Abstract
Purpose
Pazopanib is a potent, multi-targeted receptor tyrosine kinase inhibitor; however, there is limited information regarding the effects of liver function on pazopanib metabolism and pharmacokinetics (PK). The objective of this study was to establish the maximum tolerated dose (MTD) and PK profile of pazopanib in patients with varying degrees of hepatic dysfunction.
Experimental Design
Patients with any solid tumors or lymphoma were stratified into four groups based on the degree of hepatic dysfunction according to the National Cancer Institute Organ Dysfunction Working Group (NCI ODWG) criteria. Pazopanib was given orally once a day on a 21-day cycle. A modified 3+3 design was used.
Results
Ninety eight patients were enrolled. Patients in the mild group tolerated 800 mg per day. The moderate and severe groups tolerated 200 mg per day. Pharmacokinetic data in the mild group were similar to the data in the normal group. Comparison of the median Cmax and AUC(0–24) in the moderate or severe groups at 200 mg per day to the values in the normal and mild groups at 800 mg per day indicated less than dose-proportional systemic exposures in patients with moderate and severe hepatic impairment. This suggests that the lower MTD in the moderate and severe group is not due to a decrease in drug clearance or alteration in the proportion of metabolites.
Conclusions
In patients with mild liver dysfunction, pazopanib is well tolerated at the FDA-approved dose of 800 mg per day. Patients with moderate and severe liver dysfunction tolerated 200 mg per day.
Introduction
Pazopanib is a potent, multi-targeted receptor tyrosine kinase inhibitor approved for the treatment of renal cancer(1). Pazopanib inhibits angiogenesis and lymphangiogenesis by targeting multiple receptors including vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR)-α, PDGFR-β, and c-kit(2). The primary route of metabolism is hepatic, but there is limited information regarding the effects of liver function on pazopanib metabolism and pharmacokinetics (PK)(3).
In the initial phase 1 clinical trial of pazopanib in patients with advanced cancer, a total of 63 patients were treated at varying dose escalation cohorts ranging from 50 mg three times per week to 2000 mg once daily and 300–400 mg twice daily. Patients with AST or ALT greater than two times the upper limit of normal were excluded from that trial. The most common adverse events were hypertension, diarrhea, hair depigmentation and nausea. Dose-limiting toxicities observed at the 50 mg, 800 mg and 2000 mg dose levels included gastrointestinal hemorrhage, extrapyramidal involuntary movements and fatigue. Hypertension was the most common grade 3 toxicity. Abnormal liver function tests with elevated AST, ALT and bilirubin were observed in 38%, 24% and 13% of patients respectively.3 In another phase 1 study performed in patients with hepatocellular carcinoma, subjects with serum bilirubin <2.0 mg/dl (Chlids-Pugh A) were eligible. The MTD was determined to be 600 mg per day, and the dose-limiting toxicities were grade 3 AST/ALT elevations and malaise.(4) In subsequent clinical trials using pazopanib, hepatotoxicity with alanine transaminase (ALT) >3 times the upper limit of normal (ULN) was reported in 14% of patients and ALT >8 times the ULN was reported in 4% of patients. These studies suggest that pazopanib dosage has yet to be optimized and may affect patients differently based on their degree of clinical hepatic dysfunction(5).
Four pazopanib metabolites (GSK1268992, GSK1268997, GSK1071306, and GW700201) have been identified. Only one of these metabolites (GSK1268997) has been shown to inhibit the proliferation of VEGF-stimulated human umbilical vein endothelial cells with potency similar to pazopanib. The other metabolites show at least 10- to 20-fold less activity than the parent compound in the same cellular assay. The oxidative metabolism of pazopanib is mediated primarily by CYP3A4, with minor contributions from CYP1A2 and CYP2C8. In vitro studies also indicate that pazopanib is a potential inhibitor of CYP2C9, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C19, CYP2D6, CYP2E1, and CYP3A4(6). In patients with cancer, pazopanib was a weak inhibitor of CYP3A and CYP2D6 isozymes and had no effect on the PK of probe substrates for CYP1A2, CYP2C9, or CYP2C19(7). Therefore, clinical liver function may affect pazopanib PK and its subsequent pharmacodynamics. In patients with normal liver function, previous pharmacokinetic studies showed similar AUC(0–24)Cmax and C24 values after daily administration of doses from 800 mg to 2000 mg per day. This suggested that doses above 800 mg per day would not increase activity and 800 mg per day was the recommended dose for future studies. Currently there is limited information regarding the effects of liver function on pazopanib metabolism and PK. This study was designed to establish the maximal tolerated dose (MTD), dose-limiting toxicities (DLT), and PK profile of pazopanib in patients with varying degrees of hepatic dysfunction.
Patients and Methods
Eligibility Criteria
Eligible patients were ≥18 years of age with a life expectancy of >3 months and a Karnofsky Performance Status of ≥60%. All patients must have had a histologically or cytologically confirmed solid tumor or lymphoma except patients with hepatocellular carcinoma diagnosed by an elevated α-fetoprotein level (≥500 ng/mL) and positive serology for hepatitis. Other eligibility criteria included: absolute neutrophil count of ≥1.5×109/L, platelets ≥100×109/L; serum creatinine ≤ upper limit of normal or a calculated or measured level of ≥60 mL/min/1.73 m2 for creatinine levels above the institutional normal. For patients with gliomas or brain metastases, only those patients receiving a stable dose of corticosteroids and who were seizure-free for at least 1 month prior to enrollment were eligible. Patients taking CYP 450 enzyme-inducing anti-convulsant drugs were switched to other medications at least 7 days prior to the first dose of pazopanib. Patients requiring anticoagulation were required to be on a stabilized dose of low molecular weight heparin. Therapeutic anticoagulation with warfarin was not permitted. Patients with biliary obstruction were eligible if the stent had been in place for at least 10 days prior to study initiation and liver function was stable for at least 2 days without any categorical change in hepatic dysfunction stratum. Radiotherapy was required to be completed ≥ 4 weeks prior to entering the study; chemotherapy, targeted therapy or biotherapy ≥ 3 weeks; and nitrosoureas or mitomycin C ≥ 6 weeks. Agents with longer half-lives (such as suramin and bevacizumab) required longer elimination periods. Patients were not eligible if they had received prior therapy with pazopanib, had major surgery within 28 days prior to treatment, or were receiving any other concurrent investigational agents. Pregnant patients and patients with human immunodeficiency virus, or uncontrolled intercurrent illness were also excluded. The following patients were not eligible for this study: patients with a serious or non-healing wound, ulcer, or bone fracture; history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within 28 days of treatment; a cerebrovascular accident, myocardial infarction, baseline QTc ≥480 msec, recent admission for unstable angina, cardiac angioplasty, or stenting within 6 months of entry.
Drug Administration
Pazopanib was given orally once a day (1 hour before or 2 hours after a meal to minimize the effects of food on absorption) on Days 1–21 of a 21-day cycle. For days on which PK samples were obtained, patients were instructed to take their daily dose after the pretreatment blood draw to allow accurate timing of subsequent PK blood draws.
Study Design
This was a multi-institutional study conducted at 16 participating sites. Institutional Review Board (IRB) approval was obtained at each participating site and the City of Hope Comprehensive Cancer Center was the coordinating center for this National Cancer Institute Organ Dysfunction Working Group (NCI ODWG) study. Patients were stratified into four Groups [A (normal), B (mild), C (moderate), and D (severe)], using the NCI-ODWG categories of liver dysfunction for trials involving anti-cancer therapeutics (Table 1)(8). Both bilirubin and serum ALT were used to define each group; if the total bilirubin level and ALT level indicated different groups, enrollment was into the group with the greatest degree of liver dysfunction. No distinction was made between liver dysfunction due to metastases or other causes. All liver function tests were repeated within 24 hours prior to the start of treatment and patients whose degree of hepatic dysfunction changed between registration and initiation of protocol therapy were re-assigned to a different dysfunction group and dose level after discussion with the Principal Investigator. Patients in Group A (normal) were included in this study to obtain concurrent PK data in a subject population with normal hepatic function. Although AE data were recorded for Group A, there were no dose escalations because the MTD was defined in previous studies7. In the other groups, patients were evaluable for the purpose of cohort dose escalations if in the first cycle they either experienced a DLT (see below) or received at least 80% of the planned treatment dose and were followed for one full cycle without a DLT. Group B (mild) was defined according to either of two criteria (B1 and B2). Groups B1 and B2 were combined for dose level allocation and all analyses. For safety reasons, patients in Group D (severe) were enrolled only after it was possible to escalate the dose in Groups B (mild) and C (moderate).
Table 1.
NCI ODWG Liver Function Classification and Dosing Schema
| Group | Group A Normal liver function |
Group B Mild liver dysfunction |
Group C Moderate liver dysfunction |
Group D Severe liver dysfunction |
|---|---|---|---|---|
| Total Bilirubin (> 35% direct) | ≤ ULN1 | B1: ≤ ULN B2: >1.0x –1.5x ULN |
>1.5x – 3x ULN | >3x ULN |
| ALT2 | ≤ ULN | B1: > ULN B2: Any |
Any | Any |
| Dose Level | (mg/day) | (mg/day) | (mg/day) | (mg/day) |
| Level 1 | 800 (n=23) | 400 (n=9) | 200 (n=13) | 100 (n=13) |
| Level 2 | 800 (n=14) | 400 (n=7) | 200 (n=19) | |
| Level 3 | 800 | 400 | ||
| Level 4 | 800 |
ULN = upper limit of normal
ALT = alanine aminotransferase
Because treatment delays would be detrimental for patients in the eligible population and it was likely that several patients would not be evaluable for cohort dose escalation decisions, the typical 3+3 up and down dose-escalation rules were modified to allow accrual of up to 6 patients at a level if fewer than 3 patients were evaluable and fewer than 2 had experienced DLT. Dose-finding was carried out independently for each of the liver dysfunction groups; however, the dose recommended for a group with greater liver dysfunction could not be greater than that for a group with a lesser dysfunction. In each of the liver dysfunction groups, 6 patients in an expansion cohort were treated at the MTD (or the highest allowed dose) to obtain more extensive pharmacokinetic data. The MTD was defined as the highest dose at which no more than one instance of DLT was observed among the first six patients treated.
Dose-Limiting Toxicities
Toxicity was graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. First-cycle DLTs guided cohort dose escalations. DLT definitions from the NCI CTEP protocol template were modified for patient safety and defined as: a required dose reduction before 17 doses (80%) of pazopanib were administered in the first cycle of treatment; delays in next treatment cycle by ≥2 weeks due to treatment-related toxicity; grade 4 neutropenia, or occurrence of neutropenic fever with ANC <1.5×109/L; grade 4 thrombocytopenia; grade 3 nausea and vomiting if it occurred despite maximal antiemetic therapy and if hydration was required for >24 hours; grade 3 diarrhea despite patient compliance with anti-diarrheal therapy; grade 3 bleeding/hemorrhage; grade 4 hypertension and grade 4 proteinuria; and all other grade 4 non-hematologic toxicities (if an increase in grade above baseline), except hypersensitivity. Considering the nature of the patient populations, the changes in total bilirubin that constituted a DLT were specific for each group. For Group B, an increase of total bilirubin to the level defined for the Group D lasting >1 week was a DLT. For patients in Group C, a 1.5-fold increase from baseline total bilirubin to level defined for Group D lasting >1 week was a DLT. (Note: 1.5-fold increase from baseline total bilirubin which did not put a patient into Group D did not constitute a DLT). For patients in Group D, a 1.5-fold increase from baseline without recovery to <1.2×baseline lasting >2 weeks was a DLT.
Pharmacokinetics
During the dose-escalation phase of the protocol (mild, moderate, and severe cohorts), blood samples for PK analysis were collected over 6 hours during a week-3 clinic visit. During the expansion phase of the protocol at the MTD in each liver dysfunction cohort, blood samples for PK analysis were collected over 72 hours starting on Day 1 and over 24 hours during a week-3 clinic visit. Pazopanib and its metabolites (Supplemental Figure 1) were measured in plasma using HPLC-MS/MS7. The method for pazopanib was validated over the range 0.1 to 50 µg/mL. Samples above the upper limit of quantitation were diluted with blank plasma to within the validated range prior to analysis. The method for the determination of the pazopanib metabolites GSK1268992, GSK1268997, and GSK1071306 in plasma was validated over the range 0.05 to 10 µg/mL. The between run assay precision (%CV) for pazopanib and all metabolites was ≤ 15%. CmaxTmax AUC0–6) and/or AUC(0–24)and CL/F of pazopanib were calculated, as appropriate for each patient using non-compartmental methods, and summary statistics were tabulated. A normal liver function cohort was included for comparison of pharmacokinetic parameters. In addition, the non-compartmental (trapezoidal rule) AUCs of GSK1071306, GSK1268992, and GSK1268997 were calculated to compare the exposure of metabolites as a percentage of the pazopanib exposure in hepatically impaired patients to the exposure to pazopanib metabolites observed in patients with normal hepatic function.
Results
Patient Characteristics
A total of 98 patients were enrolled in the study, one of whom was not treated (Table 2). The median age of the study population was 57 years with a range between 24 and 78. A slightly higher proportion of the patients were male (54%). Colorectal cancer and liver cancer were the two most common types of primary tumor.
Table 2.
Patient Demographics by Study Group and Treatment Cohort
| Group A Normal liver function |
Group B Mild liver dysfunction |
Group C Moderate liver dysfunction |
Group D Severe liver dysfunction |
||||
|---|---|---|---|---|---|---|---|
| Starting dose of Pazopanib (mg) | 800 | 400 | 800 | 200 | 400 | 100 | 200 |
| Number of patients | 23 | 9 | 14 | 13 | 71 | 13 | 19 |
| Median Age (range) | 58 (24–78) | 56 (51–66) | 57 (47–78) | 58 (36–76) | 65 (52–73) | 56 (31–70) | 51 (39–78) |
| Gender (number and % | |||||||
| Male | 10 (43%) | 3 (33%) | 8 (57%) | 4 (31%) | 7 (100%) | 9 (69%) | 12 (63%) |
| Female | 13 (57%) | 6 (67%) | 6 (43%) | 9 (69%) | 4 (31%) | 7 (37%) | |
| Race (Number and %) | |||||||
| White | 22 (96%) | 7 (78%) | 12 (86%) | 11 (84%) | 2 (29%) | 10 (76%) | 11 (58%) |
| Black | 1 (4%) | 1 (11%) | 1 (8%) | 1 (14%) | 1 (8%) | 5 (26%) | |
| Asian | 1 (11%) | 2 (14%) | 1 (8%) | 4 (57%) | 1 (8%) | 2 (11%) | |
| Native Hawaiian or Other Pacific Islander | 1 (8%) | ||||||
| Unknown | 1 (5%) | ||||||
| Ethnicity (Number and %) | |||||||
| Not Hispanic or Latino | 22 (96%) | 9 (100%) | 13 (93%) | 9 (69%) | 5 (72%) | 13 (100%) | 15 (79%) |
| Hispanic or Latino | 1 (7%) | 4 (31%) | 1 (14%) | 4 (21%) | |||
| Unknown | 1 (4%) | 1 (14%) | |||||
| KPS2 Score (Number and %) | |||||||
| 60 | 1 (4%) | 1 (11%) | 2 (14%) | 1 (8%) | 1 (14%) | 3 (23%) | 5 (26%) |
| 70–80 | 9 (39%) | 5 (56%) | 2 (14%) | 6 (46%) | 5 (72%) | 10 (76%) | 13 (68%) |
| 90–100 | 13 (57%) | 3 (33%) | 10 (72%) | 6 (46%) | 1 (14%) | 1 (6%) | |
| Baseline Abnormalities | |||||||
| (AEs) | |||||||
| (Number and %) | |||||||
| Grade 1 | 95 (63%) | 44 (60%) | 72 (64%) | 106 (67%) | 39 (47%) | 81 (50%) | 133 (65%) |
| Grade 2 | 48 (32%) | 22 (30%) | 32 (28%) | 44 (28%) | 33 (40%) | 57 (35%) | 45 (22%) |
| Grade 3 | 7 (5%) | 7 (9%) | 9 (8%) | 9 (5%) | 11 (13%) | 20 (12%) | 21 (10%) |
| Grade 4 | 1 (1%) | 5 (3%) | 5 (3%) | ||||
| Type of Primary Tumor/Hematologic Malignance (N) | |||||||
| Bile Duct | 1 | 1 | 1 | ||||
| Breast | 4 | 1 | |||||
| Colon/ Rectum | 6 | 2 | 6 | 9 | 3 | 5 | 10 |
| Liver | 1 | 2 | 3 | 1 | 2 | 2 | 2 |
| Lung | 1 | 1 | 2 | ||||
| Pancreas | 1 | 2 | 1 | 1 | 1 | ||
| Other (n=26) | |||||||
One patient was not treated; there is no weight information for this patient.
Karnofsky Performance Status
Dose-Limiting Toxicities
Group B (Mild dysfunction)
Nine patients were enrolled at 400 mg once daily of pazopanib. Of the 6 patients evaluable for dose escalation, one experienced a DLT, grade 4 increased aspartate transaminase (AST). Including the dose-escalation and expansion patients, 13 were treated at the FDA approved dose of 800 mg per day, with one DLT (grade 5 stomach hemorrhage). One patient was accrued, but withdrew from the study prior to treatment.
Group C (Moderate dysfunction)
Three patients were enrolled at 200 mg once daily without experiencing a DLT. Seven patients were enrolled at a dose of 400 mg. Of the 4 patients evaluable for dose escalation, 2 experienced a DLT, one patient had a grade 4 AST and the other patient had grade 4 AST, grade 4 ALT, and grade 3 hyperbilirubinemia. In each case, elevations in AST and ALT decreased in grade after discontinuation of the pazopanib. The dose was de-escalated to 200 mg and 3 additional patients were accrued without a DLT, establishing the MTD. One DLT, grade 3 hyperbilirubinemia, was observed in the expansion cohort of 6 patients. (Altogether, 1 of 12 patients experienced a DLT at the MTD.)
Group D (Severe dysfunction)
At the 100 mg dose level, 1 of 6 evaluable patients experienced a DLT, grade 4 bilirubin. This patient with metastatic colon cancer initiated therapy with a total bilirubin of 7.4 mg/dL. After 2 weeks of treatment the bilirubin increased to 11.5 mg/dL. Despite a metal stent placement, the bilirubin remained greater than 1.5x > baseline. Relationship to pazopanib could not be excluded and this was considered a DLT. However, even after pazopanib was discontinued, the bilirubin continued to rise likely due to progressive disease. One of the first 6 evaluable patients experienced a DLT at the 200 mg dose level, grade 3 diarrhea. Based on the study design and the MTD established in Group C, higher doses were not tested in Group D, making 200 mg per day the recommended dose. Five evaluable patients were accrued to the expansion cohort without DLT.
Toxicity data for all cycles
The most frequently occurring treatment-related adverse event across all groups, dose levels, and cycles were fatigue, diarrhea, nausea, and increased AST, events known to be associated with pazopanib. Frequently occurring AEs were similar among all groups and dose levels. Table 3 summarizes grade 3–4 toxicities observed on trial across all cycles and Supplemental Table 1 summarizes the most frequently reported adverse events (all grades).
Table 3.
Grade 3 and 4 Adverse Events at Least Possibly Related to Pazopanib1
| Group A normal |
Group B mild |
Group C Moderate |
Group D severe |
Total | ||||
|---|---|---|---|---|---|---|---|---|
| Dose of pazopanib (mg) | 800 | 400 | 800 | 200 | 400 | 100 | 200 | |
| Number of patients (%) | 23 (100) | 9 (100) | 14 (100) | 13 (100) | 6 (100) | 13 (100) | 19 (100) | 97 (100) |
| Hematologic | ||||||||
| Lymphopenia | 1(4) | 2 (14) | 1 (17) | 1 (8) | 5 (5) | |||
| Neutrophil count decreased | 1 (7) | 1 (1) | ||||||
| Platelet count decreased | 1 (8) | 1 (1) | ||||||
| Gastrointestinal disorders | ||||||||
| Abdominal Pain | 1 (8) | 1 (1) | ||||||
| Colitis | 1 (7) | 1 (1) | ||||||
| Diarrhea | 2 (11) | 2 (2) | ||||||
| Fistula – Abdomen NOS | 1 (7) | 1 (1) | ||||||
| Nausea | 1 (11) | 1 (7) | 1 (8) | 3 (3) | ||||
| Vomiting | 1 (4) | 1 (7) | 1 (8) | 3 (3) | ||||
| Coagulation | ||||||||
| Partial Thromboplastin Time | 1 (8) | 1 (1) | ||||||
| Constitutional | ||||||||
| Anorexia | 1 (11) | 1 (17) | 2 (2) | |||||
| Dyspnea | 1 (4) | 1 (1) | ||||||
| Fatigue | 3 (13) | 1 (8) | 1 (17) | 4 (31) | 1 (5) | 10 (10) | ||
| Muscle Weakness | 1 (8) | 1 (1) | ||||||
| Liver dysfunction | ||||||||
| Alanine aminotransferase increased | 1 (11) | 1 (8) | 2 (33) | 4 (4) | ||||
| Alkaline phosphatase increased | 2 (22) | 1 (8) | 1 (8) | 1 (5) | 5 (5) | |||
| Aspartate aminotransferase increased | 3 (33) | 1 (8) | 2 (33) | 2 (15) | 3 (16) | 11 (11) | ||
| Blood bilirubin increased | 1 (11) | 4 (31) | 1 (17) | 2 (15) | 2 (11) | 10 (10) | ||
| Metabolic and other | ||||||||
| Hypokalemia | 1 (5) | 1 (1) | ||||||
| Hyponatremia | 1 (11) | 2 (15) | 3 (3) | |||||
| Hypophosphatemia | 1 (11) | 1 (8) | 1 (5) | 3 (3) | ||||
| Hyperkalemia | 1 (11) | 1 (1) | ||||||
| Hypertension | 1 (4) | 1 (11) | 1 (7) | 2 (15) | 5 (5) | |||
Maximum grade for all cycles in a given patient
Efficacy
Of 98 enrolled patients, there were no complete responses. There were four (4.1%) partial responses (PR): three in Group A, and one in Group B (all at the 800 mg dose level). The partial responses were seen in patients with fibrosarcoma, leiomyosarcoma, poorly differentiated germ cell tumor and hepatocellular carcinoma with duration of treatment ranging from 7 to 22 cycles. The fibrosarcoma and germ cell patients received 7 cycles of chemotherapy before coming off trial for progressive disease. The hepatocellular carcinoma patient received 16 cycles of treatment but came off trial due to development of an abdominal fistula. The uterine leiomyosarcoma patient received 22 cycles of treatment but required a dose reduction after 19 cycles from 800 mg per day to 400 mg per day due to grade 2 proteinuria. Treatment was stopped due to progressive disease. At the 800 mg per day dose, stable disease was seen in 19 patients with the following diagnosis: colon, fibromyxoid, osteosarcoma, bronchoalveolar, esophageal, rectal, ovarian, leiomyosarcoma, hepatocellular, breast, neuroendocrine and gastric. The duration of treatment ranged from 3 to 27 cycles. In summary, percentage of best responses in patients evaluated for response were 18% PR, 47% SD, and 35% PD in Group A; 6% PR, 61% SD, and 33% PD in Group B; 18% SD and 82% PD in Group C; and 12% SD and 88% PD in Group D.
Pharmacokinetics
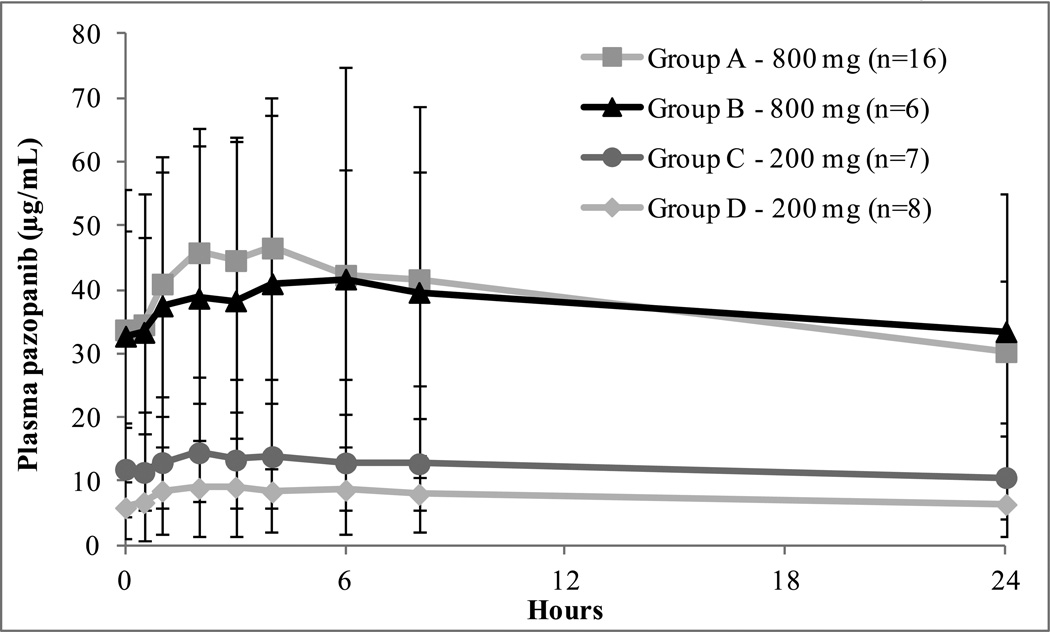
Steady-state pazopanib PK data were available in 69 patients and the results are summarized in Table 4. In addition, the average concentration-versus-time plots at steady-state for each of the Groups are depicted in Figure 1. The median steady-state Cmax in Groups A and B at a dose of 800 mg were 52.0 and 33.5 µg/mL, respectively, while the median AUC(0–24) were 888.2 and 774.2 µg/mLxhr, respectively. The median steady-state Cmax at the MTD level in patients in Groups C and D (200 mg once daily) were 22.2 and 9.4 µg/mL, respectively, and the median AUC(0–24) were 256.8 and 130.6 µg/mLxhr, respectively. Therefore, at the MTD in Group C, the median steady-state Cmax was 44% and the median AUC(0–24) was 39% of the values in the normal group at full dose. At the MTD in Group D, the median steady-state Cmax was 18% and the median AUC(0–24) 15%, of the values in the Group A at full dose, even lower than in Group C patients at the same dose. The median trough concentration (C0) for patients in Groups C and D were 16.2 and 5.7 µg/mL, respectively. As shown in Figure 1, pazopanib plasma concentrations at steady-state were highly variable in all of the Groups. There were no significant differences in the average systemic exposures in patients in Groups A and B treated at a dose of 800 mg. Likewise, there were no significant differences in subjects in Groups C and D treated with 200 mg, although there was a trend towards lower average plasma levels in Group D.
Table 4.
Steady-state Pazopanib Pharmacokinetics Measured in All Patients at Week 3 (medians and ranges)
| Group | Dose (mg) |
N | Cmax (µg/mL) |
Tmax (hr) |
C(0)1 µg/mL |
AUC(0–6) (µg/mLxhr) |
AUC(0–24) (µg/mLxhr) |
CL/F2 (L/hr) |
|---|---|---|---|---|---|---|---|---|
| A | 800 | 18 | 52.0 (17.1–85.7) | 2.8 (1.(0–24).2) | 29.8 (10.3–750.) | 260.5 (92.0–475.9) | 888.2 (345.5–1482) | 0.9 (0.5–2.3) |
| B | 3003 | 1 | 32.9 | 3.0 | 17.3 | 170.5 | n/a | n/a |
| 400 | 5 | 22.7 (14.6–39.8) | 3.0 (2.0–4.1) | 19.3 (6.8–36.6) | 125.1 (78.3–205.8) | 467.6 | 0.9 | |
| 800 | 12 | 33.5 (11.3–104.2) | 3.0 (0.5–24.4) | 24.0 (8.3–74.6) | 176.5 (41.4–518.1) | 774.2 (214.7–2034.4) | 1.0 (0.4–3.7) | |
| C | 200 | 11 | 22.2 (4.2–32.9) | 2.0 (0.0–4.0) | 16.2 (3.1–24.2) | 122.2 (21.2–182.9) | 256.8 (65.7–487.7) | 0.8 (0.4–3.0) |
| 400 | 3 | 17.6 (13.0–42.5) | 4.0 (3.0–5.9) | 16.5 (11.8–28.9) | 94.1 (74.7–220.0) | n/a | n/a | |
| D | 100 | 5 | 2.3 (0.7–12.6) | 4.0 (1.1–6.0) | 4.2 (2.3–9.4) | 12.8 (3.0–69.1) | n/a | n/a |
| 200 | 14 | 9.4 (2.4–24.3) | 3.0 (1.0–8.0) | 5.7 (1.5–18.4) | 49.2 (9.5–134.5) | 130.6 (46.9–473.2) | 1.7(0.4–4.3) |
C(0) is the pre-dose plasma concentration and is equivalent to the trough level following the previous dose.
CL/F calculated as dose/AUC(0–24) at steady-state for the 24-hour dosing interval.
Patient was dose-reduced per protocol prior to week 3.
Figure 1.
Average steady-state pazopanib plasma concentration versus time plots measured during the week 3 expansion phase
The data for the three pazopanib metabolites measured at steady-state are summarized in Supplemental Tables 2 and 3. Although the median values of each of the metabolites decreased with increasing severity of liver impairment, the ratios of the AUC0–6 for each of the metabolites to the AUC0–6 for the parent drug showed no apparent differences across all of the liver function cohorts.
First-dose pazopanib PK data, which were obtained in the expansion and normal cohorts, are available in 50 patients and the results are summarized in Table 5. The median first-dose Cmax in Groups A and B at a dose of 800 mg were 30.8 and 23.8 µg/mL, respectively, while the median AUC(0–24) were 563.1 and 462.9 µg/mLxhr, respectively. The median first-dose Cmax at the MTD level in Groups C and D (200 mg) were 4.1 and 3.7 µg/mL, respectively, and the median AUC(0–24) were 42.1 and 56.4 µg/mLxhr, respectively. As in the case of the steady-state pazopanib PK data, systemic exposures to pazopanib following a single dose at the MTD level in patients in Groups C and D were less than the systemic exposure to pazopanib after administration of 800 mg once daily in patients in Groups A and B. Univarate analyses of various steady-state and first-dose pazopanib PK parameters versus total bilirubin, ALT, and albumin revealed no significant correlations. Although the AUCs of the metabolites were lower after the first dose than at steady-state, the patterns across hepatic dysfunction groups and metabolites were similar to those at steady-state (Supplemental Table 4).
Table 5.
First-dose Pazopanib Pharmacokinetics in Expanded Cohorts and the Maximum Tolerated Dose (medians and ranges)
| Group | Dose (mg) |
N | Cmax(µg/mL) | Tmax (hr) | AUC(0–6) (µg/mLxhr) |
AUC(0–24) (µg/mLxhr) |
|---|---|---|---|---|---|---|
| A | 800 | 20 | 30.8 (4.2–66.2) | 4.0 (1.(0–24).0) | 120.6 (10.8–271.3) | 563.1 (74.4–1107) |
| B | 200 | 1 | 9.4 | 6.5 | 41.8 | 179.8 |
| 400 | 1 | 13.6 | 2.0 | 62.0 | 223.1 | |
| 800 | 6 | 23.8 (6.1–127.5) | 4.6 (2.0–8.3) | 103.0 (11.2–403.3) | 462.9 (102.0–2147) | |
| C | 200 | 7 | 4.1 (0.4–13.5) | 2.0 (2.0–3.1) | 19.7 (1.6–67.1) | 42.1 (6.1–231.2) |
| D | 100 | 3 | 2.9 (1.8–3.1) | 3.0 (2.(0–24).2) | 13.1 (0.5–13.3) | 33.6 (19.5–43.4) |
| 200 | 12 | 3.7 (0.5–13.1) | 3.5 (2.0–8.3) | 17.2 (1.8–57.3) | 56.4 (6.3–208.4) |
Discussion
Pazopanib is a multi-targeted tyrosine kinase inhibitor that is approved for the treatment of advanced renal cell carcinoma and soft tissue sarcomas. However, treatment of patients with advanced disease can be challenging due to impaired liver function from metastasis. Pazopanib’s package insert has a black box warning due to severe and fatal hepatotoxicity observed in clinical trials and currently there is limited information regarding pazopanib in patients with liver dysfunction. In a phase 1 study of pazopanib in patients with advanced hepatocellular carcinoma, the maximum tolerated dose was determined to be 600 mg per day. Evidence of antitumor activity was seen at this dose; however, the 800 mg per day dose was not tolerable due to dose-limiting toxicities of grade 3 malaise and grade 3 AST/ALT elevation. Liver abnormalities were seen in a majority of the hepatocellular carcinoma patients treated with pazopanib. AST elevation was seen in 63% of the patients. ALT elevation occurred in 41% of patients and hyperbilirubinemia in 63% of patients. We therefore conducted this clinical trial with the primary objective of determining the optimal dose of pazopanib in cancer patients with varying degrees of liver dysfunction as determined by the NCI ODWG classification system.
Steady-state pazopanib PK data were available for 69 patients. The median steady-state Cmax and AUC in Groups A and B at 800 mg are similar to the corresponding values previously reported in cancer patients with normal hepatic function(3). Therefore, patients with mild liver dysfunction would most likely derive the same benefit from this dose as patients with no hepatic impairment. However, the median steady-state Cmax and AUC(0–24) values after administration of 200 mg pazopanib once daily to patients in Group C were approximately 44% and 39%, respectively of the corresponding median values after administration of 800 mg/day in patients with normal hepatic function. Although interpretation of the data across doses is complicated by the fact that the PK of oral pazopanib are not linear in patients with normal hepatic function, the plasma concentrations at the MTD in Group C are clearly lower than the concentrations at the MTD in Groups A and B. Administration of 200 mg pazopanib once daily to patients in Group D resulted in median steady-state Cmax and AUC(0–24) values of only 18% and 15%, respectively, compared to the 800 mg daily dose in patients with no liver dysfunction. Whereas the median trough concentration for patients in Group C was within the range of plasma concentrations associated with clinical and biologic effects consistent with VEGFR inhibition of 15–20µg/mL in patients with renal cell carcinoma(3), the median trough levels in Group D was less than the desired level. These data suggest that the systemic exposure of pazopanib in Group D administered the highest dose tested in this group based on safety considerations in the study design (200 mg/day), may not provide a therapeutic benefit to these patients.
Pazopanib has been reported to exhibit non-linear PK behaviour, such that steady-state plasma levels increase in a less than dose-proportional manner(3, 4). As a result, the mean plasma AUC plateaus at doses above 800 mg. The most likely explanation for pazopanib’s non-linear PK behaviour is saturable oral absorption. Interestingly, recent studies have shown that by either taking pazopanib with food(9) or crushing the tablet(10), one can increase the oral bioavailability by as much as 2-fold. On the current trial, patients were instructed to take pazopanib on an empty stomach in accordance with the current FDA recommendations and to minimize the variable effect of food. In contrast to previously published PK in patients with normal liver biochemistry and mildly impaired liver function, our results from patients with severe hepatic impairment demonstrate a greater than dose-proportional increase in AUC. There were no differences between the Groups with respect to the metabolite to pazopanib ratios, suggesting that hepatic metabolism of pazopanib was not affected by the severity of liver dysfunction. There are limited published data at a dose of 200 mg from the initial dose finding studies. Previous steady-state PK results in patients with normal or Childs-Pugh A liver function receiving 200 mg demonstrate trough levels of 12.4 and 15.4 µg/ml, respectively. (3, 4) The average steady-state trough pazopanib concentration from the current study in subjects with severe hepatic dysfunction treated with 200 mg was 5.7 µg/ml, suggesting reduced oral absorption. The PK results following a single dose of pazopanib were consistent with the steady-state data.
Conducting a liver dysfunction clinical trial with a hepatotoxic drug can be challenging, but the information obtained from our study is critical for the management of many patients. The primary indication for pazopanib is for patients with advanced renal cell carcinoma. Since many of these patients may have had nephrectomies, there is concern for potential hepatorenal syndrome. In our study, 98 patients were treated and 7 patients had elevated creatinine (3 – grade 1; 1 – grade 2; 2 - grade 3; and 1 – grade 4). In our assessment, the grade 3 and 4 creatinine elevations were not related to treatment, but to disease progression. Overall, the PK data suggest that the lower MTD in patients with moderate or severe liver dysfunction, compared to those with no or mild liver dysfunction is not due to a decrease in drug clearance or an alteration in the metabolic pattern of pazopanib. Multiple factors are likely to have contributed to the poorer outcome for patients in the moderate and severe liver impairment groups. Concurrent with their hepatic dysfunction, the patients may have had more extensive disease when they entered the study. Therefore, they may have been less likely to have an objective response or stable disease even if they had tolerated the same drug exposure. It is not surprising that patients with baseline hepatic dysfunction did not tolerate a hepatotoxic agent well, leading to overall lower exposure to the drug and reducing further the likelihood of clinical benefit. Patients with mild hepatic dysfunction, as evidenced by total bilirubin in the range of 1.0 to 1.5-times the ULN or an ALT above the ULN, tolerated full-dose pazopanib and should be considered for therapy with this agent if otherwise appropriate. Patients with moderate or severe hepatic dysfunction tolerated a 200 mg daily dose. Furthermore, our PK data do not support the use of dose individualization for patients with impaired liver function. This study was not designed, and does not have the statistical power, to determine whether or not this reduced dose is efficacious. However, based on the low drug exposure in patients with severe hepatic dysfunction, both the US FDA package insert and the European Commission Summary of Product Characteristics do not recommend pazopanib for patients with severe hepatic dysfunction.
Supplementary Material
Statement of Translational Relevance.
Pazopanib is a potent, multi-targeted receptor tyrosine kinase inhibitor approved for the treatment of renal cancer and soft tissue sarcoma. Clinical activity has also been observed in urothelial, ovarian and non small cell lung cancer. Because patients with hepatic impairment are typically excluded from studies performed during the clinical development of a new drug, safe dosing guidelines are usually not available at the time of approval. Hepatic dysfunction is common in patients with cancer, either as a result of co-morbid conditions or because of the cancer itself. The FDA has recognized this as significant unmet clinical need, and therefore, they recently began requiring that safety and pharmacokinetic studies in patients with liver dysfunction be either completed or planned by the time of first approval. Therefore, the current study was performed to describe the pharmacokinetics and determine the maximum tolerated dose of pazopanib in patients with varying degrees of hepatic impairment.
Acknowledgement
The authors wish to acknowledge the contributions of Stella Khoo and Diana Calcanas-Perez in the Data Coordinating Center at City of Hope for their management of this multi-institution protocol and Nicola Solomon, Ph.D. for her editorial assistance and critical review. The authors also wish to acknowledge the late Stephen Shibata, M.D. and Merrill Egorin, M.D. for their hard work and dedication to this study. Also, thanks to Sridhar Mani, M.D. for his help on this study. Finally, we would like to thank all patients and families that participated as well as the support staff at each institution.
The study was supported by NIH grants: U01-CA062505 & P30-CA033572 (City of Hope, Duarte, CA); U01-CA062487 (Karmanos Cancer Institute, Detroit, MI); U01-CA099168 (University of Pittsburgh, Pittsburgh, PA); U01-CA70095 (Johns Hopkins University, Baltimore, MD); U01-CA069853 (Cancer Therapy and Research Center at University of Texas Health Science Center, San Antonio, TX); U01-CA062490 (Dana-Farber Cancer Institute, Boston, MA); U01-CA062502 (Case Western Reserve, Cleveland, OH) and U01-C 062491 (University of Wisconsin Paul P Carbone Comprehensive Cancer Center, Madison, WI). The study was also supported by the Institute for Drug Development, Cancer Therapy and Research Center at University of Texas Health Science Center San Antonio, TX: Cancer Center Support Grant P30CA054174; Johns Hopkins University Cancer Center Core Grant Support P30 CA006973.
Footnotes
Disclosures:
The following investigators do not have any disclosures: Stephen Shibata, Vincent Chung, Timothy Synold, Jeffrey Longmate, Heinz-Josef Lenz, Shivaani Kummar, R. Donald Harvey, Bert O’Neil, John Sarantopoulos, Afshin Dowlati, Daniel Mulkerin, Chandra Belani, Leena Gandhi, Cecilia Lau, S. Percy Ivy, and Edward M. Newman; A. Benjamin Suttle and Lone H. Ottessen are employed and own stock with GlaxoSmithKline; Anne Hamilton serves on the GlaxoSmithKline advisory board; Patricia LoRusso receives research funding from GlaxoSmithKline; Michelle A. Rudek’s family member employed by Amplimmune.
References
- 1.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 4.Yau T, Chen PJ, Chan P, Curtis CM, Murphy PS, Suttle AB, et al. Phase I dose-finding study of pazopanib in hepatocellular carcinoma: evaluation of early efficacy, pharmacokinetics, and pharmacodynamics. Clin Cancer Res. 2011;17:6914–6923. doi: 10.1158/1078-0432.CCR-11-0793. [DOI] [PubMed] [Google Scholar]
- 5.GlaxoSmithKline RTP, NC 27709. Pazopanib. [package insert] [Google Scholar]
- 6.Christopher Pandite LA, Howard Ball, Rocco Crescenzo, Traci Dimino, Leonora Goodman VS, Ben Suttle, Sherry Watson, Claire Wixon. Investigator's Brochure for pazopanib (GW786034) for Oncology and Ophthalmic Indications. 2011 [Google Scholar]
- 7.Goh BC, Reddy NJ, Dandamudi UB, Laubscher KH, Peckham T, Hodge JP, et al. An evaluation of the drug interaction potential of pazopanib, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, using a modified Cooperstown 5+1 cocktail in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88:652–659. doi: 10.1038/clpt.2010.158. [DOI] [PubMed] [Google Scholar]
- 8.Patel HMJE, Remick SC, Mulkerin D, Takimoto CHM, Doroshow JH, Potter D, Ivy SP, Murgo AJ, Ramanathan RK. Comparison of Child-Pugh (CP) criteria and NCI organ dysfunction working group (NCI-ODWG) criteria for hepatic dysfunction (HD): Implications for chemotherapy dosing. Journal of Clinical Oncology. 2004:22. [Google Scholar]
- 9.Heath EI, Chiorean EG, Sweeney CJ, Hodge JP, Lager JJ, Forman K, et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88:818–823. doi: 10.1038/clpt.2010.199. [DOI] [PubMed] [Google Scholar]
- 10.Heath EI, Forman K, Malburg L, Gainer S, Suttle AB, Adams L, et al. A phase I pharmacokinetic and safety evaluation of oral pazopanib dosing administered as crushed tablet or oral suspension in patients with advanced solid tumors. Invest New Drugs. 2012;30:1566–1574. doi: 10.1007/s10637-011-9725-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.