Abstract
Objectives
Studies have shown that patients harboring bicuspid aortic valve (BAV) or bovine aortic arch (BAA) are more likely to develop ascending aortic aneurysm (AsAA) than the general population. A thorough quantification of the AsAA tissue properties for these patient groups may offer insight into the underlying mechanisms of AsAA development in these patients. Thus, the objective of this study was to investigate and compare the mechanical and microstructural properties of aortic tissues from AsAA patients with and without concomitant BAV or BAA.
Materials and methods
AsAA (n = 20), BAV (n = 20) and BAA (n = 15) human tissues were obtained from patients who underwent elective AsAA surgery. Planar biaxial and uniaxial failure tests were used to characterize the mechanical and failure properties of the tissues, respectively. Histological analysis was performed to detect the medial degenerative characteristics of aortic aneurysm. Individual layer thickness and composition were quantified for each patient group.
Results
The circumferential (CIRC) response of the BAV samples was stiffer than both AsAA (p = 0.473) and BAA (p = 0.152) tissues at low load. The BAV tissues were nearly isotropic while AsAA and BAA tissues were anisotropic. The areal strain of BAV samples were significantly less than AsAA (p = 0.041) and BAA (p = 0.004) tissues at a low load. The BAA samples were similar to the AsAA samples in both mechanical and failure properties. On the microstructural level, all samples displayed moderate medial degeneration characterized by elastin fragmentation, cell loss, mucoid accumulation and fibrosis. The ultimate tensile strength of BAV and BAA tissues were also found to decrease with age.
Conclusions
The BAV tissues were stiffer than both AsAA and BAA tissues, and the BAA tissues were similar to the AsAA tissues. The BAV samples were thinnest with less elastin than AsAA and BAA samples, which may attribute to the loss of extensibility at low load of these tissues. No apparent difference in failure mechanics among the tissue groups suggests that each of the patient groups may have a similar risk of rupture.
INTRODUCTION
Thoracic aorta aneurysms represent the 18th most common cause of death in adults [1]. Approximately 6–9% of people age 65 and older in the Western world have an aortic aneurysm [2]. There are a number of conditions that have been linked to aortic dilation including Marfan syndrome, Loeys-Dietz syndrome, Ehlers-Danlos syndrome type IV, arterial tortuosity syndrome, autosomal dominant polycystic kidney disease, and autosomal recessive cutis laxa type 1 [3]. In addition to these disorders there appears to be a strong genetic link associated with aortic aneurysms, as up to 20% of patients referred for thoracic aortic aneurysm or dissection have family members with the same condition [1].
The development of ascending aortic aneurysms (AsAA) may also be linked to anatomical anomalies. It is well known that patients with a bicuspid aortic valve (BAV, a condition in which two of the three aortic leaflets fuse together) are more likely to develop AsAA than patients with a normal, tricuspid aortic valve. In the United States, approximately four million people harbor a BAV, making BAV the most common congenital malformation [4]. Approximately 50% of BAV patients will develop an AsAA and 5% will experience aortic dissection in their lifetime [5]. There is some debate on whether the high prevalence of ascending aortic aneurysms in BAV patients is due to abnormal aortic hemodynamic stresses or intrinsic material property differences rendering the aortic wall weaker than in normal patients [6–8].
More recently, a link between bovine aortic arch anatomy and thoracic aortic aneurysms has also been suggested [9]. In the majority of patients (74%), there are three great vessels branching from the aortic arch: the innominate artery, the left carotid artery, and the left subclavian artery [10]. However, there are many other possible anatomic configurations of the aortic arch – the most common being the “bovine aortic arch” (BAA) configuration, in which the innominate and the left carotid arteries originate from a common stem off the aortic arch [10]. Although, BAA is generally thought to be a benign anatomical difference, recent research shows the incidence of BAA dissection is significantly higher among patients with an AsAA than among the general population [9]. Again, it is unclear why BAA patients may be more susceptible to AsAA formation. This may be due to altered hemodynamics or possible innate aortic tissue property differences in these patients.
Rupture of the aortic wall is generally accepted as mechanical failure of the vessel due to a combination of excessive hemodynamic forces [3, 11–13] and degeneration of the medial wall [6, 8, 14–17]. The microstructural components, mainly elastin, collagen fibers and smooth muscle cells (SMCs) play an important role in maintaining proper structure and function of the aortic wall. Damage to the elastic fibers and aortic dilation might cause increased wall stiffness and stress. Eventually, acute aortic dissection and rupture can occur in response to certain dramatic events such as a spike in blood pressure during intense physical or emotional exertion [1]. Therefore, an understanding of the elastic properties and the microstructure of the vessel wall is important in predicting the risk of wall rupture.
Studies have shown that patients harboring BAV or BAA are more likely to develop ascending aortic aneurysm than the general population, however, a thorough quantification and comparison of the AsAA tissue properties for these patient groups, to our knowledge, has not been reported in the literature. Such information may offer insight into the underlying mechanisms of AsAA development in these patients. Thus, the objective of this study was to investigate and compare the mechanical and microstructural properties of aortic tissues from AsAA patients with and without concomitant BAV or BAA.
MATERIALS AND METHODS
Clinical data and aortic specimens
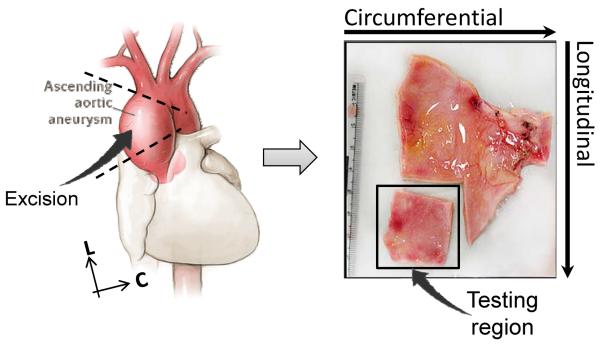
Aortic specimens were obtained from a total of 55 patients who underwent elective ascending aortic aneurysm surgery at Yale-New Haven Hospital between December 2008 and September 2010. Patients who experienced aortic dissection prior to surgery were excluded from the study. The use of human tissues in this study was approved by the Research Compliance Office of the University of Connecticut. The patients were divided into three groups: AsAA - patients without BAV or BAA (n = 20), BAV – AsAA patients with BAV but without BAA (n = 20), and BAA – AsAA patients without BAV but with BAA (n = 15). The dilated aortic section from the patients was excised during surgery, from which a small sample size was removed and preserved in a −80°C freezer, see Fig. 1. All samples were then transported to the laboratory and cryopreserved [18] at −80°C until mechanical testing.
Figure 1.
Illustration of a representative testing sample and its orientation with respect to the excising region of the ascending aortic aneurysm. C – circumferential, L – longitudinal.
Mechanical Tests
1) Planar biaxial mechanical test
Frozen samples were submerged in a 37°C water bath until totally defrosted, following the two-stage slow thawing method to remove the cryopreserving agent [18]. The tissue thickness was measured at six regions with a thickness gauge (Mitutoyo, Model 7301) and the average value was recorded. Each specimen was then biaxially tested according to the methods previously presented [19]. Briefly, all specimens were trimmed into square specimens with a side length of ~20 – 25 mm and mounted in a trampoline fashion, with the specimen circumferential (CIRC) and longitudinal (LONG) directions aligned with the primary axes of the biaxial test fixture. All specimens were tested in a Ca2+-free and glucose-free Tyrode solution (mM: NaCl 136.9, KCl 2.7, MgCl2 1.05, NaHCO3 11.9, NaHPO4 0.47, EGTA 2.0, and 0.1M papaverine) at 37°C. A stress-controlled test protocol was utilized [20] and converted to the first Piola Kirchhoff stress, P, wherein the ratio of CIRC (11) to LONG (22) that is P11 : P22 was kept constant with the shear terms P12 = P21 = 0. All specimens were stretched to a maximum membrane tension value of 120 N/m (or ~300g) [21], above which samples might be torn in biaxial testing experiments. Preconditioning was performed to minimize tissue hysteresis. Each tissue specimen was preconditioned for at least 40 continuous cycles with P11 : P22 = 1 : 1. Seven successive protocols were performed using ratios P11 : P22 = 1 : 0.3, 1 : 0.5, 1 : 0.75, 1 : 1, 0.75 : 1, 0.5 : 1, and 0.3 : 1. This range was chosen for extensive coverage of the in-plane stress state [20]. The in-plane Green strain tensor E was calculated from the deformation gradient, F, using E = ½(FTF – 1). The tensor P was calculated using P = f/(TL) where f are the current measured loads in each direction, T is the initial thickness of the specimen, and L are the unloaded reference specimen dimensions in the CIRC and LONG directions. The second Piola-Kirchhoff stress tensor S was obtained using S = F−1P.
The tissue stiffness was quantified by means of the secant modulus at both the low (60 N/m) and high membrane tension (120 N/m) regions under the equibiaxial loading protocol (P11 : P22 = 1 : 1). The extensibility of the samples was calculated via the areal strain equation, e = λ11,max λ22,max – 1, where λ11,max and λ22,max are the circumferential and longitudinal peak stretch values from the equi-biaxial protocol, respectively. The degree of anisotropy (DA) was analyzed using the ratio of peak Green strains DA = E11,max/E22,max. A DA value of 1 indicates an isotropic tissue response, whereas other values represent various degrees of anisotropy.
2) Uniaxial failure test
Following the biaxial test, each specimen was cut into strips about 15 mm × 5 mm in both the circumferential and longitudinal directions. In some cases only one uniaxial specimen was prepared in either the circumferential or the longitudinal direction due to limited tissue size. The specimens were tested with a Tinius Olsen uniaxial machine (Horsham, PA). The force and deformation in terms of stretch were measured continuously as the specimen was loaded to failure. The ultimate tensile strain (UTE) and strength (UTS) were determined from the maximum tension which correlated to the complete rupture of the specimen.
Histological analysis
A total of 10 samples in each group were selected for qualitative histological analysis of the medial degenerative characteristics of aortic aneurysm. The central region of each specimen was cut out and fixed in 10% formalin for 24 hours prior to histological processing. After a series of dehydration steps by varying alcohol concentrations, samples were embedded in paraffin, sectioned at 5 μm in thickness and then mounted on microscope slides. After deparaffinization, slides were stained with Verhoeff Van-Gieson (VVG) and Movat pentachrome stains. The VVG stain distinguishes elastic fibers as black, collagen fibers as red, and muscle fibers as a duller red or light brown color, and Movat stain is for visualization of blue mucoid material. The hematoxyline and eosin (H&E) stain was also used to visualize the nuclei of SMCs. Qualitative analysis of degenerative features of the aneurysmal tissues was performed through images obtained from an Olympus U-TVO.5xC digital camera coupled to an Olympus BX40 light microscope.
The tissue microstructure was quantitatively assessed from a histological section from the central testing region. The thickness measurements of each layer and the area fractions of elastin and collagen fibers were measured from the 2D images of histological slides using ImageJ software (Bethesda, MD). The intima, media and adventitial layer thicknesses were measured at 5 locations along the specimen length and the average values were obtained. The percent thickness of each layer was calculated relative to the overall wall thickness. The elastin, collagen and SMC/matrix components of the media layer were quantified using the color segmentation method by splitting the color channels and thresholds. The three fields of view (FOV) with equivalent area across the media thickness were selected (per 2D image). The area fractions of each component were then obtained by averaging the relative content measured from the three selected FOVs.
Statistical analyses
All measurements are presented as a mean ± standard deviation. The analysis of variance (ANOVA) test followed by the Holm-Sidak test and the Dunn's Method test were used to compare between groups. The Student's t-test was used to determine significant differences between the mean values for two variables. For comparison between circumferential and longitudinal responses, the paired Student's t-test was employed. Non-parametric tests, including the Wilcoxon signed-rank test and Mann-Whitney rank sum test, were utilized for non-normally distributed sample groups. Outliers were identified and removed when necessary for comparison across groups. Correlation was determined using the Pearson's correlation coefficient (r) and the non-parametric Spearman Rank Order. A probability, p-value, less than 0.05 was considered to indicate a statistically significant difference between the groups, with a p < 0.001 indicating high statistical significance. Statistical analyses were performed using SigmaPlot (V11.0, Systat Software Inc., San Jose, CA).
RESULTS
Sample characteristics
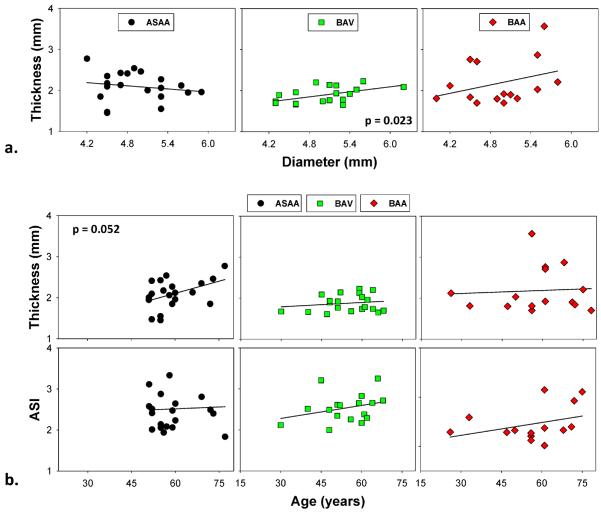
Table 1 summarizes the clinical patient data. There were no significant differences in age (p = 0.417), diameter (p = 0.877) or Aortic Size Index (ASI) [1] (p = 0.402) between the three patient groups. On average, the diameters were below 5.5 mm, and the ASI values were below the moderate risk level for all groups (see [1] for the ASI risk level classification). The percentages of hypertensive patients (systolic pressure higher than 140 mmHg) for AsAA patients was 20% (n = 4), 25% (n = 5) for BAV patients and 13% (n = 2) for BAA patients. The mean thickness of the BAV samples (1.88 ± 0.20 mm) was significantly lower than that of the AsAA samples (2.09 ± 0.35 mm, p = 0.021), but there was no difference with respect to the BAA samples (2.18 ± 0.55 mm, p = 0.072). The mean thickness of the ASAA and BAA samples were not significantly different, p = 0.566. Significant positive correlation between thickness and diameter was observed in the BAV group, r = 0.52, p = 0.023 (Fig. 2-a). No strong correlation was found in the AsAA and BAA patient groups. From Fig. 2-b, it appears that thickness and ASI increase with age, however, the correlation coefficients were not significant.
Table 1.
Summary of patients' information.
| ASAA | BAV | BAA | p-value | |
|---|---|---|---|---|
| All age (n) | 59.45 ± 7.86 (20) | 55 ± 9.65 (20) | 58.07 ± 14.70 (15) | 0.417 |
| M:F | 3:1 | 3:1 | 11:4 | - |
| Diameter (mm) | 4.94 ± 0.48 | 5.01 ± 0.50 | 4.93 ± 0.53 | 0.877 |
| ASI | 2.51 ± 0.58 | 2.54 ± 0.35 | 2.39 ± 0.37 | 0.402 |
| Hypertension (n,%) | 4 (20%) | 5 (25%) | 2 (13%) | - |
Figure 2.
(a) Diameter and thickness relationship of ASAA, BAV and BAA patient groups, the BAV group showed a strong correlation (p = 0.02); (b) a consistent trend of increasing thickness and ASI with increasing age in all groups, the ASAA group showed a near significant correlation between thickness and age (p = 0.052).
Biaxial responses
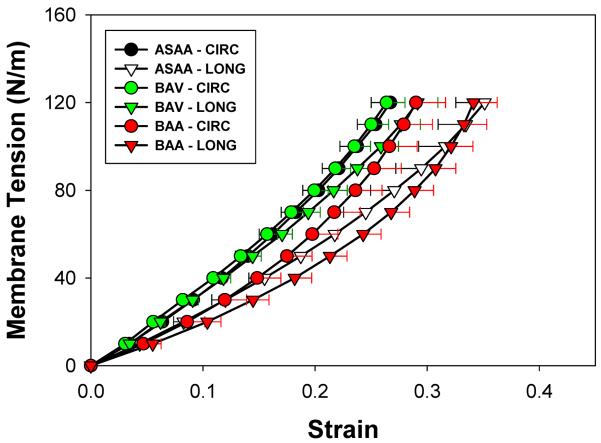
The mean equibiaxial tension-strain curves in the CIRC and LONG directions for the three patient groups are shown in Fig. 3. The mean AsAA and BAA curves exhibited a somewhat nonlinear characteristic upon a peak tension of 120 N/m, with the CIRC direction being stiffer than the LONG direction, while the BAV response was more isotropic and linear. Both directional responses of the BAV group were stiffer than those of the AsAA and BAA groups. The LONG directional responses of AsAA and BAA groups were similar.
Figure 3.
Mean and standard error equibiaxial responses of ASAA, BAV and BAA patient groups in the circumferential (CIRC) and longitudinal (LONG) directions.
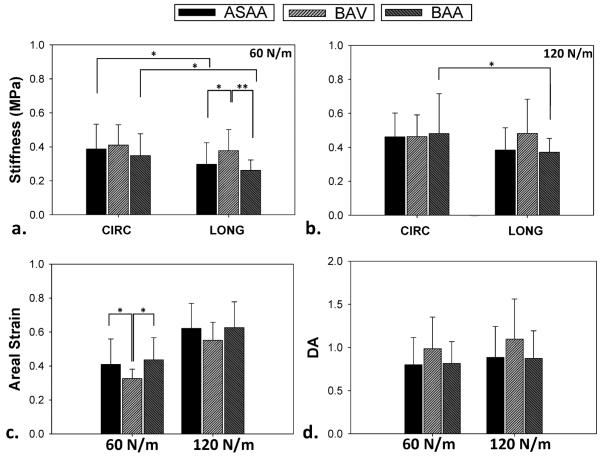
A significant difference in tissue stiffness was observed in the LONG direction at 60 N/m membrane tension where the BAV group was significantly stiffer than the AsAA group (p = 0.024) and the BAA group (p < 0.001), see Fig. 4. A similar trend was observed at 120 N/m between the BAV and AsAA groups (p = 0.072) and between the BAV and BAA groups (p = 0.072). For the CIRC tissue stiffness, the BAV group showed the highest stiffness compared to the AsAA (p = 0.473) and BAA (p = 0.152) groups at 60 N/m, but the stiffness values at 120 N/m were similar among all the groups (p = 0.890, one-way ANOVA). The CIRC direction was stiffer than the LONG direction for the AsAA (p = 0.012 at 60 N/m, p = 0.068 at 120 N/m) and BAA (p = 0.004 at 60 N/m, p = 0.025 at 120 N/m) samples, but not for the BAV samples (p = 0.407 at 60 N/m, p = 0.744 at 120 N/m).
Figure 4.
Comparison of the mean circumferential (CIRC) and longitudinal (LONG) stiffnesses at (a) low (60 N/m) and (b) high (120 N/m) membrane tensions between ASAA, BAV and BAA patient groups; (c) mean extensibility and (d) mean degree of anisotropy (DA) of ASAA, BAV and BAA tissue samples at low and high membrane tensions. (*) indicates a statistical significance with p < 0.05, (**) indicates a highly statistical significance with p < 0.001. Data presented as mean and standard deviation.
The mean areal strain of the BAV samples was significantly less than the AsAA (p = 0.041) and BAA (p = 0.004) tissues at a low membrane tension, see Fig. 4-c. The mean DA values of AsAA, BAV, and BAA samples indicated that the BAV specimens were more isotropic than the AsAA and BAA tissue samples, see Fig. 4-d.
Uniaxial mechanical responses
No significant difference in the mean UTE was found between the CIRC and LONG directions or across the groups (Fig. 5-a). The mean CIRC UTS values were larger than the mean LONG UTS in all sample groups, but the difference was significant only in the AsAA group (p = 0.016). The mean LONG UTS of the BAV group was significantly higher than the AsAA group (p = 0.006) (Fig. 5-b).
Figure 5.
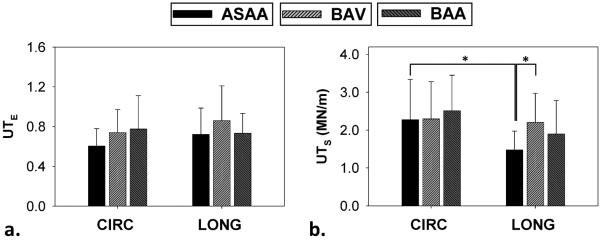
Ultimate tensile (a) strain and (b) tension for ASAA, BAV and BAA patient groups. (*) indicates a statistical significance with p < 0.05. Data presented as mean and standard deviation.
Age and mechanical data correlation
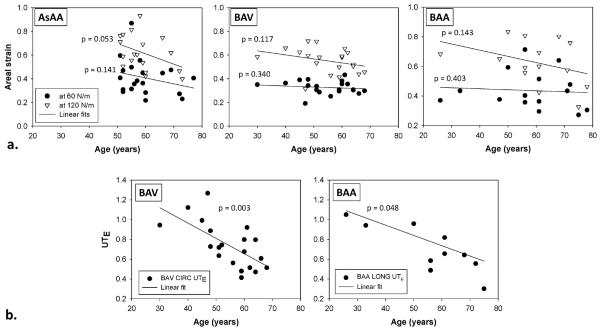
No significant correlation was found between age and biaxial mechanical data in all three groups; however, a trend was observed in all groups where the areal strain decreased with age (Fig. 6-a). There was no strong correlation between age and uniaxial data for the AsAA group. However, for the BAV group, the ultimate CIRC UTE significantly decreased with age, r = −0.630, p = 0.003 (Fig. 6-b). For the BAA group, the UTE decreased significantly with age in the LONG direction (r = −0.622, p = 0.048) but not in the CIRC direction (p = 0.060).
Figure 6.
(a) The relationship between age and areal strain at 60 and 120 N/m for AsAA, BAV and BAA patient groups, and (b) a significant negative correlation between ultimate tensile strain and age was observed in the circumferential (CIRC) direction of BAV samples and longitudinal (LONG) direction of BAA samples.
Hypertension
The LONG stiffness of the AsAA hypertensive group was significantly higher than that of the normotensive, with p = 0.017 at 60 N/m (see Table 2). Similarly, a significant difference between hypertensive and normotensive BAA groups was only observed in the LONG stiffness at 60 N/m (p = 0.049). Although statistical significance was not achieved, in general, the mean directional stiffness of hypertensive groups was greater than the normotensive groups (see Table 2) for both the AsAA and BAA groups. Although BAV samples did have on average the highest stiffness at both low and high membrane tensions, there was no significant difference between hypertensive and normotensive properties. This could be due to the highly variable data and small sample sizes. Comparison between the hypertensive and normotensive failure properties were not conducted due to small sample sizes.
Table 2.
Comparison of stiffness at 60 N/m tension between hypertensive and normotensive patients among AsAA, BAV and BAA patient groups.
| Stiffness at 60 N/m | ||||||
|---|---|---|---|---|---|---|
| n | HTN | n | NTN | p | ||
|
|
||||||
| ASAA | Circ | 3 | 453.45 ± 193.78 | 17 | 269.54 ± 96.30 | 0.427 |
| Long | 3 | 467.65 ± 275.42 | 17 | 369.89 ± 89.00 | 0.017* | |
| BAV | Circ | 5 | 375.88 ± 117.39 | 15 | 422.16 ± 121.27 | 0.466 |
| Long | 5 | 316.96 ± 294.62 | 15 | 345.62 ± 305.16 | 0.513 | |
| BAA | Circ | 2 | 379.42 ± 55.56 | 13 | 344.48 ± 136.12 | 0.732 |
| Long | 2 | 339.94 ± 43.60 | 13 | 250.15 ± 55.19 | 0.049* | |
CIRC – Circumferential, LONG – Longitudinal, HTN – Hypertensive, NTN – Normotensive.
indicates a statistical significance.
Histology
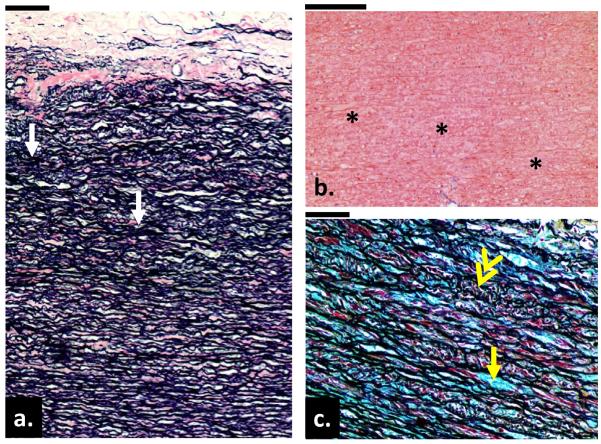
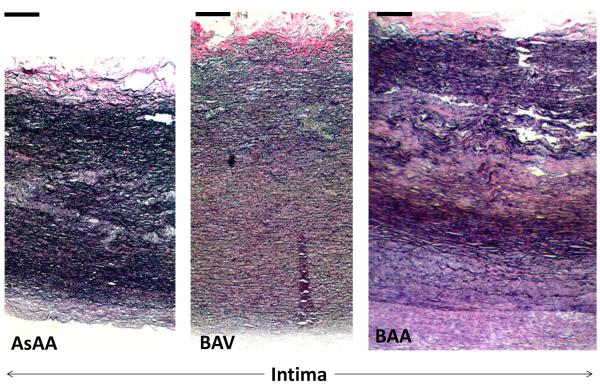
The microstructure of the aneurysmal aortic tissues from 10 samples in each group was qualitatively analyzed from the two-dimensional images. Histologic changes including cystic medial necrosis (CMN) or medial degeneration often occur in the AsAA medial wall. Within the degenerated region, disruption and disorganization of the elastic lamella resulting in collapsed lamella and elastic fiber fragmentation, focal loss of smooth muscle cell (SMC) and accumulation of mucoid material and/or the presence of cysts within basophilic substances are typically found [22–25]. Figure 7-a shows the circumferential cross section of the aortic wall from a 58-year-old patient with AsAA only. This sample exhibited the histopathologic features of tissue degeneration. According to the grading system by de Sa et al. [26], the sample had a grade 2 or a moderate degree of SMC loss, resulting in several collapsed elastic lamella (Fig. 7-a, as arrows). Loss of SMCs, indicated by the absence of the cell nuclei (see Fig. 7-b), were noted in all samples analyzed. A representative BAV sample from a 56-year-old patient is shown in Fig. 7-c, where Movat pentachrome stain revealed mucoid accumulation (grade 1 according to [26] or mild) between the elastic lamella (yellow arrows) and smooth muscle cell reorientation (double-head arrow). The most severe (or grade 3 [26]) samples for each group were displayed in Fig. 8. These samples exhibited extensive medial damages including fragmentation and focal loss of elastin fibers and SMCs, increase of ground substances or mucoid materials. Intimal and adventitial thickening of the aorta wall due to fibrosis characterized by an increase in collagen, were also observed. In summary, 20% of both AsAA and BAA samples exhibited severe degeneration, while the remaining samples displayed mild to moderate degeneration. Only one BAV sample (see Fig. 8) displayed signs of severe damage while the majority of samples had only mild to moderate damage. None of the hypertensive AsAA and BAA aortas had any severe medial wall damage, while the severely damaged BAV sample was from a hypertensive patient. No conclusion could be drawn for the hypertension effect since only a small number of hypertensive patients were histologically analyzed.
Figure 7.
(a) A circumferential cross section of the aortic wall from a 58-year-old patient with AsAA only. Note the changes in the elastic lamella including collapsed lamella (yellow arrows) and fragmentation of elastic fibers (white arrow). Image was stained with Verhoeff Van-Gieson stain. (b) Evidence of focal loss of SMC cells through loss of cell nuclei (asterisks) in the media layer from an 80-year-old AsAA only patient. (c) A cross section of the aortic wall from a 56-year-old patient with BAV and aortic stenosis. There are evidences of mucoid accumulation in between the elastic lamella (yellow arrow) throughout the media layer and the disorientation of the smooth muscle cells (double-head arrow). Image was stained with Movat pentachrome. (Bar = 200μm)
Figure 8.
A circumferential cross section through the thickness of aortic walls from (a) a 52-year-old AsAA patient (b) a 61-year-old BAV patient and (c) a 75-year-old BAA patient. Severe damages of the elastic lamella and intima thickening due to fibrosis were observed in these images. Image was stained with Verhoeff Van-Gieson stain. (Bar = 200μm).
Figure 9-a displays the percentiles of each layer thickness for AsAA, BAV and BAA groups. The BAV specimens displayed a larger media thickness than AsAA and BAA (p = 0.024) samples. Conversely, the intima thickness of BAV specimens was significantly thinner than that of the AsAA specimens (p = 0.045). Strong correlations between the dilated diameter and BAA media thickness (r = −0.736, p = 0.02) and BAA intima thickness (r = 0.728, p = 0.02) were found. The adventitial thickness was similar in all sample groups. Differences between the elastin, collagen and SMC and matrix contents between sample groups were not significant. However, a higher area density of elastin was found in BAA samples compared to AsAA and BAV samples. Less SMC and matrix contents were found in BAA samples.
Figure 9.
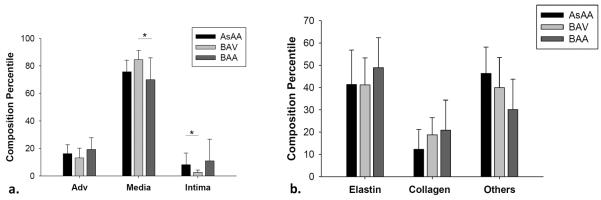
(a) The percentiles of intima, media and adventitial layers in the AsAA (n = 10), BAV (n = 10) and BAA (n = 9) patient groups, (b) the percentiles of elastin, collagen and SMCs and ground substances in the media layer of each patient group.
DISCUSSION
In this study, we investigated the mechanical and microstructural properties of aortic tissues from patients harboring ascending aortic aneurysm (AsAA) with and without concomitant bicuspid aortic valve (BAV) or bovine aortic arch (BAA) for comparison across patient groups.
Tissue properties of AsAA
There is limited data on the mechanical properties of the dilated ascending aorta subjected to planar biaxial testing. Choudhury et al. [27] performed biaxial mechanical tested in five AsAA samples but only in the circumferential direction. Their mean strain modulus at high stress of about 0.45 MPa was similar to our circumferential AsAA stiffness. Another study was done by Okamoto et al. [28] who showed biaxial stress-strain responses for one of their 65 year old AsAA patients from which the moduli were approximately 0.5 MPa in both directions.
The tensile strengths for AsAA samples obtained from uniaxial tensile test were previously reported in the literature. Vorp et al. [11] reported the mean tensile strength of their AsAA samples in the CIRC and LONG directions as 1.18 ± 0.12 and 1.21 ± 0.09 MPa, respectively. Garcia-Herrera et al. [29] found the tensile strengths of their AsAA samples to be 1.19 ± 0.13 MPa circumferentially and 0.88 ± 0.12 MPa longitudinally. The mean failure stresses of AsAA specimens found by Iliopoulos et al. [30] were about 1.70 and 1.00 MPa in the CIRC and LONG directions, respectively. The mean tensile strengths of our AsAA samples (2.27 and 1.47 MN/m, respectively) demonstrated that the tissues were stronger in the CIRC direction but similar in the LONG direction compared to other studies.
Bicuspid aortic valve effect
Overall, our study indicates that aneurysmal BAV tissues were different in both mechanical and structural properties compared to the AsAA and BAA groups. BAV samples were stiffer than the AsAA and BAA samples in both directions. Similarly, Choudhury et al. [27] reported in their study that BAV tissues were significantly stiffer than AsAA tissues at low strain, and Duprey et al. [31] also found significantly higher stiffness in the CIRC BAV response compared to their AsAA group.
BAV samples were also found to be isotropic than AsAA and BAA samples with a mean degree of anisotropy approaching 1 (0.99 ± 0.37 and 1.10 ± 0.47, p = 0.756, at low and high membrane tensions, respectively). Our findings are in agreement with Choudhury et al. and Okamoto et al. [28] who report no significant anisotropy in BAV samples. However, Duprey et al. [31] found anisotropy in the uniaxial responses of BAV tissues with the CIRC being stiffer than the LONG direction, and Garcia-Herrera et al. [29] reported a statistically significant difference in the directional responses in their BAV samples. Note that both Duprey et al. and Garcia-Herrera et al. determined BAV tissue anisotropy from uniaxial tensile tests rather than biaxial tests.
The altered mechanical properties of the BAV tissues may also be explained in part by differences in the underlying microstructure. The overall thickness of the BAV samples was found to be smallest compared to other groups, which could possibly contribute to its relatively high stiffness. However, the BAV media was thicker than that of AsAA and BAA samples. We found positive correlation between diameter and thickness in BAV tissues, which may indicate tissue remodeling to reduce the wall stress. Despite having the highest medial thickness among the sample groups, the BAV samples had the lowest elastin content, which correlates well with other reports of less elastin in the BAV aortic wall compared to that associated with normal tricuspid valves [23, 27]. The lower elastin content along with the evidence of elastin fragmentation in the BAV samples may be the result of an underlying pathology in BAV aortas and may explain the loss of extensibility at low load.
Interestingly, the tensile failure properties of BAV samples were similar to those of the AsAA and BAA groups despite the differences in mechanical properties and tissue structure. Although, patients with BAV are more likely to experience aortic dissection and rupture during their lifetimes [32], our tensile failure test results suggest that the strength of AsAA tissue is comparable in patients with and without BAV. This is in line with the conclusion of Tadros et al. [23] that BAV-associated ascending aortic aneurysms dissect and rupture at a size comparable to that of aneurysms due to other etiologies. Hence, higher risk of dissection and rupture in BAV patients may be due to the higher prevalence and earlier onset age of aortic dilation in these patients [23].
Bovine aortic arch effect
The epidemiological linkage between aneurysm and BAA was investigated by Malone et al [33] who found a statistically strong association between BAA and AsAA in patients over 70 years old and concluded that BAA should be considered a potential risk factor for thoracic aortic aneurysm. To our knowledge, this is the first study aimed to characterize the mechanical properties of aneurysmal aortic tissues in BAA patients. The BAA tissues were anisotropic with the CIRC direction being significantly stiffer than the LONG direction. We found similar mechanical and failure properties between the AsAA and BAA samples. However, the underlying trigger for ascending aortic dilation in BAA patients remains unknown.
The quantitative histological analyses showed no significant differences in medial thickness and fiber composition between BAA and AsAA tissues; however, the BAA samples had the highest elastin and collagen contents. The BAA samples also had the highest intimal and adventitial thicknesses. The medial layer, in contrast, was smallest among the groups. Moreover, there was a strong negative correlation between the medial thickness and the overall diameter, and a strong positive correlation between the intimal thickness and diameter. It may be necessary to test and compare the non-aneurysmal ascending aorta tissues in BAA and healthy patients to determine whether BAA tissues are innately different than normal.
Aging effect
Aging arteries progressively stiffen and dilate [34]. As expected, we found a consistent trend of decreasing areal strain with increasing patient age across the groups. The ultimate tensile strength of BAV and BAA tissues were also found to decrease with age. Note that the comparatively narrow age range of the AsAA patient group may have prevented us from determining an age-related decline in strength.
Hypertension effect
Prolonged hypertension generally provokes medial degeneration [35, 36] and worsen mechanical properties of the vessel wall [37, 38]. We did not see a distinct difference in the medial wall of hypertensive patients in any group. However, there was a significant increase in tissue stiffness in the longitudinal direction in hypertensive AsAA and BAA patients compared to normotensive AsAA and BAA patients. There was no difference in stiffness between the hypertensive and normotensive BAV patients. Currently there is some debate on the relationship between increased aortic stiffness and blood pressure: it may be a positive feedback relationship. It is suggested that the stiffening and dilation of the aorta act to increase systolic and decrease diastolic blood pressure; however, the initial cause of tissue stiffening may be increased systolic pressure [39]. There seems to be a linkage between severity of aortic stenosis and hypertension [40] but it cannot be determined in this study because only a small number of patients in each group acquired aortic stenosis (n < 3). There was no difference in stiffness between the hypertensive and normotensive BAV patients. Again, larger sample sizes are needed to achieve a desired level of statistical power for detecting any pathological effect on the aortic mechanical properties.
Limitation and future study
The comparison between aneurysmal tissues would be more accurate if the tissue was tested fresh, however, fresh human aneurysmal tissue is difficult to obtain. In this study, we utilized the cryopreservation technique for tissue storage which has been shown to minimize damage to the elastic components of blood vessels [18]. Our results were comparable to the results reported in the literature for tissue mechanics and strength as mentioned earlier. A dog-bone shaped sample is recommended for minimizing the boundary effect and homogenizing stress at the middle section. However, due to limited sample size, we were not able to cut the samples into a dog-bone shape, which might affect the failure stress. Other limitations of this study include the small sample sizes. Degenerative changes in the aortic wall involve complex and variable processes, and thus a larger sample size is required to determine the mechanisms of disease and its correlation with various clinical entities. A larger sample size and an extended age range could possibly demonstrate some age related differences between the groups. Other methods have been implemented for studying aneurysmal aorta tissues [41–43] where an initial tear was created in the intima layer and either force or pressure was applied to study the dissection propagation as hemodynamic forces increased. However, we did not focus on the initial tear or the dissection parameter in this study; instead we compared the tissue ultimate tensile strength between the three groups. Additionally, the quantitative analyses of specific layer thickness did not account for shrinkage after histological processing. The fiber contents were approximated based on the 2-D cross section areas at the central testing regions, thus did not represent the volume content.
We did see subtle differences in the structural layers and fiber composition between groups. Future work may include mechanical tests of each layer of the aortic wall which could possibly reveal some differences between the patient groups. Moreover, the linkages between the extracellular matrix proteins, wall remodeling and tissue mechanics in these samples require a parallel comprehensive bio-molecular study, which may help to provide a better understanding of the underlying pathologies responsible for weakening the aortic wall.
CONCLUSION
In this study, we investigated the biomechanical properties of ascending aorta aneurysmal tissues with and without bicuspid valve and bovine aortic arch conditions. We found that the BAV tissues were stiffer than both AsAA and BAA tissues, and the BAA tissues were similar to the AsAA tissues. Tissue anisotropy was found in AsAA and BAA tissues but not BAV tissues. Our histological analyses showed local aortic wall abnormalities characterized by elastin fragmentation, cystic medial necrosis, loss of smooth muscle cells and accumulation of mucoid ground substances within the medial layer. The BAV samples were thinnest with less elastin than AsAA and BAA samples, which may have attributed to the altered mechanical properties of these tissues, i.e. loss of extensibility at low load. Despite these differences, there was no apparent difference in the tissue strength found between the groups, which suggest that each of the patient groups may have a similar risk of rupture. The present findings might help to better understand the underlying mechanisms of dilation in BAV and BAA patients and aid in developing numerical and computational models for improved assessment and prediction of thoracic aortic aneurysmal rupture potential.
ACKNOWLEDGMENTS
The authors thank Stephany Santos for her technical assistance with data collection, Christine Vogel and Linda Caporale with histological data collection. We also knowledge the staffs, Laura Ondere, Mohammad Zafar and Adam Sang, at Yale University for helping us with collecting the tissues. This project was funded in part by supported in part by the NIH HL108239 and HL104080 grants. Caitlin Martin and Thuy Pham are supported by NIH NRSA pre-doctoral fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Elefteriades JA, Farkas EA. Thoracic Aortic Aneurysm. Clinically Pertinent Controversies and Uncertainties. Journal of the American College of Cardiology. 2010;55(9):841–857. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- [2].Humphrey JD, Taylor CA. Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu Rev Biomed Eng. 2008;10:221–246. doi: 10.1146/annurev.bioeng.10.061807.160439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vorp DA. Biomechanics of abdominal aortic aneurysm. Journal of Biomechanics. 2007;40(9):1887–1902. doi: 10.1016/j.jbiomech.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fedak PWM, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106(8):900–904. doi: 10.1161/01.cir.0000027905.26586.e8. [DOI] [PubMed] [Google Scholar]
- [5].Yap CH, Kim HS, Balachandran K, Weiler M, Haj-Ali R, Yoganathan AP. Dynamic deformation characteristics of porcine aortic valve leaflet under normal and hypertensive conditions. American Journal of Physiology - Heart and Circulatory Physiology. 2010;298(2):H395–H405. doi: 10.1152/ajpheart.00040.2009. [DOI] [PubMed] [Google Scholar]
- [6].Jackson V, Olsson T, Kurtovic S, Folkersen L, Paloschi V, Wågsäter D, Franco-Cereceda A, Eriksson P. Matrix metalloproteinase 14 and 19 expression is associated with thoracic aortic aneurysms. Journal of Thoracic and Cardiovascular Surgery. 2012;144(2):459–466. doi: 10.1016/j.jtcvs.2011.08.043. [DOI] [PubMed] [Google Scholar]
- [7].Mohamed SA, Aherrahrou Z, Liptau H, Erasmi AW, Hagemann C, Wrobel S, Borzym K, Schunkert H, Sievers HH, Erdmann J. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochemical and Biophysical Research Communications. 2006;345(4):1460–1465. doi: 10.1016/j.bbrc.2006.05.046. [DOI] [PubMed] [Google Scholar]
- [8].Rowe VL, Stevens SL, Reddick TT, Freeman MB, Donnell R, Carroll RC, Goldman MH. Vascular smooth muscle cell apoptosis in aneurysmal, occlusive, and normal human aortas. Journal of Vascular Surgery. 2000;31(3):567–576. [PubMed] [Google Scholar]
- [9].Hornick M, Mommiaie R, Mojibian H, Tranquilli M, Elefteriades JA. Bovine Arch - A Marker for Thoracic Aortic Aneurysm. 15th World Congress on Heart DiseaseVancouver; B.C., Canada. 2010. [Google Scholar]
- [10].Jakanani GC, Adair W. Frequency of variations in aortic arch anatomy depicted on multidetector CT. Clinical Radiology. 2010;65(6):481–487. doi: 10.1016/j.crad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- [11].Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Annals of Thoracic Surgery. 2003;75(4):1210–1214. doi: 10.1016/s0003-4975(02)04711-2. [DOI] [PubMed] [Google Scholar]
- [12].Okamoto RJ, Xu H, Kouchoukos NT, Moon MR, Sundt Iii TM. The influence of mechanical properties on wall stress and distensibility of the dilated ascending aorta. Journal of Thoracic and Cardiovascular Surgery. 2003;126(3):842–850. doi: 10.1016/s0022-5223(03)00728-1. [DOI] [PubMed] [Google Scholar]
- [13].Iliopoulos DC, Deveja RP, Kritharis EP, Perrea D, Sionis GD, Toutouzas K, Stefanadis C, Sokolis DP. Regional and directional variations in the mechanical properties of ascending thoracic aortic aneurysms. Medical Engineering and Physics. 2009;31(1):1–9. doi: 10.1016/j.medengphy.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [14].López-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. American Journal of Pathology. 1997;150(3):993–1007. [PMC free article] [PubMed] [Google Scholar]
- [15].Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(10 SUPPL):II329–II334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- [16].Mohamed SA, Noack F, Schoellermann K, Karluss A, Radtke A, Schult-Badusche D, Radke PW, Wenzel BE, Sievers HH. Elevation of matrix metalloproteinases in different areas of ascending aortic aneurysms in patients with bicuspid and tricuspid aortic valves. The Scientific World Journal. 2012;2012 doi: 10.1100/2012/806261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koullias GJ, Korkolis DP, Ravichandran P, Psyrri A, Hatzaras I, Elefteriades JA. Tissue microarray detection of matrix metalloproteinases, in diseased tricuspid and bicuspid aortic valves with or without pathology of the ascending aorta. European Journal of Cardio-thoracic Surgery. 2004;26(6):1098–1103. doi: 10.1016/j.ejcts.2004.07.050. [DOI] [PubMed] [Google Scholar]
- [18].Bia D, Pessana F, Armentano R, Perez H, Graf S, Zocalo Y, Saldıas M, Perez N, Alvarez O, Silva W, Machin D, Sueta P, Ferrin S, Acosta M, Alvarez I. Cryopreservation procedure does not modify human carotid homografts mechanical properties: an isobaric and dynamic analysis. Cell and Tissue Banking. 2006;7:183–194. doi: 10.1007/s10561-005-0655-0. [DOI] [PubMed] [Google Scholar]
- [19].Sacks MS. A method for planar biaxial mechanical testing that includes in-plane shear. J Biomech Eng. 1999;121(5):551–555. doi: 10.1115/1.2835086. [DOI] [PubMed] [Google Scholar]
- [20].Sun W, Sacks MS, Scott MJ. Effects of boundary conditions on the estimation of the planar biaxial mechanical properties of soft tissues. J Biomech Eng. 2005;127(4):709–715. doi: 10.1115/1.1933931. [DOI] [PubMed] [Google Scholar]
- [21].Vande Geest JP, Sacks MS, Vorp DA. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. Journal of Biomechanics. 2006;39(7):1324–1334. doi: 10.1016/j.jbiomech.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [22].Yuan SM, Jing H. Cystic medial necrosis: Pathological findings and clinical implications. Necrose cística da média: Manifestações patológicas com implicações clínicas. 2011;26(1):107–115. doi: 10.1590/s0102-76382011000100019. [DOI] [PubMed] [Google Scholar]
- [23].Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve. Pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119(6):880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- [24].Agozzino L, Santè P, Ferraraccio F, Accardo M, De Feo M, De Santo LS, Nappi G, Agozzino M, Esposito S. Ascending aorta dilatation in aortic valve disease: Morphological analysis of medial changes. Heart and Vessels. 2006;21(4):213–220. doi: 10.1007/s00380-005-0891-z. [DOI] [PubMed] [Google Scholar]
- [25].Schmid FX, Bielenberg K, Holmer S, Lehle K, Djavidani B, Prasser C, Wiesenack C, Birnbaum D. Structural and biomolecular changes in aorta and pulmonary trunk of patients with aortic aneurysm and valve disease: implications for the Ross procedure. Eur J Cardiothorac Surg. 2004;25(5):748–753. doi: 10.1016/j.ejcts.2004.02.028. [DOI] [PubMed] [Google Scholar]
- [26].De Sa M, Moshkovitz Y, Butany J, David TE, Robicsek F, Gardner TJ, Elkins RC, Gerosa G. Histologic abnormalities of the ascending aorta and pulmonary trunk in patients with bicuspid aortic valve disease: Clinical relevance to the Ross procedure. Journal of Thoracic and Cardiovascular Surgery. 1999;118(4):588–596. doi: 10.1016/S0022-5223(99)70002-4. [DOI] [PubMed] [Google Scholar]
- [27].Choudhury N, Bouchot O, Rouleau L, Tremblay D, Cartier R, Butany J, Mongrain R, Leask RL. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovascular Pathology. 2009;18(2):83–91. doi: 10.1016/j.carpath.2008.01.001. [DOI] [PubMed] [Google Scholar]
- [28].Okamoto RJ, Wagenseil JE, DeLong WR, Peterson SJ, Kouchoukos NT, Sundt Iii TM. Mechanical properties of dilated human ascending aorta. Annals of Biomedical Engineering. 2002;30(5):624–635. doi: 10.1114/1.1484220. [DOI] [PubMed] [Google Scholar]
- [29].García-Herrera CM, Atienza JM, Rojo FJ, Claes E, Guinea GV, Celentano DJ, García-Montero C, Burgos RL. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Medical and Biological Engineering and Computing. 2012:1–8. doi: 10.1007/s11517-012-0876-x. [DOI] [PubMed] [Google Scholar]
- [30].Iliopoulos DC, Kritharis EP, Giagini AT, Papadodima SA, Sokolis DP. Ascending thoracic aortic aneurysms are associated with compositional remodeling and vessel stiffening but not weakening in age-matched subjects. J Thorac Cardiovasc Surg. 2009;137(1):101–109. doi: 10.1016/j.jtcvs.2008.07.023. [DOI] [PubMed] [Google Scholar]
- [31].Duprey A, Khanafer K, Schlicht M, Avril S, Williams D, Berguer R. In Vitro Characterisation of Physiological and Maximum Elastic Modulus of Ascending Thoracic Aortic Aneurysms Using Uniaxial Tensile Testing. European Journal of Vascular and Endovascular Surgery. 2010;39(6):700–707. doi: 10.1016/j.ejvs.2010.02.015. [DOI] [PubMed] [Google Scholar]
- [32].Carmo M, Colombo L, Bruno A, Corsi FRM, Roncoroni L, Cuttin MS, Radice F, Mussini E, Settembrini PG. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery. 2002;23(6):543–549. doi: 10.1053/ejvs.2002.1620. [DOI] [PubMed] [Google Scholar]
- [33].Malone CD, Urbania TH, Crook SES, Hope MD. Bovine aortic arch: A novel association with thoracic aortic dilation. Clinical Radiology. 2012;67(1):28–31. doi: 10.1016/j.crad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- [34].O'Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension. 1990;15(4):339–347. doi: 10.1161/01.hyp.15.4.339. [DOI] [PubMed] [Google Scholar]
- [35].Deyl Z, Jelinek J, Macek K, Chaldakov G, Vankov VN. Collagen and elastin synthesis in the aorta of spontaneously hypertensive rats. Blood Vessels. 1987;24(6):313–320. doi: 10.1159/000158708. [DOI] [PubMed] [Google Scholar]
- [36].Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ.Res. 1970;26:507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- [37].Hu JJ, Baek S, Humphrey JD. Stress–strain behavior of the passive basilar artery in normotension and hypertension. Journal of Biomechanics. 2007;40:2559–2563. doi: 10.1016/j.jbiomech.2006.11.007. [DOI] [PubMed] [Google Scholar]
- [38].Matsumoto T, Hayashi K. Stress and strain distribution in hypertensive and normotensive rat aorta considering residual strain. J Biomech Eng. 1996;118(1):62–73. doi: 10.1115/1.2795947. [DOI] [PubMed] [Google Scholar]
- [39].Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. European Heart Journal. 1993;14(2):160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- [40].Antonini-Canterin F, Huang G, Cervesato E, Faggiano P, Pavan D, Piazza R, Nicolosi GL. Symptomatic aortic stenosis: Does systemic hypertension play an additional role? Hypertension. 2003;41(6):1268–1272. doi: 10.1161/01.HYP.0000070029.30058.59. [DOI] [PubMed] [Google Scholar]
- [41].Pasta S, Phillippi JA, Gleason TG, Vorp DA. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. Journal of Thoracic and Cardiovascular Surgery. 2012;143(2):460–467. doi: 10.1016/j.jtcvs.2011.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carson MW, Roach MR. The strength of the aortic media and its role in the propagation of aortic dissection. Journal of Biomechanics. 1990;23(6):579–588. doi: 10.1016/0021-9290(90)90050-d. [DOI] [PubMed] [Google Scholar]
- [43].Hirst AE, Jr., Johns VJ., Jr. Experimental dissection of media of aorta by pressure. Its relation to spontaneous dissecting aneurysm. Circ Res. 1962;10:897–903. doi: 10.1161/01.res.10.6.897. [DOI] [PubMed] [Google Scholar]