAEP dynamic changes and functional hearing abilities. To this end, we established psychophysical thresholds for the stimuli used in Experiment I.
Perceptual thresholds for pure tones improve throughout early infancy and into the toddler stage. Olsho et al (1988) evaluated perceptual thresholds in infants during the first year of life. They found that during the first 6 months, perceptual thresholds improve in a frequency-specific manner, by 15 dB at 250-4000 Hz but by more than 20 dB at 8 kHz. At 12 months of age, threshold variability is decreased in comparison to 6-months, but the mean thresholds are unchanged. Pure tone thresholds remain elevated by 10-15 dB with respect to adult thresholds at this age.
While there are middle ear transmission differences measured between infants and adults, these only account for a small portion of the perceptual threshold differences, and primarily in the low frequencies. Otoacoustic emissions indicate that functional cochlear maturity has been obtained by the newborn period, and so is ruled out as a source of perceptual threshold elevation (Abdala et al, 2007). Correlations between perceptual threshold and both ABR thresholds and latencies have also been investigated to account for the infant-adult threshold differences (Werner et al, 1993; 1994). ABR and perceptual thresholds for, 1.0 kHz, 4.0 and 8.0 kHz tone-pips were determined for adults and 3- and 6-month old infants. Average ABR thresholds were between 37 and 42 dB pSPL for all subjects, regardless of age or stimulus frequency. In adults, perceptual thresholds for the same stimuli were 10-20 dB lower than ABR thresholds, but in 6-monthold infants the perceptual thresholds were still elevated by 15-20 dB with respect to ABR threshold. Considering that ABR threshold is largely adult-like by 6 months of age, the contribution of brainstem immaturity to perceptual threshold elevation past the 6 month period is likely negligible. Thus, we reasoned that immaturity of the auditory system at cortical levels, may be responsible for the elevated perceptual thresholds. We hypothesized that the latency and amplitude dynamics of the CAEP would be correlated with perceptual threshold because both are dependent upon cortical areas known to be active in sound detection.
Experiment II Methods
The infants and adults who participated in Experiment II were the same as those in Experiment I. The stimuli used were the same as those in Experiment I, described above. Thresholds for all 11 stimulus types (4 tone bursts and 7 speech tokens) were determined in all 9 adult participants. Similarly all 36 infants completed at least one threshold test. There were 3 infants for whom thresholds were obtained for all 11 stimulus types, and an additional 4 infants gave thresholds for 8-10 different stimuli. There were only 4 infants who had just one threshold determination. The number of infant thresholds obtained for each stimulus type is summarized in Table 5.
Table 5.
Number of infant thresholds obtained for each stimulus token
| Token type | 0.5 kHz | 1.0 kHz | 2.0 kHz | 4.0 kHz | /a/ | /i/ | /o/ | /u/ | /m/ | /∫/ | /s/ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 11 | 22 | 14 | 13 | 30 | 16 | 14 | 8 | 19 | 33 | 13 |
Infants were tested using visually-reinforced operant procedures with observer-based psychophysics (Werner, 1995). Infants were seated on their parent's lap or in a high chair with the loudspeaker at a 45-degree angle, and elevated by 50 cm above eye level. A test assistant in the booth manipulated toys to keep the infant quiet and alert. The parent and test assistant listened to music presented through earphones to keep them masked as to when the stimuli were presented. Infants were trained to detect the presence of a tone or speech token by pairing the presentation of the stimulus (at 50 dB SPL) with a visual reinforcer. The visual reinforcer was a 4 s animated cartoon that was presented on a video monitor at eye level placed within 100 cm of the infant. The pairing of the reinforcer with the stimulus trial was used to teach the infant make a response that could be judged by the observer during the testing phase. This response could be a head turn, an eye movement towards the reinforcer, a change in facial expression or cessation of, or increase in body movement. Training consisted of up to 5 pairings of the stimulus with the reinforcer. After 5 pairings, a probe trial was used in which the stimulus was presented, but the reinforcer was withheld until the infant made an observable motor response. When the infant did so within the 4 s response period, the reinforcer was introduced. The observer had to make two correct judgments to probe trials before testing was begun.
The observer controlled when a trial was initiated, but was masked as whether the trial they contained a stimulus token or not. Trials that did not contain a stimulus token were control trials. The threshold search procedure was automated using the Optimized Hearing Threshold Algorithm (OHTA) (Eilers et al 1990, 1991), as implemented in Intelligent Hearing Systems (IHS) Smart-IVRA system. The OHTA algorithm is based upon parameter estimation by sequential testing (PEST) rules (Taylor & Creelman, 1967). Infants received visual reinforcement when the observer made a correct detection that a stimulus was present. When the observer made a correct rejection (no response on a control trial) there was no reinforcer. Likewise, there was no reinforcer for a miss (failure to detect a token) or a false alarm (voting that a token was present during a control trial). The observer received feedback for all trials and observer responses were recorded for each trial. The no-stimulus control trials, occurring at a rate of 25%, were used to estimate the false alarm rate. The threshold searches started with an initial stimulus level of 50 or 60 dBA SPL. The stopping rule employed was 3 reversals around the estimated threshold level.
Adults were tested using similar procedures. Adult subjects were told to “raise your hand when you hear the sound that turns the cartoon on.” Only 2 pairings of the stimulus change with the reinforcer were used to train adult subjects. Only 1 probe was used prior to initiating testing.
Experiment II Data Analyses
Two methods of estimating threshold were employed, one for individual infants, and one for the group data. For an individual, the lowest level at which the observer detected a stimulus present (from the infant behavior) in 50% of the trials was designated to be threshold. This is method of threshold estimation is equivalent to what is done in clinically.
For the group data, threshold was determined from the psychometric function, derived in the following manner: first, the responses (hit, miss, false alarm, correct rejection) for each trial as a function of stimulus type and level for each threshold search were entered onto a spreadsheet. Second, the responses were pooled over all participants to estimate the group's hit rate, P(Hit | xi), and false alarm rate, P(FA | xi), at each stimulus level. The group data were used rather than individual data due to the relatively modest number of trials (on average, 22) available for each individual threshold determination,. Third, at each stimulus level, the sensitivity index, d', was calculated from each pair of the estimated P(Hit | xi) and P(FA | xi), following the standard procedure of signal-detection theory for deriving d' from hit and false-alarm rates (e.g., Green and Swets, 1966; Macmillan and Creelman, 2005). Fourth, the obtained d' values at all the stimulus levels were fitted by a psychometric function (Dai, 1995). From the fitted psychometric function, the stimulus level corresponding to d’ = 1, denoted by x|d' = 1, was obtained. Following the convention of the signal-detection theory, this stimulus level was designated as the threshold. Defined on the basis of d' rather than a hit rate, the threshold is independent of response criteria of the listeners.
Experiment II Results
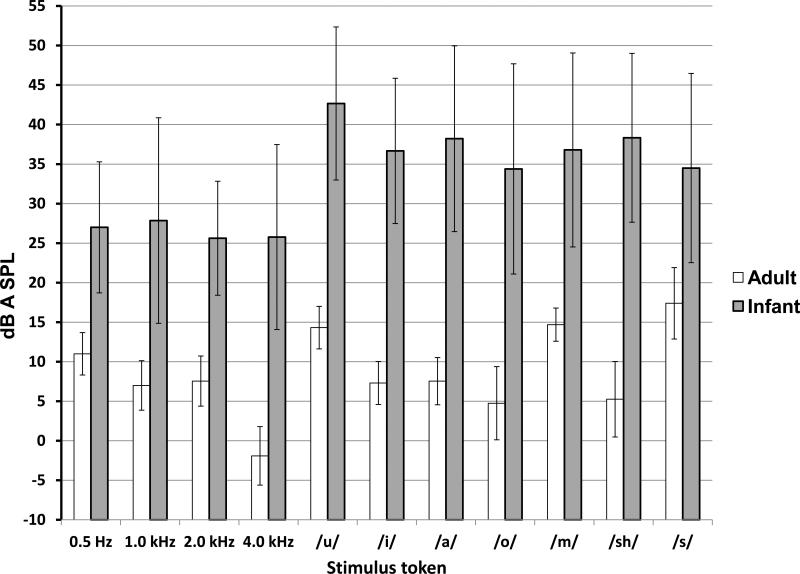
The mean thresholds for tone bursts are shown for infant and adult subjects in Figure 5. These thresholds were estimated from the lowest level at which a response was detected 50% of the time for individual subjects. The infant thresholds are, on average 21 dB greater than those of adults. A 2-x-4 factor analysis of variance was completed to establish the statistical significance for the factors of group (infant vs. adult) and tone burst frequency (0.5, 1.0, 2.0, and 4.0 kHz) on perceptual detection threshold. The threshold differences for subject group were statistically significant (F 1,91= 137.2; p<.0001), as they were also for tone burst frequency (F 3, 91= 3.061, p = 0.03). There were no significant group-X-tone burst frequency interactions. The infant-adult threshold difference is greatest at 4.0 kHz at 27 dB, and smallest threshold difference between infants and adults was 17 dB at 2.0 kHz. Adult thresholds, when converted to audiometric 0 for sound field presentation (Wilber & Burkard, 2009), are between 0-7 dB HL.
Figure 5.
Perceptual thresholds for tone burst and speech tokens in infants and adults. Thresholds were determined as the lowest level at which the subject responded for 50% of trials.
The detection thresholds for the individual speech sounds also indicate significant differences (F 1,199 = 438.4, p<.0001) between infants and adults (Figure 5) when tested using a, 2-X-7 analysis of variance for group (infant vs. adult) and speech token type. The mean threshold for speech sounds in infants was 36 dBA SPL, and in adults it was 10 dBA SPL. Adult thresholds for /u/, /m/ and /s/ were elevated with respect to those for /i/, /a/, /o/ and /∫/ and these differences were statistically significant (F 6,82 = 26.7, p< .0001). Infant thresholds did not appear to vary with speech sound, nor did they vary between consonant vs. vowel, nor manner of consonant production.
For adults, the average difference between the tone burst thresholds (6 dBA SPL) and speech sound thresholds (10 dBA SPL) was only 4 dB. In contrast, the difference for infants was, on average 10 dB (36 dB [speech sound threshold average] – 26 dB [tone burst threshold average]).
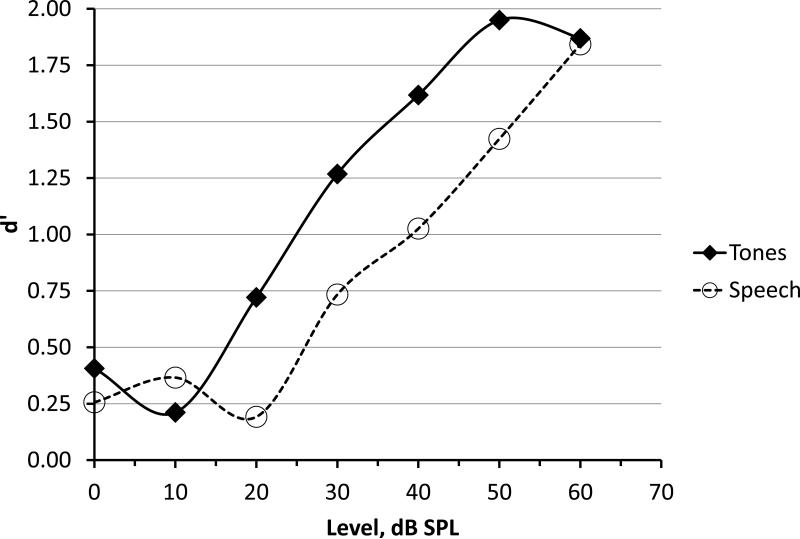
Figure 6 presents the infant psychometric functions for tone burst and speech tokens. The d'prime value is plotted as a function of stimulus level for each stimulus type. Using the d’ value of 1.0 as the criterion for threshold results in a group mean detection threshold of 28 dBA SPL for tone bursts and 41 dBA SPL for speech tokens.
Figure 6.
Psychometric functions for tone burst and speech tokens in infants. Thresholds can be estimated using d’ as a criterion. For example, using d’=1.0, indicates a threshold of 44 dB SPL for speech tokens.
Summary of Experiment II Results
There are significant differences between infants and adults in perceptual thresholds for tone bursts and speech sounds. The mean difference in the 4-frequency tone burst threshold average was 22 dB SPL. Adults demonstrate speech sound detection thresholds that are within 4 dB of their tone burst threshold average. Infant thresholds for speech sounds are elevated by 10-13 dB with respect to their threshold for tone bursts.
Discussion of Experiment II
The perceptual thresholds for the tone-burst stimuli obtained with observer-based psychophysical techniques are comparable to findings of others who have used this and clinical visual reinforcement audiometry (VRA) methods in this age group. The four frequency threshold average for the tone-bursts (0.5-4.0 kHz) was 28 dBA SPL when based upon a d’ criterion of 1.0. There were no significant differences in infant threshold as a function of frequency found in the present study, although Olsho et al (1988) and Parry et al (2003) found elevated thresholds at 0.5 kHz in comparison to those at 1.0, 2.0 or 4.0 kHz. The Parry et al thresholds, when converted to dB SPL for insert phones in an occluded (adult) ear canal, were 26, 19, 19, and 22 dB SPL for 0.5, 1.0, 2.0 and 4 kHz, respectively, or a 4-frequency average of 21.5 dB SPL. These thresholds were somewhat lower than those found in the present study. Olsho et al, measured thresholds of 30, 22, 10 and 15 dB SPL for the same frequencies in a 6-8 month old age group. Sinnott & Aslin (1985) found sound-field pure tone thresholds of between 30-37 dB SPL for a 7-11 month old age group.
Notably, Sinnott & Aslin (1985) used pure tones of 1.0 and 0.5 s duration. They found that thresholds measured for the 0.5 s duration tones were elevated with respect to the 1.0 s tones, consistent with immaturity in temporal integration of energy. The tone tokens used in the present research were of 50 ms duration, whereas Sinnot & Aslin used tones of at least an order of magnitude longer in duration. Thus, it is likely that the brevity of the stimuli in the present work led to threshold elevations with respect to those established for longer duration tones. That infant thresholds are elevated for brief duration tokens was also shown by Werner et al (1993) who measured psychophysical thresholds for tone-pips at 1.0, 4.0 and 8.0 kHz. The durations of these tone-pips were less than 10 ms. The infant thresholds for these brief signals were elevated by 20-30 dB with respect to thresholds found in adult listeners, and by as much as 45 dB when compared to thresholds established for long duration tones in infants at the same age (Olsho et al, 1988).
The difference between the infant pure tone threshold average, 28 dBA, and their detection threshold for speech tokens, 41 dBA, was unexpected as it is common clinical procedure to “cross check” pure tone threshold against speech awareness threshold and obtain similar levels. Yet, there are few published studies that have explicitly measured thresholds for speech sound tokens in infants. Tharpe and Ashmead (2001) reported infant thresholds for speech-weighted noise bursts with a 232 ms duration, which were, on average, 38 dB SPL for 4 month old infants. This threshold decreased to 28-30 dB SPL when the same infants were re-tested at 6 months of age. Similarly, Nozza and Wilson (1986) tested 7-8 month-old infants using a 300 ms synthesized speech token /ba/, and obtained a mean threshold of 29.6 dB SPL. Sabo et al (2003) measured speech awareness thresholds for live-voice speech in 6-9 month old infants and found them to be 6 dB better than for pure tone threshold averages: speech awareness threshold was 14 dB HL whereas the pure tone average was 20 dB HL. However, the temporal and spectral properties of the Sabo et al speech stimuli, were very different from those of the pure tone stimuli. In the Sabo et al study, as is the case in most clinical practice, “live voice speech” signals are used to test infant's speech awareness. The tester speaks highly inflected phrases (“look here” “where am I?”) or canonical babbling (/ba-ba-ba-ba/) over a microphone circuit of the audiometer and varies the level systematically to obtain the speech awareness threshold. Given the well-established infant listening preferences for speech (Vouloumanos & Werker, 2004), and its complex spectral and temporal characteristics (in comparison to pure tones) it is not surprising that thresholds for “running speech” are better than those obtained for pure tones.
In the present study, the thresholds for the speech sound tokens were elevated by 10-13 dB with respect to the average threshold for the tone bursts. This was surprising because the speech sound tokens had the same duration as the tone-bursts (50 ms) and were presented in the same manner as the tone-bursts during threshold tests. It may be that the brief duration of the speech sounds in combination with their spectral spread of energy may have presented a more challenging detection task than for tone bursts (Bargones & Werner, 1994), given immature temporal integration abilities.
It appears that thresholds for individual speech sound tokens or phonemes cannot be easily predicted from pure tone sensitivity, even when the tone and speech tokens are similar in duration and presentation method, such as they were in the current study. In this study and also in previously published data from other labs, there appears to be a discrepancy between tonal and speech thresholds. For example, considering the Nozza and Wilson (1986) speech token threshold of 30 dB SPL, and the Olsho et al (1988) 4-frequency pure tone average of 19 dB SPL, there exists a threshold difference of 11 dB. These findings have implications for hearing aid fitting algorithms used for infants such as DSL-I/O (Bagatto, 2008; Bagatto et al, 2010). The presumption is that pure tone thresholds can be used to predict audibility of speech sounds. This does not appear to be the case in infants under the age of 12 months, who appear to require higher stimulus levels to detect speech sounds.
Comparison of CAEP and Perceptual Results
A third goal of this investigation was to correlate the findings from cortical electrophysiology (Experiment I) with infant perception (Experiment II). We had hypothesized that the immaturity in the CAEP latency and amplitude input-output functions would be related to the immaturity in infant detection threshold, because both responses share cortical response areas.
The CAEP latency input-output functions were steeper in infants compared to adults (Table 2), whereas the amplitude input-output functions were similar in infants and adults when plotted with respect to dBA SPL. Another view of the CAEP amplitude input-output functions can be appreciated when the data are analyzed with respect to the thresholds derived from behavioral tests. Using rule-bound visual detection methods, infant CAEP components are detected 50% of the time at stimulus levels of 30 dBA SPL, whereas behavioral thresholds for tones and speech tokens are 28 dBA and 41 dBA, respectively, from the psychometric function estimates. Table 4 also provides the mean amplitudes of the P1-N1, N1-P2 and P2-N2 components for 0 and 10 dB sensation level (SL). Sensation level is determined from using perceptual threshold as the 0 dB reference. The slope calculations (μV/dB) for 0-10 dB SL are also provided in Table 2. It is apparent that the infant responses show a steeper amplitude growth at their (perceptual) threshold levels, compared to adults. The slopes of the amplitude growth functions in infants are 3 to over 4 times as steep in infants compared to adults.
The steep CAEP amplitude I/O functions in infants were similar to those obtained in adults when ipsilateral masking was introduced (Davis et al, 1968). These steep slopes were suggestive of a recruitment-like phenomenon. The physiological mechanisms of recruitment caused by noise masking of tones are different from those caused by cochlear damage, and appear to involve “central neural processing” (for review, see Phillips, 1987). Could the steep slope of the I/O functions (when plotted with respect to psychophysical thresholds) be indicative of infant listening behavior? From the CAEP amplitude I/O functions it appears as if significant portions of neural resources are recruited to the response, once the noise floor has been overcome. Likewise, infant psychophysical responses have been modeled using masking models (Nozza, 1987; Nozza & Henson, 1999; Werner & Boike, 2001) and differences in the amount of internal noise have been proposed as a mechanism that accounts for a portion of infant-adult perceptual threshold differences. As is the case in psychophysics, the detection of the CAEP is dependent upon the signal-to-noise ratio of CAEP signal to the background EEG and myogenic noise. In this case, we are suggesting that once there is a sufficient CAEP signal-to-noise level, a response will occur, and the magnitude of that response grows steeply once above the criterion level. This may reflect the way in which infants perceive loudness. Furthermore, Leibold & Werner (2002) suggested that reaction time as a function of stimulus level could be used to estimate loudness and found that infants had steeper reaction time vs. level functions compared to adults. In the present study, we found that the latency I/O functions for CAEP components P1 and N1 and P2 were steeper for infants. Considering CAEP response latency as an indicator of neural processing speed, the latency I/O findings are congruent with the behavioral results of Leibold & Werner. So, there is converging evidence from the CAEP amplitude and latency I/O functions to suggest that loudness perception may grow more quickly in infants than adults; however, experiments testing this hypothesis must be undertaken.
It is possible to estimate CAEP threshold from a given point or intercept of the amplitude I/O function. For example, if the point at which the amplitude growth function has changed by 10% of its total slope is used as a criterion for CAEP threshold, then adult and infant CAEP thresholds are equal. The amplitude I/O functions as function of SPL suggest that peripheral sensitivity is comparable in infants and adults. This current study was specifically aimed at evaluating the dynamic changes in CAEP latency and amplitude as a function of stimulus level, and so it is appropriate to estimate threshold from the I/O functions. An alternative strategy for measuring CAEP threshold would require increasing the number of averages at lower stimulus levels to obtain a criterion response-to-noise ratio, and also decreasing the level step size to smaller than the 10 dB using to define the input-output functions.
The results of the present study are similar to those of Werner et al (1994) who measured both psychophysical and ABR thresholds for 1.0, 4.0 and 8.0 kHz tone pips in infants and adults. The ABR thresholds for adults and infants were comparable but there were substantial differences between adult and infant psychophysical thresholds for the tone pips. In the present work, CAEPs for tone bursts and speech sounds could be obtained at levels or below perceptual threshold in infants, but in adults, CAEPs were found at 10-15 dB above perceptual threshold. CAEP amplitude I/O functions are more closely tied to stimulus SPL, not perceptual levels, in the same way as more peripheral responses such as the ABR.
Brainstem evoked responses such as the ABR or the 80-Hz auditory steady state response (ASSR) are routinely used to estimate threshold in infants. The ABR threshold for transients, ranges from 5-20 dB nHL, or 40-55 dB peSPL. Thresholds for ASSR are in the range of 20-40 dB HL, depending on the carrier frequency. When expressed in dB SPL, the ASSR thresholds are close to 30-50 dB. ABR and ASSR at these threshold levels have low amplitudes response-to-noise ratios. In comparison, the infant CAEPs for tone bursts and speech tokens at 40-50 dB SPL are robust. This suggests that there may be some “gain” or amplification of the neural signal as it ascends the afferent pathway. An alternative hypothesis is that the immature auditory cortex has not yet developed the intra-cortical or efferent inhibitory processes that provide for a more gradual growth of the I/O function.
The mechanisms involved in infant sound detection and perception are still a matter of speculation. Why are infant perceptual thresholds elevated with respect to electrophysiologic thresholds? Certainly, “attention” or its corollary “distraction” have been posited as mechanisms that affect perceptual threshold in infants. This has been studied using psychophysical methods in which infant thresholds are determined in the presence of broadband noise, in narrow band remote noise and in “informational masking” noise. (Leibold & Werner, 2006; Werner & Bargones, 1991). It should be possible to duplicate these listening paradigms while measuring CAEP and so have an electrophysiologic assay of cortical mechanisms involved in sound detection.
Infant CAEPs are robust at 35 dB SPL, a level that is at or below their perceptual threshold for the same sounds. CAEP amplitude growth as a function of SPL appears remarkably adult-like in 7month old infants, especially for the earliest component, P1-N1. In contrast, infant N1-P2 and P2-N2 I/O functions show steep growth above perceptual threshold and latency I/O functions are very steep in infants compared to adults suggesting immature processing of stimulus level.
The findings from the current study can be translated to the clinical setting. It is possible to use tonal or speech sound tokens to evoke CAEPs in an awake, passively alert infant, and thus determine whether these sounds activate the auditory cortex. This test result could be used in the verification of hearing aid or cochlear implant benefit. The results of CAEP tests may also be of benefit for determining whether infants with normal peripheral sensitivity, but who are at risk for developmental disability have auditory responses at higher levels of the auditory neuraxis (Cone-Wesson, Kurtzberg and Vaughan, 1987). The amplitude I/O function may provide additional information as to how stimulus level is perceived.
Acknowledgements
The authors acknowledge the support of NIH-NIDCD Grant # NIH-NIDCD K24 DC 008826. The authors wish to thank Dr. Huanping Dai for his contribution to analyses of psychophysical data. Portions of this paper have been presented at the Association for Research in Otolaryngology Mid-Winter Meeting, 2010.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barbara Cone, University of Arizona Department of Speech, Language and Hearing Sciences P.O. Box 210071 Tucson, AZ 85721 520-626-3710 conewess@u.arizona.edu.
Richard Whitaker, Hearing Science of Rancho Cucamonga 6283 Grove Avenue Suite 104 Rancho Cucamonga, CA 91730 909-920-9906 rapscal36@hotmail.com.
References
- Abdala C, Keefe DH, Oba SI. Distortion product otoacoustic emission suppression tuning and acoustic admittance in human infants: birth through 6 months. J Acoust Soc Am. 2007;121(6):3617–27. doi: 10.1121/1.2734481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatto MP. Page 10-Baby waves and hearing aids: Using ABR to fit hearing aids to infants. Hearing J. 2008;6(2):10–16. [Google Scholar]
- Bagatto MP, Scollie SD, Hyde M, Seewald R. Protocol for the provision of amplification within the Ontario infant hearing program. Int J Audiol. 2010;49:S70–S79. doi: 10.3109/14992020903080751. [DOI] [PubMed] [Google Scholar]
- Bargones JY, Werner LA. Adults listen selectively; infants do not,”. Psychol. Sci. 1994;5:170–174. [Google Scholar]
- Barnet AB, Lodge A. Click evoked EEG responses in normal and developmentally retarded infants. Nature. 1967;214:252–255. doi: 10.1038/214252a0. [DOI] [PubMed] [Google Scholar]
- Barnet A, Ohlrich E, Weiss I, Shanks B. Auditory evoked potentials during sleep in normal children from ten days to three years of age. Electroencephalog and Clin Neurophysiol. 1975;39:29–41. doi: 10.1016/0013-4694(75)90124-8. [DOI] [PubMed] [Google Scholar]
- Baudhuin J, Cadieux J, Firszt JB, Reeder RM, Maxson JL. Optimization of programming parameters in children with the advanced bionics cochlear implant. Journal of the American Academy of Audiology. 2012;23(5):302–312. doi: 10.3766/jaaa.23.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P. Praat, a system for doing phonetics by computer. Glot International. 2001;5:341–345. [Google Scholar]
- Carter L, Golding M, Dillon H, Seymour J. The detection of infant cortical auditory evoked potentials (CAEPs) using statistical and visual detection techniques. J Am Acad Audiol. 2010;21(5):347–56. doi: 10.3766/jaaa.21.5.6. [DOI] [PubMed] [Google Scholar]
- Cone-Wesson B, Kurtzberg D, Vaughan HG., Jr Electrophysiologic assessment of auditory pathways in high risk infants. Int J Pediatr Otorhinolaryngol. 1987;14(2-3):203–14. doi: 10.1016/0165-5876(87)90032-2. [DOI] [PubMed] [Google Scholar]
- Dai H. On measuring psychometric functions: A comparison of constant-stimulus and adaptive up-down methods. J Acoust Soc Amer. 1995;98(6):3135–3139. doi: 10.1121/1.413802. [DOI] [PubMed] [Google Scholar]
- Davidson LS, Skinner MW, Holstad BA, Fears BT, Richter MK, Matusofsky M, Brenner C, Holden T, Birath A, Kettel JL, Scollie S. The effect of instantaneous input dynamic range setting on the speech perception of children with the nucleus 24 implant. Ear & Hearing. 2009;30(3):340–349. doi: 10.1097/AUD.0b013e31819ec93a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H, Bowers C, Hirsh SK. Relations of the human vertex potential to acoustic input: loudness and masking. J Acoust Soc Am. 1968;43(3):431–8. doi: 10.1121/1.1910849. 1968 Mar. [DOI] [PubMed] [Google Scholar]
- Davis H, Zerlin S. Acoustic relations of the human vertex potential. J Acoust Soc Am. 1966;39(1):109–16. doi: 10.1121/1.1909858. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with changes in structure and speech perception. Acta Otolaryngol (Stockh) 2003;123(2):249–52. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- Eilers RE, Ozdamar O, Miskiel E, Widen J. Classification of audiograms by sequential testing testing using a dynamic Bayesian procedure. J Acoust Soc Am. 1990;88(5):2171–9. doi: 10.1121/1.400114. [DOI] [PubMed] [Google Scholar]
- Eilers RE, Widen JE, Urbano R, Hudson T, Gonzales L. Otimization of automated hearing test algorithms: a comparison of data from simulations and young children. Ear Hear. 1991;12(3):199–204. doi: 10.1097/00003446-199106000-00007. [DOI] [PubMed] [Google Scholar]
- Golding M, Dillon H, Seymour J, et al. the detection of adult cortical auditory evoked potentials (CAEP) using an automated statistic and visual detection. Int. J. Audiology. 2009;48:833–842. doi: 10.3109/14992020903140928. [DOI] [PubMed] [Google Scholar]
- Golding M, Pearce W, Seymour, Cooper J, King A, Ching T, Dillon H. The relationship between obligatory cortical auditory evoked potentials (CAEPs) and functional measures in young infants. J Amer Acad Audiol. 2007;18(2):117–25. doi: 10.3766/jaaa.18.2.4. [DOI] [PubMed] [Google Scholar]
- Graziani LJ, Weitzman ED, Velasco MS. Neurologic maturation and auditory evoked responses in low birth weight infants. Pediatrics. 1968;41(2):483–494. [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Krieger; New York: 1966. [Google Scholar]
- Hecox K, Galambos R. Brain stem auditory evoked responses in human infants and adults. Arch Otolaryngol. 1974;99(1):30–3. doi: 10.1001/archotol.1974.00780030034006. [DOI] [PubMed] [Google Scholar]
- Huotilainen M, Kujala A, Hotakainen M, Shestakova A, Kushnerenko E, Parkkonen L, Fellman V, Naatanen R. Auditory magnetic responses of healthy newborns. Neuroreport. 2003;14(14):1871–1875. doi: 10.1097/00001756-200310060-00023. [DOI] [PubMed] [Google Scholar]
- Kiang NYS. Discharge Patterns of Single Nerve Fibers in the Cat's Auditory Nerve. MIT; Cambridge MA: 1965. [Google Scholar]
- Klein AJ, Teas D. Acoustically dependent shifts of BSER (wave V) in man. J Acoust Soc Amer. 1978;63:1887–1895. doi: 10.1121/1.381930. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Hilpert PL, Kreuzer JA, Vaughan HG., Jr Differential maturation of cortical auditory evoked potentials to speech sounds in normal fullterm and very low-birth weight infants. Dev Med Child Neurol. 1984;26:466–475. doi: 10.1111/j.1469-8749.1984.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Hilpert PL, Kreuzer JA, Stone CL, Vaughan HG., Jr McCallum WC, Zappoli R, Denoth F, editors. Topographical analysis of auditory evoked potentials to speech sounds in infants. Cerebral Psychophysiology: Studies in Event-Related Potentials. 1986;(EEG Supplement 38):326–328. [Google Scholar]
- Leibold LJ, Werner LA. Relationship between intensity and reaction time in normal-hearing infants and adults. Ear & Hearing. 2002;23(2):92–97. doi: 10.1097/00003446-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Leibold LJ, Werner LA. Effect of masker-frequency variability on the detection performance of infants and adults. J Acoust. Soc. Am. 2006;119(6):3960–3970. doi: 10.1121/1.2200150. [DOI] [PubMed] [Google Scholar]
- Little VM, Thomas DG, Letterman MR. Single-trial analyses of developmental trends in infant auditory event-related potentials. Developmental Neuropsychology. 1999;16(3):455–478. [Google Scholar]
- MacMillan NA, Creelman CD. Detection theory: A user's guide. 2nd ed. Erlbaum; Mahwah, NJ: 2005. [Google Scholar]
- May BJ. Physiological and psychophysical assessments of the dynamic range of vowel representations in the auditory periphery. Speech Communication. 2003;41:49–57. [Google Scholar]
- Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain and Language. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Moore DR. Development of the cat peripheral auditory system: Input-output functions of cochlear potentials. Brain Research. 1981;219:29–44. doi: 10.1016/0006-8993(81)90265-1. [DOI] [PubMed] [Google Scholar]
- Moore JK, Perazzo LM, Braun A. Time course of axonal myelination in the human brainstem auditory pathway. Hear Res. 1995;87(1-2):21–31. doi: 10.1016/0378-5955(95)00073-d. [DOI] [PubMed] [Google Scholar]
- Moore JK, Guan Y-L. Cytoarchitectural and axonal maturation in human auditory cortex. JARO. 2001;2(4):297–311. doi: 10.1007/s101620010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G. Stimulus duration and input-output function of the different components of the slow auditory evoked potential. Audiology. 1973;12(4):250–61. [PubMed] [Google Scholar]
- Munro KJ, Purdy SC, Ahmed S, Begum R, Dillon H. Obligatory cortical auditory evoked potential waveform detection and differentiation using a commercially available clinical system: HEARLab™. Ear and Hearing. 2011;32(6):782–786. doi: 10.1097/AUD.0b013e318220377e. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiol. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Novak GP, Kurtzberg D, Kreuzer JA, Vaughan HG., Jr Cortical responses to speech sounds and their formants in normal infants: maturational sequence and spatiotemporal analysis. Electroencephalog and Clin Neurophysiol. 1989;73:295–305. doi: 10.1016/0013-4694(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Nozza RJ. Infant speech-sound discrimination testing: effects of stimulus intensity and procedural model on measures of performance. J Acoust Soc Amer. 1987;81(6):1928–39. doi: 10.1121/1.394757. [DOI] [PubMed] [Google Scholar]
- Nozza RJ, Henson AM. Unmasked thresholds and minimum masking in infants and adults: separating sensory from non-sensory contributions to infant-adult differences in behavioral thresholds. Ear Hear. 1999;20(6):483–96. doi: 10.1097/00003446-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Nozza RJ, Wilson WR. Masked and unmasked pure-tone thresholds of infants and adults: development of auditory frequency selectivity and sensitivity. Speech Hear Res. 1986;27(4):613–22. doi: 10.1044/jshr.2704.613. [DOI] [PubMed] [Google Scholar]
- Ohlrich ES, Barnet AB. Auditory evoked responses during the first year of life. Electroencephalog and Clin Neurophysiol. 1972;32:161–169. doi: 10.1016/0013-4694(72)90138-1. [DOI] [PubMed] [Google Scholar]
- Ohlrich ES, Barnet AB, Weiss IP, Shanks BL. Auditory evoked potential development in early childhood: a longitudinal study. Electroencephalog and Clin Neurophysiol. 1978;44:411–423. doi: 10.1016/0013-4694(78)90026-3. [DOI] [PubMed] [Google Scholar]
- Olsho LW, Koch EG, Carter EA, Halpin CF, Spetner NB. Pure tone sensitivity of human infants. J Acoust. Soc. Am. 1988;84(4):1316–1324. doi: 10.1121/1.396630. [DOI] [PubMed] [Google Scholar]
- Parry G, Hacking C, Bamford J, Day J. Minimal response levels for visual reinforcement audiometry in infants. Int J Audiol. 2003;42(7):413–7. doi: 10.3109/14992020309080050. 2003. [DOI] [PubMed] [Google Scholar]
- Phillips DP. Stimulus intensity and loudness recruitment: neural correlates. J Acoust Soc Am. 1987;82(1):1–12. doi: 10.1121/1.395547. [DOI] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech and cortical event related potentials in children with auditory neuropathy. Ear Hear. 2002;23(3):239–253. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Rapin I, Graziani LJ. Auditory-evoked responses in normal, brain-damaged, and deaf infants. Neurology. 1967;17:881–894. doi: 10.1212/wnl.17.9.881. [DOI] [PubMed] [Google Scholar]
- Rose JE, Hind JE, Anderson JE, Brugge JF. Some effects of stimulus intensity on response of auditory nerve fibers in the squirrel monkey. J Neurophysiol. 1971;34:685–699. doi: 10.1152/jn.1971.34.4.685. [DOI] [PubMed] [Google Scholar]
- Rotteveel JJ, Colon EJ, Stegeman DF, Visco YM. The maturation of the central auditory conduction in preterm infants until three months post term. IV. Composite group averages of the cortical auditory evoked responses (ACRs). Hear Res. 1987a;27:85–93. doi: 10.1016/0378-5955(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Rotteveel JJ, de Graaf R, Stegeman DF, Colon EJ, Visco YM. The maturation of the central auditory conduction in preterm infants until three months post term. V. The auditory cortical response (ACR). Hear Res. 1987b;27:95–110. doi: 10.1016/0378-5955(87)90029-3. [DOI] [PubMed] [Google Scholar]
- Sabo DL, Paradise JL, Kurs-Lasky M, Smith CG. Hearing levels in Infants and young children in relation to testing technique, age group, and the presence or absence of middle-ear effusion. Ear Hear. 2003;24:38–47. doi: 10.1097/01.AUD.0000051988.23117.91. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Abbas PJ. Rate versus level functions for auditory nerve fibers in cats: Tone burst stimuli. J. Acoust. Soc. Amer. 1974;56:1835–1847. doi: 10.1121/1.1903521. [DOI] [PubMed] [Google Scholar]
- Schimmel H. The (±) reference: accuracy of mean components in average response studies. Science. 1967;157:92–94. doi: 10.1126/science.157.3784.92. [DOI] [PubMed] [Google Scholar]
- Schimmel H, Rapin I, Cohen M. Improving evoked response audiometry with special reference to the use of machine scoring. Audiology. 1974;13:33–65. doi: 10.3109/00206097409089335. [DOI] [PubMed] [Google Scholar]
- Schimmel H, Rapin I, Cohen M. Improving evoked response audiometry. Audiology. 1975;14:486–479. [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203:134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Shucard DW, Shucard JL, Thomas DG. Auditory event-related potentials in waking infants and adults: a developmental perspective. Electroencephalog and Clin Neurophysiol. 1987;68:303–310. doi: 10.1016/0168-5597(87)90051-7. [DOI] [PubMed] [Google Scholar]
- Sinnott JM, Aslin RN. Frequency and intensity discrimination in human infants and adults. J Acoust Soc Am. 1985;78(6):1986–1992. doi: 10.1121/1.392655. [DOI] [PubMed] [Google Scholar]
- Statview for Windows V 5.0.1. SAS Institute; 1992-1998. [Google Scholar]
- Taylor MM, Creelman CD. PEST: Efficient estimates on probability functions. J Acoust Soc Am. 1967;41:782–787. [Google Scholar]
- Thomas DG, Whitaker E, Crow CD, Little V, Love L, Lykins S, Letterman M. Event-related potential variability as a measure of information storage in infant development. Developmental Neuropsych. 1997;13(2):205–232. [Google Scholar]
- Tharpe AM, Ashmead DH. A longitudinal investigation of infant auditory sensitivity. Am J Audiol. 2001;10(2):104–12. doi: 10.1044/1059-0889(2001/011). [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Morrongiello BA, Thorpe LA. Auditory sensitivity in school-age children. J Exp Child Psychol. 1988;46(2):273–85. doi: 10.1016/0022-0965(88)90060-4. [DOI] [PubMed] [Google Scholar]
- Von Bekesy G. Experiments in Hearing. McGraw Hill; New York: 1960. [Google Scholar]
- Voulamanos A, Werker JF. Tuned to the signal: the privileged status of speech for young infants. Developmental Science. 2004;7(3):270–276. doi: 10.1111/j.1467-7687.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Graziani L, Duhamel L. Maturation of the auditory evoked response of the prematurely born infant. Electroenceph and Clin Neurophysiol. 1967;23:82–83. [PubMed] [Google Scholar]
- Weitzman ED, Graziani LJ. Maturation and topography of the auditory evoked response of the prematurely born infant. Developmental Psychobiology. 1968;1(2):79–89. [PubMed] [Google Scholar]
- Werner LA. Observer-based approaches to human infant psychoacoustics. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Birkhauser; Boston: 1995. pp. 135–146. [Google Scholar]
- Werner LA, Boike K. Infants’ sensitivity to broadband noise. J Acoust Soc Am. 2001;109(5):2103–11. doi: 10.1121/1.1365112. [DOI] [PubMed] [Google Scholar]
- Werner LA, Bargones JY. Sources of auditory masking in infants: Distraction effects. Percept. Psychophys. 1991;50:405–412. doi: 10.3758/bf03205057. [DOI] [PubMed] [Google Scholar]
- Werner LA, Folsom RC, Mancl LR. The relationship between auditory brainstem response latencies and behavioral thresholds in normal hearing infants and adults. Hear Res. 1994;77:88–98. doi: 10.1016/0378-5955(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Werner LA, Folsom RC, Mancl LR. The relationship between auditory brainstem response and behavioral thresholds in normal hearing infants and adults. Hear Res. 1993;68:131–141. doi: 10.1016/0378-5955(93)90071-8. [DOI] [PubMed] [Google Scholar]
- Wilber LA, Burkard R. Calibration: Puretone, speech and noise signals. In: Katz J, Burkard R, Hood L, editors. Handbook of Clinical Audiology. 6th Ed. Lippincott Williams & Wilkins; Philadephia: 2009. pp. 7–29. [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Maturation of CAEP in infants and children: a review. Hear Res. 2006;212(1-2):212–223. doi: 10.1016/j.heares.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children. Hear Res. 2006;212(1-2):185–202. doi: 10.1016/j.heares.2005.11.010. [DOI] [PubMed] [Google Scholar]