Abstract
Bioactive and biodegradable hydrogels that mimic the extracellular matrix and regulate valve interstitial cells (VIC) behavior are of great interest as three dimensional (3D) model systems for understanding mechanisms of valvular heart disease pathogenesis in vitro and the basis for regenerative templates for tissue engineering. However, the role of stiffness and adhesivity of hydrogels in VIC behavior remains poorly understood. This study reports synthesis of oxidized and methacrylated hyaluronic acid (Me-HA and MOHA) and subsequent development of hybrid hydrogels based on modified HA and methacrylated gelatin (Me-Gel) for VIC encapsulation. The mechanical stiffness and swelling ratio of the hydrogels were tunable with molecular weight of HA and concentration/composition of precursor solution. The encapsulated VIC in pure HA hydrogels with lower mechanical stiffness showed more spreading morphology comparing to stiffer counterparts and dramatically upregulated alpha smooth muscle actin expression indicating more activated myofibroblast properties. The addition of Me-Gel in Me-HA facilitated cell spreading, proliferation and VIC migration from encapsulated spheroids and better maintained VIC fibroblastic phenotype. The VIC phenotype transition during migration from encapsulated spheroids in both Me-HA and Me-HA/Me-Gel hydrogel matrix was also observed. These findings are important for the rational design of hydrogels for controlling VIC morphology, and for regulating VIC phenotype and function. The Me-HA/Me-Gel hybrid hydrogels accommodated with VIC are promising as valve tissue engineering scaffolds and 3D model for understanding valvular pathobiology.
Keywords: Stiffness, cell spreading, microenvironment, tissue engineering, phenotype
1. Introduction
The physiochemical similarity to the native extracellular matrix (ECM) and permeability to nutrients make hydrophilic hydrogels advantageous for tissue engineering applications [1, 2]. By carefully selecting a hydrogel material, crosslinking method, and conjugatable biomolecules, hydrogels can support two dimensional (2D) surface-seeded cell culture and three dimensional (3D) cell encapsulation as well [3, 4]. Numerous efforts are being made to investigate the role of 3D microenvironments on cell behavior, especially the effects of stiffness, matrix components, and chemical stimuli [5, 6].
Among various biodegradable synthetic and natural polymers, hyaluronic acid (HA), a glycosaminoglycan (GAG) component richly present in connective tissues, has been reported to mediate its activity in cell signaling [7], cell migration [8], wound repair [9], and morphogenesis [10]. HA based hydrogels are thus widely used as wound healing dressing, tissue engineering scaffolds, and cell/molecule delivery carriers [11, 12]. The mechanical properties of hyaluronic acid based hydrogels are tunable by varying crosslinking degree [13–16], photoinitiator concentration [17], or crosslinker concentration [18, 19]. The stiffness of HA based hydrogels can regulate phenotypic changes and differentiation of human hepatic stem cells [18], mesenchymal stem cells (MSC) [15] and neural progenitor cells [13]. With combination of matrix metalloproteinase (MMP) degradable crosslinkers and arginine-glycine-aspartic acid (RGD) peptide, HA based hydrogels can modulate the spreading and migration of mouse MSC [20].
Valve interstitial cells (VIC) are the major cell type found in heart valve leaflets and play a critical role in maintaining homeostasis and driving tissue remodeling within the dynamic environment of the valve [21]. VIC are a heterogeneous population with up to five distinct phenotypes [22]. The predominant VIC phenotype in healthy adult valves is quiescent fibroblasts, with only 2–5% expressing myofibroblast markers [23]. VIC are activated and show myofibroblastic phenotype with more alpha smooth muscle actin (aSMA) expression during remodeling and disease states [24, 25]. Prolonged activation of this myofibroblast phenotype may result in pathological heart valve matrix remodeling, formation of calcific nodules on the valvular leaflets and further stenosis of heart valve [23, 26, 27]. The characterization of VIC in response to substrate stiffness in 2D culture has been widely investigated [28–32]. For 3D culture, VIC are usually encapsulated in collagen hydrogels [33, 34] and poly(ethylene glycol) (PEG) based hydrogels [35, 36], which are model biomaterials rather than serving for tissue engineering purpose. Collagen hydrogels can well support the spreading and proliferation of VIC, but they are easily contracted and are mechanically inadequate [37]. PEG hydrogels are considered as blank slate and can control the cell fate through co-polymerization with other macromolecules and multiple functional moieties [38], but PEG are unable to totally degrade and be remodeled. VIC in 3D methacrylated hyaluronic acid (Me-HA) based hydrogels had very limited spreading morphology without incorporation of any cell adhesion motif [39], and the encapsulated VIC may have different phenotype from native tissue. Although VIC were able to achieve their native morphology within methacrylated gelatin (Me-Gel) hydrogels [40], the role of hydrogel stiffness on VIC behavior in 3D environment remains unclear. In addition, hybrid hydrogels such as collagen and glycosaminoglycan (GAG) are better ECM analog to investigate the role of 3D microenvironment on VIC physiological and pathological phenotypes [41]. It is thus important to develop appropriate 3D hydrogels with biochemical and biophysical tunability for mimicking ECM to better regulate VIC activity and for living valve replacements as well.
In this study, we developed photocrosslinked hydrogels formed from oxidized and methacrylated HA with different molecular weight and tunable stiffness. Combining the modified HAs with Me-Gel, we assessed the roles of stiffness and adhesion on VIC proliferation, spreading, and phenotype. Furthermore, we studied the migration rate and phenotype of VIC from encapsulated VIC spheroids in HA based hydrogels as well as in hybrid hydrogels.
2. Materials and Methods
2.1 Modification of HA and gelatin
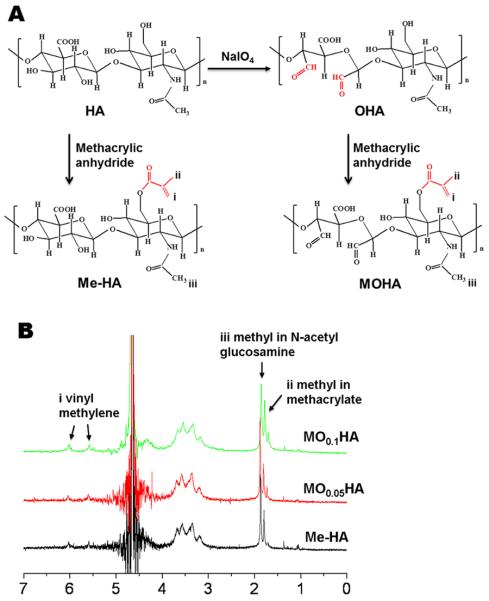
Oxidized HA (OHA) with different molecular weight was first prepared by reacting HA (Novozymes) with sodium periodate (NaIO4, Sigma). Briefly, 10 ml sodium periodate aqueous solution (0.05 mol/ml or 0.1 mol/ml) was added into HA solution (0.5 g in 100 ml distilled water) with continuous stirring in the dark at room temperature. The reaction was stopped after 10 min by the addition of ethylene glycol (Sigma; molar ratio of ethylene glycol:sodium periodate=1:1). The OHA was purified by dialysis (MWCO 1000; Spectra/por, Spectrum) against distilled water for 3 days. Then the methacrylated HA and OHA (Me-HA and MOHA) were synthesized by the addition of a methacrylate functional group to the HA as described by Smeds et al and Brigam et al [42, 43]. Briefly, 10 ml methacrylic anhydride (MA, Sigma) was reacted with HA (or obtained OHA) solution at 40 °C for 6 h and the pH of mixture during reaction was maintained at 8.5 by adding 5N NaOH. The Me-HA and MOHA solution was dialyzed for 3 days and lyophilized.
The molecular weight and polydispersity of HA and various modified HA were determined by a Waters gel-permeation chromatography (GPC) system equipped with three Ultrahydrogel columns (i.e. 2000, 500 and 250 Å in series, Waters) in series, a 1515 isocratic HPLC pump (Waters), and a 2414 refractive index detector (Waters). The mobile phase employed was 0.1 M phosphate buffer saline (PBS, pH=7.4) with 0.125% sodium azide at a rate of 0.8 ml/min calibrated with eight individual pullulan standards. Degree of methacrylation was determined using 1H-nuclear magnetic resonance (1H NMR) spectroscopy. Lyophilized modified HA were dissolved in D2O at a concentration of 10 mg/ml at 40 °C and spectra were recorded on a Bruker ARX-300 spectrometer using tetramethylsilane (TMS) as internal standard. The degree of methacrylation was calculated as the ratio of the relative peak integration of the double bond protons in methacrylate groups (peaks at ~5.6 and ~6.1 ppm) to the methyl protons of HA (peak at ~1.9 ppm) normalized according to the number of protons per group.
Me-Gel was synthesized as previously described [44]. Briefly, type A porcine skin gelatin (Sigma) was dissolved at 10% (w/v) into distilled water at 40 °C and then MA (1:5 v/v to gelatin solution) was added drop by drop under stirred conditions at 40 °C for 1 h. The solution was dialyzed at 40 °C to remove methacrylic acid and lyophilized for 3 days.
2.2 Preparation and characterization of HA based hydrogels
Photocrosslinkable HA based polymers (Me-HA or MOHA, 0.75% w/v) with or without Me-Gel (1% w/v) were dissolved in cell culture medium with 0.03% w/v 2-hydroxy-1(4-(hydroxyethox)pheny)-2-methyl-1-propanone (Irgacure 2959; BASF The Chemical Company). The gel precursor solution was transferred into molds and subsequently exposed to 365 nm UV light (EN-280L, Spectroline, 2.0 mW/cm2) for 5 min. Six types of hydrogels were fabricated by using methacrylated HA with varying molecular weight (MO0.1HA, MO0.05HA and Me-HA) with addition of Me-Gel, and were denoted as MO0.1HA, MO0.05HA, Me-HA, MO0.1HA/Me-Gel, MO0.05HA/Me-Gel and Me-HA/Me-Gel.
Uniaxial compressive test of different hydrogels (8 mm in diameter, 2 mm in thickness, n=4–6) were performed using an ELF 3200 (Bose EnduraTEC) mechanical test-frame. A 250 g load cell (Sensotec) was attached to the bottom plate and a displacement sensor to the top plate with the cross-head speed of 0.01 mm/s. The bulk compressive moduli were calculated from the slope of the initial linear region (5%–10% strain for compressive test) of the respective stress-strain curves.
For mass swelling ratio measurement, different hydrogel samples were dried and weighed (weight Wd) and re-immersed in DMEM at 37 °C for 48 h. The weight of the samples (n=5) in the swollen state (Ws) was measured. The mass swelling ratio (Q) was calculated by Q=(Ws-Wd)/Wd.
2.3 Cell culture and cell encapsulation in 3D hydrogels
Porcine aortic valve interstitial cells (VIC) were isolated from porcine aortic valves obtained at a slaughterhouse (Shirk Meats, Himrod NY) using collagenase digestion of the leaflets as previously described [37]. Cells were used at passages 5–8.
VIC were encapsulated in the 3D hydrogels using two different strategies: homogeneous encapsulation with individual cells throughout the hydrogel, and spheroid encapsulation with a single cluster of cells inside the hydrogel. The hydrogels containing homogeneously encapsulated cells were used to study VIC spreading and phenotype while the encapsulated VIC spheroids were used to study cell migration. For the homogeneous encapsulation, VIC were resuspended in different precursor solutions (polymer plus culture medium with Irgacure at concentration described previously) at the density of 5×106 cells/ml and then 50 μl of cell encapsulated precursor solution was transferred into molds and subject to photopolymerization with UV light for 5 min. VIC spheroids were prepared by the hanging drop method. Briefly, 20 μl cell resuspension with the density of 5×106 cells/ml was deposited onto the lid of 48-well plate and cultured at 37 °C in a cell culture incubator overnight. For VIC spheroid encapsulation, a poly(ethylene glycol) diacrylate (PEGDA, Mw 3350) layer was first prepared from 30 μl precursor solution (with 0.03% w/v Irgacure) to ensure that cells would not migrate out of the gel and grow onto the dish. Then 50 μl precursor solutions were transferred onto PEGDA base gels and VIC spheroids were incorporated into the HA gels by placing them inside the precursor solutions. The hydrogels with encapsulated VIC spheroids were photopolymerized as described above. The cell-hydrogel constructs were washed with PBS and cultured in 6 well culture plates. The medium was changed after 24 h culture and then refreshed every two days. At the predetermined time points, spheroid laden hydrogels were imaged with an inverted microscope to record the phase pictures for quantifying cell migration out of spheroid within the hydrogels. The area of migrated cells from encapsulated spheroid was measured and averaged using Image J.
2.5 Cell viability and proliferation
The viability of encapsulated cells was determined using Live/Dead cell viability assays (Invitrogen) and confocal microscopy (CLSM, LSM 710, Carl Zeiss) as previously described [45]. Cell viability was measured via counting live (green) and dead (red) cells and average cell circularity was calculated based on Live/Dead images (at least six images were analyzed for each hydrogel sample) using ImageJ [45]. Cell proliferation was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays [46].
2.6 Sulfated GAG assay
Dimethylmethylene blue (DMMB) assay was performed to measure the sulfated GAG production in the hydrogels [47, 48]. The constructs (n=4–5) were digested with 300 μg/ml papain in 50 mM phosphate buffer (pH 6.5), containing 5 mM cysteine and 5 mM EDTA for 16 h at 60 °C. GAG concentration was calculated by calibrating against a standard curve obtained with shark chondroitin sulfate (Sigma). To assess the biosynthetic activity of the cells, results of GAG were expressed as the ratio of GAG amount to DNA amount which was assessed via the PicoGreen double strand DNA assay (Invitrogen).
2.7 Immunohistochemistry
Immunohistochemistry was performed to stain for α-smooth muscle actin (αSMA) and vimentin using monoclonal anti-αSMA-Cy3 antibody (1:200, Sigma) and mouse monoclonal anti-vimentin primary antibody (Invitrogen), respectively. Secondary fluorescent antibodies and nuclear counterstaining (via Draq 5, 1: 1000, Biostatus) were performed for 30 minutes at room temperature, and samples were imaged with Zeiss 710 CLSM. To visualize actin cytoskeletal filaments of encapsulated VIC spheroids, the hydrogels were stained by Alexa Fluor 488 conjugated phalloidin (1:40, Invitrogen), and the images were captured using a Zeiss Discovery v20 stereo microscope (Spectra Services, Inc.).
2.8 RNA isolation and quantitative real time polymerase chain reaction (PCR)
Total RNA was extracted from cell-laden hydrogels after 14 day culture using QIA-Shredder and RNeasy mini-kits (QIAgen) according to the manufactures' instructions. Thirty nanograms of total RNA was synthesized into first strand cDNA in a 20 μL reaction using iScript cDNA synthesis kit (BioRad Laboratories). Real-time PCR analysis was performed in a CFX Connect Real-Time PCR detection system (Bio-Rad) using SsoAdvanced SYBR Green Supermix (Bio-Rad). All primers used in this study are listed in Supplementary Table S1. cDNA samples were analyzed for gene of interest and for the housekeeping gene 18S rRNA. The level of expression of each target gene was calculated using comparative Ct method (also known as 2 −ΔΔCt method).
2.9 Statistical analysis
All quantitative data are expressed as the mean±standard deviation (SD). Statistical analysis was performed using ANOVA with Scheffé post-hoc tests. A value of p<0.05 was considered to be statistically significant.
3. Results
3.1 Tunable mechanical properties and swelling ratio of HA based hydrogels
In order to obtain HA with different molecular weight, sodium periodate aqueous solution was first used to oxidize HA. As listed in Table 1, the molecular weight of oxidized HA, i.e. O0.05HA and O0.1HA, decreased with increasing the sodium periodate concentration from 0.05 mol/ml to 0.1 mol/ml. Then photocrosslinkable macromers were prepared by the methacrylation of HA or OHA. The whole modification process (including both oxidization and methacrylation) is shown in Fig. 1A. The 1H NMR spectra (Fig. 1B) confirmed the methacrylation of HA and OHA showing distinctive peaks in the double bond region (~5.6 ppm and ~6.1 ppm) and the peak that corresponds to -CH3 group of the methacrylate group (~1.85 ppm) on the methacrylated HA (OHA) spectra. The methacrylation process slightly decreased the molecular weight of HA and OHA (Table 1). Degree of methacrylation for Me-HA was calculated to be 22.5% and slightly higher degrees of methacrylation of 29.0% and 30.7% for MO0.05HA and MO0.1HA, respectively, were obtained.
Table 1.
Molecular weight and degree of methacrylation of HA and modified HA
| Code | Number average molecular weight (Da) | Polydiversity | Degree of methacrylation (%) |
|---|---|---|---|
| HA | 1,236,388 | 1.77 | |
| O0.05HA | 787,099 | 1.87 | |
| O0.1HA | 564,866 | 1.76 | |
| Me-HA | 1,168,916 | 1.66 | 22.5 |
| MO0.05HA | 765,549 | 1.87 | 29.0 |
| MO0.1HA | 539,989 | 1.85 | 30.7 |
Fig. 1.
(A) Schematic illustration of the synthesis of Me-HA and MOHA. (B) 1H NMR spectra of synthesized Me-HA and MOHA in D2O. (i) Vinyl groups of the Me-HA and MOHA identified at ~5.6 and 6.1 ppm; (ii) methyl groups located at ~1.85 ppm; (iii) methyl in N-acetyl glucosamine found at ~1.95 ppm.
Photocrosslinked Me-HA and MOHA hydrogels were prepared with or without Me-Gel in the presence of Irgacure. Bulk compressive properties of HA based hydrogels were measured (Fig. 2). Strength versus strain curves for different investigated networks are shown in Fig. 2A. Generally, the modulus correlates with the molecular weight of HA. With decreasing the HA molecular weight by oxidization, the stiffness of HA based hydrogels increased. Significantly higher compressive modulus was seen for MO0.1HA hydrogels (2.74±0.20 kPa) compared to Me-HA hydrogels (1.86±0.20 kPa, p<0.05). As the macromer concentration increased with the addition of Me-Gel, the resulting moduli for all the hydrogels statistically increased (p<0.01). The hybrid hydrogels with lowest HA molecular weight (MO0.1HA/Me-Gel) showed substantially higher stiffness (5.19±1.00 kPa) than Me-HA/Me-Gel (3.93±0.11 kPa, p<0.05).
Fig. 2.
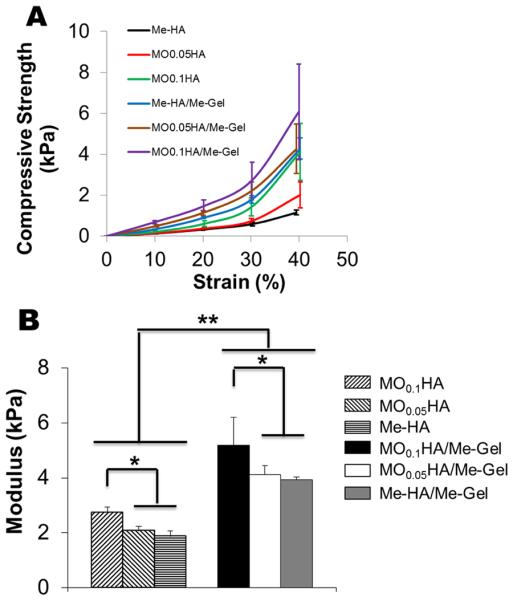
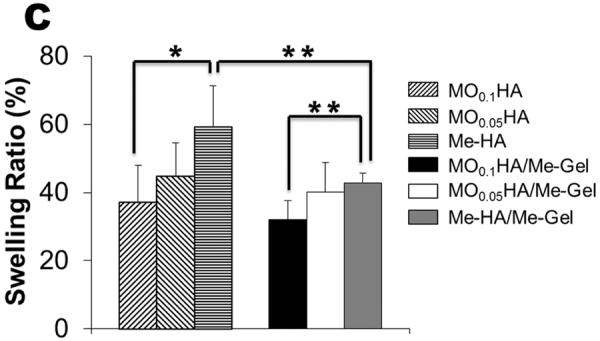
Tunable properties of different hydrogels. (A) Typical compressive strength-strain curves; (B) modulus; (C) swelling ratio. (*p<0.05, **p<0.01; n=4–6 for compressive test; n=5 for swelling ratio)
The swelling ratio of HA based hydrogels in vitro was assessed by immersing the hydrogels in DMEM. All the HA based hydrogels reached equilibrium swelling within 2 days and the equilibrium mass swelling ratio for different hydrogels was shown in Fig. 2C. For Me-HA and MOHA alone, the swelling ratio increased with decreasing the stiffness which was caused by increasing the HA molecular weight. We observed that the hydrogels with the lowest stiffness exhibited the highest swelling ratio (Q=59.2±12.2%). With addition of Me-Gel, the hybrid hydrogels showed decreasing swelling ratio comparing to pure HA hydrogels, indicating increased levels of crosslinking. Similarly, the equilibrated swelling ratio of Me-HA/Me-Gel was significantly higher than that of MO0.1HA/Me-Gel (p<0.01).
3.2 Effects of stiffness and addition of Me-Gel on morphology and proliferation of encapsulated VIC
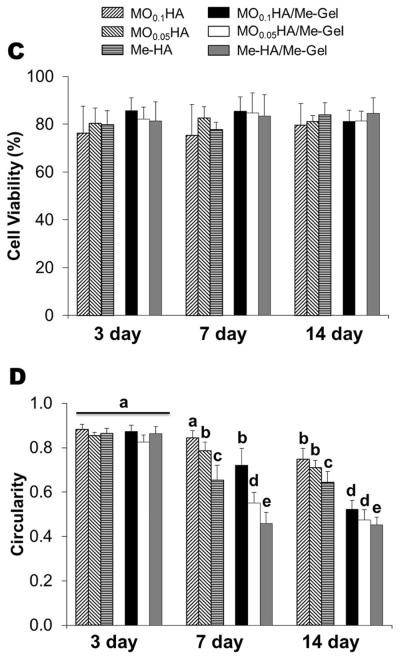
Fig. 3A and B show that the encapsulated VIC in different HA hydrogels with and without Me-Gel were alive (green) at different time points. Fig. 3C and D summarizes the overall encapsulated cell viability and cell circularity in different hydrogels. Generally, the cell viability was greater than 75% for all the hydrogels throughout 14 day culture and the addition of Me-Gel slightly increased cell viability without significant difference. Most of encapsulated VIC cells remained spherical after 3 day culture and more cells showed spreading morphology in the hydrogels with increasing the culture time. At day 7 and 14, the circularity for VIC in Me-HA hydrogel with low stiffness was significantly lower than that in stiffer HA hydrogels (MO0.1HA and MO0.05HA) as shown in Fig. 3A and D. However, the encapsulated VIC in Me-HA hydrogels still showed limited spreading morphology with some protruding filopodia (Fig. 3A). Extensive VIC spreading was found only in the hydrogels with addition of Me-Gel. Similarly, the stiffer Me-Gel based hydrogels (MO0.1HA/Me-Gel) lagged cell spreading and exhibited significantly higher cell circularity than softer hydrogels (MO0.05HA/Me-Gel and Me-HA/Me-Gel). Majority of VIC in Me-HA/Me-Gel hydrogels spread and showed spindle-like morphology (Fig. 3B).
Fig. 3.
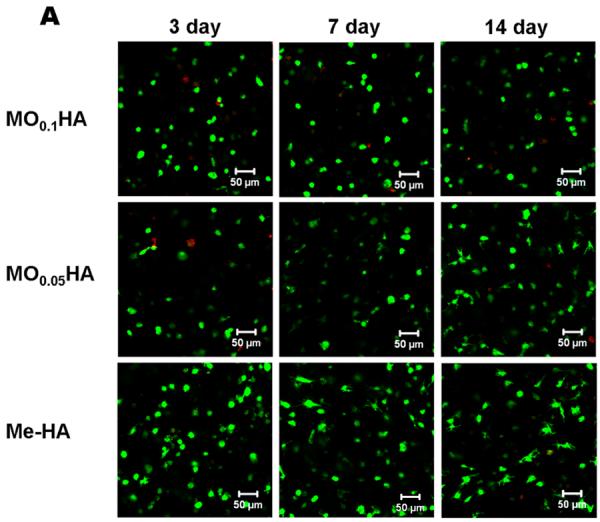
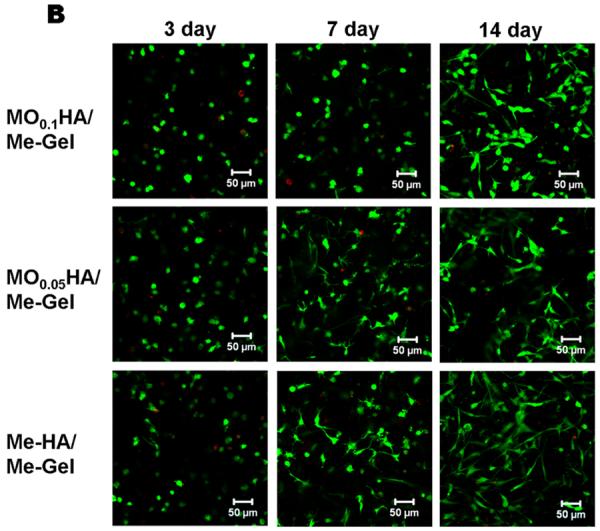
Live/Dead assay for encapsulated VIC within different hydrogels. (A) pure HA based hydrogels; (B) HA based hydrogels with addition of Me-Gel (3day, left column; 7days, middle column; 14days, right column); (C) cell viability measured based on Live/Dead images (n=6); (D) cell circularity measured based on Live/Dead images (n=6, bars that do not share letters are significantly different from each other).
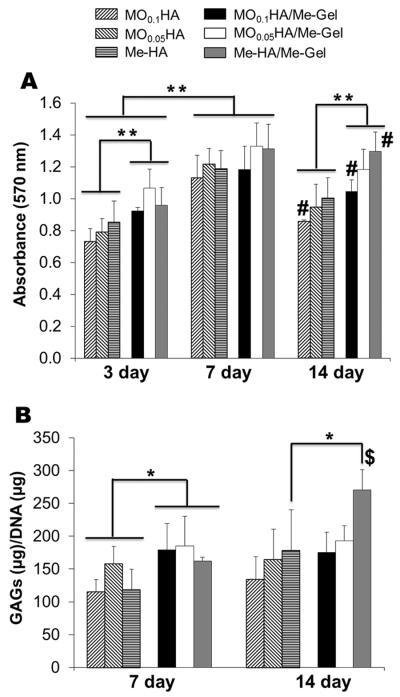
To quantify the proliferation of VIC within the 3D hydrogels, cell viability during 14 day culture was measured using MTT assay for different hydrogels. As shown in Fig. 4A, VIC in all the hydrogels proliferated during 14-day study period, with significant increase in MTT absorbance (p<0.01 for day 7 and day 14). The encapsulated cells proliferated rapidly during the first week and then the viability plateaued or slightly declined somewhat thereafter. During the first 7 day there was no significant difference in proliferation rate for HA hydrogels with different stiffness, and at day 14 the MTT absorption for softer hydrogels (MO0.05HA, Me-HA for pure HA hydrogels and MO0.05HA/Me-Gel, Me-HA/Me-Gel for Me-Gel based hydrogels) was significantly higher than that of stiffer hydrogels (MO0.1HA for pure HA hydrogels and MO0.1HA/Me-Gel for Me-Gel based hydrogels). When comparing pure HA hydrogels with Me-Gel based hydrogels, the addition of Me-Gel remarkably increased cell proliferation through the 14-day cell culture period (p<0.05 for day 3; p<0.01 for day 7 and 14).
Fig. 4.
Encapsulated VIC proliferation and GAG deposition within different hydrogels. (A) MTT assay showed the proliferative capacity of VIC encapsulated within different hydrogels (n=5, **p< 0.01, # indicated significantly difference between 3-day and 14-day culture); (B) GAG content detected by DMMB-based assay and normalized to DNA content (n=4–5, *p< 0.05, $ indicated significantly difference between 7-day and 14-day culture).
The level of sulfated GAG produced by encapsulated VIC in different hydrogels after 7 and 14 day culture was quantitatively determined by modified DMMB assay. The GAG content of Me-Gel based hydrogels was measured to be significantly higher on day 7 (p<0.05) compared with that of HA hydrogels without Me-Gel (Fig. 4B). However, only Me-HA/Me-Gel showed significant increase of GAG content after 14 day culture, and it remained unchanged for other hydrogels from day 7 to day 14.
3.3 VIC phenotypes in response to hydrogel stiffness and adhesivity
As shown in Fig. 5, the encapsulated VIC express both αSMA and vimentin within all the hydrogel samples after 14 day culture. Again, the encapsulated VIC were more extensively spread in compliant hydrogels and Me-Gel based hydrogels. The immunohistochemical imaging results were complemented by quantitative analysis of gene expression using real-time PCR, normalized to 18S, and expressed relative to MO0.1HA. After 14 day culture, αSMA, periostin and hyaluronidase I gene expression was dramatically increased with decreasing stiffness of different HA hydrogels (p<0.01) (Fig. 6A, C and D). The upregulation of hyaluronidase I could explain the cell spreading morphology within Me-HA hydrogels. Vimentin expression was significantly upregulated in Me-HA hydrogels compared to MO0.1HA (p<0.05), but no significant difference was observed between MO0.05HA and MO0.1HA hydrogels (Fig. 6B). The addition of Me-Gel did not affect αSMA expression (1.63±0.62 fold for MO0.1HA/Me-Gel, 1.08±0.07 fold for MO0.05HA/Me-Gel and 1.53±0.48 fold for Me-HA/Me-Gel, no difference), but elevated vimentin expression by about 5-fold for Me-HA/Me-Gel. This indicates that extensive cell spreading in 3D culture could better maintain a quiescent VIC fibroblastic phenotype. The expression of periostin and hyaluronidase I showed an increasing trend with decreasing stiffness of Me-Gel based hydrogels, but was downregulated comparing to their HA counterparts.
Fig. 5.
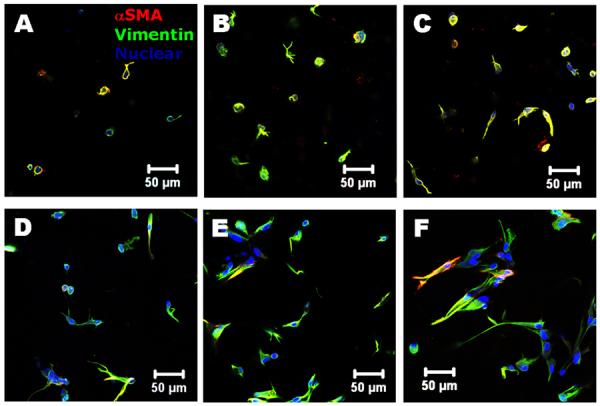
Immunohistochemical staining for encapsulated VIC after 14 day culture. (A) MO0.1HA; (B) MO0.05HA; (C) Me-HA; (D) MO0.1HA/Me-Gel; (E) MO0.05HA/Me-Gel; (F) Me-HA/Me-Gel; aSMA (red); vimentin (green) and nuclei (blue).
Fig. 6.
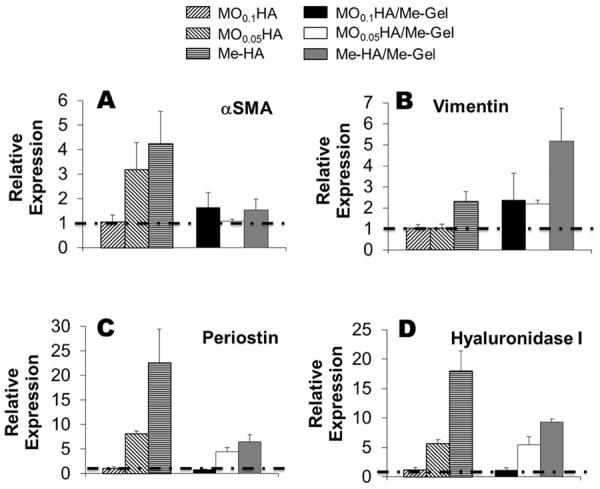
Real-time PCR analysis of (A) αSMA; (B) vimentin; (C) periostin and (D) hyaluronidase I of VIC encapsulated in different hydrogels after 14-day culture. Relative gene expression is presented as normalized to 18S and expressed relative to MO0.1HA.
3.4 Migration of VIC from spheroids within hydrogels
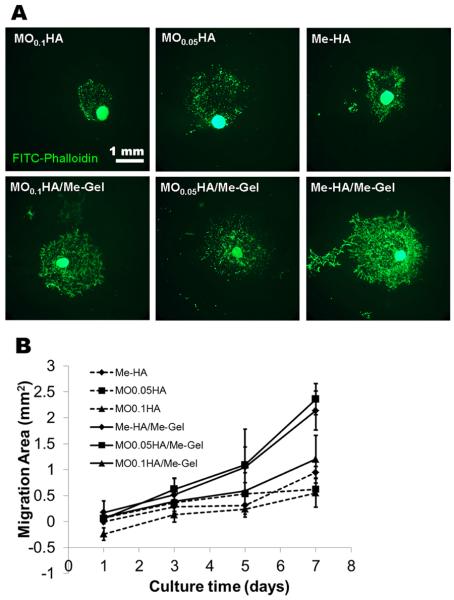
To test whether the hydrogels are capable of supporting VIC migration and whether the migration is sensitive to hydrogel stiffness and composition, VIC spheroids were encapsulated within different hydrogels. While VIC started to migrate from the spheroids in all the hydrogels after 1 day culture, there were clear differences in terms of migration kinetics and the morphology of migrated VIC between different hydrogels. The migrated VIC showed less spreading morphology in HA based hydrogels without Me-Gel, while they showed extensive cell spreading with faster migration rate in the HA hydrogels with Me-Gel (Fig. 7A). However, the stiffness of hydrogels did not significantly affect the migration starting time and rate (Fig. 7B). The αSMA and vimentin expression of migrated VIC and VIC in spheroids encapsulated in both Me-HA and Me-HA/Me-Gel hydrogels was observed using immunohistochemical staining. As shown in Fig. 8A and B, αSMA was strongly expressed in VIC inside spheroid encapsulated within Me-HA hydrogels, while the migrated VIC showed more vimentin (Fig. 8C), indicating the phenotype transformation during migration process. In contrast, VIC inside spheroid within Me-HA/Me-Gel hydrogels expressed more vimentin and less αSMA, but VIC showed more αSMA expression during migration process (Fig. 8D and E). After migration, αSMA expression was down regulated and the migrated VIC showed extensive spreading morphology (Fig. 8F).
Fig. 7.
VIC migration from encapsulated spheroid within different hydrogels after 7-day culture. (A) Representative images of phalloidin staining for F-actin (green); (B) migration kinetic curve.
Fig. 8.
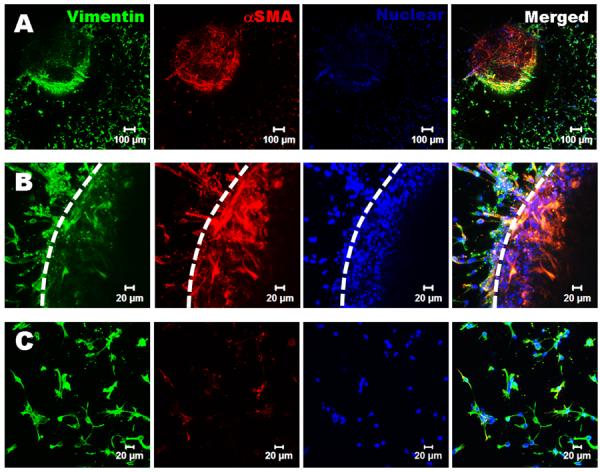
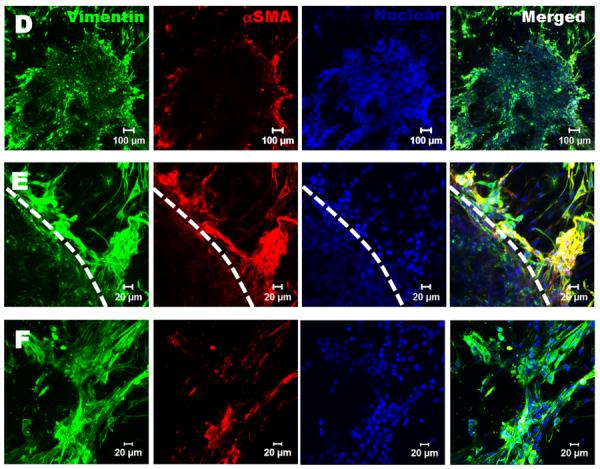
Immunohistochemical staining for encapsulated VIC spheroids within Me-HA and Me-HA/Me-Gel after 7 day culture. Vimentin (green), αSMA (red) and nuclei (blue). (A) The VIC in spheroid within Me-HA hydrogels expressed more αSMA, while the migrated VIC expressed less αSMA and showed less spreading morphology; (B) closed view of (A), dash line indicates the boundary of spheroid; (C) the migrated VIC within Me-HA expressed less αSMA; (D) the VIC within Me-HA/Me-Gel hydrogels expressed more vimentin inside the spheroid and showed more αSMA expression during migration; (E) closed view of (B), dash line indicates the boundary of spheroid; (F) after migration within Me-HA/Me-Gel hydrogels, the expression of αSMA was down regulated and migrated VIC showed extensive spreading morphology.
4. Discussion
It is of great importance to establish a 3D model to study the functional and dysfunctional behavior of VIC in vitro, since conventional 2D culture of VIC on the stiff culture plate stimulates pathological phenotype [33]. Bioactive hydrogels can provide an appropriate 3D microenvironment that mimics ECM and regulates VIC behavior. In addition, bioactive hydrogels are also appealing for use as tissue engineering heart valve (TEHV) scaffolds in layering method because they have tunable structure and mechanics and can mimic the layered microstructure of the valve leaflet [49]. The aim of this study was to investigate the ability of hydrogels based on modified HA and gelatin to support VIC spreading and proliferation and to investigate the effect of the 3D hydrogel environment on VIC phenotype and migration.
First, HA with relatively high molecular weight was partially degraded by sodium peroxidate, which is well known for its role as an oxidant, cleaving the C2–C3 hydroxyl groups of vicinal diol to form dialdehyde groups [50, 51]. By varying the concentration of oxidant and oxidization time, the molecular weight of oxidized HA was totally controllable. With further methacrylation, the modified HA with different molecular weight was photocrosslinkable with presence of photoinitiator (such as Irgacure 2959). The physical properties of photocrosslinked HA hydrogels (MO0.1HA, MO0.05HA and Me-HA), including the mechanical properties, swell ratio, and degradation rate are tunable with molecular weight. Similar results were also reported by another study that used HA with different molecular weights to control the properties of hydrogel networks for cartilage tissue engineering [52]. The hybrid hydrogels consisting of modified HA and Me-Gel had similar trend of increasing/decreasing properties with decreasing HA molecular weight and showed higher stiffness and lower swell ratio compared to HA hydrogels alone.
Cell spreading inside hydrogels required that the encapsulated cells be capable of interacting with the hydrogel matrix and displacing the polymer chains to make more space for their movement [53]. The stiffness of hydrogels is known to dictate local cell behavior, and plays an important role through mechanotransduction during cell spreading and differentiation [54]. HA based hydrogels were completely degradable by hyaluronidase (Supplementary Figure S1) in a dose and stiffness dependent manner. The degradation rate can affect cellular remodeling and cell proliferation, which are essential for the success of the tissue engineered valve conduits. In 2D culture, the effects of modulus of substrates on VIC behavior have been extensively studied. VIC cultured on soft polyacrylamide (PA) gels (with storage modulus of 0.3 kPa) exhibit a small rounded morphology, significantly smaller and less spread than those on stiff substrates [32]. On high stiffness substrates (>15 kPa), VIC had a higher population of myofibroblast-like cells with elevated αSMA expression [28, 30]. Myofibroblastic VIC can be de-activated to fibroblasts in situ by decreasing the tissue modulus of photodegradable hydrogels from ~32 kPa to ~7 kPa using UV light [28, 31]. External forces and internal mechanical rigidity both influence VIC phenotype in a manner somewhat dependent on age and anatomic region [29]. In the current study, the stiffness of HA based hydrogels varied from 1.86±0.20 kPa (Me-HA) to 2.74±0.20 kPa (MO0.1HA) and it increased to 3.93±0.11 kPa and 5.19±1.00 kPa with addition of Me-Gel correspondingly. The stiffness of hydrogels is comparable to modulus of ventricularis (ranging from around 0.25 to 3 kPa) and fibrosa (ranging from around 0.5 to 5 kPa) layers in porcine aortic heart valves measured by micropipette aspiration method [55]. Comparing to compliant hydrogels (Me-HA and Me-HA/Me-Gel), stiffer hydrogels (MO0.1HA and MO0.1HA/Me-Gel) had correspondingly denser networks and delayed the cell spreading process. This relationship between hydrogel mechanics and cell spreading agrees with previous reports that used smooth muscle cells in dextran/gelatin hydrogels [56] or mesenchymal stem cells in HA based hydrogels [20]. Different from 2D culture (including both plastic culture plate and 3D surface culture), these results show that VIC spreading is very sensitive to hydrogel mechanics. However, Live/Dead staining and proliferation assays demonstrated that the encapsulated cells maintained similar viability and were able to proliferate. The encapsulated VIC in Me-HA hydrogels showed certain spreading morphology, indicating the interaction between VIC and HA matrix. In contrast, the addition of Me-Gel promoted extensive VIC spreading and proliferation likely due to the increased cell adhesion supported by integrin binding sites. The spreading VIC showed higher proliferation rate and expressed more GAGs during first 7 day culture. For VIC spheroid encapsulation, VIC gradually migrated out from the spheroid within all the hydrogel samples and the hydrogel stiffness of current range did not significantly affect the VIC migration rate. The migrated VIC showed more extensive cell spreading morphology with faster migration rate in the HA hydrogels with Me-Gel. However, the migration process is dependent on cell type and hydrogel matrix properties. For example, we conducted a chick aortic ring outgrowth assay to test the migration of endothelial cells (Supplementary Figure S3). No detectable (for MO0.1HA and MO0.05HA) or very limited (for Me-HA) angiogenic sprouting was observed for HA based hydrogels. Similarly, no cell migration was observed in a fibrin/mouse mesenchymal stem cells clot encapsulated in HA hydrogel without RGD peptide [20]. These results support that hyaluronidases released by VIC could degrade the HA matrix and promote cell migration.
For the VIC phenotypes, expression of αSMA is the primary indicator of activation of VIC from fibroblastic to myofibroblastic phenotype [23, 37]. In their quiescent state, VIC have a fibroblast phenotype, characterized by the presence of vimentin and very low αSMA expression [22]. Periostin is a marker for mature valve cell phenotype and plays distinct roles in different valve layers, impacting different aspects of calcific aortic valve disease [33, 57]. Compliant Me-HA hydrogels promoted all the targeted gene expression (i.e. αSMA, vimentin, periostin and hyaluronidase I) with dramatic stimulation of αSMA expression, indicating more activated myofibroblast properties. These results demonstrate that hydrogel stiffness in 2D culture and 3D culture has different effects on VIC phenotypes [58]. The hydrogel stiffness for fibroblast-myofibroblast transition of VIC in 3D culture is lower than that presented in 2D culture (4.80–9.60 kPa [30] or ~7 kPa [28]). The addition of Me-Gel promoted VIC spreading and maintained VIC quiescent phenotype. Durst et al. reported that majority of VIC encapsulated within PEGDA hydrogels were positive for αSMA [36], and this is probably due to the lack of cell spreading within the hydrogels.
VIC are induced to migrate and differentiate into myofibroblasts in response to valve injury [59]. The activation of VIC is an important feature of the wound repair process and is associated with expression of MMPs and repairing and replacing damaged ECM [22]. The myofibroblasts deactivate when the original structure of the ECM is reconstituted [60]. For in vitro, linear scratch wound model is usually used to study the VIC morphology, migration and repair mechanism [61]. In this study, we presented an encapsulated spheroid model in hydrogels. The 3D migration model with encapsulation of VIC spheroids within Me-HA and Me-HA/Me-Gel hydrogels presented fibroblast-myofibroblast phenotype transition during VIC migration process. Comparing to Me-HA hydrogels, Me-HA/Me-Gel hydrogels can also better maintain the fibroblastic phenotype of encapsulated spheroid with less expression of αSMA. During migration, VIC were activated and strongly expressed more αSMA. The migrated VIC in both Me-HA and Me-HA/Me-Gel hydrogels were then partially deactivated from more myofibroblastic phenotype to more fibroblastic phenotype, which is also observed during normal valve repair process. Tamura et al. [62] showed that spindle-shaped cells that take on the characteristics of myofibroblasts appeared at the site of injury in an in vivo sheep model of wound healing in the mitral valve. In addition, spreading cells were reported to be primarily involved in wound repair, especially the early stages [63]. These results suggest that Me-HA/Me-Gel hybrid hydrogel with encapsulated VIC spheroid is better 3D model to investigate the valvular response to injury process.
HA comprises 60% of the total GAG content in the heart valve, and is abundant in the spongiosa layer of the valve leaflet [54, 55]. Previous studies showed that exogenous HA in 2D VIC culture suppressed nodule formation in a molecular weight-dependent manner, and disruption of VIC-HA interactions was unregulated markers of VIC disease and induced leaflet mineralization [59]. The addition of Me-Gel, which contains both cell adhesion and cellular degradation motifs, promoted cell adhesion and cell-matrix interaction. The hybrid hydrogels with tunable physical properties are also promising scaffolds for heart valve tissue engineering. Me-HA/Me-Gel hydrogels with comparable stiffness to ventricularis and fibrosa layers can be laminated and serve as layer-specific valve ECM analog. The laminate hydrogel strategy allows direct control of layer-specific cellularity and material properties in an effort to mimic the layered microstructure of the valve leaflet [49]. Me-HA/Me-Gel hydrogels could provide a hybrid layer to support initial VIC phenotype or even promote stem cell differentiation towards valve cells in the presence of biochemical stimuli, while other layers provide mechanical strength [49, 64]. In addition, with suitable viscosity and composition, Me-HA/Me-Gel hybrid hydrogels could be bioprinted into cell laden valve conduits [65, 66].
5. Conclusions
We have engineered photocrosslinkable hydrogels with tunable physical properties based on oxidization and methacrylation of HA and gelatin. The hydrogels with high mechanical stiffness delayed the encapsulated VIC spreading process. Introducing Me-Gel enabled the cells to interact with the hybrid hydrogels and stimulate cell spreading, proliferation, GAG secretion and VIC migration from encapsulated spheroids. VIC cultured in our 3D hydrogels showed evidence of phenotype modulation by the stiffness and adhesivity of the hydrogels. Compliant Me-HA hydrogels promoted all the targeted gene expression with dramatic stimulation of αSMA expression, while Me-HA/Me-Gel hydrogels with extensive VIC spreading morphology could better maintain a quiescent VIC fibroblastic phenotype. The cellular phenotype transition during VIC migration from encapsulated spheroids in both Me-HA and Me-HA/Me-Gel hydrogel matrix was also observed. The information obtained in this study can provide important insight into the design of hydrogels for regulating VIC activity. In addition, the hybrid hydrogels could mimic layer-specific valve ECM and serve as 3D scaffolds for heart valve tissue engineering.
Supplementary Material
Acknowledgments
This work was funded by the Morgan Family Foundation, The Hartwell Foundation, the National Science Foundation (CBET-0955172) NSF Graduate Research Fellowship, Fondation LeDucq MITRAL Transnational Network, and the National Institutes of Health (HL110328). We thank Dr. David Putnam, Bailey Cooper and Dr. Jingjing Zhou for the help on GPC analysis and chick embryo isolation. We would like to thank Microscopy and Imaging Facility, Life Sciences Core Laboratories Center in Cornell University for their assistance with CLSM imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures.
References
- 1.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 2.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 3.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 4.Jabbari E. Bioconjugation of hydrogels for tissue engineering. Curr Opin Biotechnol. 2011;22:655–660. doi: 10.1016/j.copbio.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater. 2010;22:3484–3494. doi: 10.1002/adma.200904179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos E, Hernandez RM, Pedraz JL, Orive G. Novel advances in the design of three-dimensional bio-scaffolds to control cell fate: translation from 2D to 3D. Trends Biotechnol. 2012;30:331–341. doi: 10.1016/j.tibtech.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Lokeshwar VB, Selzer MG. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. J Biol Chem. 2000;275:27641–27649. doi: 10.1074/jbc.M003084200. [DOI] [PubMed] [Google Scholar]
- 8.Gomes JAP, Amankwah R, PowellRichards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004;88:821–825. doi: 10.1136/bjo.2003.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi S, Griffin CT, Wang SS, Bissell DM. Role of CD44 in epithelial wound repair - Migration of rat hepatic stellate cells utilizes hyaluronic acid and CD44v6. J Biol Chem. 2005;285:15398–15404. doi: 10.1074/jbc.M414048200. [DOI] [PubMed] [Google Scholar]
- 10.Rosines E, Schmidt HJ, Nigam SK. The effect of hyaluronic acid size and concentration on branching morphogenesis and tubule differentiation in developing kidney culture systems: Potential applications to engineering of renal tissues. Biomaterials. 2007;28:4806–4817. doi: 10.1016/j.biomaterials.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kogan G, Soltes L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 12.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 14.Ananthanarayanan B, Kim Y, Kumar S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials. 2011;32:7913–7923. doi: 10.1016/j.biomaterials.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toh WS, Lim TC, Kurisawa M, Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33:3835–3845. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 16.Bencherif SA, Srinivasan A, Horkay F, Hollinger JO, Matyjaszewski K, Washburn NR. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials. 2008;29:1739–1749. doi: 10.1016/j.biomaterials.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 17.Hachet E, Van Den Berghe H, Bayma E, Block MR, Auzely-Velty R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules. 2012;13:1818–1827. doi: 10.1021/bm300324m. [DOI] [PubMed] [Google Scholar]
- 18.Lozoya OA, Wauthier E, Turner RA, Barbier C, Prestwich GD, Guilak F, et al. Regulation of hepatic stem/progenitor phenotype by microenvironment stiffness in hydrogel models of the human liver stem cell niche. Biomaterials. 2011;32:7389–7402. doi: 10.1016/j.biomaterials.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehfeldt F, Brown AEX, Raab M, Cai SS, Zajac AL, Zemel A, et al. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr Biol. 2012;4:422–430. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
- 20.Lei YG, Gojgini S, Lam J, Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials. 2011;32:39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks MS, Schoen FJ, Mayer JEJ. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 22.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 24.Taylor PA, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 2003;35:113–118. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 25.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 26.Mohler ER. Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Butcher JT, Nerem RM. Valvular endothelial cells and the mechanoregulation of valvular pathology. Philos. Trans R Soc B-Biol Sci. 2007;362:1445–1457. doi: 10.1098/rstb.2007.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens EH, Durst CA, West JL, Grande-Allen KJ. Mitral valvular interstitial cell responses to substrate stiffness depend on age and anatomic region. Acta Biomater. 2011;7:75–82. doi: 10.1016/j.actbio.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinlan AMT, Billiar KL. Investigating the role of substrate stiffness in the persistence of valvular interstitial cell activation. J Biomed Mater Res Part A. 2012;100A:2474–2482. doi: 10.1002/jbm.a.34162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One. 2012;7:e39969. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinlan AMT, Sierad LN, Capulli AK, Firstenberg LE, Billiar KL. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PLoS One. 2011;6:e23272. doi: 10.1371/journal.pone.0023272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyss K, Yip CYY, Mirzaei Z, Jin XF, Chen JH, Simmons CA. The elastic properties of valve interstitial cells undergoing pathological differentiation. J Biomech. 2012;45:882–887. doi: 10.1016/j.jbiomech.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Gould RA, Chin K, Santisakultarm TP, Dropkin A, Richards JM, Schaffer CB, et al. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 2012;8:1710–1719. doi: 10.1016/j.actbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30:6593–6603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durst CA, Cuchiara MP, Mansfield EG, West JL, Grande-Allen KJ. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomater. 2011;7:2467–2476. doi: 10.1016/j.actbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: Comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13:478–485. [PubMed] [Google Scholar]
- 38.Lin C, Anseth K. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Benton JA, DeForest CA, Vivekanandan V, Anseth KS. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15:3221–3230. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flanagan TC, Wilkins B, Black A, Jockenhoevel S, Smith TJ. A collagen-glycosaminoglycan co-culture model for heart valve tissue engineering applications. Biomaterials. 2006;27:2233–2246. doi: 10.1016/j.biomaterials.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 42.Smeds KA, Pfister-Serres AM, K DD, Inoue M, Hatchell DL, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;55:254–255. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2009;15:1645–1653. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res Part A. 2012 doi: 10.1002/jbm.a.34420. DOI: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010;6:4495–4505. doi: 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 47.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 48.Hui TY, Cheung KMC, Cheung WL, Chan D, Chan BP. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: Influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201–3212. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Tseng H, Cuchiara ML, Durst CA, Cuchiara MP, Lin CJ, West JL, et al. Fabrication and mechanical evaluation of anatomically-inspired quasilaminate hydrogel structures with layer-specific formulations. Ann Biomed Eng. 2012;41:398–407. doi: 10.1007/s10439-012-0666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kristiansen KA, Potthast A, Christensen BE. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr Res. 2010;345:1264–1271. doi: 10.1016/j.carres.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Su WY, Chen YC, Lin FH. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010;6:3044–3055. doi: 10.1016/j.actbio.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 52.Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials. 2007;28:134–146. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 54.DeForest CA, Anseth KS. Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng. 2012;3:421–444. doi: 10.1146/annurev-chembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 55.Zhao RG, Sider KL, Simmons CA. Measurement of layer-specific mechanical properties in multilayered biomaterials by micropipette aspiration. Acta Biomater. 2011;7:1220–1227. doi: 10.1016/j.actbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Chan-Park MB. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials. 2010;31:1158–1170. doi: 10.1016/j.biomaterials.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 57.Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, et al. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008;316:200–213. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13:478–486. [PubMed] [Google Scholar]
- 59.Durbin AD, Gotlieb AI. Advances towards understanding heart valve response to in injury. Cardiovasc Pathol. 2002;11:69–77. doi: 10.1016/s1054-8807(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 60.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 61.Gotlieb AI, Rosenthal A, Kazemian P. 2002 SJOTACSVIPDmPS. Fibroblast growth factor 2 regulation of mitral valve interstitial cell repair in vitro. J Thorac Cardiovasc Surg. 2002;124:591–597. doi: 10.1067/mtc.2002.123812. [DOI] [PubMed] [Google Scholar]
- 62.Tamura K, Jones M, Yamada I, Ferrans VJ. Wound healing in the mitral valve. J Heart Valve Dis. 2000;9:53–63. [PubMed] [Google Scholar]
- 63.Fayet C, Bendeck MP, Gotlieb AI. Cardiac valve interstitial cells secrete fibronectin and form fibrillar adhesions in response to injury. Cardiovasc Pathol. 2007;16:203–211. doi: 10.1016/j.carpath.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Tedder ME, Simionescu A, Chen J, Liao J, Simionescu DT. Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering. Tissue Eng Part A. 2011;17:25–36. doi: 10.1089/ten.tea.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skardal A, Zhang JX, McCoard L, Xu XY, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A. 2010;16:2675–2685. doi: 10.1089/ten.tea.2009.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hockaday LA, Kang KH, Colangelo NW, Cheung PYC, Duan B, Malone E, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4:035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.