Abstract
BACKGROUND
Uterine serous carcinoma (USC) is a subtype of endometrial cancer associated with chemoresistance and poor outcome. Overexpression of tubulin-β-III and p-glycoprotein has been linked to paclitaxel resistance in many cancers but has been undercharacterized among USC. Epothilones have demonstrated activity in certain paclitaxel-resistant malignancies. In this study, we clarify in USC relative to ovarian serous carcinomas (OSC) the relationships between tubulin-β-III and p-glycoprotein expression, clinical outcome, and in vitro chemoresponsiveness to epothilone B, ixabepilone and paclitaxel.
METHODS
Tubulin-β-III and p-glycoprotein were quantified by real time polymerase chain reaction (qRT-PCR) in 48 fresh-frozen tissue samples and 13 cell lines. Copy number was correlated with immunohistochemistry and overall survival. IC50 was determined using viability and metabolic assays. Impact of tubulin-β-III knockdown on IC50 was assessed with siRNAs.
RESULTS
USC overexpressed tubulin-β-III but not p-glycoprotein relative to OSC in both fresh-frozen tissues (552.9±106.7 versus 202.0±43.99, p=0.01) and cell lines (1701.0±.376.4 versus 645.1±157.9, p=0.02). Tubulin-β-III immunohistochemistry reflected qRT-PCR copy number and overexpression stratified patients by overall survival (copy number ≤400: 615 days; copy number >400: 165 days, p=0.049); p-glycoprotein did not predict clinical outcome. USC remained exquisitely sensitive to patupilone in vitro despite tubulin-β-III overexpression (IC50, USC 0.245±0.11 nM versus IC50, OSC 1.01±0.13 nM, p=0.006).
CONCLUSIONS
Tubulin-β-III overexpression in USC discriminates poor prognosis, serves as a marker for sensitivity to epothilones, and may contribute to paclitaxel resistance. Immunohistochemistry reliably identifies tumors with overexpression of tubulin-β-III, and a subset of individuals likely to respond to patupilone and ixabepilone. Epothilones warrant clinical investigation for treatment of USC.
Keywords: uterine serous carcinoma, tubulin-β-III, paclitaxel resistance, epothilone, ovarian serous carcinoma
INTRODUCTION
In 2012, a projected 29,520 deaths will occur from gynecologic malignancies.1 Among gynecologic cancers, endometrial cancer is the most common and may be broadly dichotomized into two classes with separate underlying molecular pathogenesis, clinical behavior, and histopathology.2 Type I endometrial cancers comprise 80% of cases, and are associated with endometrioid histology (grade 1 or 2) and favorable prognosis.3 Type II endometrial cancers are characterized by an aggressive clinical course with relatively poor prognosis.3–4 Uterine serous carcinomas (USC) represent the most common form of type II disease and are poorly differentiated by definition. As many as 37–70% of patients with USC demonstrate extrauterine disease at time of diagnosis,5–6 sometimes in the setting of scant or no myometrial invasion.7–8 So while USC represents only 10% of all uterine cancers, it accounts for 39% of deaths.3
Ovarian cancer remains the leading cause of death from gynecologic malignancy in the United States and most developed countries.1,9 Among epithelial subtypes, ovarian serous carcinomas (OSC) represent 75% of all cases and can be organized into two tiers.10Low-grade OSC develop indolently from non-invasive borderline precursors.11 The origin of high-grade OSC is more controversial; genetic analyses suggest that rapid transformation of intraepithelial carcinoma of the fallopian tube may underlie as many as 50% of cases, in addition to those which evolve primarily from peritoneal or ovarian surface epithelium.7 OSC has one of the highest death-to-incidence ratios, attributable largely to a lack of effective screening modalities with subsequent initial diagnosis at late stages.
High-grade serous carcinomas of the uterus and ovary may be morphologically indistinguishable.8 Advanced-stage disease is associated with grim 5-year overall survival (OS) rates of 18.5–32.5%5 and 34%,11 respectively. Though a growing body of molecular evidence delineates the distinctness of these entities,12–13 first-line therapy for both begins with surgical staging followed by platinum/taxane combination chemotherapy for advanced disease.14–17 Initial response rates of advanced USC to this regimen are as low as 60%,18 which compare unfavorably to rates of 80% for OSC.19 Responses in USC are generally non-durable.20 In patients treated with intravenous carboplatin/paclitaxel for advanced disease, median progression-free survival in USC is 13 months,17 compared to 20.7 months in OSC.15
Resistance to paclitaxel has been linked to overexpression of class III β-tubulin,21 one of 9 β-isoforms22 capable of heterodimerizing with α subunits to form microtubules critical to cell division. Paclitaxel binds preferentially to the tubulin-β-I isoform,23 which differs from tubulin-β-III at paclitaxel-binding site positions 275 (Ser→Ala) and 364-5 (Ala-Val→Ser-Ser).22 High tubulin-β-III expression reduces the rate of microtubule assembly24 and correlates with poorer OS in ovarian serous,25 colon,26 non-small cell lung,27 and breast28 cancers. This clinicopathologic association has not yet been demonstrated in endometrial cancers.29
Epothilones (EPOxide THIazoLe ketONEs) are novel microtubule-stabilizing macrolides isolated from Sorangium cellulosum30 with activity in paclitaxel-resistant malignancies putatively due to their unique ability to bind class III and I isoforms with at least equal affinity.23 Epothilones generally do not share with paclitaxel overlapping mechanisms of resistance,31 and are not substrates for the drug efflux pump p-glycoprotein, the overexpression of which has been linked to multidrug resistance to cytotoxic agents including taxanes.32 Despite encouraging in vitro data and strong biologic plausibility, p-glycoprotein expression often does not reliably correlate with clinical outcome and trials of p-glycoprotein inhibitors have failed to demonstrate improved survival benefit.33 This underscores the need to identify novel biomarkers that predict clinical response.
In this study, we sought to (1) quantify by qRT-PCR tubulin-β-III and p-glycoprotein expression in a large number of USC in comparison to high-grade OSC within solid tissues and cell lines, (2) describe the association of tubulin-β-III and p-glycoprotein with in vitro chemoresponsiveness to epothilone B (patupilone), ixabepilone and paclitaxel, (3) characterize the contribution of tubulin-β-III to paclitaxel resistance, (4) correlate tubulin-β-III immunohistochemistry with qRT-PCR copy number, and (5) examine the prognostic implications of tubulin-β-III overexpression on clinical outcome.
METHODS
Tissue procurement, establishment of cell lines, and characterization of growth rate
As approved by the institutional review boards at Yale University and the University of Arkansas, fresh-frozen tissues and patient characteristics were obtained at time of primary debulking. Tumors were staged according to criteria established by the AJCC 7th ed./FIGO. As per WHO guidelines for epithelial tumors,34 only those that contained <10% of a second malignant component were considered ‘pure;’ those which contained >10% were considered ‘mixed.’ Carcinoma and normal human ovarian surface epithelial cell lines were established from tissue biopsies and maintained as described previously.35–36 Cellular growth rate was determined per established protocol. 37 Survival data were obtained through review of electronic medical records and/or public records.
RNA extraction, purification, and reverse-transcription
Total RNA extraction from cell culture lysates and fresh-frozen tissues was performed using AllPrep DNA/RNA/Protein Minikit (Qiagen, Germantown, MD). Quantitative real time polymerase chain reaction (qRT-PCR) was performed with a 7500 RealTime PCR System per the manufacturer’s protocol (Applied Biosystems, Foster City, CA). All RNA samples were treated with Turbo DNase enzyme (Applied Biosystems). Total RNA (5μg) was reverse-transcribed using Superscript III (Invitrogen, Carlsbad, CA). Five microliters of reverse-transcribed RNA was amplified using Taqman PCR Master Mix (Applied Biosystems). Tubulin-β-III (Hs00964962_g1) and p-glycoprotein (Hs00184491_m1) specific primers were obtained (Assay-On-Demand, Carlsbad, CA), with GAPDH (Hs99999905_m1) as an internal control. Gene expression was analyzed using the comparative threshold method and normalized to levels observed in lymphoid cell lines.
Chemosensitivity and viability assays
Patupilone (Novartis Pharma, Basel, Switzerland), Ixabepilone (Bristol-Myers Squibb Company, Princeton, NJ) and paclitaxel (T7402: Sigma-Aldrich, St. Louis, MO) were dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) as 10mM stock solutions protected from light exposure and stored at −20°C prior to serial dilution. Metabolic assays were performed after drug exposure using MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assays (Promega Corporation, Madison, WI). Briefly, cells at log phase of growth were seeded in 96-well culture plates at optimum density, incubated, and exposed to drug after 24 hours. Tetrazolium was added after 72 hours of additional incubation. Absorbance at a reference wavelength of 490 nm was measured with a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). For cell viability assays, cells at log phase of growth were seeded in 6-well microtiter plates at optimum density and exposed to drug at 24 hours. After 48–72 hours of additional incubation, well contents were harvested in entirety, centrifuged then stained with either trypan blue for manual counts or propidium iodide (2 μL of a 500 μg/mL stock solution in PBS with 0.1% sodium azide and 2% fetal bovine serum) for flow cytometric counts. Unless stated otherwise, all IC50 values presented have been determined by flow cytometry.
Immunohistochemistry
All slides were reviewed by a gynecologic pathologist (NB). Formalin-fixed sections were cut at 4 μm and stained with anti-tubulin-β-III monoclonal antibody (TUJ1; Covance, Berkeley, CA) at 1:500 dilution. Staining intensity was assessed using a semi-quantitative scoring system: 0, negative (no staining observed); 1+, focal/weak staining; 2+, diffuse weak/focal moderate staining; 3+, diffuse moderate/focal strong staining; 4+, diffuse strong immunoreactivity. For mixed specimens, only the serous component was examined.
Small-interfering RNA (siRNA)
Tubulin-β-II-specific (5′>3′ GAUCAAUGCUGCAUCCUUAtt) and tubulin-β-III-specific small-interfering RNA oligonucleotides (5′>3′ CCAUUCUGGUGGUGGACCUGGAtt) were purchased from Ambion, Inc. (Silencer Select, Life Technologies, Carlsbad, CA). Cells were seeded in 6-well plates at a concentration of 200,000 per well and transfected with siRNA duplexes at 10 nM with 5 μL Lipofectamine RNAiMAX (Invitrogen) in OptiMem (Life Technologies) and RPMI with 10% fetal bovine serum. Mock transfections with nonspecific siRNA duplexes were used as negative controls. Cells were exposed to drug 24 hours after plating. Cells remained in culture for a total of 72 hours prior to harvest.
Statistical analyses
Dose response curves were analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc., LaJolla, CA). IC50, absolute was defined as the concentration of drug required to result in reduction of cell viability to 50% of untreated control. IC50, relative was defined as the concentration at 50% between maximum and minimum points of the dose-response curve. IC50 was determined through interpolation of sigmoidal curves fit with a standard Hill slope of −1.0. Unless stated otherwise, all values presented reflect IC50, absolute and mean ± standard error of the mean (SEM). Kaplan-Meier curves were analyzed with the log-rank test. All student’s t-tests and Pearson correlations employed 2-tails and assumed equal variance. Non-parametric Mann-Whitney analyses were applied to examine the distributions of immunohistochemistry scores. P values <0.05 were considered statistically significant.
RESULTS
Uterine serous carcinomas overexpress tubulin-β-III but not p-glycoprotein in fresh-frozen tissues and cell lines by qRT-PCR
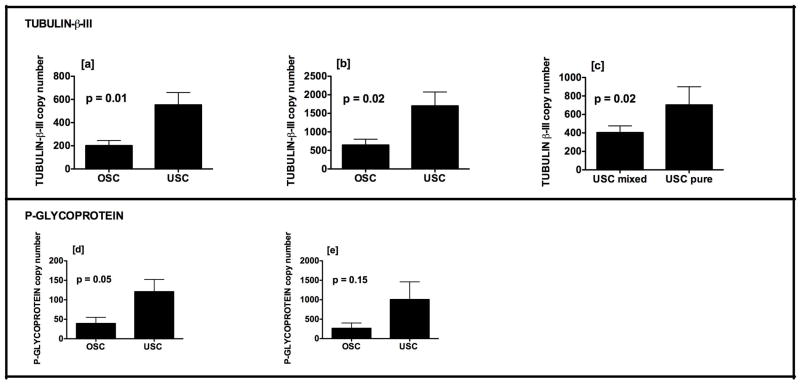
Fresh-frozen tissue specimens representing 28 USC and 20 OSC were analyzed. A total of 6 USC and 7 OSC primary cell lines were established. Source patient characteristics are provided in Table 1a/b. Mixed specimens all contained endometrioid in addition to serous components; in one specimen, clear cell features were also present. USC overexpressed tubulin-β-III relative to OSC in both fresh-frozen tissues (Figure 1a; 552.9±106.7 versus 202.0±43.99, p=0.01) and cell lines (Figure 1b; 1701±376.4 versus 645.1±157.9, p=0.02). Tubulin-β-III expression in fresh-frozen tissues was greater for patients with pure versus mixed histology (703.2±196.4 versus 403.4±72.57, p = 0.02; Figure 1c). There was no difference in tubulin-β-III expression among those with early versus advanced stage disease (533.2±134.3 versus 595.8±183.3, p = 0.79; data not shown). Tubulin-β-III was not expressed at high levels in normal human ovarian surface epithelial cell lines (133.3±22.37, n=3; data not shown).
Table 1a.
Source patient characteristics: fresh-frozen tissues.
| USC (n = 28) | % | OSC (n = 20) | % | |
|---|---|---|---|---|
| STAGE | ||||
| I | 7 | 25.0 | 0 | 0.0 |
| II | 2 | 7.1 | 2 | 10.0 |
| III | 10 | 35.7 | 18 | 90.0 |
| IV | 9 | 32.1 | 0 | 0.0 |
| HISTOLOGY | ||||
| Pure | 14 | 50.0 | 20 | 100.0 |
| Mixed | 14 | 50.0 | 0 | 0.0 |
| RACE | ||||
| White | 19 | 67.9 | 18 | 90.0 |
| Black | 9 | 32.1 | 2 | 10.0 |
| AGE, mean [range] (y) | 69.2 [36–88] | 60.6 [33–90] | ||
USC-uterine serous carcinoma; OSC-ovarian serous carcinoma
Table 1b.
Source patient characteristics: cell lines.
| RACE | STAGE | CELL LINE HISTOLOGY | |
|---|---|---|---|
| OSC-1 | WHITE | IIIC | Pure |
| OSC-2 | WHITE | IV | Pure |
| OSC-3 | WHITE | IV | Pure |
| OSC-6 | WHITE | IIIC | Pure |
| OSC-7 | WHITE | IIIC | Pure |
| OSC-8 | WHITE | IC | Pure |
| OSC-9 | WHITE | IIIC | Pure |
| USC-1 | BLACK | IVA | Pure |
| USC-2 | BLACK | IVB | Pure |
| USC-3 | BLACK | IVB | Pure |
| USC-5 | BLACK | IIIC | Pure |
| USC-7 | WHITE | IIC | Pure |
| USC-12 | WHITE | IVB | Pure |
USC-uterine serous carcinoma; OSC-ovarian serous carcinoma
Fresh frozen tissue specimens were available for PCR only for USC cell lines 5 and 7.
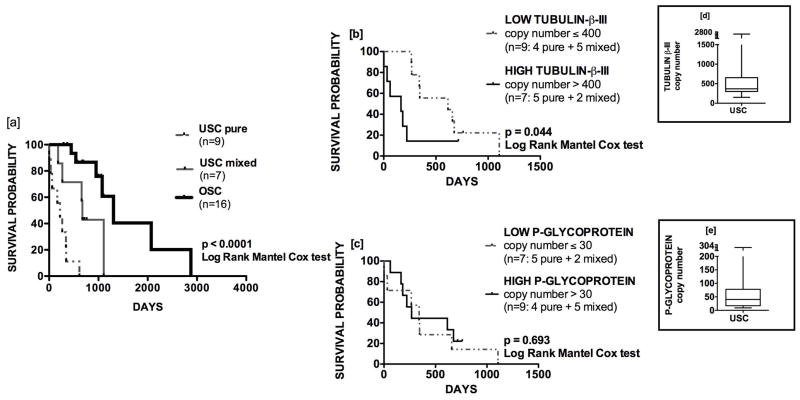
Figure 1.
Expression of tubulin-β-III mRNA (y-axis) is greater for USC relative to OSC in [a] fresh-frozen tissue and [b] chemo-naive cell lines. Among fresh-frozen tissues, expression is higher in pure compared to mixed serous histology [c]. USC exhibit a trend towards overexpression of p-glycoprotein in fresh-frozen tissues [d] and cell lines [e]. Mean ± SEM are shown.
Compared to OSC, USC exhibited a trend towards higher p-glycoprotein expression in fresh-frozen tissues (Figure 1d; 120.9±31.39 versus 39.22±15.82, p = 0.05) and cell lines (Figure 1e: 1005±455.4 versus 266.5±136.5, p = 0.15). The greater expression of both tubulin-β-III and p-glycoprotein in cell lines compared to fresh-frozen tissues is thought to reflect heterogeneity of the tissue specimens, which might contain vessels and stromal components.
Immunohistochemistry staining for tubulin-β-III correlates with qRT-PCR copy number
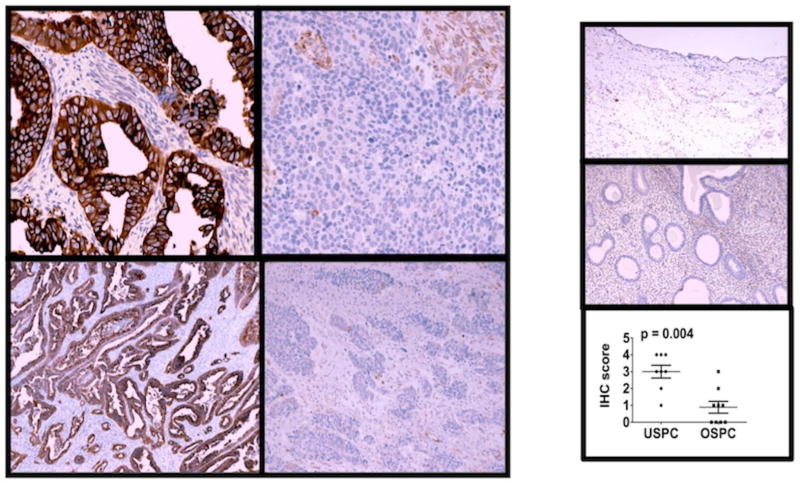
Approximately 35% of specimens included in the qRT-PCR analysis were also examined with immunohistochemistry (IHC) (5 pure UPSC, 3 mixed USC, 9 OSC). USC demonstrated stronger staining (median IHC score: 3+) relative to OSC (median IHC score: 0 to 1+) (p = 0.004). qRT-PCR copy number among these specimens was 631.12±193.31 versus 92.98±25.53 (p = 0.01). Normal ovary and endometrium did not express tubulin-β-III by IHC; this reflects qRT-PCR copy number among unmatched normal ovary, endometrium, and myometrium (32.63±6.51, n=3). Representative slides and distribution of IHC scores are shown in Figure 2.
Figure 2.
Immunohistochemistry correlates with tissue expression determined by qRT-PCR. Representative slides are shown at original magnification for USC (score: 4+, left) and OSC (score: 0, right) at 400x (top) and 100x (bottom). Corresponding qRT-PCR copy numbers for these specimens are 685.01 and 134.67, respectively. Normal ovary (inset-top) and endometrium (inset-middle) do not express tubulin-β-III as shown at 100x. Median IHC scores are higher among USC compared to OSC (p=0.004) (inset-bottom).
Uterine serous carcinomas are highly sensitive to patupilone relative to ovarian serous carcinomas
Next, we performed chemosensitivity profiling of 4 USC and 3 OSC cell lines, which were selected given their similarity in growth rates as this may influence response to cytotoxic agents (mean doubling time ± SEM USC versus OSC: 24.5±4.25 versus 35.0±8.5 hours, respectively, p=0.28; data not shown). USC were found to be highly sensitive to patupilone. As determined by flow cytometric assays of cell viability, IC50 values for patupilone among 4 USC and 3 OSC cell lines were 0.25±0.11 nM [range: 0.02–0.53] and 1.01±0.13 nM [range: 0.81–1.24], respectively (p = 0.006) (Figure 3). Corresponding IC50 values for paclitaxel were not significantly different (26.41±27.14 [range: 48.42–182.52] versus 95.64±75.34 [4.89–65.52]; p=0.14). Within these 7 cell lines, tubulin-β-III expression was higher among USC compared to OSC with a trend towards statistical significance (2297±462.2 versus 834.0±289.5, p=0.058) (Figure 3-inset). p-glycoprotein expression was not significantly different (p=0.27; not shown). Flow cytometric findings were robust across both metabolic assays and manual counts by trpyan blue exclusion. IC50 values determined by flow cytometry are presented given superior sensitivity and dynamic range of the assay; results of these independent validation studies have been described previously (submitted for publication).
Figure 3.
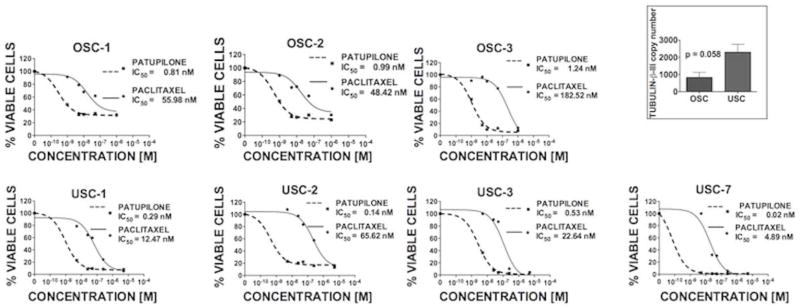
USC cell lines are highly sensitive to patupilone. Representative dose-response curves and IC50 values as determined by flow cytometry after exposure to drug for 72 hours in OSC (top) and USC (bottom) cell lines are shown. IC50 values (mean ± SEM) for patupilone among OSC and USC cell lines are 1.01 ± 0.12 and 0.25 ± 0.11 nM, respectively (p = 0.006). There was no statistical difference between IC50 values for paclitaxel or p-glycoprotein expression within the cells lines shown. Tubulin-β-III copy number was higher among USC compared to OSC with a trend towards statistical significance (inset).
Uterine serous carcinomas are significantly more sensitive to patupilone and ixabepilone when compared to paclitaxel
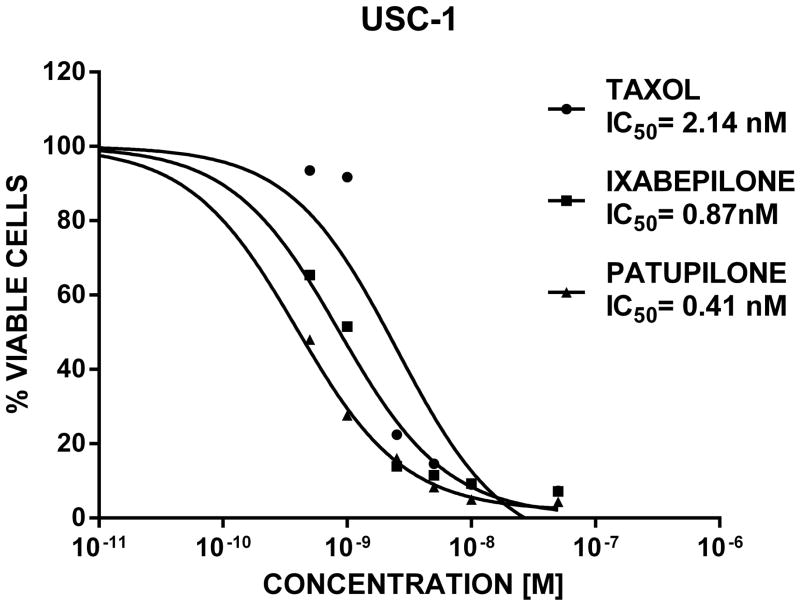
We next compared the efficacy of ixabepilone (a commercially available and FDA-approved epothilone) to patupilone and paclitaxel in a limited number of USC cell lines (i.e., USC-1 and USC-2). As representatively shown in figure 4 for USC-1, both USC cell lines tested demonstrated significantly higher sensitivity to patupilone and ixabepilone when compared to paclitaxel. Indeed, as determined by flow cytometric assays of cell viability, IC50 values were 0.41 nM (range 0.3–0.55 nM) for patupilone and 0.87 nM [range: 0.58–1.27 nM] for ixabepilone, respectively, while IC50 values were 2.12 nM [range: 1.44–3.05 nM] for paclitaxel (paclitaxel vs patupilone: p = 0.003, paclitaxel vs ixabepilone p = 0.02, Figure 4). In multiple parallel experiments, we consistently noted higher sensitivity of USC cell lines to patupilone when compared to ixabepilone (p = 0.02, Figure 4).
Figure 4.
USC cell lines are significantly more sensitive to epothilones when compared to paclitaxel. Representative dose-response curves and IC50 values as determined by flow cytometry after exposure to patupilone, ixabepilone and paclitaxel for 72 hours in USC-1 cell line are shown. IC50 values (mean) for patupilone, ixabepilone and paclitaxel are 0.41, 0.87 and 2.14 nM, respectively (paclitaxel vs patupilone: p = 0.003, paclitaxel vs ixabepilone: p = 0.02; patupilone vs ixabepilone: p = 0.02).
Chemoresistance of uterine serous carcinomas to paclitaxel and patupilone varies with tubulin-β-III expression
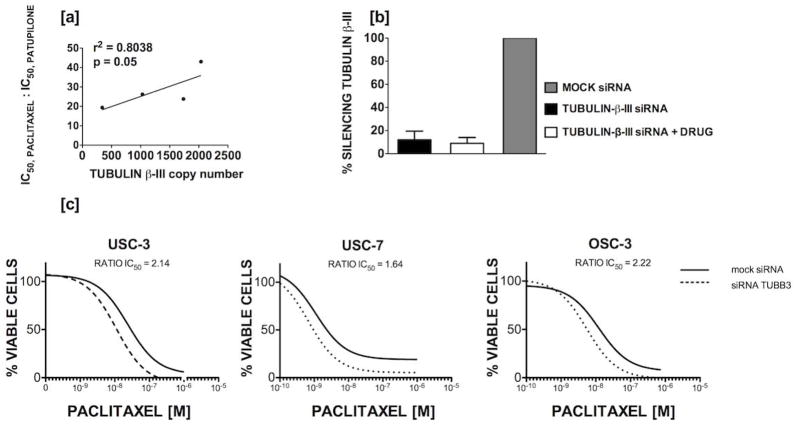
We investigated the relationship of tubulin-β-III as a marker of patupilone sensitivity and paclitaxel resistance in USC by plotting by qRT-PCR copy number against the ratio of IC50, paclitaxel to IC50, patupilone (Figure 5a; r2=0.8038, p=0.05). P-glycoprotein expression in the USC (n=4) and OSC (n=3) cell lines did not correlate with patupilone IC50 (R2=0.357, p=0.16, data not shown). To investigate the role of tubulin-β-III in paclitaxel and patupilone resistance, siRNAs were then used to down-regulate expression prior to exposure to drug. Transfection successfully produced reductions in tubulin-β-III of 83–93%, and efficiency was not affected by addition of chemotherapy (Figure 5b). Knockdown of tubulin-β-III enhanced sensitivity of both USC and OSC cell lines to paclitaxel (Figure 5c); based on IC5050, relative values, sensitivity in USC-3, USC-7, and OSC-1 increased by 2.14, 1.64, and 2.22-fold, respectively. In contrast, down-regulation of tubulin-β-II (i.e., a tubulin used as additional control in our experiments), did not enhanced sensitivity to paclitaxel (Figure 5d). Knockdown of tubulin-β-III expression sensitized USC cells to patupilone (Figure 5e).
Figure 5.
[a] Tubulin-β-III copy number correlates with paclitaxel resistance and sensitivity to patupilone among uterine serous papillary carcinomas. The ratio of IC50, paclitaxel to IC50, patupilone increases (y-axis) with higher tubulin-β-III copy number (x-axis). Data for 4 cell lines is shown. [b] siRNA transfection resulted in reductions in tubulin-β-III expression by 83–93% and transfection efficiency was not affected by addition of drug; data for three cell lines with replicates is shown. Knockdown of tubulin-β-III [c] but not tubulin-β-II [d] increases sensitivity to paclitaxel in both USC and OSC cell lines. Representative dose-response curves are shown for cells treated with mock (solid line) and tubulin-β-III (dotted line) siRNA. [e] Knockdown of tubulin-β-III increases sensitivity to patupilone in a representative USC cell line.
Tubulin-β-III but not p-glycoprotein expression stratifies among patients with advanced stage disease poorer OS after platinum/taxane combination chemotherapy
Kaplan-Meier estimates of OS were generated for patients with stage III/IV USC (67.9% white vs 32.1% black) and OSC (90% white vs 10% black) (Table 1a and Figure 6a). All patients received adjuvant therapy with intravenous carboplatin and paclitaxel with or without radiation therapy. None of the patients received neoadjuvant chemotherapy. Follow-up was available for 16/19 patients with advanced USC and 16/18 patients with advanced OSC. Median OS was much worse for patients with either pure USC (221 days) or mixed component USC (675 days) compared to OSC (1306 days) (p<0.0001). The prognostic value of tubulin-β-III and p-glycoprotein copy number was then assessed in patients with USC. Using a cutoff of 400, median OS among patients with low and high tubulin-β-III was 615 and 165 days, respectively (Figure 6b; p=0.049). Of patients with high tubulin-β-III expression, 71% demonstrated pure histology compared to 44% in those with low expression but this difference was not statistically different (p=0.33). The hazard ratio for death (HR) was 3.929 (95% CI 1.04–14.84). Within this same group, no differences in OS were found between patients with low and high p-glycoprotein expression using a cutoff of 30 (Figure 6c) (median OS: 268 versus 341 days, respectively, p=0.69; HR: 0.79, 95% CI 0.26–2.438). Among patients with high and low p-glycoprotein expression, there was not a difference in distribution of patients with mixed and pure histology (p=0.31). Among the USC cohort, mean tubulin-β-III and p-glycoprotein copy numbers were 643.31 (range: 150–2837) and 72.63 (range: 9–304), respectively (Figure 6d/e). Median follow-up interval in the cohort was only 345 days, but only 2 patients remained alive at time of last follow-up. In the OSC cohort, mean tubulin-β-III and p-glycoprotein copy numbers were 212.59 (range: 12–839) and 32.69 (range: 1–118), respectively; median follow-up was 693 days, and 9 patients were alive at time of censoring.
Figure 6.
[a] Median OS of patients with stage III/IV pure USC, mixed USC, and OSC histologies was 221, 675, and 1306 days, respectively (p<0.0001). [b] Among patients with USC, tubulin-β-III overexpression stratifies poor clinical outcome. Median OS in patients with low and high expression was 615 versus 165 days, respectively (p=0.044). [c] In this same cohort, p-glycoprotein overexpression had no prognostic utility (p=0.69). Box-plots demonstrate median, interquartile range, and overall range of copy number values for [d] tubulin-β-III and [e] p-glycoprotein.
DISCUSSION
Though uterine and ovarian serous carcinomas represent two morphologically similar diseases often treated with identical chemotherapeutic regimens, they differ in their biologic pathogenesis and clinical behavior. In accordance with this diversity, we have shown for the first time using quantitative methods within a large number of fresh-frozen tissues and cell lines that USC overexpress tubulin-β-III relative to high-grade OSC. We also demonstrate correlation between qRT-PCR copy number and immunostaining. This validation is crucial since mRNA transcript copy number and protein expression may be discordant due to post-transcriptional modifications in mammalian cells.38
The pathways leading to chemoresistance in USC are incompletely elucidated. A brisk growth rate likely permits rapid tumor regrowth despite initial intrinsic sensitivity to chemotherapeutic agents.39 These tumors are also associated with a distinct milieu of autocrine factors including upregulation of IL-6,40 which has been shown to confer resistance to paclitaxel in gynecologic malignancies.41 Though p-glycoprotein has been shown in vitro to maintain low intracellular concentrations of paclitaxel,33 expression has not shown to be clinically meaningful for endometrial cancers.29,42–43 The design of rational therapeutic strategies requires a more thorough understanding of the mechanisms that contribute to resistance as well as isolation of novel prognostic markers for response.
Tubulin-β-III has been proposed as a biomarker of chemoresistance to paclitaxel44 as well as response to patupilone.37 In this study, USC exhibited greater tubulin-β-III expression and were 4.1-fold more sensitive (p=0.006) to patupilone relative to OSC cell lines, despite equal sensitivity to paclitaxel. As chemosensitivity is multifactorial, the similar sensitivity to paclitaxel may reflect the concurrent absence of differential p-glycoprotein expression across the uterine and ovarian serous cell lines tested. Chemosensitivities were confirmed rigorously across assays based on both cell viability (trypan blue exclusion and flow cytometry) and metabolism (MTS) since the latter methodology may be greatly influenced by variables such as media composition.45 Given that heightened sensitivity to paclitaxel in response to tubulin-β-III downregulation had not yet been demonstrated in USC cell lines, we used siRNA to reduce tubulin-β-III expression; consistent with previous studies, we witnessed improved in vitro sensitivity of both USC and OSC to paclitaxel by approximately 2-fold. Tubulin-β-III downregulation has been previously shown to improve sensitivity to multiple chemotherapeutic agents,46 including epothilone B47 by predisposing cells to apoptosis through mechanisms other than reduction in mitotic block induced by chemotherapeutic agent.46–47 The results of our siRNA tubulin-β-III knockdown experiments in USC are in agreement with this view and suggest that tubulin-β-III may not only serve as a marker for sensitivity to epothilones but also as a mediator of multiple cell survival pathways. 46–47
In this study, overexpression of tubulin-β-III had prognostic relevance. Among patients with advanced-stage disease, we have shown for the first time in USC that tubulin-β-III copy number may identify patients who are at risk of poorer OS. Consistent with prior publications, we found reduced OS in uterine cancers with increasing volume of the serous component.48 Our findings differ from the report by Vandenput et al.29 and by Zhu et al. 49 who failed to identify a relationship between tubulin-β-III expression and outcome in patients who had received paclitaxel for endometrial cancer, however, their analyses employed IHC alone.
Patupilone has shown encouraging activity in the treatment of gynecologic malignancies, but has not been tested beyond phase II in endometrial cancers.50,51 Ixabepilone, which differs from patupilone by a single moiety, has been evaluated in GOG-129P, a study of 50 patients with recurrent or persistent endometrial cancer who received one prior line of taxane-based chemotherapy including 40% with serous and 2% with clear cell histology. An overall response rate of 12% was achieved using 40mg/m2 every 21d; disease stabilization for at least 8 weeks occurred in 60%. Median progression-free and overall survival was 2.9 months and 8.7 months, respectively.51 Among the various epothilones, patupilone was chosen for most of our work because our institution previously participated in EPO2303, a multi-center phase III trial of patupilone versus pegylated liposomal doxorubicin in taxane- and/or platinum-refractory/resistant recurrent ovarian cancer. In our study, an in vitro comparison alongside of the sensitivity of USC cell lines to patupilone and ixabepilone versus paclitaxel demonstrated a consistent higher sensitivity of USC to Epothilones. Patupilone however had significantly higher potency against USC cell lines when compared to ixabepilone. These results are consistent with published reports comparing patupilone to ixabepilone activity against human colorectal cell lines.52
In conclusion, we have demonstrated that tubulin-β-III overexpression in USC may discriminate poor prognosis among patients with advanced disease, serves as a marker for sensitivity to patupilone, and may contribute to paclitaxel resistance. IHC applied to surgical specimens may be a reliable adjunct to identify tumors with overexpression of tubulin-β-III, and accordingly the subset of individuals at risk of poor outcome in the setting of platinum/taxane chemotherapy who may instead respond to patupilone. Epothilones warrant further clinical investigation in the treatment of these aggressive, chemoresistant tumors, especially in the setting of recurrent or persistent disease and high tubulin-β-III expression.
Footnotes
FINANCIAL DISCLOSURES/FUNDING: none
SOURCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics-2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Bohkman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goff BA, Kato D, Schmidt RA, et al. Uterine papillary serous carcinoma: pattern of metastatic spread. Gynecol Oncol. 1994;54:264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- 5.Slomovitz BM, Burke TW, Eifel PJ, et al. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol. 2003;91(3):463–469. doi: 10.1016/j.ygyno.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Podratz KC, Mariani A. Uterine papillary serous carcinomas: the exigency for clinical trials. Gynecol Oncol. 2003;91:461–462. doi: 10.1016/j.ygyno.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Crumm CP. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Molec Oncol. 2009;3:165–170. doi: 10.1016/j.molonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendrickson M, Ross J, Eifel P, et al. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Global Cancer Facts & Figures. 2. Atlanta, GA: [Google Scholar]
- 10.Malpica A, Deavers MT, Karen Lu, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Vang R, Shih le-M, Jurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16(5):267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santin AD, Zhan F, Bellone S, et al. Discrimination between uterine serous papillary carcinomas and ovarian serous papillary tumours by gene expression profiling. Br J Cancer. 2004;90:1814–1824. doi: 10.1038/sj.bjc.6601791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zorn KK, Bonome T, Gangi L, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–6430. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 14.Dizon DS. Treatment options for advanced endometrial carcinoma. Gynecol Oncol. 2010;117:373–381. doi: 10.1016/j.ygyno.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 16.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22(11):2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 17.Sovak MA, Dupont J, Hensley ML, et al. Paclitaxel and carboplatin in the adjuvant treatment of patients with high-risk stage III and IV endometrial cancer: a retrospective study. Gynecol Oncol. 2006;103(2):451–457. doi: 10.1016/j.ygyno.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Hoskins PJ, Swenerton KD, Pike JA, et al. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol. 2001;19:4048–4053. doi: 10.1200/JCO.2001.19.20.4048. [DOI] [PubMed] [Google Scholar]
- 19.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9(3):167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 20.Nicklin JL, Copeland LJ. Endometrial papillary serous carcinoma: patterns of spread and treatment. Clin Obstet Gynecol. 1996;39(3):686–695. doi: 10.1097/00003081-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Kavallaris M, Kuo DY, Burkhart CA, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100(5):1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferlini C, Raspaglio G, Cicchillitti L, et al. Looking at drug resistance mechanisms for microtubule interacting drugs: does TUBB3 work? Curr Cancer Drug Targets. 2007;7(8):704–712. doi: 10.2174/156800907783220453. [DOI] [PubMed] [Google Scholar]
- 23.Magnani M, Ortuso F, Soro S, et al. The β-I/β-III-tubulin isoforms and their complexes with antimitotic agent: docking and molecular dynamics studies. FEBS J. 2006;273:3301–3310. doi: 10.1111/j.1742-4658.2006.05340.x. [DOI] [PubMed] [Google Scholar]
- 24.Hari M, Yang H, Zeng C, et al. Expression of class III-β tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil Cytoskeleton. 2003;56:45–56. doi: 10.1002/cm.10132. [DOI] [PubMed] [Google Scholar]
- 25.Ferrandina G, Zannoni GF, Matrinelli E, et al. Class III β-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res. 2006;12:2774–2779. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 26.Mariani M, Zannoni GF, Sioletic S, et al. Gender influences the class III and V β-tubulin ability to predict poor outcome in colorectal cancer. Clin Cancer Res. 2012;18(10):2964–2975. doi: 10.1158/1078-0432.CCR-11-2318. [DOI] [PubMed] [Google Scholar]
- 27.Seve P, Dumontet C, Reiman T. Role of tubulin-β-III in predicting chemoresistance in non-small cell lung cancer. Lung Cancer. 2010;67(2):136–143. doi: 10.1016/j.lungcan.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Tommasi S, Mangia A, Lacalamita R, et al. Cytoskeleton and paclitaxel sensitivity in breast cancer: the role of β-tubulins. Int J Cancer. 2007;120:2078–2085. doi: 10.1002/ijc.22557. [DOI] [PubMed] [Google Scholar]
- 29.Vandenput I, Capoen A, Coenegrachts L, et al. Expression of ERCC1, p53, and class III β-tubulin do not reveal chemoresistance in endometrial cancer. Int J Gynecol Cancer. 2011;21(6):1071–1077. doi: 10.1097/IGC.0b013e318218f28b. [DOI] [PubMed] [Google Scholar]
- 30.Bollag DM, McQueney PA, Zhu J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 31.Mozzetti S, Iantomasi R, De Maria I, Pri, et al. Molecular mechanisms of patupilone resistance. Cancer Res. 2008;68:10197–101204. doi: 10.1158/0008-5472.CAN-08-2091. [DOI] [PubMed] [Google Scholar]
- 32.Nobili S, Landini I, Mazzei T, Mini E. Overcoming tumor multidrug resistance using drugs able to evade p-glycoprotein or to exploit its expression. Med Res Rev. 2011 doi: 10.1002/med.20239. [DOI] [PubMed] [Google Scholar]
- 33.Leonard GD, Fojo T, Bates SE. The role of ABCB1 transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 34.Tavassoli FA, Devilee P, editors. World Health Organization: Tumours of the Breast and Female Genital Organs. Chapter 2. Lyon: IARC Press; 2003. Tumours of the Ovary and Peritoneum; pp. 113–145. [Google Scholar]
- 35.El-Sahwi K, Bellone S, Cocco E, et al. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer. 2010;102(1):134–143. doi: 10.1038/sj.bjc.6605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tassi RA, Bignotti E, Rossi E, et al. Overexpression of mammaglobin B in epithelial ovarian carcinomas. Gynecol Oncol. 2007;105:578–585. doi: 10.1016/j.ygyno.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 37.Carrara L, Guzzo F, Roque DM, et al. Differential in vitro sensitivity to patupilone versus paclitaxel in uterine and ovarian carcinosarcoma cell lines is linked to tubulin-beta-III expression. Gynecol Oncol. 2012;125(1):231–236. doi: 10.1016/j.ygyno.2011.12.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian Q, Stepaniants SB, Mao M, et al. Integrated genomic and proteomic analyses of gene expression in mammalian cells. Mol Cell Proteomics. 2004;3:960–969. doi: 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Cross SN, Cocco E, Bellone S, et al. Differential sensitivity to platinum-based chemotherapy in primary uterine serous papillary carcinoma cell lines with high versus low HER-2/neu expression in vitro. Am J Obstet Gynecol. 2010;203(2):162e1–8. doi: 10.1016/j.ajog.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellone S, Watts K, Cane S, et al. High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer. Gynecol Oncol. 2005;98:92–98. doi: 10.1016/j.ygyno.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Niua XL, Qu Y, et al. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295:110–123. doi: 10.1016/j.canlet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Kuo DY, Mallek S, Shen H, et al. Analysis of MDR1 expression in normal and malignant endoemtrium by reverse-transcription polymerase chain reaction. Clin Cancer Res. 1996;2:1981–1992. [PubMed] [Google Scholar]
- 43.Kodama J, Ryoji H, Okuda H, et al. Reverse correlation between P-glycoprotein expression and proliferative activity in endometrial adenocarcinoma. Eur J Obstet Gynecol Rep Biol. 1995;59:45–51. doi: 10.1016/0028-2243(94)02024-9. [DOI] [PubMed] [Google Scholar]
- 44.Umezu T, Shibata K, Kajiyama H, et al. Taxol resistance among the different histological subtypes of ovarian cancer may be associated with the expression of class III β-tubulin. Int J Gynaecol Pathol. 2008;27(2):207–122. doi: 10.1097/PGP.0b013e318156c838. [DOI] [PubMed] [Google Scholar]
- 45.Wang P, Henning SM, Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLOS One. 2010;5(4):e10202. doi: 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gan PP, Pasquier E, Kavallaris M. Class III tubulin mediates sensitivity to chemotherapeutic drugs in non-small cell lung cancer. Cancer Res. 2007;67:9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 47.Gan PP, McCarroll JA, Bryne FL, et al. Specific-β-III tubulin isotypes can functionally enhance or diminish epothilone B sensitivity in non-small cell lung cancer cells. PLOS One. 2011;6(6):e21717. doi: 10.1371/journal.pone.0021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boruta DM, Gehrig PA, Groben PA, et al. Uterine serous and grade 3 endometrioid carcinomas: is there a survival difference? Cancer. 2004;101:2214–2221. doi: 10.1002/cncr.20645. [DOI] [PubMed] [Google Scholar]
- 49.Zhu c, Luo J, Shi H, Xie X, Ding Z. Expression of tubulin, p53, ki67, receptor for estrogen, and progesterone in endometrial cancer. Eur J Gynaecol Oncol. 2009;30:514–7. [PubMed] [Google Scholar]
- 50.Dizon DS, Blessing JA, McMeekin DS, et al. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: Gynecologic Oncology Group Trial 129-P. J Clin Oncol. 2009;27:3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin EH, Rothermel J, Tesfaye F, et al. Phase I dose-finding study of weekly single-agent patupilone in patients with advanced solid tumors. J Clin Oncol. 2005;23:9120–9129. doi: 10.1200/JCO.2005.03.0981. [DOI] [PubMed] [Google Scholar]
- 52.O’Reilly T, Wartmann M, Brueggen J, et al. Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother Pharmacol. 2008;62:1045–1054. doi: 10.1007/s00280-008-0695-9. [DOI] [PubMed] [Google Scholar]