Abstract
Protein kinase R (PKR), a regulator of translation in mammalian cells, possesses two ds-RNA binding domains responsible for kinase activation. Protein kinase Z (PKZ), a PKR-like kinase present in fish, possesses two Z-DNA binding domains. A complementation strategy with cells stably deficient in PKR was used to compare the functions of PKR and PKZ. We found reporter expression was inhibited by wildtype (WT) PKR but not by either catalytic (K296R) or RNA-binding (K64E) mutants. PKZ, like PKR, more potently inhibited 5’ cap-dependent compared to IRES-dependent reporter expression. However, in contrast to PKR-expressing cells, phosphorylation of initiation factor eIF2α was not detectably increased in PKZ-expressing cells. Furthermore, virus-induced stress granule formation was observed in PKR-deficient cells complemented with WT PKR but not K296R mutant PKR or WT PKZ. These results suggest that PKR and PKZ function by distinguishable mechanisms to modulate host responses including protein synthesis inhibition and stress granule formation.
Keywords: PKR, PKZ, protein kinase, stress granule, double-stranded RNA, innate immunity
INTRODUCTION
The interferon (IFN) inducible protein kinase regulated by RNA (PKR) functions as a mediator of antiviral innate immunity (Garcia et al., 2006; Pfaller et al., 2011; Samuel, 2001). In addition to possessing antiviral activity, PKR is implicated in the regulation of cellular stress-induced responses and apoptosis (Pindel and Sadler, 2011; Toth et al., 2006). The domain organization of PKR includes kinase catalytic subdomains in the C-terminal region and a repeated RNA-binding domain in the N-terminal region. Binding of double-stranded (ds) RNA or structured single-stranded RNA by inactive PKR protein can lead to kinase autoactivation by a mechanism involving dimerization and autophosphorylation of the PKR protein (McCormack et al., 1992; McKenna et al., 2007; Thomis and Samuel, 1993). Following autoactivation, PKR catalyzes the phosphorylation of the alpha subunit of protein synthesis initiation factor 2 (eIF2α), the best characterized substrate of PKR (Garcia et al., 2006; Nallagatla et al., 2011; Samuel, 1979; Samuel, 1993). Phosphorylation of eIF2α at serine 51 leads to an inhibition of translation by impairing the activity of guanine nucleotide exchange factor eIF2B, a limiting component of the translation initiation process (Barber, 2005; Sudhakar et al., 1999). In addition to serine 51, serine 48 of eIF2α also has been described as a phosphorylation site that affects interaction with eIF2B (Kaufman et al., 1989; Kramer, 1990; Sudhakar et al., 1999). The universal nature of the translation control mechanism mediated by eIF2α phosphorylation is illustrated by the fact that three additional protein kinases are known that phosphorylate eIF2α at serine 51: the general control nonderepressible kinase 2 (GCN2) activated by amino acid starvation; the heme-regulated inhibitor kinase (HRI) activated by hemin deficiency and oxidative stress; and, the PKR-like endoplasmic reticulum-resident kinase (PERK) activated by protein misfolding and ER-stress (Samuel, 1993; Wek et al., 2006).
Although phosphorylation of eIF2α leads to the inhibition of 7-methylguanosine cap-dependent translation initiation, some viral and cellular mRNAs are translated in the presence of eIF2α phosphorylation through a 5’ cap-independent mechanism of initiation that involves ribosome recruitment to an RNA structure known as the internal ribosome entry site (IRES) (Balvay et al., 2009; Bushell and Sarnow, 2002; Deniz et al., 2009; Garrey et al., 2010; Komar and Hatzoglou, 2005). Viruses such as hepatitis C virus utilize the cap-independent, IRES-dependent mechanism of initiation to circumvent an inhibition of viral protein synthesis mediated by activated PKR (Garaigorta and Chisari, 2009). For some IRES elements, such as that found in cricket paralysis virus (CrPV), the IRES element directly binds the ribosome to initiate mRNA translation in the absence of translation initiation factors typically required during cap-dependent translation including eIF2α (Deniz et al., 2009; Jan and Sarnow, 2002; Pestova and Hellen, 2003).
A PKR-like kinase known as PKZ has been described in fish that is similar to PKR in the C-terminal kinase domain, but within the N-terminal region contains two copies of the binding domain for the Z-DNA (Zα) instead of the RNA binding domains found in PKR (Hu et al., 2004; Rothenburg et al., 2005; Toth et al., 2006). While PKR is the founding member of a protein family that possess the dsRNA-binding motif R (Fierro-Monti and Mathews, 2000; Katze et al., 1991; McCormack et al., 1992), the IFN-inducible form of the RNA adenosine deaminase ADAR1 is the founding protein where the Z-DNA binding motif was first identified (Herbert et al., 1997; Patterson et al., 1995). In addition to ADAR1 and PKZ, two additional Z-DNA-binding proteins that play a role in virus-host interactions and the innate immune response are known: the cellular cytosolic sensor of foreign DNA (DAI/DLM-1/ZBP1) (Schwartz et al., 2001; Takaoka et al., 2007) and the poxvirus E3L protein (Kim et al., 2004). It has been established that the Z-DNA binding activity of E3L protein is necessary for vaccinia virus pathogenicity in the mouse model of infection (Kim et al., 2003).
As an approach to analyze the functions of PKR and PKZ, we used a complementation assay strategy in which either wildtype or mutant PKR or PKZ were expressed in PKR-deficient human cells in culture. Our results reveal that cap-dependent compared to IRES-dependent reporter protein synthesis in PKR-deficient cells was significantly reduced when complemented with either wildtype PKR or PKZ but not with their respective catalytic subdomain II mutants nor an RNA-binding mutant of PKR. The ability of PKR to inhibit gene expression correlated with PKR autoactivation and increased eIF2α serine 51 phosphorylation. Although potent inhibition of cap-dependent protein synthesis was observed in PKZ-complemented cells, no increase in the level of phosphorylated eIF2α was detected. Furthermore, phosphorylation-defective forms of eIF2α did not reverse the inhibition of reporter synthesis in PKZ-expressing cells but did in PKR-complemented cells. The formation of virus-induced stress granules associated with inhibition of translation initiation and eIF2α phosphorylation was observed in PKR-but not in PKZ-complemented cells following measles virus infection and was dependent upon PKR kinase catalytic activity.
MATERIALS and METHODS
Cells and virus
Parental human amnion U cells and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% (vol/vol) fetal bovine serum (HyClone), 100 µg/ml of penicillin and 100 units/ml of streptomycin (GIBCO/Invitrogen) as previously described (McAllister et al., 2010; Zhang et al., 2009). HeLa cells and U cells made stably deficient in PKR by an integrated short hairpin silencing RNA interference strategy, designated PKRkd and UPKRkd , respectively, were as previously described (Zhang et al., 2009; Zhang and Samuel, 2007). Knockdown cells were maintained in medium containing 1 µg/ml puromycin (Sigma). Virus infections were carried out with the C-deficient (Cko) mutant of the recombinant Moraten measles virus vaccine (MVvac) strain (Devaux et al., 2007) as previously described (Taghavi and Samuel, 2012; Toth et al., 2009). The MVvac Cko virus was generously provided by R. Cattaneo (Mayo Clinic, Rochester, MN) and includes the green fluorescent protein (GFP) gene inserted downstream of the viral H gene. Infections were at a multiplicity of infection (moi) 50% tissue culture dose (TCID50) per cell of 1.
Plasmids
The pcDNA6 constructs used for expression of the wildtype (WT) human PKR protein and mutants defective for either catalytic activity (K296R) or dsRNA binding activity (K64E) (McCormack et al., 1994; Thomis and Samuel, 1992) were as previously described (Taghavi and Samuel, 2012). To generate the double KE-KR mutant defective in both catalytic and dsRNA-binding activities (KE-KR), the lysine (K) to glutamic acid (E) substitution at position 64 in the K296R plasmid background was generated using the following forward 5’-GTAGATCAAAGAAGGAAGCAG AAAATGCCGCAGCCAAATTA - 3’ and reverse 5’- TAATTTGGCTGCGGCATTT CTGCTTCCTTCTTTGATCTAC −3’ primers. In order to circumvent knockdown by the stably expressed silencing RNA while still preserving the PKR amino acid sequence, the PKR expression plasmid constructs were made to possess four synonymous mutations (shown by the underline italics font) in the shRNA target sequence GCAGGGAGTAGTCTTAAAGTA located in the PKR open reading frame (Zhang et al., 2009). The expression plasmids for WT and the catalytic domain K199R mutant of PKZ (Rothenburg et al., 2005) were generously provided by S. Rothenburg (National Institutes of Health, Bethesda, MD).
The luciferase reporter constructs utilized are summarized schematically in figure 1. The monocistronic reporter constructs, PGL3-SV40 expressing firefly (5’ cap-FF) and phRL-CMV expressing Renilla (5’ cap-RL) luciferase, both by a 5’ cap-dependent translation initiation mechanism, were from Promega. The monocistronic reporter construct expressing firefly luciferase by a CrPV IRES-dependent translation initiation mechanism (IRES-FF) was provided by K. Gustin (University of Arizona College of Medicine, Phoenix, AZ) and the bicistronic reporter construct expressing Renilla and firefly luciferases under the control of 5’ cap- and IRES-dependent translation initiation mechanisms (5’ cap-RL -IRES-FF), respectively, was provided by M. Hatzoglou (Case Western University, Cleveland, OH).
FIGURE 1. Schematic organization of luciferase reporter transcripts.
Monocistronic transcripts encoding either firefly (FF) or Renilla (RL) luciferase (luc) by a cap-dependent (5’ cap-FF and 5’ cap-RL) or an IRES-dependent (IRES-FF) translation initiation mechanism; bicistronic transcript (5’ cap-RL - IRES-FF) encoding RL and FF by a 5’ cap- and an internal IRES- dependent translation initiation mechanism, respectively.
The pMSCV-HA3iresGFP constructs for expression of human eIF2α protein containing serine 51 mutated to either alanine (S51A) or aspartic acid (S51D) were also generously provided by M. Hatzoglou (Case Western University, Cleveland, OH). The eIF2α double mutant with both serine 48 and 51 mutated to alanine (S 48,51A) and the empty pMSCV-HA3iresGFP vector (Vec) were as described (McAllister et al., 2012). For generation of the wildtype (WT) eIF2α expression plasmid, the alanine 51 residue of the S51A construct was mutated to serine using the Quick-Change site-directed mutagenesis strategy (Stratagene) and the following primers (mutations are designated by underlined italics font): forward 5’- GATTCTTCTTAGTGAATTGTCCAGAAGGCGTATCCGTTC - 3’ and reverse 5’-GAACGGATACGCCTTCTGGACAATTCACTAAGAAGAATC - 3’. Constructs were verified by direct sequencing and restriction enzyme analysis.
Plasmid transfections and luciferase reporter assays
HeLa PKRkd or amnion UPKRkd cells were co-transfected with the indicated luciferase (0.05 µg) and PKR or PKZ (0.5 µg for wildtype, 0.1 µg for mutants, or as indicated) expression constructs, either with or without an eIF2α expression construct (1 µg), using FuGENE HD transfection reagent (Roche) according to the manufacturer’s protocol as described previously (Taghavi and Samuel, 2012). Accordingly, the total amount of transfected plasmid DNA was made equivalent by addition of empty vector plasmid as necessary, dependent upon the amount of expression construct utilized. At 24 h after transfection cell extracts were prepared and analyzed for luciferase activity according to the manufacturer’s protocol (Promega) using an OPTOCOMP I luminometer. Luciferase activity was normalized to total extract protein.
Western immunoblot analyses
At 24 h post-transfection or as indicated, extracts were prepared with lysis buffer [20 mM Hepes (pH 7.9), 400 mM NaCl, 1mM DTT, 1mM EDTA, and 0.5% NP-40] containing 50 mM NaF, 1mM Na2VO3, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% (vol/vol) protease and phosphatase inhibitor cocktails (Sigma) as previously described (Toth et al., 2009). Protein concentration of extracts was determined by the Bradford method. Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%), transferred to nitrocellulose membranes, and blocked for 1 h at room temperature in Tris-buffered saline containing 5% (wt/vol) of either bovine serum albumin (for detection of phosphoproteins) or non-fat milk (for detection of total proteins). Primary antibody incubation was performed overnight at 4°C. Rabbit polyclonal antibodies were used to detect PKR (Santa Cruz Biotechnology), eIF2α (Cell Signaling Technology) and GFP (Santa Cruz Biotechnology). Rabbit monoclonal antibodies were used to detect phospho-PKR Thr446 (Epitomics) and phospho-eIF2α Ser51 (Epitomics); mouse monoclonal antibody was used to detect α-actin (Sigma). Anti-Myc antibody (Roche) was used for detection of epitope-tagged PKZ. Western immunoblot detection was performed with IRDye 800CW-conjugated anti-rabbit immunoglobulin G or IRDye 680-conjugated anti-mouse IgG secondary antibody according to the manufacturer's protocols. Immunoreactive bands were quantified using an Odyssey infrared imaging system (Li-COR Biosciences) and the obtained values normalized to β-actin as a loading control.
Real-time PCR analysis
Firefly luciferase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript levels were measured by quantitative real-time PCR (qPCR) as previously described (Wang and Samuel, 2009). RNA was isolated from cells 24 h after co-transfection with the 5’ cap-FF reporter construct (Fig. 1) and either PKR or PKZ plasmids by the RNeasy (Qiagen) protocol. In order to eliminate residual DNA, on-column RNase-free DNase digestion was performed. Random hexamer-primed cDNA was prepared using 0.5 µg RNA and Superscript II (Invitrogen). qPCR analyses were performed in duplicate using IQ SYBR Green Supermix (Bio-Rad) and a Bio-Rad MyIQ multicolor real-time qPCR instrument.
Immunofluorescence and stress granule analyses
Briefly, cells were grown on 18 mm glass coverslips and then transfected. After 24 h cells then were either left uninfected or infected with the MVvac Cko virus at an moi of 1 TCID50/cell for 24 h. Fixation for immunofluorescent microscopy was with neutral-buffered 10% (v/v) formalin (Sigma) at room temperature for 15 m and blocking with donkey serum (Jackson ImmunoResearch) and permeabilization with Triton X-100 were as described (Okonski and Samuel, 2013). Permeabilized cells were incubated with primary antibody overnight at 4°C using mouse monoclonal anti-G3BP1 1:500 (Sigma) and chicken IgY anti-GFP 1:300 (Molecular Probes). GFP is encoded by the recombinant Moraten vaccine strain of virus and is a marker of infection. Ras GAP SH3-domain-binding protein 1 (G3BP1) is a cell marker for stress granule (SG) formation (Anderson and Kedersha, 2008; Tourriere et al., 2003; White and Lloyd, 2012). Incubation of coverslips with donkey secondary antibodies, Alexa Fluor 594 anti-mouse (Molecular Probes) and AffiniPure FITC-conjugated anti-chicken (Jackson ImmunoResearch), was as described (Okonski and Samuel, 2013). Nuclei were stained by DAPI (blue). Images were captured using an Olympus IX71 microscope with Q-Capture PRO software (QImaging). To quantify SG formation, a minimum of 7–10 wide-field 40X images was examined per experiment with ~100–200 cells per field. Cells displaying punctate immunofluorescent foci of the G3BP1 marker protein were designated as SG-positive. Percentages were determined as the number of SG-positive cells divided by the total number of cells × 102 . The infection efficiency was estimated above 95% determined by detecting GFP expressed by virus.
RESULTS
5’ Cap-dependent and IRES-dependent reporter expression are differentially sensitive to inhibition by PKR
To test the ability of PKR to inhibit gene expression, PKR-deficient cells (UPKRkd) were complemented with PKR and transfected with either a 5’ cap-dependent or an IRES-dependent reporter construct (Fig. 1). The PKR-deficient UPKRkd cells, stably knocked down for PKR by a shRNAi silencing strategy, contain less than 10% of the PKR protein expressed in PKR-sufficient parental U cells (Zhang et al., 2009). To circumvent knockdown of the ectopically expressed PKR by the stably expressed shRNA, the PKR cDNA sequence was mutated at the RNAi target site without altering the amino acid sequence of the encoded PKR protein.
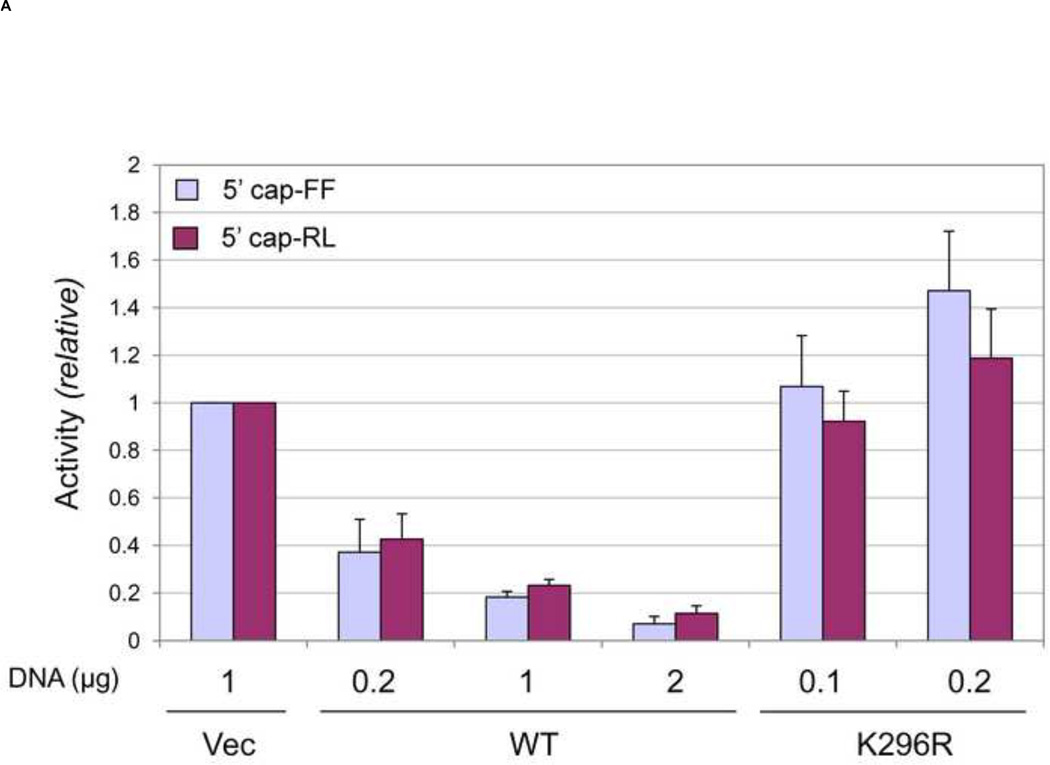
As shown in figure 2, expression of WT PKR protein (WT) in UPKRkd cells inhibited cap-dependent reporter gene expression from monocistronic luciferase (both 5’ cap-FF firefly and 5’ cap-RL Renilla) reporter constructs (Fig. 1) in a plasmid concentration dependent manner (Fig. 2A). Inhibition of luciferase activity required catalytic activity of PKR, because the K296R mutant lacking enzymatic activity did not inhibit reporter gene expression. Rather, a slight increase in expression was observed in cells that expressed the K296R mutant (Fig. 2A), consistent with a dominant negative effect of the ectopically expressed mutant PKR protein on the residual endogenous PKR. Because PKR proteins mutant for either catalytic (K296) or RNA binding (K64E) activity are expressed more efficiently than WT protein due to PKR autoregulatory effects seen in transfected cells (Barber et al., 1993; McCormack et al., 1994; Thomis and Samuel, 1992), different amounts of PKR plasmid were required to achieve comparable expression levels of the WT and mutant PKR proteins as measured by western blot analysis (Fig. 2–4). The ectopic PKR protein expression in PKRkd cells complemented with 1 µg WT construct or 0.2 µg K296R mutant construct was comparable to the level of endogenous PKR in parental cells not transfected (Fig. 2B). The inhibition of reporter expression observed in cells complemented with WT PKR (Fig. 2A) correlated with increased phosphorylation of both PKR and initiation factor eIF2α (Fig. 2B, 2C). Cells expressing the K296R mutant PKR or transfected with vector (Vec) alone did not show either detectable PKR autophosphorylation or an increase in eIF2α phosphorylation (Fig. 2B, 2C).
FIGURE 2. Inhibition of 5’ cap-dependent reporter gene expression by complementation of PKR-deficient human U cells with PKR requires catalytic activity.
PKR-deficient human amnion U cells (UPKRkd) were co-transfected with reporter constructs expressing either firefly (5’ cap- FF) or Renilla (5’ cap-RL) luciferase together with either empty vector (Vec) or the indicated DNA amount (µg) of PKR expression plasmid encoding either wildtype (WT) or catalytic mutant (K296R) PKR. At 24 h after transfection, cell extracts were prepared and analyzed. (A) Relative luciferase activity of PKR-transfected compared to vector-transfected cells. (B) Representative western blots are shown for PKR, Thr446 phospho-PKR (P-PKR), eIF2α, and Ser51 phospho-eIF2α (P-eIF2α), and β-actin as a loading control. (C) Levels of PKR (upper), P-PKR (middle), and P-eIF2α (lower) in transfected PKR-deficient (UPKRkd) cells normalized to the untransfected (NT) PKR-sufficient cells (U) determined by quantification of western blots using infrared imaging. The quantified results shown in (A) and (C) are means with standard deviation determined from three to four independent experiments.
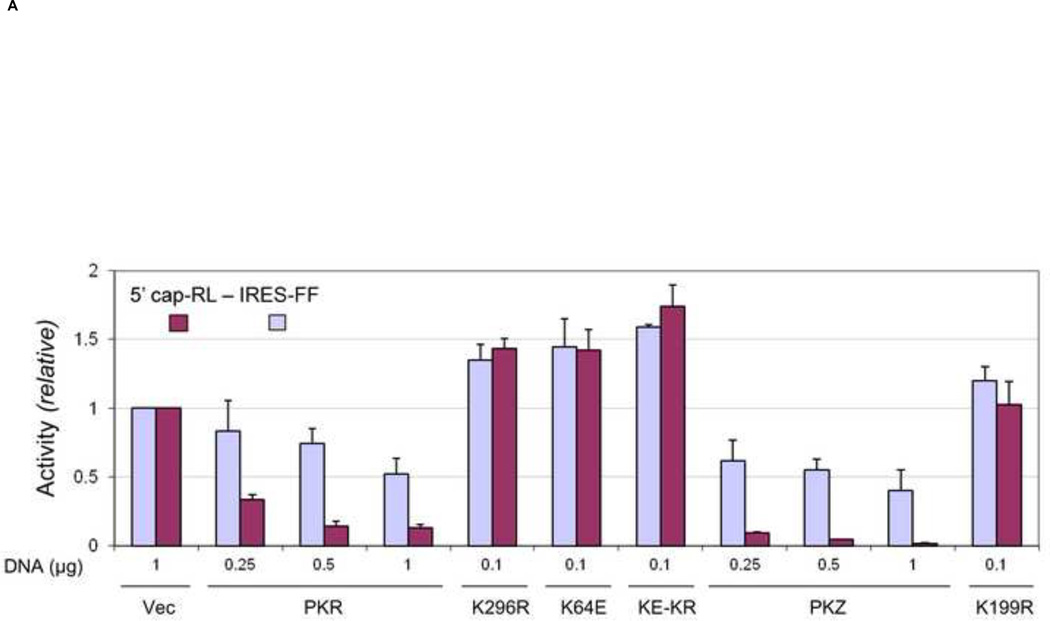
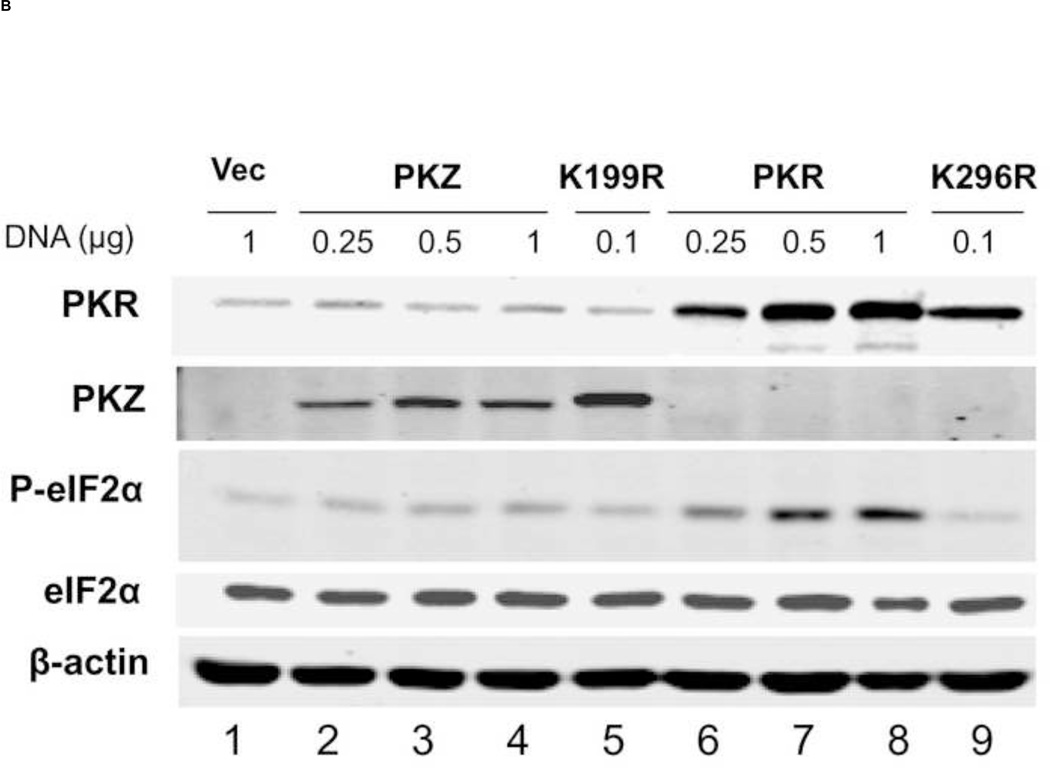
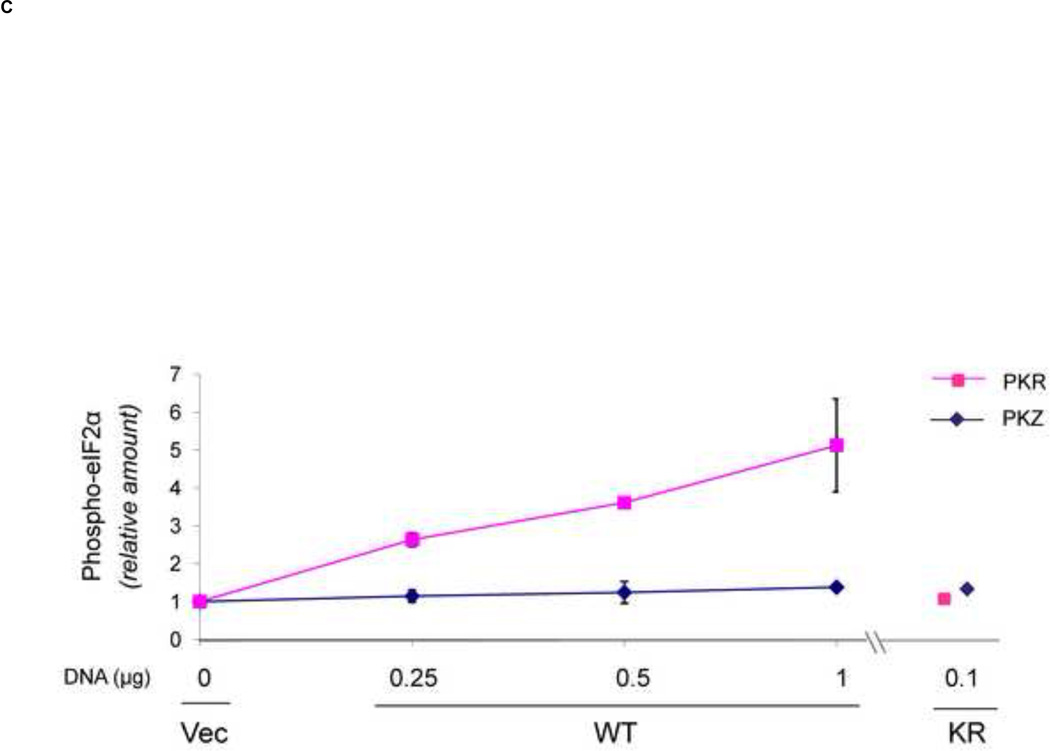
FIGURE 4. Inhibition by PKR and PKZ of cap-dependent compared to IRES-dependent reporter protein expression encoded by a bicistronic transcript.
PKR-deficient U cells (UPKRkd) were co-transfected with a bicistronic reporter construct (5 cap-RL- IRES-FF) expressing Renilla (5’ cap) and firefly (IRES) luciferase together with either empty vector (Vec) or the indicated DNA amount (µg) of PKR expression plasmid encoding: wildtype (PKR), catalytic mutant (K296R), RNA-binding mutant (K64E), or catalytic and RNA-binding double mutant (KE-KR) of PKR; wildtype (PKZ) or catalytic mutant (K199R) of PKZ. The total amount of transfected plasmid DNA was adjusted to 1 µg by addition of empty vector as necessary. At 24 h after transfection cell extracts were prepared and analyzed. (A) Luciferase activity of PKR- or PKZ-transfected cells compared to vector-transfected cells. The absolute values for Renilla (5’-cap) and firefly (IRES) bicistronic reporter expression were 7.9 × 106 RLU for Renilla and 3.7 × 104 RLU for firefly luciferase in the vector-transfected cells which were normalized to 1.0. (B) Representative western blots of extracts prepared from transfected cells using antibodies against PKR, Myc for epitope-tagged-PKZ, Ser 51 phospho-eIF2α (P-eIF2α), and eIF2α, and β-actin as a loading control. (C) Quantitation of phospho-eIF2α in PKR-and PKZ-transfected cells determined from western blots normalized to the vector (Vec)-transfected cells: WT, wildtype PKR or PKZ; KR, the catalytic mutant PKR (K296R) or PKZ (K199R). Results shown in A and C are means with standard deviation determined from three independent experiments.
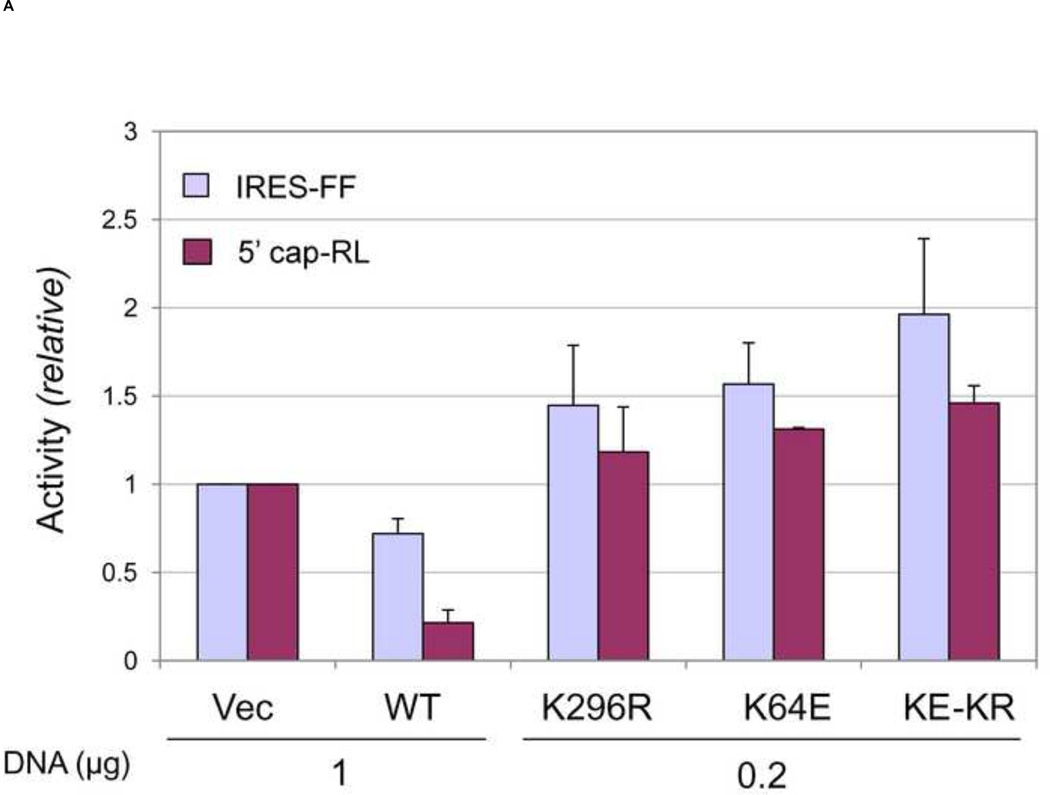
The IRES element of cricket paralysis virus (CrPV) can mediate translation initiation in the absence of initiation factor eIF2α (Deniz et al., 2009; Jan and Sarnow, 2002; Kieft, 2008). To test whether the RNA-activated PKR kinase differentially affects 5’ cap-dependent and IRES-dependent protein expression, the activity of a monocistronic firefly expression construct (IRES-FF) (Fig. 1) possessing the CrPV IRES was compared to the 5’ cap-dependent reporter (5’ cap-RL) (Fig. 1). As shown in figure 3, figure 5’ cap-dependent Renilla expression was more sensitive to inhibition by WT PKR than was IRES-dependent firefly expression compared to the vector-transfected cells (Fig. 3A). An RNA binding defective mutant protein (K64E), tested either as a single mutant or as a double mutant that also lacked catalytic activity (K64E-K296R), like the single K296R mutant seen earlier (Fig. 2A) did not inhibit 5’ cap-dependent reporter expression compared to WT PKR (Fig. 3A). Furthermore, none of the mutant PKR proteins (K296R, K64E, and K64E-K296R) inhibited IRES-dependent reporter expression (Fig. 3A). The inhibition of cap-dependent expression in cells expressing WT PKR correlated with higher levels of phosphorylated PKR and eIF2α relative to either vector-transfected cells or cells expressing mutant PKR (Fig. 3B).
FIGURE 3. Effect of PKR on reporter expression mediated by IRES-dependent compared to 5’ cap-dependent translation initiation.
PKR-deficient U cells (UPKRkd) were co-transfected with monocistronic reporter constructs expressing either firefly (IRES-FF) or Renilla (5’ cap-RL) luciferase, along with either empty vector (Vec) or the indicated DNA amount (µg) of PKR expression plasmid encoding either wildtype (WT), catalytic mutant (K296R), RNA-binding mutant (K64E) or the catalytic and RNA-binding double mutant ( KE-KR) PKR protein. At 24 h after transfection extracts were prepared and analyzed. (A) Relative luciferase activity of PKR-transfected compared to vector-transfected cells; the mean and standard deviation were determined from three independent experiments. The absolute values for Renilla (5’-cap) and firefly (IRES) monocistronic reporter expression were 2.3 × 106 RLU for Renilla and 9.7 × 103 RLU for firefly luciferase in the vector-transfected cells which were normalized to 1.0. (B) Representative western blots showing the levels of PKR, Thr446 phospho-PKR (P-PKR), eIF2α, and Ser51 phospho-eIF2α (P-eIF2α), and β-actin as a loading control.
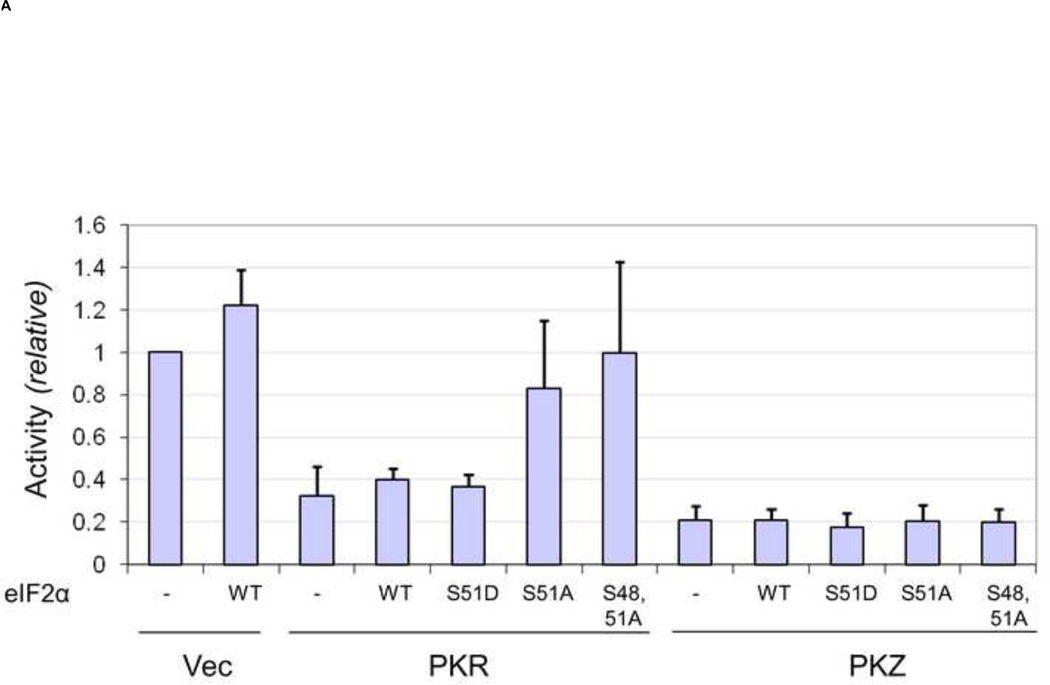
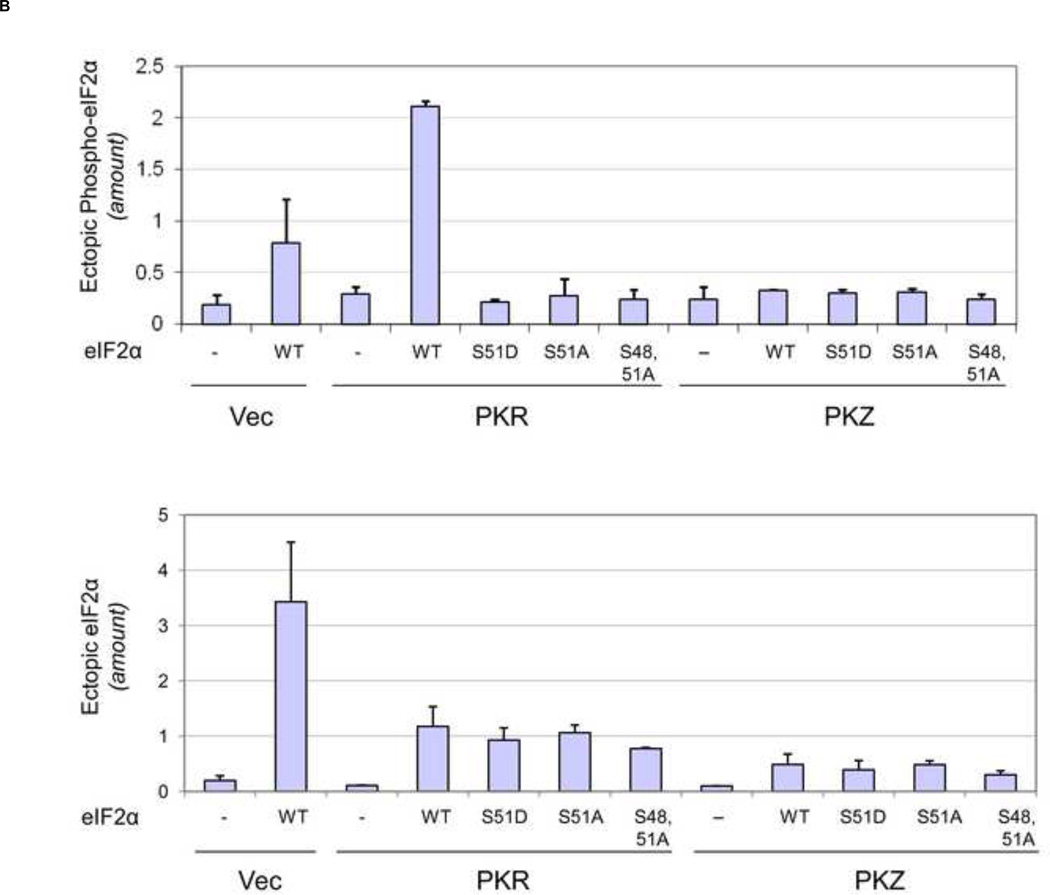
FIGURE 5. Expression of mutant eIF2α increases reporter protein expression in PKR-complemented but not PKZ-complemented PKR-deficient cells.
PKR-deficient U cells (UPKRkd) were co-transfected with the monocistronic reporter construct (5’ cap-FF) together with either empty vector (Vec) or the expression plasmid encoding wildtype PKR (PKR) or PKZ (PKZ) and an expression plasmid encoding either wildtype (WT) or mutant S51D, S51A, S48,51A eIF2α or vector alone (−). At 24 h after transfection, extracts were prepared and analyzed. (A). Relative luciferase activity in PKR- or PKZ-complemented cells compared to vector-transfected cells. (B) Quantitation by western analysis of ectopically expressed eIF2α and Ser 51 phospho-eIF2α . Results are means and standard deviation determined from three independent experiments.
PKZ inhibits cap-dependent compared to IRES-dependent reporter expression but does not detectably increase eIF2α serine 51 phosphorylation
The kinase domain of PKZ, an orthologue of mammalian PKR, is closely related to that of the PKR, but PKZ possesses two Z-DNA binding domains in the N- terminal region in place of the two RNA binding domains found in PKR (Hu et al., 2004; Rothenburg et al., 2005). PKZ, like PKR, is known to inhibit expression of a 5’ cap-dependent monocistronic reporter gene (Liu et al., 2011; Rothenburg et al., 2005; Taghavi and Samuel, 2012). To test the ability of PKZ to inhibit IRES-dependent compared to cap-dependent expression, a bicistronic luciferase reporter construct (5’ cap-RL - IRES-FF) was utilized (Fig. 1). The bicistronic reporter expresses Renilla luciferase from the first cistron via a 5’ cap-dependent mechanism, and firefly luciferase from the second cistron by an IRES-dependent translation mechanism.
The bicistronic reporter construct was co-expressed in UPKRkd cells together with either WT or mutant forms of PKR and PKZ. When compared to vector-transfected cells, PKZ and PKR when expressed as WT proteins both inhibited cap-dependent expression more strongly compared to IRES-dependent expression from the bicistronic reporter plasmid (Fig. 4A). The inhibition of 5’ cap-dependent expression required PKZ catalytic activity because the K199R mutant did not inhibit reporter expression (Fig. 4A); the K296R mutant of PKR also did not inhibit cap-dependent expression with the bicistronic reporter (Fig. 4A) as was seen with the monocistronic reporter (Fig. 3A). Inhibition of cap-dependent expression by WT PKZ occurred in a plasmid DNA concentration-dependent manner. Furthermore, the extent of inhibition by PKZ compared to PKR was typically greater (~10- to 50-fold by PKZ versus ~ 3- to 8-fold by PKR) relative to vector-transfected cells (Fig. 4A). By contrast, IRES-dependent reporter expression from the bicistronic construct was only modestly reduced by either PKZ or PKR even at the highest (1 µg) concentration of plasmid DNA tested. As a control, when a bicistronic construct possessing a mutation in the IRES structure that is defective for proper ribosome loading at the IRES (Jan and Sarnow, 2002) was tested, firefly expression by the mutant IRES construct was impaired compared to that of the WT IRES construct, whereas Renilla expression from the 5’ cistron was similar between them (data not shown).
Cells expressing K199R mutant PKZ protein did not increase 5’ cistron reporter gene expression characteristic of the dominant negative effect observed with cells complemented with mutant (K296R, K64E, K64E-K296R) PKR proteins (Fig. 4A). This suggests that the ectopically expressed PKZ likely was not able to dimerize with the residual endogenous PKR present in the knockdown cells to exert a dominant negative effect on the residual endogenous PKR.
Although PKZ inhibited cap-dependent translation more strongly than PKR, western blot analysis showed increased eIF2α phosphorylation detectable only in PKR, and not PKZ, complemented cells or in vector- transfected control cells (Fig. 4B, lanes, 1–4 and 6–8). Quantitation of western blot data revealed that WT PKR increased the phosphorylation of eIF2α ~ 3- to 7-fold in a plasmid DNA concentration dependent manner (Fig. 4C). By contrast, no significant increase in eIF2α phosphorylation was observed in cells expressing either WT PKZ or the mutant form of either PKR (K296R) or PKZ (K199R).
Phosphorylation-defective mutant forms of eIF2α block the inhibitory effect of PKR but not PKZ in reporter expressing cells
Both WT PKZ and WT PKR inhibited luciferase reporter expression from the 5’ cap-FF monocistronic construct (Fig. 2A, 5A) consistent with earlier observations (Taghavi and Samuel, 2012). As a test of whether the inhibition occurred at the translational level rather than an earlier step, for example transcription, luciferase reporter transcript levels were measured by qPCR. The quantitative difference in ectopically expressed firefly luciferase transcript levels was less than 2-fold in WT PKR or WT PKZ transfected cells compared to vector-transfected cells (average Ct 19.1, 20.3, 19.9, respectively). GAPDH endogenous transcript levels likewise were comparable in cells co-transfected with the firefly reporter and either PKR (Ct 14.5), PKZ (Ct 14.4) or vector (Ct 14.3).
In order to further test the role of eIF2α in PKZ-mediated inhibition of reporter gene expression, we next examined whether the phosphorylation-defective mutant forms of eIF2α were capable of reversing the inhibition of reporter gene expression caused by PKR and PKZ. We first tested the S51A mutant of eIF2α. When S51A eIF2α was coexpressed with PKR, the PKR-mediated inhibition of 5’ cap-FF reporter activity was largely prevented, with activity comparable to vector (Vec) expressing cells (Fig. 5A). By contrast, expression of S51A mutant eIF2α in cells together with PKZ was unable to detectably reverse the PKZ-mediated inhibition of reporter activity (Fig. 5A). We next considered whether Ser48 of eIF2α was a potential target site for phosphorylation by PKZ as opposed to Ser51 which is the established site of phosphorylation by PKR (Samuel, 1993). To test this possibility, an expression construct possessing Ser to Ala mutations at both serine 51 and 48 of eIF2α was generated. The S48,51A eIF2α double mutant reversed the inhibition of reporter expression caused by PKR to an extent similar to that seen with the S51A mutant, but the PKZ-mediated inhibition of expression still was not reversed by the S48,51A mutant (Fig. 5A). Two additional eIF2α expression constructs were examined as controls; neither wildtype (WT) eIF2α nor the Ser to Asp mutant (S51D) that mimics phosphorylated Ser51 reversed the PKR-mediated inhibition of expression. The level of reporter activity also remained low in PKZ-expressing cells that expressed WT or S51D eIF2α, similar to the low level seen with S51A or S48,51A expressing cells (Fig. 5A). In the absence of PKR or PKZ complementation of the PKRkd cells, WT eIF2α modestly increased reporter activity.
Quantitation of western blots revealed that, in PKR expressing cells with ectopically expressed eIF2α, the level of Ser51-phosphorylated eIF2α was ~ 5- to 10-fold higher in cells expressing WT eIF2α relative to that observed in cells expressing the phosphorylation defective eIF2α mutants (Fig. 5B). In addition, WT eIF2α phosphorylation was increased ~3 fold in PKR complemented cells compared to vector-transfected cells (Fig. 5B). GFP protein expressed by an IRES element from the eIF2α construct was used as a reporter for transfection efficiency, which with FUGENE HD typically ranged from 50 to 75%. Finally, the results obtained for eIF2α phosphorylation in PKR- or PKZ-complemented Hela PKRkd cells (data not shown) were similar to that observed in UPKRkd cells (Fig. 5A, 5B).
PKR catalytic activity is required for induction of stress granule formation following virus infection
Stress granule (SG) formation is a recognized hallmark of translation inhibition and represents a cellular response to infection (Anderson and Kedersha, 2008; Courtney et al., 2012; Garaigorta et al., 2012; Lindquist et al., 2011; White and Lloyd, 2012). We previously found that SG formation induced by measles virus MVvac Cko infection of HeLa cells was PKR-dependent (Okonski and Samuel, 2013). We now have examined whether PKR expression in PKR-deficient cells could restore SG formation following MV infection. HeLa cells stably deficient for PKR expression (PKRkd) were complemented with either WT PKR, or K296R or K64E mutant PKR, and then either left uninfected or infected with Cko virus. G3BP1 then was used as a marker to monitor SG formation (Garaigorta et al., 2012; Tourriere et al., 2003).
Immunofluorescent microscopy revealed that G3BP1 formed cytoplasmic punctuate foci characteristic of SG (Anderson and Kedersha, 2008; Okonski and Samuel, 2013) following infection of PKRkd cells complemented with WT PKR protein (Fig. 6A). By contrast, the SG response was not observed with the control vector-transfected cells (Fig. 6A). Quantitation revealed that the level of SG-positive cells in infected-PKR complemented cells was ~ 5 fold higher compared to the level seen in uninfected cells (Fig. 6B). Furthermore, neither the catalytic-defective K296R mutant nor the RNA-binding K64E mutant was able to restore the SG formation following infection; the levels of SG-positive cells in the mutant PKR expressing cells were comparable to the low level that was seen in control vector-transfected cells (Fig. 6A,B). These results establish that only a catalytically active form of PKR that is capable of catalyzing eIF2α phosphorylation can efficiently restore the induction of SG in PKRkd cells following infection.
FIGURE 6. PKR kinase catalytic activity is required for induction of stress granule formation following measles virus infection.
PKRkd HeLa cells were complemented by transfection with plasmid expression construct for wildtype PKR, catalytic mutant (K296R) PKR or RNA-binding mutant (K64E) PKR, or with empty vector (Vec) alone, as indicated. At 24 h after transfection cells were either infected with Cko measles virus (Cko) or left uninfected (UI). (A) At 24 h after infection, cells were analyzed by immunofluorescence microscopy using G3BP1 antibody as a marker for stress granule formation (punctate white foci). (B). Quantitation of stress granule positive cells. Wide-field 40X images were obtained, and the total number of cells in each field was analyzed per experiment for the presence of stress granules as shown. The results shown are means and standard errors from three independent experiments. (C) Western immunoblot analysis for ectopic PKR expression. At 24 h after infection, whole-cell extracts were prepared and analyzed using antibodies against PKR and β-actin as indicated.
PKZ is unable to restore stress granule formation following virus infection of PKR deficient cells
To assess the ability of PKZ compared to PKR to restore SG formation following infection, either WT PKZ, K199R mutant PKZ or WT PKR were expressed in PKRkd cells which then were left uninfected or infected with MVvac Cko virus. As shown in figure 7A, only PKR and not PKZ had the ability to rescue the capacity to form SG following infection. SG formation was readily observed in PKR-complemented cells compared to vector-transfected cells and was significantly enhanced by virus infection (Fig. 6A,B and 7A,B). By contrast, very few SG-positive cells were observed following complementation with PKZ, either infected or uninfected cells (Fig. 7A,B). The low levels (<5%) of SG-positive cells were comparable between vector, K199R mutant and WT PKZ transfected cells (Fig. 7B), whereas SG formation reached a much higher level (~40 to 45%) following complementation with WT PKR (Fig. 6B, 7B). These results indicate that PKZ is not functionally able to complement PKR deficiency in PKRkd cells as measured by infection-induced formation of SG, a process believed to require eIF2α phosphorylation, and are consistent with the observations that PKZ was unable to phosphorylate eIF2α (Fig. 4, 5).
FIGURE 7. Measles virus infection induces stress granule formation in PKR-deficient cells complemented with PKR but not PKZ.
PKRkd HeLa cells were complemented by transfection with the plasmid expression construct for wildtype PKZ, catalytic mutant (K199R) PKZ or wildtype PKR, or with empty vector (Vec) alone, as indicated. At 24 h after transfection cells were either infected with Cko measles virus (Cko) or left uninfected (UI). (A) At 24 h after infection, cells were analyzed by immunofluorescence microscopy using G3BP1 antibody as a marker for stress granule formation (punctate white foci). (B) Quantitation of stress granule positive cells. Wide-field 40X images were obtained, and the total number of cells in each field was analyzed per experiment for the presence of stress granules as shown. The results shown are means and standard errors from three independent experiments. (C) Western immunoblot analysis for ectopic PKZ and PKR expression. At 24 h after infection, whole-cell extracts were prepared and analyzed using antibodies against Myc-tagged PKZ, PKR and β-actin as indicated.
DISCUSSION
The importance of PKR in regulation of translation is best understood in the context of cellular responses to viral infection or stress-induced signaling cascades involved in apoptosis (Toth et al., 2006; Williams, 2001). While PKR is one among four known protein kinases that phosphorylate eIF2α at serine 51 in response to different kinds of physiologic stress (Samuel, 1993; Wek et al., 2006), it is unclear whether the PKR protein may have activities independent of its kinase catalytic activity, and whether the PKZ orthologue protein has the capacity to complement functionally the activities of PKR in PKR-deficient cells. We provide evidence herein that the two activities of PKR, inhibition of reporter expression and formation of stress granules following infection, are dependent upon catalytic activity and eIF2α phosphorylation. While PKZ, like PKR, inhibited 5’ cap-dependent reporter expression, PKZ was not able to complement other PKR functions: PKZ was unable to mediate SG formation following infection; PKZ was unable to mediate detectable eIF2α phosphorylation; and, mutant forms of eIF2α were unable to reverse PKZ-mediated reporter inhibition but did reverse PKR-mediated inhibition. Several important points emerge from these findings that extend prior observations and provide new insights.
PKZ, a PKR-like kinase in fish, has been reported to inhibit 5’ cap-dependent reporter expression in tranfected cells (Liu et al., 2011; Rothenburg et al., 2005). We confirmed using both monocistronic and bicistronic constructs that PKZ inhibits reporter protein expression in human PKRkd cells in a manner that is dependent upon the catalytic subdomain Lys199 of PKZ. The inhibition of reporter expression in PKR-complemented cells correlated with an increase in eIF2α phosphorylation at serine 51. Surprisingly, expression of PKZ, in spite of showing a strong inhibition of reporter expression, did not detectably increase the phosphorylation of eIF2α in PKZ-complemented PKRkd human cells. eIF2α is one of several eukaryotic translational initiation factors required for cap-dependent translation initiation, but not required for IRES-dependent initiation (Deniz et al., 2009; Jan and Sarnow, 2002; Kieft, 2008). Reporter protein expression, using either a monocistronic or bicistronic construct, revealed that both PKR and PKZ potently inhibited cap-dependent expression but only modestly affected IRES-dependent translation. Kinase catalytic activity was required for the inhibition of 5’ cap-dependent reporter expression by both PKR and PKZ, as neither the k296R nor K199R mutant, respectively, were inhibitory. The differential inhibition of expression of luciferase reporter activity by cap- versus IRES-dependent mechanisms suggests that both PKR and PKZ affect reporter expression at the translational level rather than at an earlier step, for example reporter transcript formation. Consistent with this conclusion, direct measurement of the transcript levels by qPCR confirmed that comparable amounts of luciferase reporter mRNA were expressed in the PKRkd cells transfected with vector, PKR or PKZ.
An intriguing question arises concerning the mechanism by which activation of ectopically expressed PKR or PKZ is triggered, thereby leading to the subsequent inhibition of reporter protein expression. Cells transfected with a WT PKR expression construct showed high levels of PKR phosphorylation as well as eIF2α phosphorylation compared to vector-transfected cells. The fact that activation of PKR occurred during the transfection process in the absence of any additional exogenous stimuli such as pIpC transfection or virus infection might be the consequence of aberrant dsRNA generated by RNA polymerase III following the plasmid transfection process (Chiu et al., 2009; Wang and Samuel, 2009). RNA polymerase III, a cytosolic DNA sensor, is known to trigger induction of IFN-β by generating 5’- triphosphate-containing dsRNA from transfected plasmid templates with dA-dT rich sequences (Ablasser et al., 2009; Chiu et al., 2009). The possibility cannot be excluded that binding of PKZ to RNA generated by plasmid transfection also occurs, thereby leading to activation of PKZ, as some Z-DNA binding proteins reportedly can bind to Z-RNA as well as Z-DNA (Herbert et al., 1998; Kim et al., 2004; Lushnikov et al., 2004; Rothenburg et al., 2005).
Consistent with the results that showed eIF2α serine 51 phosphorylation in vivo by PKR but not PKZ, in vitro assays carried out with purified eIF2α substrate protein added to cell-free extracts of PKRkd cells revealed that only cells complemented by PKR and not by PKZ expression showed increased phosphorylation of eIF2α at serine 51 (data not shown). What then is the mechanism of reporter inhibition by PKZ, when expressed in human PKRkd cells? We considered the possibility that phosphorylation of eIF2α by PKZ might occur at a site other than serine 51, for example serine 48 (Kaufman et al., 1989; Kramer, 1990; Sudhakar et al., 1999). However, the inability of the S48,51A double mutant to reverse the inhibitory effect of PKZ, as was observed for PKR, argues against this possibility. Because PKZ transfection reduced the expression of eIF2α variants by −50% relative to the expression seen with PKR transfection, we cannot exclude the possibility that this modest expression difference contributes to the lack of an effect seen with the S51A or S48,51A mutants. However, the inability of transfected PKZ to restore stress granule formation with the endogenous eIF2α argues against this possibility.
Unlike PKR, the mechanism of PKZ action and its substrate specificity are not well resolved. While weak phosphorylation of eIF2α by PKZ has been reported in carp CAB cells (Liu et al., 2011), it cannot be excluded that the observed increase was due to PKR also present in the CAB cells. We were not able to detect an increase in eIF2α phosphorylation in PKZ-transfected cells that are PKR-deficient. Alternative potential explanations for the PKZ-mediated inhibition reporter protein expression invoke impairment of a function other than that affecting eIF2α activity. For example, cellular kinases have been demonstrated to phosphorylate eIF4G, eIF4E, and eIF3F translation factors (He et al., 2001; Ling et al., 2005; Shi et al., 2009). PKR also has been reported to modulate the activation status of translation factor eIF-4E and to modulate translation through increasing the activation of the PP2A phosphatase which in turn dephosphorylates eIF4E (Williams, 2001; Xu and Williams, 2000), although our findings with mutant forms of eIF2α that reverse the PKR phenotype suggest the PP2A effect is secondary at least in transfected cells. However, PKZ, in contrast to PKR, conceivably might have as its primary effect the modulation of eIF4E or another translation factor other than eIF2α, as the eIF2α mutants were unable to reverse the PKZ inhibitory activity.
Although, the kinase domain of PKZ shows homology to that of the mammalian PKR, only some of the key residues within the catalytic domain important for mediating contact with eIF2α (αG helix) are conserved between PKR and PKZ (Dar et al., 2005; Rothenburg et al., 2008). In addition, the kinase insert domain that is important for eIF2α phosphorylation is extended in PKZ compared to that of PKR (Dar et al., 2005; Garcia et al., 2006; Rothenburg et al., 2008). On the other hand, the eIF2α-subunit substrate is highly conserved among different species; human and zebrafish factors, and human and rabbit factors, display 94% and 99% sequence identity respectively, and they are especially well conserved in the residues surrounding serine 51 (data not shown). These sequence and structural considerations of PKR, PKZ and eIF2α are consistent with the notion that eIF2α is not likely the principle substrate of PKZ.
Characteristics of virus-induced stress granule formation as a host response to infection also suggest that eIF2α is not likely a main substrate of PKZ. SG formation in response to virus infection, in the case of several viruses including measles virus (Okonski and Samuel, 2013), is known to be PKR-dependent and associated with eIF2α phosphorylation and protein synthesis inhibition (Courtney et al., 2012; Ruggieri et al., 2012; Smith et al., 2006; White and Lloyd, 2012). We earlier observed that measles virus infection led to increased phosphorylation of eIF2α in PKRkd cells complemented with WT but not K64E or K296R mutant PKR (Taghavi and Samuel, 2012). We now have found that measles virus induction of SG was restored in PKRkd cells complemented with PKR but not PKZ. That PKR-complemented cells displayed a robust induction of SGs after viral infection but that PKZ-complemented cells did not is a further indication that PKR and PKZ inhibit reporter protein expression by different mechanisms. In addition, PKR-dependent amplification of IFN induction following MV infection is dependent upon eIF2α phosphorylation (McAllister et al., 2012) which occurs in PKR but not in PKZ expressing PKRkd cells (Taghavi and Samuel, 2012), observations consistent with our present finding of an eIF2α-independent mode of translation inhibition by PKZ.
Highlights.
Protein kinase PKR and the Z-DNA binding orthologue PKZ inhibit protein expression.
PKR but not PKZ acted through an eIF2α-dependent mechanism of inhibition.
PKR but not PKZ mediated virus-induced stress granule formation.
Virus induced stress granule formation is dependent upon catalytically active PKR.
ACKNOWLEDGEMENTS
This work was supported in part by research grants AI-12520 and AI-20611 to CES from the National Institute of Allergy and Infectious Diseases, NIH, U.S. Public Health Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Balvay L, Soto Rifo R, Ricci EP, Decimo D, Ohlmann T. Structural and functional diversity of viral IRESes. Biochim. Biophys. Acta. 2009;1789:542–557. doi: 10.1016/j.bbagrm.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Barber GN. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 2005;12:563–570. doi: 10.1038/sj.cdd.4401643. [DOI] [PubMed] [Google Scholar]
- Barber GN, Wambach M, Wong ML, Dever TE, Hinnebusch AG, Katze MG. Translational regulation by the interferon-induced double-stranded-RNA-activated 68-kDa protein kinase. Proc. Natl. Acad. Sci. U. S. A. 1993;90:4621–4625. doi: 10.1073/pnas.90.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell M, Sarnow P. Hijacking the translation apparatus by RNA viruses. J. Cell Biol. 2002;158:395–399. doi: 10.1083/jcb.200205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SC, Scherbik SV, Stockman BM, Brinton MA. West nile virus infections suppress early viral RNA synthesis and avoid inducing the cell stress granule response. J. Virol. 2012;86:3647–3657. doi: 10.1128/JVI.06549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2αlpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Deniz N, Lenarcic EM, Landry DM, Thompson SR. Translation initiation factors are not required for Dicistroviridae IRES function in vivo. RNA. 2009;15:932–946. doi: 10.1261/rna.1315109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, von Messling V, Songsungthong W, Springfeld C, Cattaneo R. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology. 2007;360:72–83. doi: 10.1016/j.virol.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Fierro-Monti I, Mathews MB. Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem. Sci. 2000;25:241–246. doi: 10.1016/s0968-0004(00)01580-2. [DOI] [PubMed] [Google Scholar]
- Garaigorta U, Chisari FV. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe. 2009;6:513–522. doi: 10.1016/j.chom.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaigorta U, Heim MH, Boyd B, Wieland S, Chisari FV. Hepatitis C virus (HCV) induces formation of stress granules whose proteins regulate HCV RNA replication and virus assembly and egress. J. Virol. 2012;86:11043–11056. doi: 10.1128/JVI.07101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrey JL, Lee YY, Au HH, Bushell M, Jan E. Host and viral translational mechanisms during cricket paralysis virus infection. J. Virol. 2010;84:1124–1138. doi: 10.1128/JVI.02006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Tan SL, Tareen SU, Vijaysri S, Langland JO, Jacobs BL, Katze MG. Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A. J. Virol. 2001;75:5090–5098. doi: 10.1128/JVI.75.11.5090-5098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Schade M, Lowenhaupt K, Alfken J, Schwartz T, Shlyakhtenko LS, Lyubchenko YL, Rich A. The Zalpha domain from human ADAR1 binds to the Z-DNA conformer of many different sequences. Nucleic Acids Res. 1998;26:3486–3493. doi: 10.1093/nar/26.15.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CY, Zhang YB, Huang GP, Zhang QY, Gui JF. Molecular cloning and characterisation of a fish PKR-like gene from cultured CAB cells induced by UV-inactivated virus. Fish Shellfish Immunol. 2004;17:353–366. doi: 10.1016/j.fsi.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Jan E, Sarnow P. Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus. J. Mol. Biol. 2002;324:889–902. doi: 10.1016/s0022-2836(02)01099-9. [DOI] [PubMed] [Google Scholar]
- Katze MG, Wambach M, Wong ML, Garfinkel M, Meurs E, Chong K, Williams BR, Hovanessian AG, Barber GN. Functional expression and RNA binding analysis of the interferon-induced, double-stranded RNA-activated, 68,000-Mr protein kinase in a cell-free system. Mol. Cell. Biol. 1991;11:5497–5505. doi: 10.1128/mcb.11.11.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ, Davies MV, Pathak VK, Hershey JW. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol. Cell. Biol. 1989;9:946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft JS. Viral IRES RNA structures and ribosome interactions. Trends Biochem. Sci. 2008;33:274–283. doi: 10.1016/j.tibs.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Lowenhaupt K, Oh DB, Kim KK, Rich A. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1514–1518. doi: 10.1073/pnas.0308260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Muralinath M, Brandt T, Pearcy M, Hauns K, Lowenhaupt K, Jacobs BL, Rich A. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar AA, Hatzoglou M. Internal ribosome entry sites in cellular mRNAs: mystery of their existence. J. Biol. Chem. 2005;280:23425–23428. doi: 10.1074/jbc.R400041200. [DOI] [PubMed] [Google Scholar]
- Kramer G. Two phosphorylation sites on eIF-2 alpha. FEBS Lett. 1990;267:181–182. doi: 10.1016/0014-5793(90)80919-a. [DOI] [PubMed] [Google Scholar]
- Lindquist ME, Mainou BA, Dermody TS, Crowe JE., Jr Activation of protein kinase R is required for induction of stress granules by respiratory syncytial virus but dispensable for viral replication. Virology. 2011;413:103–110. doi: 10.1016/j.virol.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Morley SJ, Traugh JA. Inhibition of cap-dependent translation via phosphorylation of eIF4G by protein kinase Pak2. Embo J. 2005;24:4094–4105. doi: 10.1038/sj.emboj.7600868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TK, Zhang YB, Liu Y, Sun F, Gui JF. Cooperative roles of fish protein kinase containing Z-DNA binding domains and double-stranded RNA-dependent protein kinase in interferon-mediated antiviral response. J. Virol. 2011;85:12769–12780. doi: 10.1128/JVI.05849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushnikov AY, Brown BA, Oussatcheva EA, 2nd, Potaman VN, Sinden RR, Lyubchenko YL. Interaction of the Zalpha domain of human ADAR1 with a negatively supercoiled plasmid visualized by atomic force microscopy. Nucleic Acids Res. 2004;32:4704–4712. doi: 10.1093/nar/gkh810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CS, Taghavi N, Samuel CE. Protein kinase PKR amplification of interferon beta induction occurs through initiation factor eIF-2alpha-mediated translational control. J. Biol. Chem. 2012;287:36384–36392. doi: 10.1074/jbc.M112.390039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CS, Toth AM, Zhang P, Devaux P, Cattaneo R, Samuel CE. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J. Virol. 2010;84:380–386. doi: 10.1128/JVI.02630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack SJ, Ortega LG, Doohan JP, Samuel CE. Mechanism of interferon action motif I of the interferon-induced, RNA-dependent protein kinase (PKR) is sufficient to mediate RNA-binding activity. Virology. 1994;198:92–99. doi: 10.1006/viro.1994.1011. [DOI] [PubMed] [Google Scholar]
- McCormack SJ, Thomis DC, Samuel CE. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase. Virology. 1992;188:47–56. doi: 10.1016/0042-6822(92)90733-6. [DOI] [PubMed] [Google Scholar]
- McKenna SA, Lindhout DA, Kim I, Liu CW, Gelev VM, Wagner G, Puglisi JD. Molecular framework for the activation of RNA-dependent protein kinase. J. Biol. Chem. 2007;282:11474–11486. doi: 10.1074/jbc.M700301200. [DOI] [PubMed] [Google Scholar]
- Nallagatla SR, Toroney R, Bevilacqua PC. Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr. Opin. Struct. Biol. 2011;21:119–127. doi: 10.1016/j.sbi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonski KM, Samuel CE. Stress Granule Formation Induced by Measles Virus Is Protein Kinase PKR Dependent and Impaired by RNA Adenosine Deaminase ADAR1. J. Virol. 2013;87:756–766. doi: 10.1128/JVI.02270-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Thomis DC, Hans SL, Samuel CE. Mechanism of interferon action: double-stranded RNA-specific adenosine deaminase from human cells is inducible by alpha and gamma interferons. Virology. 1995;210:508–511. doi: 10.1006/viro.1995.1370. [DOI] [PubMed] [Google Scholar]
- Pestova TV, Hellen CU. Translation elongation after assembly of ribosomes on the Cricket paralysis virus internal ribosomal entry site without initiation factors or initiator tRNA. Genes Dev. 2003;17:181–186. doi: 10.1101/gad.1040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller CK, Li Z, George CX, Samuel CE. Protein Kinase PKR and RNA Adenosine Deaminase ADAR1: New Roles for Old Players as Modulators of the Interferon Response. Curr. Opin. Immunol. 2011;23:573–582. doi: 10.1016/j.coi.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindel A, Sadler A. The role of protein kinase R in the interferon response. J. Interferon Cytokine Res. 2011;31:59–70. doi: 10.1089/jir.2010.0099. [DOI] [PubMed] [Google Scholar]
- Rothenburg S, Deigendesch N, Dey M, Dever TE, Tazi L. Double-stranded RNA-activated protein kinase PKR of fishes and amphibians: varying the number of double-stranded RNA binding domains and lineage-specific duplications. BMC Biol. 2008;6:12. doi: 10.1186/1741-7007-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenburg S, Deigendesch N, Dittmar K, Koch-Nolte F, Haag F, Lowenhaupt K, Rich A. A PKR-like eukaryotic initiation factor 2alpha kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1602–1607. doi: 10.1073/pnas.0408714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri A, Dazert E, Metz P, Hofmann S, Bergeest JP, Mazur J, Bankhead P, Hiet MS, Kallis S, Alvisi G, Samuel CE, Lohmann V, Kaderali L, Rohr K, Frese M, Stoecklin G, Bartenschlager R. Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe. 2012;12:71–85. doi: 10.1016/j.chom.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Mechanism of interferon action. Kinetics of interferon action in mouse L929 cells: phosphorylation of protein synthesis initiation factor elF-2 and ribosome-associated protein P1. Virology. 1979;93:281–285. doi: 10.1016/0042-6822(79)90300-3. [DOI] [PubMed] [Google Scholar]
- Samuel CE. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J. Biol. Chem. 1993;268:7603–7606. [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat. Struct. Biol. 2001;8:761–765. doi: 10.1038/nsb0901-761. [DOI] [PubMed] [Google Scholar]
- Shi J, Hershey JW, Nelson MA. Phosphorylation of the eukaryotic initiation factor 3f by cyclin-dependent kinase 11 during apoptosis. FEBS Lett. 2009;583:971–977. doi: 10.1016/j.febslet.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Schmechel SC, Raghavan A, Abelson M, Reilly C, Katze MG, Kaufman RJ, Bohjanen PR, Schiff LA. Reovirus induces and benefits from an integrated cellular stress response. J. Virol. 2006;80:2019–2033. doi: 10.1128/JVI.80.4.2019-2033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar A, Krishnamoorthy T, Jain A, Chatterjee U, Hasnain SE, Kaufman RJ, Ramaiah KV. Serine 48 in initiation factor 2 alpha (eIF2 alpha) is required for high-affinity interaction between eIF2 alpha(P) and eIF2B. Biochemistry. 1999;38:15398–15405. doi: 10.1021/bi991211n. [DOI] [PubMed] [Google Scholar]
- Taghavi N, Samuel CE. Protein kinase PKR catalytic activity is required for the PKR-dependent activation of mitogen-activated protein kinases and amplification of interferon beta induction following virus infection. Virology. 2012;427:208–216. doi: 10.1016/j.virol.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- Thomis DC, Samuel CE. Mechanism of interferon action: autoregulation of RNA-dependent P1/eIF-2 alpha protein kinase (PKR) expression in transfected mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10837–10841. doi: 10.1073/pnas.89.22.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomis DC, Samuel CE. Mechanism of interferon action: evidence for intermolecular autophosphorylation and autoactivation of the interferon-induced, RNA-dependent protein kinase PKR. J. Virol. 1993;67:7695–7700. doi: 10.1128/jvi.67.12.7695-7700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Devaux P, Cattaneo R, Samuel CE. Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C proteindeficient measles virus. J. Virol. 2009;83:961–968. doi: 10.1128/JVI.01669-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Zhang P, Das S, George CX, Samuel CE. Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase. Prog. Nucleic Acid Res. Mol. Biol. 2006;81:369–434. doi: 10.1016/S0079-6603(06)81010-X. [DOI] [PubMed] [Google Scholar]
- Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang Y, Samuel CE. Adenosine deaminase ADAR1 increases gene expression at the translational level by decreasing protein kinase PKR-dependent eIF-2alpha phosphorylation. J. Mol. Biol. 2009;393:777–787. doi: 10.1016/j.jmb.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- White JP, Lloyd RE. Regulation of stress granules in virus systems. Trends Microbiol. 2012;20:175–183. doi: 10.1016/j.tim.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR. Signal integration via PKR. Sci STKE 2001. 2001 doi: 10.1126/stke.2001.89.re2. re2. [DOI] [PubMed] [Google Scholar]
- Xu Z, Williams BR. The B56alpha regulatory subunit of protein phosphatase 2A is a target for regulation by double-stranded RNA-dependent protein kinase PKR. Mol. Cell. Biol. 2000;20:5285–5299. doi: 10.1128/mcb.20.14.5285-5299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Langland JO, Jacobs BL, Samuel CE. Protein kinase PKR-dependent activation of mitogen-activated protein kinases occurs through mitochondrial adapter IPS-1 and is antagonized by vaccinia virus E3L. J. Virol. 2009;83:5718–5725. doi: 10.1128/JVI.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Samuel CE. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J. Virol. 2007;81:8192–8200. doi: 10.1128/JVI.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]